The Role of RhoH in TCR Signalling and Its Involvement in Diseases
Abstract
1. Introduction
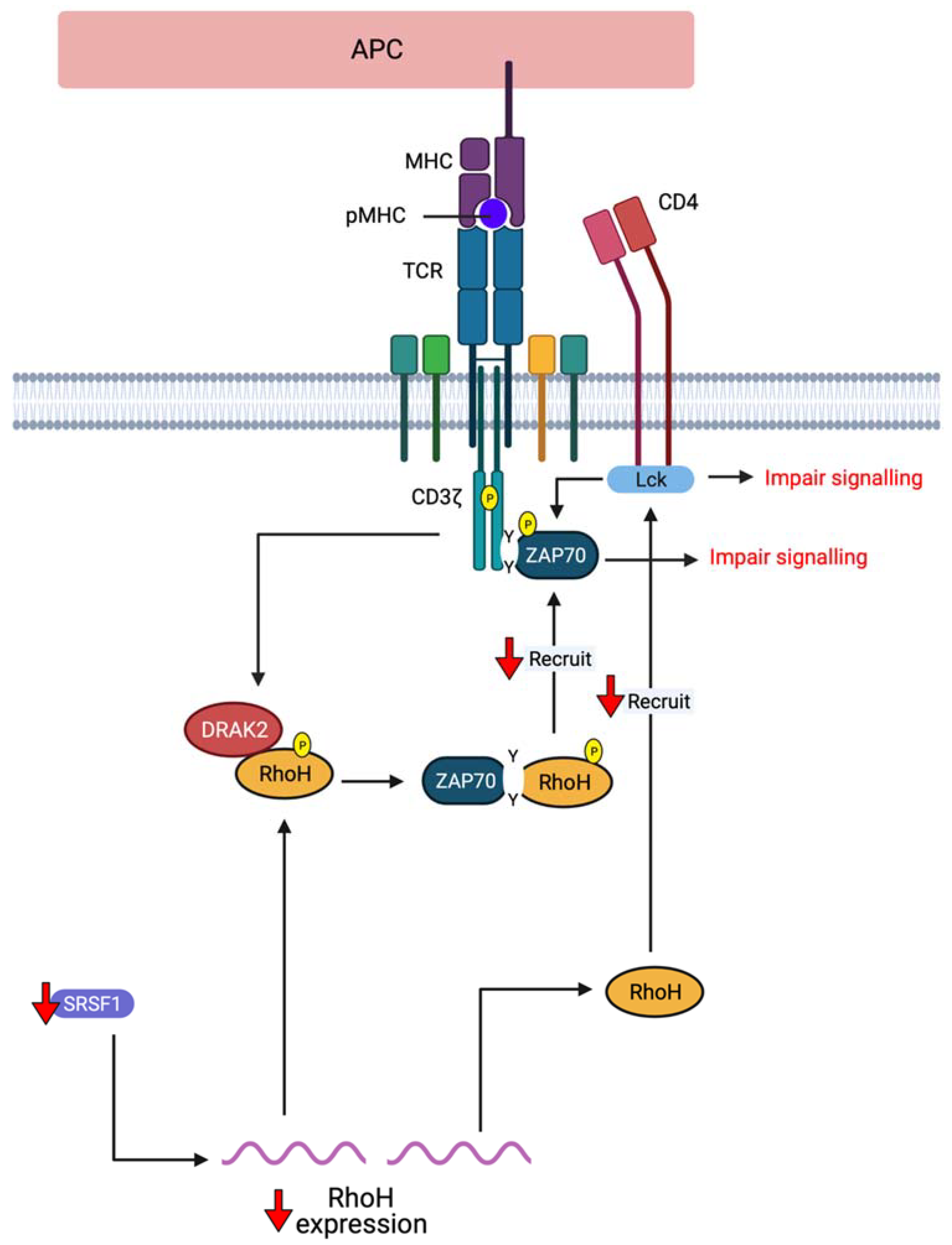
2. RhoH, An Atypical Rho Family Small GTPase
3. Deregulation of RhoH in Diseases
3.1. RhoH in B-Cell Malignancies
3.2. Immune-Related Diseases
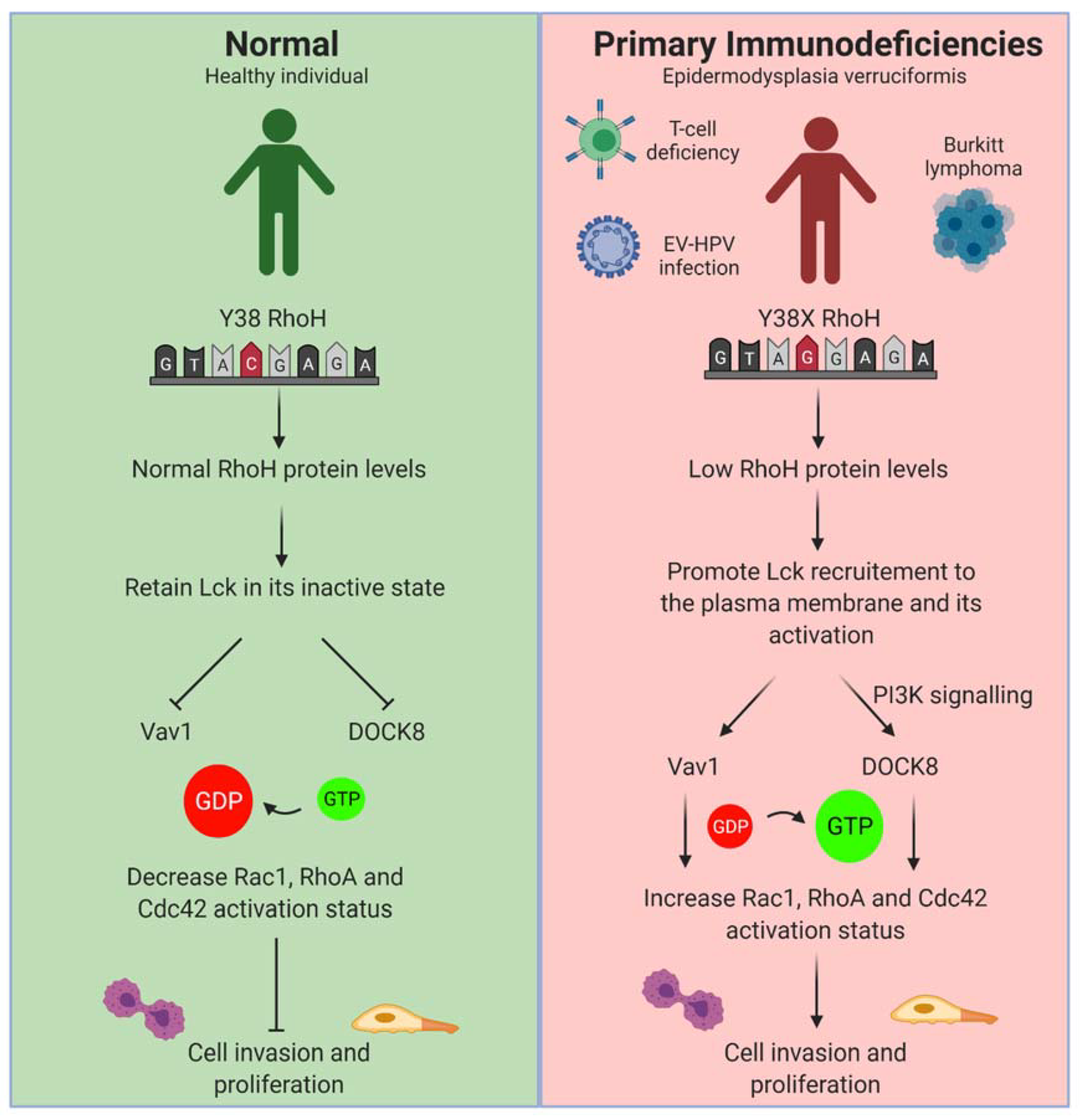
3.2.1. Primary Immunodeficiencies (PIDs)
3.2.2. Autoimmune-Related Diseases
4. RhoH as a Therapeutic Target
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TCR | T cell receptor |
| CMA | chaperone-mediated autophagy |
| LAT | linker of activated T cells |
| DLBCL | diffuse large B-cell lymphoma |
| aSHM | aberrant somatic hypermutation |
| AID | activation-induced deaminase |
| CLL | Chronic lymphocytic leukaemia |
| FL | follicular lymphoma |
| CSK | C-terminal Src kinase |
| HCL | hairy cell leukaemia |
| AML | acute myeloid leukaemia |
| PIDs | primary immunodeficiencies |
| SLE | systemic lupus erythematosus |
| BCR | B cell receptor |
| LCK | lymphocyte-specific protein tyrosine kinase |
| SRSF1 | serine/arginine-rich splicing factor 1 |
| GEF | guanine nucleotide exchange factors |
| GDI | Guanosine nucleotide dissociation inhibitor |
References
- Hodge, R.G.; Ridley, A.J. Regulating Rho GTPases and their regulators. Nat. Rev. Mol. Cell Biol. 2016, 17, 496–510. [Google Scholar] [CrossRef]
- Fransson, Å.; Ruusala, A.; Aspenström, P. Atypical Rho GTPases Have Roles in Mitochondrial Homeostasis and Apoptosis. J. Biol. Chem. 2003, 278, 6495–6502. [Google Scholar] [CrossRef] [PubMed]
- Boureux, A.; Vignal, E.; Faure, S.; Fort, P. Evolution of the Rho Family of Ras-Like GTPases in Eukaryotes. Mol. Biol. Evol. 2006, 24, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Aspenström, P.; Ruusala, A.; Pacholsky, D. Taking Rho GTPases to the next level: The cellular functions of atypical Rho GTPases. Exp. Cell Res. 2007, 313, 3673–3679. [Google Scholar] [CrossRef]
- Valencia, A.; Chardin, P.; Wittinghofer, A.; Sander, C. The ras protein family: Evolutionary tree and role of conserved amino acids. Biochemistry 1991, 30, 4637–4648. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, K.; Der, C.J. Rho-family GTPases: It’s not only Rac and Rho (and I like it). J. Cell Sci. 2004, 117, 1301–1312. [Google Scholar] [CrossRef]
- Citalán-Madrid, A.F.; García-Ponce, A.; Vargas-Robles, H.; Betanzos, A.; Schnoor, M. Small GTPases of the Ras superfamily regulate intestinal epithelial homeostasis and barrier function via common and unique mechanisms. Tissue Barriers 2013, 1, e26938. [Google Scholar] [CrossRef]
- Michaelson, D.; Silletti, J.; Murphy, G.; D’Eustachio, P.; Rush, M.; Philips, M.R. Differential localization of Rho GTPases in live cells: Regulation by hypervariable regions and RhoGDI binding. J. Cell Biol. 2001, 152, 111–126. [Google Scholar] [CrossRef]
- Choy, E.; Chiu, V.K.; Silletti, J.; Feoktistov, M.; Morimoto, T.; Michaelson, D.; Philips, M.R. Endomembrane Trafficking of Ras: The CAAX Motif Targets Proteins to the ER and Golgi. Cell 1999, 98, 69–80. [Google Scholar] [CrossRef]
- Adamson, P.; Paterson, H.F.; Hall, A. Intracellular localization of the P21rho proteins. J. Cell Biol. 1992, 119, 617–627. [Google Scholar] [CrossRef]
- Ridley, A.J. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006, 16, 522–529. [Google Scholar] [CrossRef]
- Roberts, P.J.; Mitin, N.; Keller, P.J.; Chenette, E.J.; Madigan, J.P.; Currin, R.O.; Der, C.J. Rho Family GTPase modification and dependence on CAAX motif-signaled posttranslational modification. J. Biol. Chem. 2008, 283, 25150–25163. [Google Scholar] [CrossRef] [PubMed]
- Aspenström, P. Fast-cycling Rho GTPases. Small Gtpases 2020, 11, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Dovas, A.; Couchman, J.R. RhoGDI: Multiple functions in the regulation of Rho family GTPase activities. Biochem. J. 2005, 390, 1–9. [Google Scholar] [CrossRef]
- Jaiswal, M.; Fansa, E.K.; Dvorský, R.; Ahmadian, M.R.; Communication, S.; Jaiswal, M.; Fansa, E.K.; Dvorsky, R.; Ahmadian, M.R. New insight into the molecular switch mechanism of human Rho family proteins: Shifting a paradigm. Biol. Chem. 2012, 394, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Shutes, A.; Berzat, A.C.; Cox, A.D.; Der, C.J. Atypical Mechanism of Regulation of the Wrch-1 Rho Family Small GTPase. Curr. Biol. 2004, 14, 2052–2056. [Google Scholar] [CrossRef] [PubMed]
- Shutes, A.; Berzat, A.C.; Chenette, E.J.; Cox, A.D.; Der, C.J.B.T.-M. Biochemical Analyses of the Wrch Atypical Rho Family GTPases. Regul. Eff. Small Gtpases Rho Fam. 2006, 406, 11–26. [Google Scholar]
- Lin, R.; Bagrodia, S.; Cerione, R.; Manor, D. A novel Cdc42Hs mutant induces cellular transformation. Curr. Biol. 1997, 7, 794–797. [Google Scholar] [CrossRef]
- Aspenström, P. Activated Rho GTPases in Cancer-The Beginning of a New Paradigm. Int. J. Mol. Sci. 2018, 19, 3949. [Google Scholar] [CrossRef]
- Prive, G.G.; Milburn, M.V.; Tong, L.; de Vos, A.M.; Yamaizumi, Z.; Nishimura, S.; Kim, S.H. X-ray crystal structures of transforming p21 ras mutants suggest a transition-state stabilization mechanism for GTP hydrolysis. Proc. Natl. Acad. Sci. USA 1992, 89, 3649–3653. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Maldonado, C.; Zimmer, Y.; Medová, M. A Comparative Analysis of Individual RAS Mutations in Cancer Biology. Front. Oncol. 2019, 9, 1088. [Google Scholar] [CrossRef]
- Fueller, F.; Kubatzky, K.F. The small GTPase RhoH is an atypical regulator of haematopoietic cells. Cell Commun. Signal. 2008, 6, 6. [Google Scholar] [CrossRef]
- Chae, H.-D.; Siefring, J.E.; Hildeman, D.A.; Gu, Y.; Williams, D.A. RhoH regulates subcellular localization of ZAP-70 and Lck in T cell receptor signaling. PLoS ONE 2010, 5, e13970. [Google Scholar] [CrossRef]
- Tamehiro, N.; Oda, H.; Shirai, M.; Suzuki, H. Overexpression of RhoH Permits to Bypass the Pre-TCR Checkpoint. PLoS ONE 2015, 10, e0131047. [Google Scholar] [CrossRef]
- Sunshine, H.; Iruela-Arispe, M.L. Membrane lipids and cell signaling. Curr. Opin. Lipidol. 2017, 28, 408–413. [Google Scholar] [CrossRef]
- Troeger, A.; Chae, H.-D.; Senturk, M.; Wood, J.; Williams, D.A. A Unique Carboxyl-terminal Insert Domain in the Hematopoietic-specific, GTPase-deficient Rho GTPase RhoH Regulates Post-translational Processing. J. Biol. Chem. 2013, 288, 36451–36462. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bu, X.; Lu, B.; Avraham, H.; Flavell, R.A.; Lim, B. The Hematopoiesis-Specific GTP-Binding Protein RhoH Is GTPase Deficient and Modulates Activities of Other Rho GTPases by an Inhibitory Function. Mol. Cell. Biol. 2002, 22, 1158–1171. [Google Scholar] [CrossRef]
- Horiguchi, H.; Ciuculescu, M.F.; Troeger, A.; Xu, H.; Brendel, C.; Williams, D.A. Deletion of Murine Rhoh induces More Aggressive Diffuse Large B Cell Lymphoma (DLBCL) Via Interaction with Kaiso and Regulation of BCL-6 Expression. Blood 2018, 132, 1574. [Google Scholar] [CrossRef]
- Gu, Y.; Chae, H.-D.; Siefring, J.E.; Jasti, A.C.; Hildeman, D.A.; Williams, D.A. RhoH GTPase recruits and activates Zap70 required for T cell receptor signaling and thymocyte development. Nat. Immunol. 2006, 7, 1182. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.A.J.; Vousden, K.H.; Norman, J.C. p53 and its mutants in tumor cell migration and invasion. J. Cell Biol. 2011, 192, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Tajadura-Ortega, V.; Garg, R.; Allen, R.; Owczarek, C.; Bright, M.D.; Kean, S.; Mohd-Noor, A.; Grigoriadis, A.; Elston, T.C.; Hahn, K.M.; et al. An RNAi screen of Rho signalling networks identifies RhoH as a regulator of Rac1 in prostate cancer cell migration. Bmc Biol. 2018, 16, 1–20. [Google Scholar] [CrossRef]
- Dorn, T.; Kuhn, U.; Bungartz, G.; Stiller, S.; Bauer, M.; Ellwart, J.; Peters, T.; Scharffetter-Kochanek, K.; Semmrich, M.; Laschinger, M.; et al. RhoH is important for positive thymocyte selection and T-cell receptor signaling. Blood 2007, 109, 2346–2355. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Aguilera, A.; Rattmann, I.; Drew, D.Z.; Müller, L.U.W.; Summey, V.; Lucas, D.M.; Byrd, J.C.; Croce, C.M.; Gu, Y.; Cancelas, J.A.; et al. Involvement of RhoH GTPase in the development of B-cell chronic lymphocytic leukemia. Leukemia 2010, 24, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Tybulewicz, V.L.J.; Henderson, R.B. Rho family GTPases and their regulators in lymphocytes. Nat. Rev. Immunol. 2009, 9, 630–644. [Google Scholar] [CrossRef] [PubMed]
- Kay, L. Characterisation of Atypical Human GTPases: Elucidation of Molecular Functions and Interactors. Ph.D. Thesis, Northumbria University, Newcastle, UK, 2016. [Google Scholar]
- Yablonski, D. Bridging the Gap: Modulatory Roles of the Grb2-Family Adaptor, Gads, in Cellular and Allergic Immune Responses. Front. Immunol. 2019, 10, 1704. [Google Scholar] [CrossRef]
- Wang, H.; Zeng, X.; Fan, Z.; Lim, B. RhoH modulates pre-TCR and TCR signalling by regulating LCK. Cell. Signal. 2011, 23, 249–258. [Google Scholar] [CrossRef]
- Preudhomme, C.; Roumier, C.; Hildebrand, M.P.; Dallery-Prudhomme, E.; Lantoine, D.; Laï, J.L.; Daudignon, A.; Adenis, C.; Bauters, F.; Fenaux, P.; et al. Nonrandom 4p13 rearrangements of the RhoH/TTF gene, encoding a GTP-binding protein, in non-Hodgkin’s lymphoma and multiple myeloma. Oncogene 2000, 19, 2023–2032. [Google Scholar] [CrossRef]
- Hiraga, J.; Katsumi, A.; Iwasaki, T.; Abe, A.; Kiyoi, H.; Matsushita, T.; Kinoshita, T.; Naoe, T. Prognostic analysis of aberrant somatic hypermutation of RhoH gene in diffuse large B cell lymphoma. Leukemia 2007, 21, 1846–1847. [Google Scholar] [CrossRef]
- Deutsch, A.J.A.; Frühwirth, M.; Aigelsreiter, A.; Cerroni, L.; Neumeister, P. Primary Cutaneous Marginal Zone B-Cell Lymphomas Are Targeted by Aberrant Somatic Hypermutation. J. Investig. Dermatol. 2009, 129, 476–479. [Google Scholar] [CrossRef]
- Voena, C.; Chiarle, R. RHO Family GTPases in the Biology of Lymphoma. Cells 2019, 8, 646. [Google Scholar] [CrossRef]
- Pasqualucci, L.; Neumeister, P.; Goossens, T.; Nanjangud, G.; Chaganti, R.S.K.; Küppers, R.; Dalla-Favera, R. Hypermutation of multiple proto-oncogenes in B-cell diffuse large-cell lymphomas. Nature 2001, 412, 341–346. [Google Scholar] [CrossRef]
- Reiniger, L.; Bödör, C.; Bognár, Á.; Balogh, Z.; Csomor, J.; Szepesi, Á.; Kopper, L.; Matolcsy, A. Richter’s and prolymphocytic transformation of chronic lymphocytic leukemia are associated with high mRNA expression of activation-induced cytidine deaminase and aberrant somatic hypermutation. Leukemia 2006, 20, 1089–1095. [Google Scholar] [CrossRef]
- Rossi, D.; Berra, E.; Cerri, M.; Deambrogi, C.; Barbieri, C.; Franceschetti, S.; Lunghi, M.; Conconi, A.; Paulli, M.; Matolcsy, A.; et al. Aberrant somatic hypermutation in transformation of follicular lymphoma and chronic lymphocytic leukemia to diffuse large B-cell lymphoma. Haematologica 2006, 91, 1405–1409. [Google Scholar]
- Cattoretti, G.; Pasqualucci, L.; Ballon, G.; Tam, W.; Nandula, S.V.; Shen, Q.; Mo, T.; Murty, V.V.; Dalla-Favera, R. Deregulated BCL6 expression recapitulates the pathogenesis of human diffuse large B cell lymphomas in mice. Cancer Cell 2005, 7, 445–455. [Google Scholar] [CrossRef]
- Gruber, T.A.; Chang, M.S.; Sposto, R.; Müschen, M. Activation-induced cytidine deaminase accelerates clonal evolution in BCR-ABL1-driven B-cell lineage acute lymphoblastic leukemia. Cancer Res. 2010, 70, 7411–7420. [Google Scholar] [CrossRef]
- Perona, R.; Montaner, S.; Saniger, L.; Sánchez-Pérez, I.; Bravo, R.; Lacal, J.C. Activation of the nuclear factor-kappaB by Rho, CDC42, and Rac-1 proteins. Genes Dev. 1997, 11, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Gastonguay, A.; Berg, T.; Hauser, A.D.; Schuld, N.; Lorimer, E.; Williams, C.L. The role of Rac1 in the regulation of NF-κB activity, cell proliferation, and cell migration in non-small cell lung carcinoma. Cancer Biol. Ther. 2012, 13, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Tergaonkar, V. Rho protein GTPases and their interactions with NFκB: Crossroads of inflammation and matrix biology. Biosci. Rep. 2014, 34, e00115. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Katsumi, A.; Kiyoi, H.; Tanizaki, R.; Ishikawa, Y.; Ozeki, K.; Kobayashi, M.; Abe, A.; Matsushita, T.; Watanabe, T.; et al. Prognostic implication and biological roles of RhoH in acute myeloid leukaemia. Eur. J. Haematol. 2008, 81, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-R.; Byeon, Y.; Kim, D.; Park, S.-G. Recent insights of T cell receptor-mediated signaling pathways for T cell activation and development. Exp. Mol. Med. 2020, 52, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Lambe, T.; Crawford, G.; Johnson, A.L.; Crockford, T.L.; Bouriez-Jones, T.; Smyth, A.M.; Pham, T.H.M.; Zhang, Q.; Freeman, A.F.; Cyster, J.G.; et al. DOCK8 is essential for T-cell survival and the maintenance of CD8+ T-cell memory. Eur. J. Immunol. 2011, 41, 3423–3435. [Google Scholar] [CrossRef]
- Rodríguez-Fdez, S.; Bustelo, X.R. The Vav GEF Family: An Evolutionary and Functional Perspective. Cells 2019, 8, 465. [Google Scholar] [CrossRef]
- Mino, A.; Troeger, A.; Brendel, C.; Cantor, A.; Harris, C.; Ciuculescu, M.F.; Williams, D.A. RhoH participates in a multi-protein complex with the zinc finger protein kaiso that regulates both cytoskeletal structures and chemokine-induced T cells. Small Gtpases 2018, 9, 260–273. [Google Scholar] [CrossRef]
- Antoni, A.; Ray, C.; Kohn, R.; Andreyko, D.; Levine, J. Analysis of the misregulation of RhoA and RhoH in autoimmune mice. (HUM7P.302). J. Immunol. 2014, 192. [Google Scholar]
- Stoeckle, C.; Geering, B.; Yousefi, S.; Rožman, S.; Andina, N.; Benarafa, C.; Simon, H.-U. RhoH is a negative regulator of eosinophilopoiesis. Cell Death Differ. 2016, 23, 1961–1972. [Google Scholar] [CrossRef] [PubMed]
- Salloum, G.; Jaafar, L.; El-Sibai, M. Rho A and Rac1: Antagonists moving forward. Tissue Cell 2020, 65, 101364. [Google Scholar] [CrossRef]
- Galiègue-Zouitina, S.; Delestré, L.; Dupont, C.; Troussard, X.; Shelley, C.S. Underexpression of RhoH in Hairy Cell Leukemia. Cancer Res. 2008, 68, 4531–4540. [Google Scholar] [CrossRef] [PubMed]
- Troeger, A.; Johnson, A.J.; Wood, J.; Blum, W.G.; Andritsos, L.A.; Byrd, J.C.; Williams, D.A. RhoH is critical for cell-microenvironment interactions in chronic lymphocytic leukemia in mice and humans. Blood 2012, 119, 4708–4718. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Mende, J.; Geering, B.; Yousefi, S.; Simon, H.-U. Lysosomal degradation of RhoH protein upon antigen receptor activation in T but not B cells. Eur. J. Immunol. 2010, 40, 525–529. [Google Scholar] [CrossRef]
- Delestré, L.; Berthon, C.; Quesnel, B.; Figeac, M.; Kerckaert, J.-P.; Galiègue-Zouitina, S.; Shelley, C.S. Repression of the RHOH gene by JunD. Biochem. J. 2011, 437, 75–88. [Google Scholar] [CrossRef]
- Gazon, H.; Lemasson, I.; Polakowski, N.; Césaire, R.; Matsuoka, M.; Barbeau, B.; Mesnard, J.-M.; Peloponese, J.-M. Human T-Cell Leukemia Virus Type 1 (HTLV-1) bZIP Factor Requires Cellular Transcription Factor JunD To Upregulate HTLV-1 Antisense Transcription from the 3′ Long Terminal Repeat. J. Virol. 2012, 86, 9070–9078. [Google Scholar] [CrossRef] [PubMed]
- Galiègue-Zouitina, S.; Fu, Q.; Carton-Latreche, C.; Poret, N.; Cheok, M.; Leprêtre, F.; Figeac, M.; Quesnel, B.; El Bouazzati, H.; Shelley, C.S. Bimodal expression of RHOH during myelomonocytic differentiation: Implications for the expansion of AML differentiation therapy. EJHaem 2021. [Google Scholar] [CrossRef]
- Nicolaou, F.; Teodoridis, J.M.; Park, H.; Georgakis, A.; Farokhzad, O.C.; Böttinger, E.P.; Da Silva, N.; Rousselot, P.; Chomienne, C.; Ferenczi, K.; et al. CD11c gene expression in hairy cell leukemia is dependent upon activation of the proto-oncogenes ras andjunD. Blood 2003, 101, 4033–4041. [Google Scholar] [CrossRef]
- Umit, E.G.; Baysal, M.; Durmus, Y.; Demir, A.M. CD11c expression in chronic lymphocytic leukemia revisited, related with complications and survival. Int. J. Lab. Hematol. 2017, 39, 552–556. [Google Scholar] [CrossRef]
- Park, H.; Shelley, C.S.; Arnaout, M.A. The zinc finger transcription factor ZBP-89 is a repressor of the human β2-integrin CD11b gene. Blood 2003, 101, 894–902. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pilling, D.; Fan, T.; Huang, D.; Kaul, B.; Gomer, R.H. Identification of Markers that Distinguish Monocyte-Derived Fibrocytes from Monocytes, Macrophages, and Fibroblasts. PLoS ONE 2009, 4, e7475. [Google Scholar] [CrossRef]
- Wagner, M.; Oelsner, M.; Moore, A.; Götte, F.; Kuhn, P.-H.; Haferlach, T.; Fiegl, M.; Bogner, C.; Baxter, E.J.; Peschel, C.; et al. Integration of innate into adaptive immune responses in ZAP-70–positive chronic lymphocytic leukemia. Blood 2016, 127, 436–448. [Google Scholar] [CrossRef]
- Burger, J.A.; Chiorazzi, N. B cell receptor signaling in chronic lymphocytic leukemia. Trends Immunol. 2013, 34, 592–601. [Google Scholar] [CrossRef]
- Kohlhaas, V.; Blakemore, S.J.; Al-Maarri, M.; Nickel, N.; Pal, M.; Roth, A.; Hövelmeyer, N.; Schäfer, S.C.; Knittel, G.; Lohneis, P.; et al. Active Akt signaling triggers CLL toward Richter transformation via overactivation of Notch1. Blood 2021, 137, 646–660. [Google Scholar] [CrossRef]
- Bousfiha, A.; Jeddane, L.; Picard, C.; Al-Herz, W.; Ailal, F.; Chatila, T.; Cunningham-Rundles, C.; Etzioni, A.; Franco, J.L.; Holland, S.M.; et al. Human Inborn Errors of Immunity: 2019 Update of the IUIS Phenotypical Classification. J. Clin. Immunol. 2020, 40, 66–81. [Google Scholar] [CrossRef]
- Crequer, A.; Troeger, A.; Patin, E.; Ma, C.S.; Picard, C.; Pedergnana, V.; Fieschi, C.; Lim, A.; Abhyankar, A.; Gineau, L.; et al. Human RHOH deficiency causes T cell defects and susceptibility to EV-HPV infections. J. Clin. Investig. 2012, 122, 3239–3247. [Google Scholar] [CrossRef]
- Gaidano, G.; Pasqualucci, L.; Capello, D.; Berra, E.; Deambrogi, C.; Rossi, D.; Larocca, L.M.; Gloghini, A.; Carbone, A.; Dalla-Favera, R. Aberrant somatic hypermutation in multiple subtypes of AIDS-associated non-Hodgkin lymphoma. Blood 2003, 102, 1833–1841. [Google Scholar] [CrossRef] [PubMed]
- Vakiani, E.; Basso, K.; Klein, U.; Mansukhani, M.M.; Narayan, G.; Smith, P.M.; Murty, V.V.; Dalla-Favera, R.; Pasqualucci, L.; Bhagat, G. Genetic and phenotypic analysis of B-cell post-transplant lymphoproliferative disorders provides insights into disease biology. Hematol. Oncol. 2008, 26, 199–211. [Google Scholar] [CrossRef]
- Finn, O.J. Immuno-oncology: Understanding the function and dysfunction of the immune system in cancer. Ann. Oncol. 2012, 23, viii6–viii9. [Google Scholar] [CrossRef] [PubMed]
- Satgé, D. A Tumor Profile in Primary Immune Deficiencies Challenges the Cancer Immune Surveillance Concept. Front. Immunol. 2018, 9, 1149. [Google Scholar] [CrossRef] [PubMed]
- Swann, J.B.; Smyth, M.J. Immune surveillance of tumors. J. Clin. Investig. 2007, 117, 1137–1146. [Google Scholar] [CrossRef]
- Chae, H.-D.; Lee, K.E.; Williams, D.A.; Gu, Y. Cross-talk between RhoH and Rac1 in regulation of actin cytoskeleton and chemotaxis of hematopoietic progenitor cells. Blood 2008, 111, 2597–2605. [Google Scholar] [CrossRef]
- Troeger, A.; Williams, D.A. Hematopoietic-specific Rho GTPases Rac2 and RhoH and human blood disorders. Exp. Cell Res. 2013, 319, 2375–2383. [Google Scholar] [CrossRef]
- Shaverdashvili, K.; Padlo, J.; Weinblatt, D.; Jia, Y.; Jiang, W.; Rao, D.; Laczkó, D.; Whelan, K.A.; Lynch, J.P.; Muir, A.B.; et al. KLF4 activates NFκB signaling and esophageal epithelial inflammation via the Rho-related GTP-binding protein RHOF. PLoS ONE 2019, 14, e0215746. [Google Scholar] [CrossRef]
- Itan, Y.; Casanova, J.-L. Novel Primary Immunodeficiency Candidate Genes Predicted by the Human Gene Connectome. Front. Immunol. 2015, 6, 142. [Google Scholar] [CrossRef]
- Côté, J.-F.; Vuori, K. GEF what? Dock180 and related proteins help Rac to polarize cells in new ways. Trends Cell Biol. 2007, 17, 383–393. [Google Scholar] [CrossRef]
- Han, J.; Das, B.; Wei, W.; Van Aelst, L.; Mosteller, R.D.; Khosravi-Far, R.; Westwick, J.K.; Der, C.J.; Broek, D. Lck regulates Vav activation of members of the Rho family of GTPases. Mol. Cell. Biol. 1997, 17, 1346–1353. [Google Scholar] [CrossRef]
- Cho, H.J.; Kim, J.-T.; Baek, K.E.; Kim, B.-Y.; Lee, H.G. Regulation of Rho GTPases by RhoGDIs in Human Cancers. Cells 2019, 8, 1037. [Google Scholar] [CrossRef]
- Ahmad Mokhtar, A.M. Investigating the Functional Interaction between RhoGDI Family Proteins and Activated Cdc42 Associated-Kinase (ACK). Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2020. [Google Scholar]
- Tamehiro, N.; Nishida, K.; Sugita, Y.; Hayakawa, K.; Oda, H.; Nitta, T.; Nakano, M.; Nishioka, A.; Yanobu-Takanashi, R.; Goto, M.; et al. Ras homolog gene family H (RhoH) deficiency induces psoriasis-like chronic dermatitis by promoting TH17 cell polarization. J. Allergy Clin. Immunol. 2019, 143, 1878–1891. [Google Scholar] [CrossRef]
- Lowes, M.A.; Suárez-Fariñas, M.; Krueger, J.G. Immunology of Psoriasis. Annu. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef]
- Oda, H.; Tamehiro, N.; Patrick, M.S.; Hayakawa, K.; Suzuki, H. Differential requirement for RhoH in development of TCRαβ CD8αα IELs and other types of T cells. Immunol. Lett. 2013, 151, 1–9. [Google Scholar] [CrossRef]
- Timlin, H.; Syed, A.; Haque, U.; Adler, B.; Law, G.; Machireddy, K.; Manno, R. Fevers in Adult Lupus Patients. Cureus 2018, 10, e2098. [Google Scholar]
- Anaya, J.-M. Common mechanisms of autoimmune diseases (the autoimmune tautology). Autoimmun. Rev. 2012, 11, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Katsuyama, T.; Li, H.; Krishfield, S.M.; Kyttaris, V.C.; Moulton, V.R. Splicing factor SRSF1 limits IFN-γ production via RhoH and ameliorates experimental nephritis. Rheumatology 2021, 60, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.L.; Guoliu, R.N.; Qin, H.; Shen, Y.Y.; Wang, B.; Zhai, Z.M. Elevated plasma levels of IL-12 and IFN-γ in systemic lupus erythematosus. Int. J. Clin. Exp. Pathol. 2017, 10, 3286–3291. [Google Scholar]
- Mojic, M.; Takeda, K.; Hayakawa, Y. The Dark Side of IFN-γ: Its Role in Promoting Cancer Immunoevasion. Int. J. Mol. Sci. 2017, 19, 89. [Google Scholar] [CrossRef] [PubMed]
- Harigai, M.; Kawamoto, M.; Hara, M.; Kubota, T.; Kamatani, N.; Miyasaka, N. Excessive Production of IFN-γ in Patients with Systemic Lupus Erythematosus and Its Contribution to Induction of B Lymphocyte Stimulator/B Cell-Activating Factor/TNF Ligand Superfamily-13B. J. Immunol. 2008, 181, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Lowin, T.; Anssar, T.M.; Bäuml, M.; Classen, T.; Schneider, M.; Pongratz, G. Positive and negative cooperativity of TNF and Interferon-γ in regulating synovial fibroblast function and B cell survival in fibroblast/B cell co-cultures. Sci. Rep. 2020, 10, 780. [Google Scholar] [CrossRef]
- Morimoto, S.; Nakano, S.; Watanabe, T.; Tamayama, Y.; Mitsuo, A.; Nakiri, Y.; Suzuki, J.; Nozawa, K.; Amano, H.; Tokano, Y.; et al. Expression of B-cell activating factor of the tumour necrosis factor family (BAFF) in T cells in active systemic lupus erythematosus: The role of BAFF in T cell-dependent B cell pathogenic autoantibody production. Rheumatology 2007, 46, 1083–1086. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-J.; Jiang, W.-Q.; Rao, H.-L.; Huang, J.-J.; Xia, Y.; Huang, H.-Q.; Lin, T.-Y.; Xia, Z.-J.; Li, S.; Li, Z.-M. Expression of BAFF and BAFF-R in Follicular Lymphoma: Correlation with Clinicopathologic Characteristics and Survival Outcomes. PLoS ONE 2012, 7, e50936. [Google Scholar] [CrossRef]
- Yang, S.; Li, J.-Y.; Xu, W. Role of BAFF/BAFF-R axis in B-cell non-Hodgkin lymphoma. Crit. Rev. Oncol. Hematol. 2014, 91, 113–122. [Google Scholar] [CrossRef]
- Vásquez, A.; Baena, A.; González, L.A.; Restrepo, M.; Muñoz, C.H.; Vanegas-García, A.; Ortiz-Reyes, B.; Abdoel, N.; Rojas, M.; García, L.F.; et al. Altered recruitment of Lyn, Syk and ZAP-70 into lipid rafts of activated B cells in Systemic Lupus Erythematosus. Cell. Signal. 2019, 58, 9–19. [Google Scholar] [CrossRef]
- Jury, E.C.; Kabouridis, P.S.; Abba, A.; Mageed, R.A.; Isenberg, D.A. Increased ubiquitination and reduced expression of LCK in T lymphocytes from patients with systemic lupus erythematosus. Arthritis Rheum. 2003, 48, 1343–1354. [Google Scholar] [CrossRef]
- Moulton, V.R.; Tsokos, G.C. T cell signaling abnormalities contribute to aberrant immune cell function and autoimmunity. J. Clin. Investig. 2015, 125, 2220–2227. [Google Scholar] [CrossRef]
- Xavier, R.; Brennan, T.; Li, Q.; McCormack, C.; Seed, B. Membrane Compartmentation Is Required for Efficient T Cell Activation. Immunity 1998, 8, 723–732. [Google Scholar] [CrossRef]
- Packard, T.A.; Cambier, J.C. B lymphocyte antigen receptor signaling: Initiation, amplification, and regulation. F1000prime Rep. 2013, 5, 40. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, W.; Xiong, S. Blockade of Notch1 signaling alleviates murine lupus via blunting macrophage activation and M2b polarization. J. Immunol. 2010, 184, 6465–6478. [Google Scholar] [CrossRef]
- Kang, J.-A.; Kim, W.-S.; Park, S.-G. Notch1 is an important mediator for enhancing of B-cell activation and antibody secretion by Notch ligand. Immunology 2014, 143, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Calamito, M.; Srivastava, B.; Maillard, I.; Pear, W.S.; Allman, D. Notch activity synergizes with B-cell-receptor and CD40 signaling to enhance B-cell activation. Blood 2007, 109, 3342–3350. [Google Scholar] [CrossRef] [PubMed]
- Karrar, S.; Cunninghame Graham, D.S. Abnormal B Cell Development in Systemic Lupus Erythematosus: What the Genetics Tell Us. Arthritis Rheumatol. 2018, 70, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Sadras, T.; Cutler, J.; Aguade-Gorgorio, J.; Chen, Z.; Cosgun, K.N.; Pandey, A.; Muschen, M. Cooperation between SYK and ZAP70 Kinases As a Driver of Oncogenic BCR-Signaling in B-Cell Malignancies. Blood 2018, 132, 3922. [Google Scholar] [CrossRef]
- Chen, J.; Moore, A.; Ringshausen, I. ZAP-70 Shapes the Immune Microenvironment in B Cell Malignancies. Front. Oncol. 2020, 10, 2188. [Google Scholar] [CrossRef]
- Pollyea, D.; Gore, L.; Gutman, J.; Eckhardt, S.G.; Hagelstrom, N.; Coutre, S.; Thirman, M.; Byrd, J. A Dose Escalation Study of Ibrutinib with Lenalidomide for Relapsed and Refractory Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma. Ann. Oncol. 2013, 24, i33. [Google Scholar] [CrossRef]
- Robert, G.; Jacquel, A.; Auberger, P. Chaperone-Mediated Autophagy and Its Emerging Role in Hematological Malignancies. Cells 2019, 8, 1260. [Google Scholar] [CrossRef]
- Anguiano, J.; Garner, T.P.; Mahalingam, M.; Das, B.C.; Gavathiotis, E.; Cuervo, A.M. Chemical modulation of chaperone-mediated autophagy by retinoic acid derivatives. Nat. Chem. Biol. 2013, 9, 374–382. [Google Scholar] [CrossRef]
- Visperas, P.R.; Wilson, C.G.; Winger, J.A.; Yan, Q.; Lin, K.; Arkin, M.R.; Weiss, A.; Kuriyan, J. Identification of Inhibitors of the Association of ZAP-70 with the T Cell Receptor by High-Throughput Screen. SLAS Discov. Adv. Life Sci. R D 2017, 22, 324–331. [Google Scholar] [CrossRef]
- Boohaker, R.J.; Lee, M.W.; Vishnubhotla, P.; Perez, J.M.; Khaled, A.R. The use of therapeutic peptides to target and to kill cancer cells. Curr. Med. Chem. 2012, 19, 3794–3804. [Google Scholar] [CrossRef] [PubMed]
- Cooper, B.M.; Iegre, J.; O’ Donovan, D.H.; Ölwegård Halvarsson, M.; Spring, D.R. Peptides as a platform for targeted therapeutics for cancer: Peptide–drug conjugates (PDCs). Chem. Soc. Rev. 2021, 50, 1480–1494. [Google Scholar] [CrossRef] [PubMed]
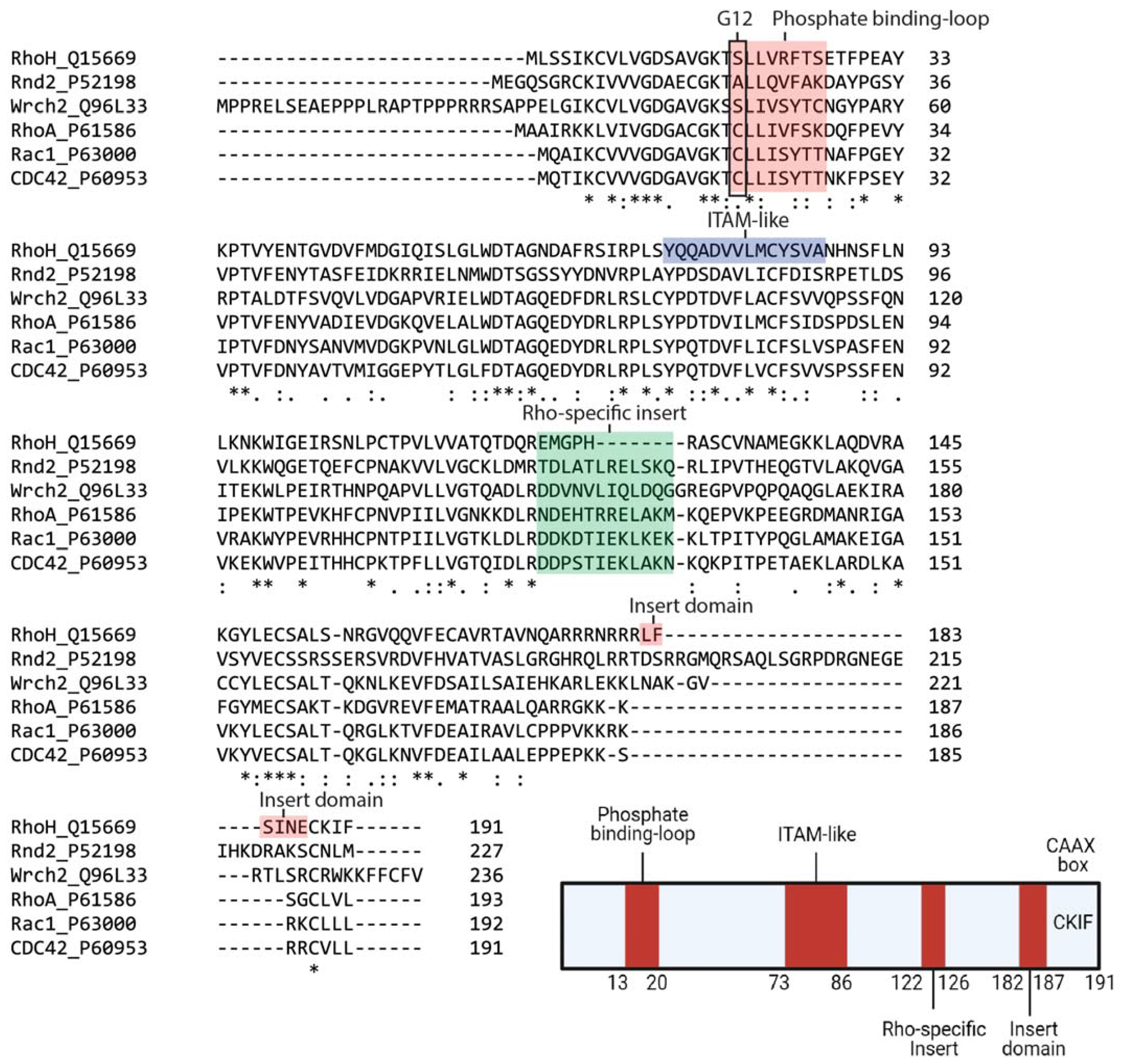

| Rho BTB1 | Rho BTB2 | RhoH | Rnd1 | Rnd2 | Rnd3 | RhoD | RhoF | RhoA | RhoC | RhoB | Wrch2 | Wrch1 | TC10 | TCL | Cdc42 | RhoG | Rac2 | Rac1 | Rac3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RhoBTB1 | 70 | 34 | 33 | 31 | 30 | 34 | 28 | 38 | 37 | 38 | 34 | 32 | 38 | 35 | 40 | 40 | 41 | 42 | 41 | |
| RhoBTB2 | 70 | 32 | 32 | 31 | 29 | 34 | 28 | 39 | 37 | 38 | 33 | 32 | 39 | 37 | 40 | 40 | 42 | 42 | 41 | |
| RhoH | 34 | 32 | 29 | 32 | 36 | 38 | 33 | 40 | 40 | 41 | 41 | 38 | 40 | 39 | 42 | 40 | 40 | 41 | 40 | |
| Rnd1 | 32 | 32 | 29 | 53 | 61 | 37 | 39 | 41 | 42 | 41 | 31 | 32 | 36 | 34 | 37 | 37 | 39 | 39 | 38 | |
| Rnd2 | 31 | 31 | 32 | 53 | 63 | 39 | 41 | 46 | 47 | 43 | 28 | 31 | 36 | 35 | 37 | 41 | 40 | 41 | 39 | |
| Rnd3 | 29 | 29 | 36 | 61 | 63 | 37 | 40 | 48 | 48 | 47 | 31 | 32 | 39 | 35 | 38 | 41 | 39 | 42 | 40 | |
| RhoD | 34 | 35 | 38 | 37 | 39 | 37 | 49 | 49 | 49 | 49 | 39 | 36 | 42 | 38 | 43 | 44 | 46 | 49 | 49 | |
| RhoF | 28 | 28 | 33 | 39 | 41 | 40 | 49 | 47 | 48 | 47 | 36 | 37 | 46 | 43 | 43 | 46 | 50 | 59 | 47 | |
| RhoA | 38 | 39 | 40 | 41 | 46 | 48 | 49 | 47 | 92 | 85 | 40 | 44 | 51 | 48 | 53 | 55 | 53 | 57 | 55 | |
| RhoC | 37 | 37 | 40 | 42 | 47 | 48 | 49 | 48 | 92 | 85 | 40 | 44 | 50 | 49 | 51 | 55 | 53 | 57 | 54 | |
| RhoB | 38 | 38 | 41 | 41 | 43 | 47 | 49 | 47 | 85 | 85 | 42 | 45 | 51 | 48 | 50 | 53 | 54 | 55 | 54 | |
| Wrch2 | 34 | 33 | 41 | 31 | 28 | 31 | 39 | 36 | 40 | 40 | 42 | 59 | 51 | 48 | 53 | 46 | 51 | 52 | 53 | |
| Wrch1 | 32 | 32 | 37 | 32 | 31 | 32 | 36 | 37 | 44 | 44 | 45 | 59 | 50 | 46 | 56 | 48 | 54 | 54 | 54 | |
| TC10 | 38 | 39 | 40 | 36 | 36 | 39 | 42 | 46 | 51 | 50 | 51 | 51 | 50 | 76 | 66 | 54 | 60 | 62 | 61 | |
| TCL | 35 | 37 | 39 | 34 | 35 | 35 | 38 | 44 | 48 | 49 | 48 | 48 | 46 | 76 | 63 | 53 | 58 | 60 | 59 | |
| Cdc42 | 40 | 40 | 42 | 37 | 37 | 38 | 43 | 43 | 53 | 51 | 50 | 53 | 56 | 66 | 63 | 61 | 69 | 71 | 70 | |
| RhoG | 39 | 40 | 40 | 37 | 41 | 41 | 44 | 46 | 55 | 55 | 53 | 46 | 48 | 54 | 53 | 60 | 72 | 72 | 70 | |
| Rac2 | 41 | 42 | 40 | 39 | 40 | 39 | 46 | 50 | 53 | 53 | 54 | 50 | 54 | 60 | 58 | 69 | 72 | 92 | 89 | |
| Rac1 | 42 | 42 | 41 | 39 | 41 | 42 | 49 | 49 | 57 | 57 | 55 | 52 | 54 | 62 | 60 | 71 | 72 | 92 | 93 | |
| Rac3 | 41 | 41 | 40 | 38 | 39 | 40 | 49 | 47 | 55 | 54 | 54 | 53 | 54 | 61 | 59 | 70 | 70 | 89 | 93 |
| Group | Rho Protein | C-Terminal Sequence | Lipid Modification | Ref |
|---|---|---|---|---|
| Typical | RhoA | KDGVREVFEMATRAALQARRGKKKSGCLVL | GG | [11] |
| RhoB | VREVFETATRAALQKRYGSQNGCINCCKVL | GG, F, P | ||
| RhoC | KEGVREVFEMATRAGLQVRKNKRRRGCPIL | GG | ||
| Rac1 | RGLKTVFDEAIRAVLCPPPVKKRKRKCLLL | GG, P | ||
| Rac2 | RGLKTVFDEAIRAVLCPQPTRQQKRACSLL | GG | ||
| Rac3 | RGLKTVFDEAIRAVLCPPPVKKPGKKCTVF | GG | ||
| RhoG | QDGVKEVFAEAVRAVLNPTPIKRGRSCILL | GG | ||
| Cdc42 | QKGLKNVFDEAILAALEPPEPKKSRRCVLL | GG | ||
| TCL | AVFDEAILTIFHPKKKKKRCSEGHSCCSII | F | ||
| TC10 | DEAIIAILTPKKHTVKKRIGSRCINCCLIT | F, P | ||
| Atypical | RhoU | QQQPKKSKSRTPDKMKNLSKSWWKKYCCFV | P | [11] |
| RhoV | EHKARLEKKLNAKGVRTLSRCRWKKFFCFV | P | ||
| RhoD | AVFQEAAEVALSSRGRNFWRRITQGFCVVT | F, GG | [12] | |
| RhoF | EDVFREAAKVALSALKKAQRQKKRRLCLLL | F, GG | ||
| Rnd1/ RhoS | LSKRLLHLPSRSELISSTFKKEKAKSCSIM | F | [11] | |
| Rnd2/ RhoN | MQRSAQLSGRPDRGNEGEIHKDRAKSCNLM | F | ||
| Rnd3/ RhoE | KRISHMPSRPELSAVATDLRKDKAKSCTVM | F | ||
| RhoH/ TTF | VFECAVRTAVNQARRRNRRRLFSINECKIF | GG, F | [11,12] | |
| RhoBTB1 | KREREKEDIALNKHRSRRKWCFWNSSPAVA | Unknown | N/A | |
| RhoBTB2 | KRRWLFWNSPSSPSSSAASSSSPSSSSAVV | Unknown |
| Amino Acids 12, 59 and 61 | |||
|---|---|---|---|
| Group | Subfamily | Member | Sequence |
| Classic | Cdc42 | Cdc42 | 12 59 61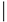
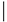
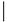 |
| GDGAV---AGQED | |||
| Fast-cycling | RhoU/RhoV | RhoU | GDGAV---AGQED |
| RhoV | GDGAV---AGQDE | ||
| RhoD/RhoF | RhoD | GDGGC---AGQDD | |
| RhoF | GDGGC---AGQED | ||
| GTPase defective | RhoBTB | RhoBTB−1 | GDNAV---FGDHH |
| RhoBTB−2 | GDNAV---FGDHH | ||
| Rnd | Rnd1 | GDVQC---SGSPY | |
| Rnd2 | GDAEC---SGSSY | ||
| Rnd3 | GDVQC---SGSPY | ||
| RhoH | GDSAV---AGNDA | ||
| Amino acids 28 | |||
| Classic | Rac | Rac1 | 28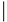 |
| SYTTNAFPGEYIP | |||
| Fast-cycling | RhoU/RhoV | RhoU | SYTTNGYPTEYIP |
| RhoV | SYTCNGYPARYRP | ||
| RhoD/RhoF | RhoD | VFADGAFPESYTP | |
| RhoF | VYSQGSFPEHYAP | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad Mokhtar, A.M.; Hashim, I.F.; Mohd Zaini Makhtar, M.; Salikin, N.H.; Amin-Nordin, S. The Role of RhoH in TCR Signalling and Its Involvement in Diseases. Cells 2021, 10, 950. https://doi.org/10.3390/cells10040950
Ahmad Mokhtar AM, Hashim IF, Mohd Zaini Makhtar M, Salikin NH, Amin-Nordin S. The Role of RhoH in TCR Signalling and Its Involvement in Diseases. Cells. 2021; 10(4):950. https://doi.org/10.3390/cells10040950
Chicago/Turabian StyleAhmad Mokhtar, Ana Masara, Ilie Fadzilah Hashim, Muaz Mohd Zaini Makhtar, Nor Hawani Salikin, and Syafinaz Amin-Nordin. 2021. "The Role of RhoH in TCR Signalling and Its Involvement in Diseases" Cells 10, no. 4: 950. https://doi.org/10.3390/cells10040950
APA StyleAhmad Mokhtar, A. M., Hashim, I. F., Mohd Zaini Makhtar, M., Salikin, N. H., & Amin-Nordin, S. (2021). The Role of RhoH in TCR Signalling and Its Involvement in Diseases. Cells, 10(4), 950. https://doi.org/10.3390/cells10040950







