Anticancer Effects of Sublingual Type I IFN in Combination with Chemotherapy in Implantable and Spontaneous Tumor Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Reagents
2.2. Mice
2.3. Genetic Screening of the NeuT Mouse Colony and Monitoring of Tumors Onset
2.4. Treatment Protocol
2.5. Primary Cells
2.6. IFN-γ ELISpot
2.7. Flow Cytometry
2.8. Intracellular Staining
2.9. mRNA Extraction and Real Time PCR
2.10. Magnetic Resonance Imaging
2.11. Histology
2.12. Statistical Analysis
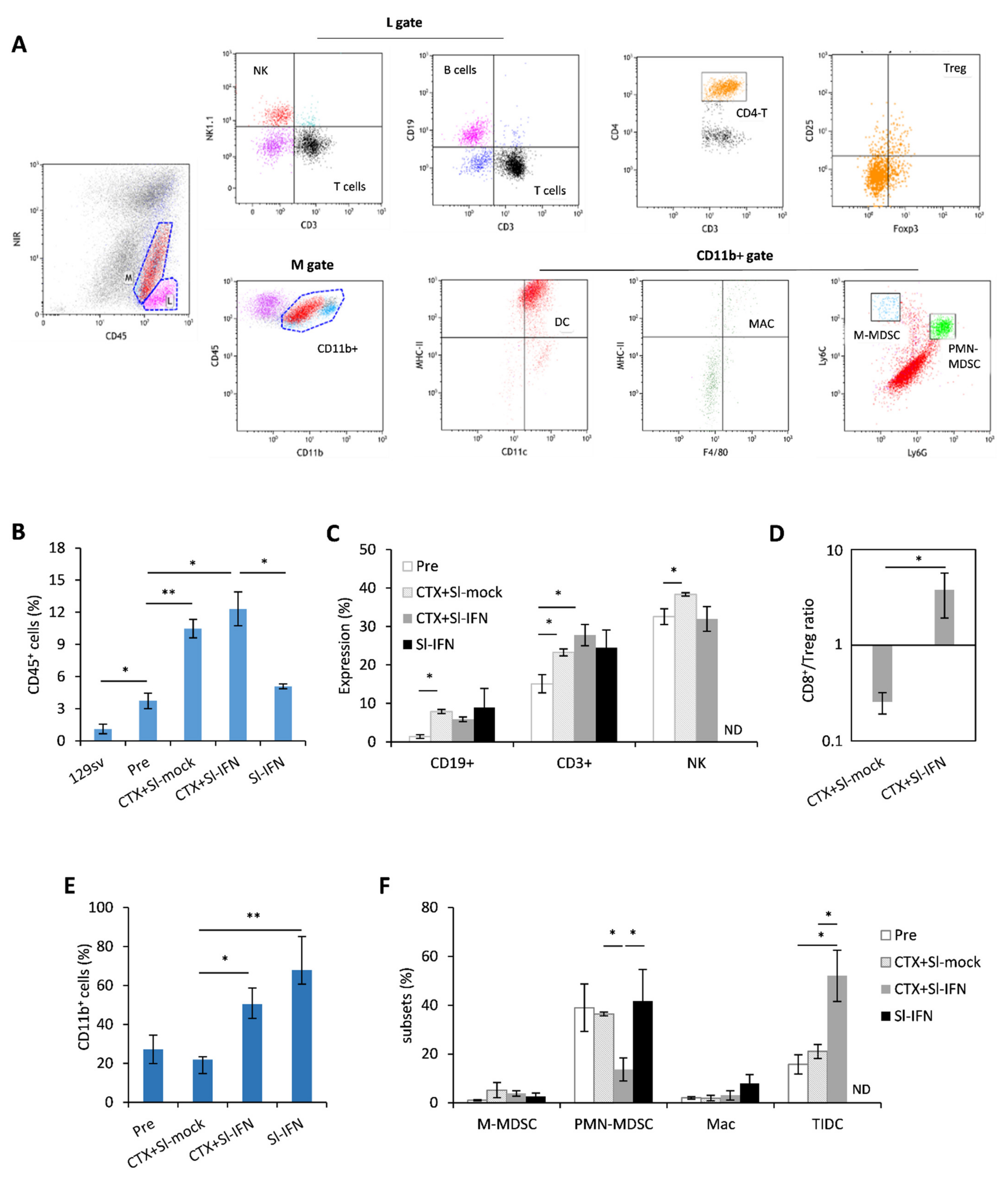
3. Results
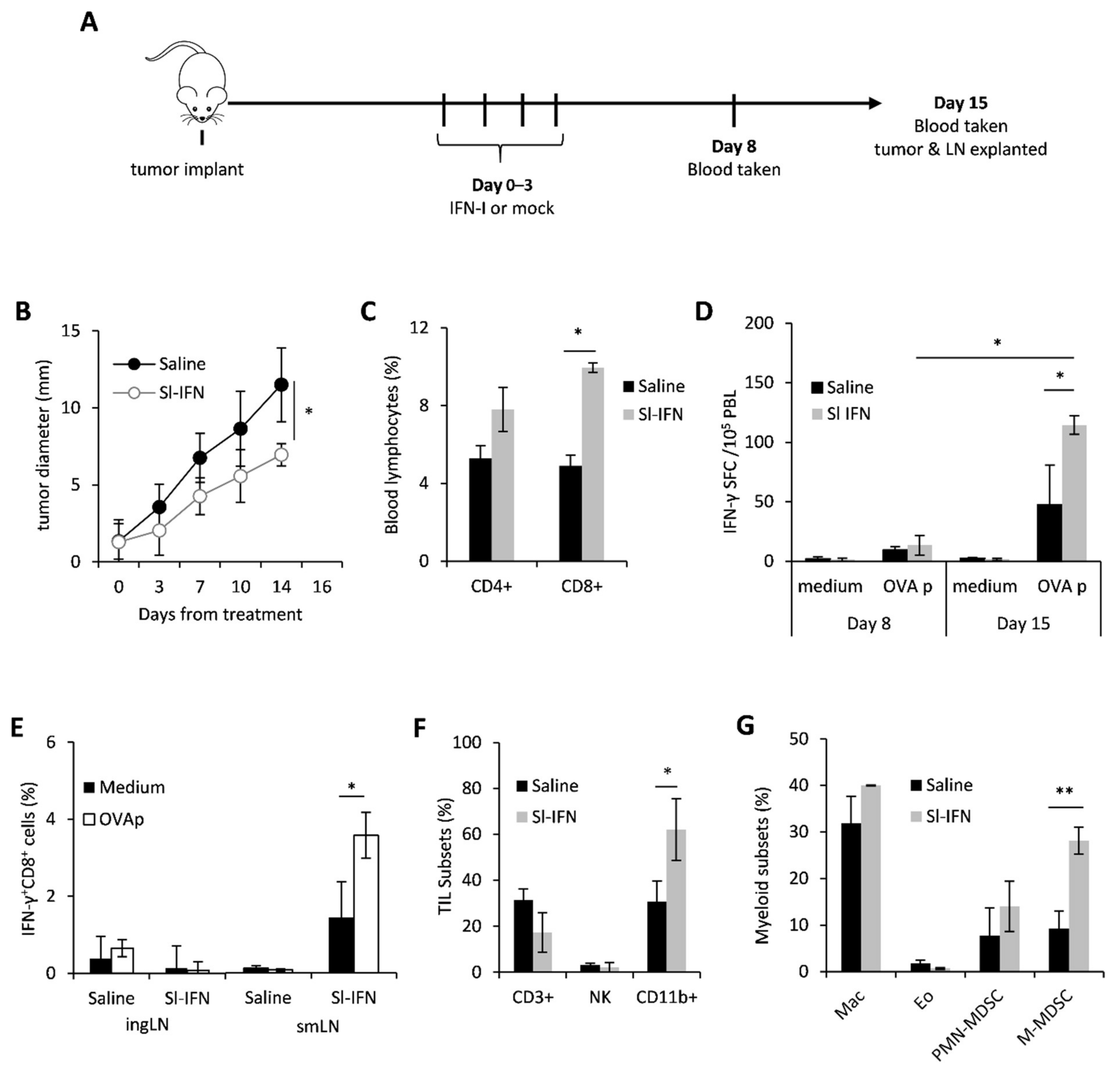
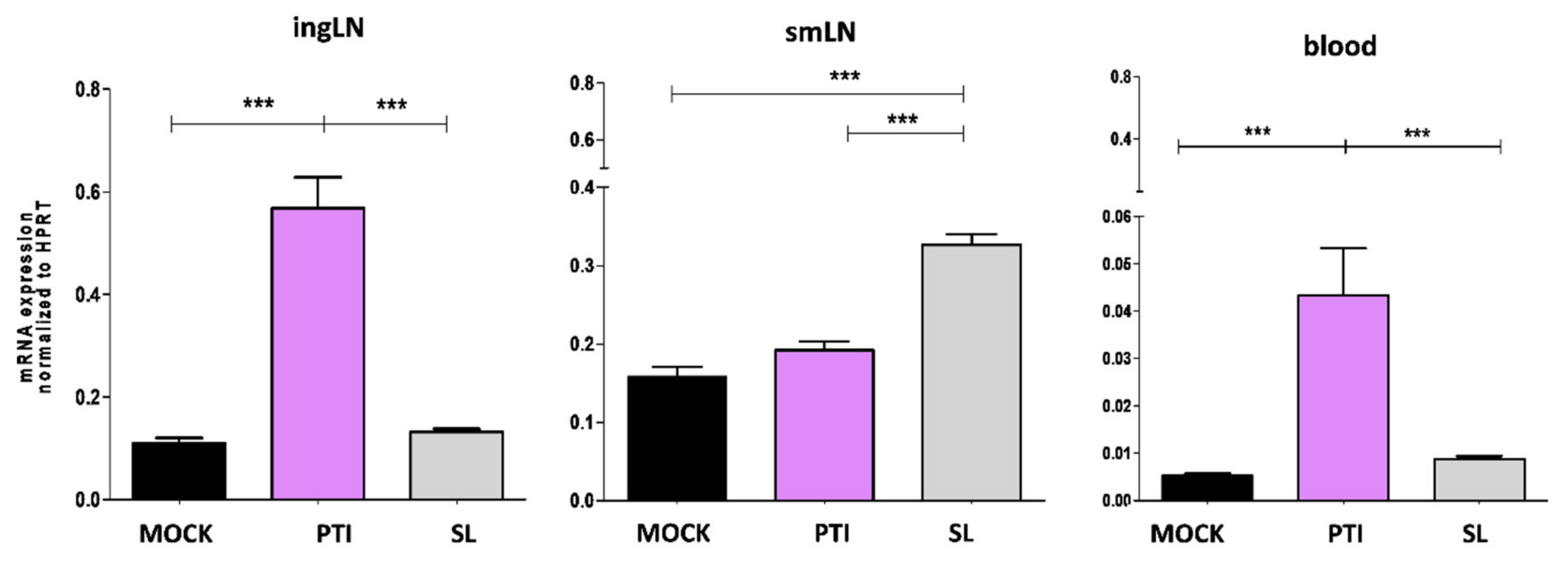
3.1. In Vivo Anticancer Effect of Sublingual IFN-I
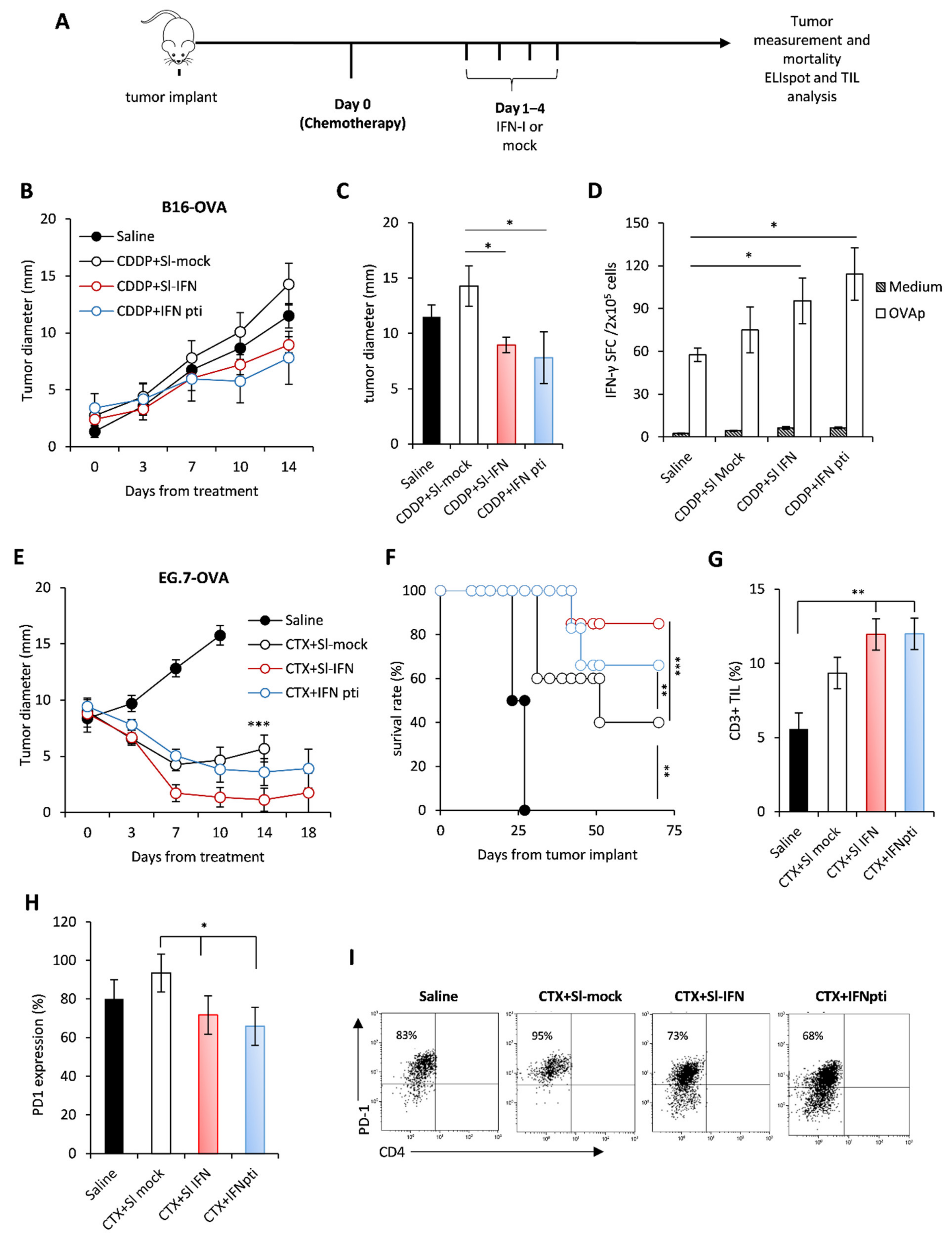
3.2. Anticancer Effect of Sl-IFN in Combination with Chemotherapy in Transplantable Tumors
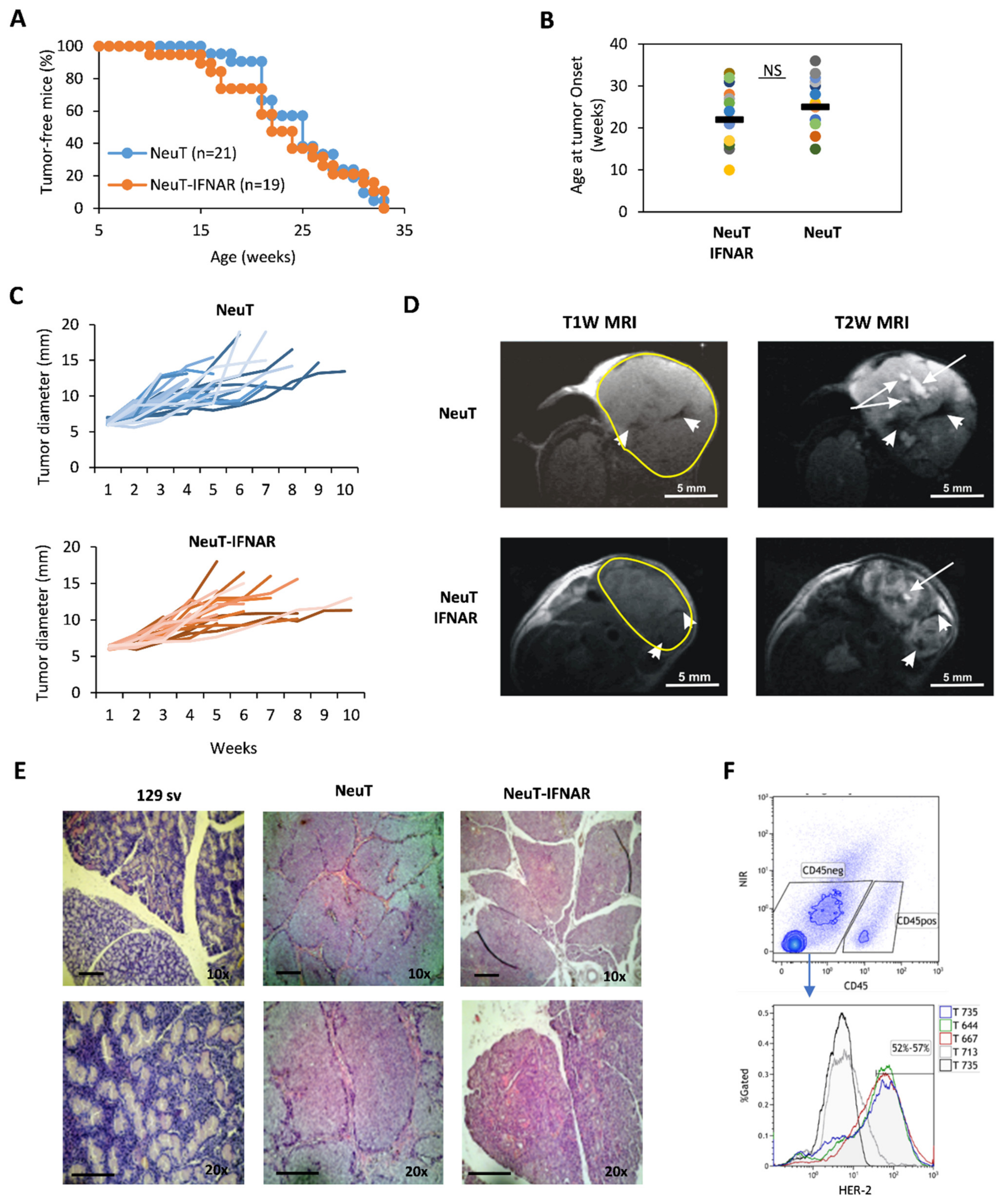
3.3. Characterization of the Salivary Gland Tumor Model
3.4. Anticancer Effect of Combined CTX/Sl-IFN Treatment in SDC
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2021. [Google Scholar] [CrossRef]
- Kleinsasser, O.; Klein, H.J.; Hübner, G. Duct carcinoma of the salivary glands. Arch. Klin. Exp. Ohren. Nasen. Kehlkopfheilkd. 1968, 192, 100–115. [Google Scholar] [CrossRef]
- Skálová, A.; Stárek, I.; Kuerová, V.; Szépe, P.; Plank, L. Salivary duct carcinoma—A highly aggressive salivary gland tumor with HER-2/neu oncoprotein overexpression. Pathol. Res. Pract. 2001, 197, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Felix, A.; El-Naggar, A.K.; Press, M.F.; Ordonez, N.G.; Fonseca, I.; Tucker, S.L.; Luna, M.A.; Batsakis, J.G. Prognostic significance of biomarkers (c-erbB-2, p53, proliferating cell nuclear antigen, and DNA content) in salivary duct carcinoma. Hum. Pathol. 1996, 27, 561–566. [Google Scholar] [CrossRef]
- Skálová, A.; Stárek, I.; Vanecek, T.; Kucerová, V.; Plank, L.; Szépe, P.; Di Palma, S.; Leivo, I. Expression of HER-2/neu gene and protein in salivary duct carcinomas of parotid gland as revealed by fluorescence in-situ hybridization and immunohistochemistry. Histopathology 2003, 42, 348–356. [Google Scholar] [CrossRef]
- Heliquist, H.B.; Karlsson, M.G.; Nilsson, C. Salivary duct carcinoma—A highly aggressive salivary gland tumour with overexpression of c-erB-2. J. Pathol. 1994, 172, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Barnes, L.; Rao, U.; Contis, L.; Krause, J.; Schwartz, A.; Scalamogna, P. Salivary duct carcinoma. Part II. Immunohistochemical evaluation of 13 cases for estrogen and progesterone receptors, cathepsin D, and c-erbB-2 protein. Oral Surg. Oral Med. Oral Pathol. 1994, 78, 74–80. [Google Scholar] [CrossRef]
- Alotaibi, A.M.; Alqarni, M.A.; Alnobi, A.; Tarakji, B. Human Epidermal Growth Factor Receptor 2 ( HER2/neu ) in Salivary Gland Carcinomas: A Review of Literature. J. Clin. Diagn. Res. 2015, 9, ZE04-8. [Google Scholar] [CrossRef] [PubMed]
- Perissinotti, A.J.; Pierce, M.L.; Pace, M.B.; El-Naggar, A.; Kies, M.S.; Kupferman, M. The role of trastuzumab in the management of salivary ductal carcinomas. Anticancer Res. 2013, 33, 2587–2592. [Google Scholar]
- Di Villeneuve, L.; Souza, I.L.; Tolentino, F.D.S.; Ferrarotto, R.; Schvartsman, G. Salivary Gland Carcinoma: Novel Targets to Overcome Treatment Resistance in Advanced Disease. Front. Oncol. 2020, 10, 580141. [Google Scholar] [CrossRef]
- Terhaard, C.H.J.; Lubsen, H.; Rasch, C.R.N.; Levendag, P.C.; Kaanders, H.H.À.M.; Tjho-Heslinga, R.E.; Van Den Ende, P.L.A.; Burlage, F. The role of radiotherapy in the treatment of malignant salivary gland tumors. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Osborn, V.; Givi, B.; Lee, A.; Sheth, N.; Roden, D.; Schwartz, D.; Schreiber, D. Characterization, treatment and outcomes of salivary ductal carcinoma using the National Cancer Database. Oral Oncol. 2017, 71, 41–46. [Google Scholar] [CrossRef]
- Ruzich, J.C.; Ciesla, M.C.; Clark, J.I. Response to paclitaxel and carboplatin in metastatic salivary gland cancer: A case report. Head Neck 2002, 24, 406–410. [Google Scholar] [CrossRef]
- Laurie, S.A.; Licitra, L. Systemic therapy in the palliative management of advanced salivary gland cancers. J. Clin. Oncol. 2006, 24, 2673–2678. [Google Scholar] [CrossRef] [PubMed]
- Chintakuntlawar, A.V.; Okuno, S.H.; Price, K.A. Systemic therapy for recurrent or metastatic salivary gland malignancies. Cancers Head Neck 2016, 1, 11. [Google Scholar] [CrossRef]
- Uijen, M.J.M.; Lassche, G.; van Engen-van Grunsven, A.C.H.; Tada, Y.; Verhaegh, G.W.; Schalken, J.A.; Driessen, C.M.L.; van Herpen, C.M.L. Systemic therapy in the management of recurrent or metastatic salivary duct carcinoma: A systematic review. Cancer Treat. Rev. 2020, 89, 102069. [Google Scholar] [CrossRef]
- Vital, D.; Ikenberg, K.; Moch, H.; Rössle, M.; Huber, G.F. The expression of PD-L1 in salivary gland carcinomas. Sci. Rep. 2019, 9, 12724. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.B.; Delord, J.P.; Doi, T.; Piha-Paul, S.A.; Liu, S.V.; Gilbert, J.; Algazi, A.P.; Damian, S.; Hong, R.L.; Le Tourneau, C.; et al. Pembrolizumab for the treatment of advanced salivary gland carcinoma. Proc. Am. J. Clin. Oncol. Cancer Clin. Trials 2018, 41, 1083–1088. [Google Scholar] [CrossRef]
- Niwa, K.; Kawakita, D.; Nagao, T.; Takahashi, H.; Saotome, T.; Okazaki, M.; Yamazaki, K.; Okamoto, I.; Hirai, H.; Saigusa, N.; et al. Multicentre, retrospective study of the efficacy and safety of nivolumab for recurrent and metastatic salivary gland carcinoma. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Porcheri, C.; Meisel, C.T.; Mitsiadis, T.A. Molecular and cellular modelling of salivary gland tumors open new landscapes in diagnosis and treatment. Cancers 2020, 12, 3107. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, E.; Mastini, C.; Forni, G.; Cavallo, F. ErbB2 transgenic mice: A tool for investigation of the immune prevention and treatment of mammary carcinomas. Curr. Protoc. Immunol. 2008. [Google Scholar] [CrossRef]
- Diodoro, M.G.; Di Carlo, E.; Zappacosta, R.; Iezzi, M.; Coletti, A.; Modesti, A.; D’Antuono, T.; Forni, G.; Musiani, P. Salivary carcinoma in HER-2/neu transgenic male mice: An angiogenic switch is not required for tumor onset and progression. Int. J. Cancer 2000, 88, 329–335. [Google Scholar] [CrossRef]
- Masuelli, L.; Fantini, M.; Benvenuto, M.; Sacchetti, P.; Giganti, M.G.; Tresoldi, I.; Lido, P.; Lista, F.; Cavallo, F.; Nanni, P.; et al. Intratumoral delivery of recombinant vaccinia virus encoding for ErbB2/Neu inhibits the growth of salivary gland carcinoma cells. J. Transl. Med. 2014, 12, 1–14. [Google Scholar] [CrossRef]
- Focaccetti, C.; Benvenuto, M.; Ciuffa, S.; Fazi, S.; Scimeca, M.; Nardi, A.; Miele, M.T.; Battisti, A.; Bonanno, E.; Modesti, A.; et al. Curcumin enhances the antitumoral effect induced by the recombinant vaccinia neu vaccine (RV-NEUT) in mice with transplanted salivary gland carcinoma cells. Nutrients 2020, 12, 1417. [Google Scholar] [CrossRef] [PubMed]
- Sistigu, A.; Viaud, S.; Chaput, N.; Bracci, L.; Proietti, E.; Zitvogel, L. Immunomodulatory effects of cyclophosphamide and implementations for vaccine design. Semin. Immunopathol. 2011, 33, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Aricò, E.; Sestili, P.; Carpinelli, G.; Canese, R.; Cecchetti, S.; Schiavoni, G.; D’Urso, M.T.; Belardelli, F.; Proietti, E. Chemo-immunotherapy induces tumor regression in a mouse model of spontaneous mammary carcinogenesis. Oncotarget 2016, 7, 59754–59765. [Google Scholar] [CrossRef]
- Salem, M.L.; Kadima, A.N.; El-Naggar, S.A.; Rubinstein, M.P.; Chen, Y.; Gillanders, W.E.; Cole, D.J. Defining the ability of cyclophosphamide preconditioning to enhance the antigen-specific CD8+ T-cell response to peptide vaccination: Creation of a beneficial host microenvironment involving type I IFNs and myeloid cells. J. Immunother. 2007, 30, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, A.; Ogino, A.; Yachi, K.; Ohta, T.; Fukushima, T.; Watanabe, T.; Katayama, Y.; Okamoto, Y.; Naruse, N.; Sano, E. Effect of IFN-β on human glioma cell lines with temozolomide resistance. Int. J. Oncol. 2009, 35, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Schiavoni, G.; Sistigu, A.; Valentini, M.; Mattei, F.; Sestili, P.; Spadaro, F.; Sanchez, M.; Lorenzi, S.; D’Urso, M.T.; Belardelli, F.; et al. Cyclophosphamide synergizes with type I interferons through systemic dendritic cell reactivation and induction of immunogenic tumor apoptosis. Cancer Res. 2011, 71. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, C.; Perez-Gracia, J.L.; Suarez, N.; Rodriguez, J.; Fernandez de Sanmamed, M.; Sangro, B.; Martin-Algarra, S.; Calvo, A.; Redrado, M.; Agliano, A.; et al. Pilot Clinical Trial of Type 1 Dendritic Cells Loaded with Autologous Tumor Lysates Combined with GM-CSF, Pegylated IFN, and Cyclophosphamide for Metastatic Cancer Patients. J. Immunol. 2011, 187, 6130–6142. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Guo, C.C.; Wang, J.; Qiu, Z.K.; Sai, K.; Yang, Q.Y.; Chen, Y.S.; Chen, F.R.; Wang, J.; Panasci, L.; et al. Interferon-α/β enhances temozolomide activity against MGMT-positive glioma stem-like cells. Oncol. Rep. 2015, 34, 2715–2721. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.R.; Guo, C.C.; Yu, Y.J.; Yu, Z.H.; Cai, H.P.; Wu, W.C.; Ma, J.X.; Chen, F.R.; Wang, J.; Chen, Z.P. Combination of levetiracetam and IFN-α increased temozolomide efficacy in MGMT-positive glioma. Cancer Chemother. Pharmacol. 2020, 86, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, G.; Scagnolari, C.; Moschella, F.; Proietti, E. Twenty-five years of type I interferon-based treatment: A critical analysis of its therapeutic use. Cytokine Growth Factor Rev. 2015, 26, 121–131. [Google Scholar] [CrossRef]
- Moschos, S.; Kirkwood, J.M. Present role and future potential of type I interferons in adjuvant therapy of high-risk operable melanoma. Cytokine Growth Factor Rev. 2007, 18, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Aricò, E.; Castiello, L.; Capone, I.; Gabriele, L.; Belardelli, F. Type i interferons and cancer: An evolving story demanding novel clinical applications. Cancers 2019, 11, 1943. [Google Scholar] [CrossRef]
- Cummins, J.M.; Krakowka, G.S.; Thompson, C.G. Systemic effects of interferons after oral administration in animals and humans. Am. J. Vet. Res. 2005, 66, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Kweon, M.N. Sublingual mucosa: A new vaccination route for systemic and mucosal immunity. Cytokine 2011, 54, 1–5. [Google Scholar] [CrossRef]
- Narang, N.; Sharma, J. Sublingual mucosa as a route for systemic drug delivery. Int. J. Pharm. Pharm. Sci. 2011, 3, 18–22. [Google Scholar]
- Çuburu, N.; Kweon, M.N.; Song, J.H.; Hervouet, C.; Luci, C.; Sun, J.B.; Hofman, P.; Holmgren, J.; Anjuère, F.; Czerkinsky, C. Sublingual immunization induces broad-based systemic and mucosal immune responses in mice. Vaccine 2007, 25, 8598–8610. [Google Scholar] [CrossRef]
- Nagai, Y.; Shiraishi, D.; Tanaka, Y.; Nagasawa, Y.; Ohwada, S.; Shimauchi, H.; Aso, H.; Endo, Y.; Sugawara, S. Transportation of sublingual antigens across sublingual ductal epithelial cells to the ductal antigen-presenting cells in mice. Clin. Exp. Allergy 2015, 45, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Tovey, M.G.; Begon-Lours, J.; Gresser, I. A Method for the Large Scale Production of Potent Interferon Preparations. Exp. Biol. Med. 1974, 146, 809–815. [Google Scholar] [CrossRef]
- Castiello, L.; Sestili, P.; Schiavoni, G.; Dattilo, R.; Monque, D.M.; Ciaffoni, F.; Iezzi, M.; Lamolinara, A.; Sistigu, A.; Moschella, F.; et al. Disruption of IFN-I Signaling Promotes HER2/Neu Tumor Progression and Breast Cancer Stem Cells. Cancer Immunol. Res. 2018, 6, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Negri, D.; Sestili, P.; Borghi, M.; Ciccolella, M.; Bracci, L. Enzyme-linked immunospot assay to monitor antigen-specific cellular immune responses in mouse tumor models. Methods Enzymol. 2020, 632, 457–477. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, V.; Buccione, C.; Ziccheddu, G.; Peschiaroli, F.; Sestili, P.; Puglisi, R.; Mattia, G.; Zanetti, C.; Parolini, I.; Bracci, L.; et al. Combining Type I Interferons and 5-Aza-2′-Deoxycitidine to Improve Anti-Tumor Response against Melanoma. J. Investig. Dermatol. 2017, 137, 159–169. [Google Scholar] [CrossRef]
- Canese, R.; Palombelli, G.; Chirico, M.; Sestili, P.; Bagnoli, M.; Canevari, S.; Mezzanzanica, D.; Podo, F.; Iorio, E. Integration of MRI and MRS approaches to monitor molecular imaging and metabolomic effects of trabectedin on a preclinical ovarian cancer model. NMR Biomed. 2019, 32. [Google Scholar] [CrossRef]
- Harrell, M.I.; Iritani, B.M.; Ruddell, A. Lymph node mapping in the mouse. J. Immunol. Methods 2008, 332, 170–174. [Google Scholar] [CrossRef]
- Carretero, R.; Sektioglu, I.M.; Garbi, N.; Salgado, O.C.; Beckhove, P.; Hämmerling, G.J. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8+ T cells. Nat. Immunol. 2015, 16, 609–617. [Google Scholar] [CrossRef]
- Lucarini, V.; Ziccheddu, G.; Macchia, I.; La Sorsa, V.; Peschiaroli, F.; Buccione, C.; Sistigu, A.; Sanchez, M.; Andreone, S.; D’Urso, M.T.; et al. IL-33 restricts tumor growth and inhibits pulmonary metastasis in melanoma-bearing mice through eosinophils. Oncoimmunology 2017, 6, e1317420. [Google Scholar] [CrossRef]
- Andreone, S.; Spadaro, F.; Buccione, C.; Mancini, J.; Tinari, A.; Sestili, P.; Gambardella, A.R.; Lucarini, V.; Ziccheddu, G.; Parolini, I.; et al. IL-33 promotes CD11b/CD18-mediated adhesion of eosinophils to cancer cells and synapse-polarized degranulation leading to tumor cell killing. Cancers 2019, 11, 1664. [Google Scholar] [CrossRef]
- Rossi, A.; Lucarini, V.; Macchia, I.; Sestili, P.; Buccione, C.; Donati, S.; Ciccolella, M.; Sistigu, A.; Teresa D’urso, M.; Pacca, A.M.; et al. Tumor-Intrinsic or Drug-Induced Immunogenicity Dictates the Therapeutic Success of the PD1/PDL Axis Blockade. Cells 2020, 9, 940. [Google Scholar] [CrossRef] [PubMed]
- Buccione, C.; Fragale, A.; Polverino, F.; Ziccheddu, G.; Aricò, E.; Belardelli, F.; Proietti, E.; Battistini, A.; Moschella, F. Role of interferon regulatory factor 1 in governing Treg depletion, Th1 polarization, inflammasome activation and antitumor efficacy of cyclophosphamide. Int. J. Cancer 2018, 142, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Pandha, H.S.; Heinemann, L.; Simpson, G.R.; Melcher, A.; Prestwich, R.; Errington, F.; Coffey, M.; Harrington, K.J.; Morgan, R. Synergistic Effects of Oncolytic Reovirus and Cisplatin Chemotherapy in Murine Malignant Melanoma. Clin Cancer Res 2009, 15, 6158–6166. [Google Scholar] [CrossRef] [PubMed]
- Sistigu, A.; Yamazaki, T.; Vacchelli, E.; Chaba, K.; Enot, D.P.; Adam, J.; Vitale, I.; Goubar, A.; Baracco, E.E.; Remédios, C.; et al. Cancer cell–autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat. Med. 2014, 20, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Bracci, L.; Moschella, F.; Sestili, P.; Sorsa, V.L.; Valentini, M.; Canini, I.; Baccarini, S.; Maccari, S.; Ramoni, C.; Belardelli, F.; et al. 644 Cancer Research. Clin. Cancer Res. 2007, 13. [Google Scholar] [CrossRef]
- Schworer, S.A.; Kim, E.H. Sublingual immunotherapy for food allergy and its future directions. Immunotherapy 2020, 12, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, C.S.; Blazevic, A.; Killoran, E.A.; Morris, M.S.; Hoft, D.F. Induction of mycobacterial protective immunity by sublingual BCG vaccination. Vaccine 2019, 37, 5364–5370. [Google Scholar] [CrossRef] [PubMed]
- Negri, D.R.M.; Riccomi, A.; Pinto, D.; Vendetti, S.; Rossi, A.; Cicconi, R.; Ruggiero, P.; Del Giudice, G.; Magistris, M.T. De Persistence of mucosal and systemic immune responses following sublingual immunization. Vaccine 2010, 28, 4175–4180. [Google Scholar] [CrossRef]
- Song, J.H.; Nguyen, H.H.; Cuburu, N.; Horimoto, T.; Ko, S.Y.; Park, S.H.; Czerkinsky, C.; Kweon, M.N. Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc. Natl. Acad. Sci. USA 2008, 105, 1644–1649. [Google Scholar] [CrossRef]
- Singh, S.; Yang, G.; Schluns, K.S.; Anthony, S.M.; Sastry, K.J. Sublingual vaccination induces mucosal and systemic adaptive immunity for protection against lung tumor challenge. PLoS ONE 2014, 9, e90001. [Google Scholar] [CrossRef]
- Tovey, M.G.; Maury, C. Oromucosal Interferon Therapy: Marked Antiviral and Antitumor Activity. J. Interf. Cytokine Res. 1999, 19, 145–155. [Google Scholar] [CrossRef]
- Eid, P.; Meritet, J.-F.; Maury, C.; Lasfar, A.; Weill, D.; Tovey, M.G. Oromucosal Interferon Therapy: Pharmacokinetics and Pharmacodynamics. J. Interf. Cytokine Res. 1999, 19, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Sprent, J.; Zhang, X.; Sun, S.; Tough, D. T-cell turnover in vivo and the role of cytokines. Proc. Immunol. Lett. 1999, 65, 21–25. [Google Scholar] [CrossRef]
- Tessmer, M.S.; Reilly, E.C.; Brossay, L. Salivary gland NK cells are phenotypically and functionally unique. PLoS Pathog. 2011, 7. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nagashima, H.; Bando, K.; Lu, L.; Ozaki, A.; Morita, Y.; Fukumoto, S.; Ishii, N.; Sugawara, S. Oral CD103− CD11b+ classical dendritic cells present sublingual antigen and induce Foxp3+ regulatory T cells in draining lymph nodes. Mucosal Immunol. 2017, 10, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, S.; Mattei, F.; Sistigu, A.; Bracci, L.; Spadaro, F.; Sanchez, M.; Spada, M.; Belardelli, F.; Gabriele, L.; Schiavoni, G. Type I IFNs Control Antigen Retention and Survival of CD8α+ Dendritic Cells after Uptake of Tumor Apoptotic Cells Leading to Cross-Priming. J. Immunol. 2011, 186, 5142–5150. [Google Scholar] [CrossRef]
- Brehm, M.A.; Daniels, K.A.; Welsh, R.M. Rapid Production of TNF-α following TCR Engagement of Naive CD8 T Cells. J. Immunol. 2005, 175, 5043–5049. [Google Scholar] [CrossRef]
- Lutsiak, M.E.C.; Semnani, R.T.; De Pascalis, R.; Kashmiri, S.V.S.; Schlom, J.; Sabzevari, H. Inhibition of CD4+25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood 2005, 105, 2862–2868. [Google Scholar] [CrossRef]
- Zilio, S.; Serafini, P. vaccines Neutrophils and Granulocytic MDSC: The Janus God of Cancer Immunotherapy. Vaccines 2016, 4, 31. [Google Scholar] [CrossRef]
- Pisanu, M.E.; Ricci, A.; Paris, L.; Surrentino, E.; Liliac, L.; Bagnoli, M.; Canevari, S.; Mezzanzanica, D.; Podo, F.; Iorio, E.; et al. Monitoring response to cytostatic cisplatin in a HER2+ ovary cancer model by MRI and in vitro and in vivo MR spectroscopy. Br. J. Cancer 2014, 110, 625–635. [Google Scholar] [CrossRef]
- Ali, H.R.; Provenzano, E.; Dawson, S.J.; Blows, F.M.; Liu, B.; Shah, M.; Earl, H.M.; Poole, C.J.; Hiller, L.; Dunn, J.A.; et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12 439 patients. Ann. Oncol. 2014, 25, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Sato, F.; Ono, T.; Kawahara, A.; Matsuo, K.; Kondo, R.; Sato, K.; Akiba, J.; Kawaguchi, T.; Kakuma, T.; Chitose, S.I.; et al. Prognostic Value of Tumor Proportion Score in Salivary Gland Carcinoma. Laryngoscope 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, C.; de Arruda, J.A.A.; de Faria, A.C.R.; Oliveira, G.A.Q.; de Paula, H.M.; Fonseca, F.P.; Mesquita, R.A.; Silva, T.A.; Mendonça, E.F.; Batista, A.C. Immune microenvironment and evasion mechanisms in adenoid cystic carcinomas of salivary glands. Oral Oncol. 2019, 88, 95–101. [Google Scholar] [CrossRef]
- Li, J.-Y.; Chen, Y.-P.; Li, Y.-Q.; Liu, N.; Ma, J. Chemotherapeutic and targeted agents can modulate the tumor microenvironment and increase the efficacy of immune checkpoint blockades. Mol. Cancer 2021, 20, 27. [Google Scholar] [CrossRef] [PubMed]
- Guidi, A.; Codecà, C.; Ferrari, D. Chemotherapy and immunotherapy for recurrent and metastatic head and neck cancer: A systematic review. Med. Oncol. 2018, 35. [Google Scholar] [CrossRef] [PubMed]
- Rolla, S.; Nicoló, C.; Malinarich, S.; Orsini, M.; Forni, G.; Cavallo, F.; Ria, F. Distinct and Non-Overlapping T Cell Receptor Repertoires Expanded by DNA Vaccination in Wild-Type and HER-2 Transgenic BALB/c Mice. J. Immunol. 2006, 177, 7626–7633. [Google Scholar] [CrossRef]
- Landuzzi, L.; Antognoli, A.; Nicoletti, G.; Croci, S.; Palladini, A.; Ianzano, M.L.; Murgo, A.; Stivani, V.; Grosso, V.; Nanni, P.; et al. HER-2/neu tolerant and non-tolerant mice for fine assessment of antimetastatic potency of dendritic cell-tumor cell hybrid vaccines. Vaccine 2011, 29, 4690–4697. [Google Scholar] [CrossRef]
- Ko, H.J.; Kim, Y.J.; Kim, Y.S.; Chang, W.S.; Ko, S.Y.; Chang, S.Y.; Sakaguchi, S.; Kang, C.Y. A combination of chemoimmunotherapies can efficiently break self-tolerance and induce antitumor immunity in a tolerogenic murine tumor model. Cancer Res. 2007, 67, 7477–7486. [Google Scholar] [CrossRef]
- Guo, F.F.; Cui, J.W. The role of tumor-infiltrating B cells in tumor immunity. J. Oncol. 2019, 2019. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciccolella, M.; Andreone, S.; Mancini, J.; Sestili, P.; Negri, D.; Pacca, A.M.; D’Urso, M.T.; Macchia, D.; Canese, R.; Pang, K.; et al. Anticancer Effects of Sublingual Type I IFN in Combination with Chemotherapy in Implantable and Spontaneous Tumor Models. Cells 2021, 10, 845. https://doi.org/10.3390/cells10040845
Ciccolella M, Andreone S, Mancini J, Sestili P, Negri D, Pacca AM, D’Urso MT, Macchia D, Canese R, Pang K, et al. Anticancer Effects of Sublingual Type I IFN in Combination with Chemotherapy in Implantable and Spontaneous Tumor Models. Cells. 2021; 10(4):845. https://doi.org/10.3390/cells10040845
Chicago/Turabian StyleCiccolella, Maria, Sara Andreone, Jacopo Mancini, Paola Sestili, Donatella Negri, Anna Maria Pacca, Maria Teresa D’Urso, Daniele Macchia, Rossella Canese, Ken Pang, and et al. 2021. "Anticancer Effects of Sublingual Type I IFN in Combination with Chemotherapy in Implantable and Spontaneous Tumor Models" Cells 10, no. 4: 845. https://doi.org/10.3390/cells10040845
APA StyleCiccolella, M., Andreone, S., Mancini, J., Sestili, P., Negri, D., Pacca, A. M., D’Urso, M. T., Macchia, D., Canese, R., Pang, K., SaiYing Ko, T., Decadt, Y., Schiavoni, G., Mattei, F., Belardelli, F., Aricò, E., & Bracci, L. (2021). Anticancer Effects of Sublingual Type I IFN in Combination with Chemotherapy in Implantable and Spontaneous Tumor Models. Cells, 10(4), 845. https://doi.org/10.3390/cells10040845








