KLF4 Regulates Metabolic Homeostasis in Response to Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture, Reagents, and Drug Treatments
2.2. Analysis of Metabolic Parameters
2.3. Western Blot Analysis
2.4. Immunostaining
2.5. qPCR
2.6. ROS Assay
2.7. Flow Cytometry
2.8. Statistical Analysis
3. Results
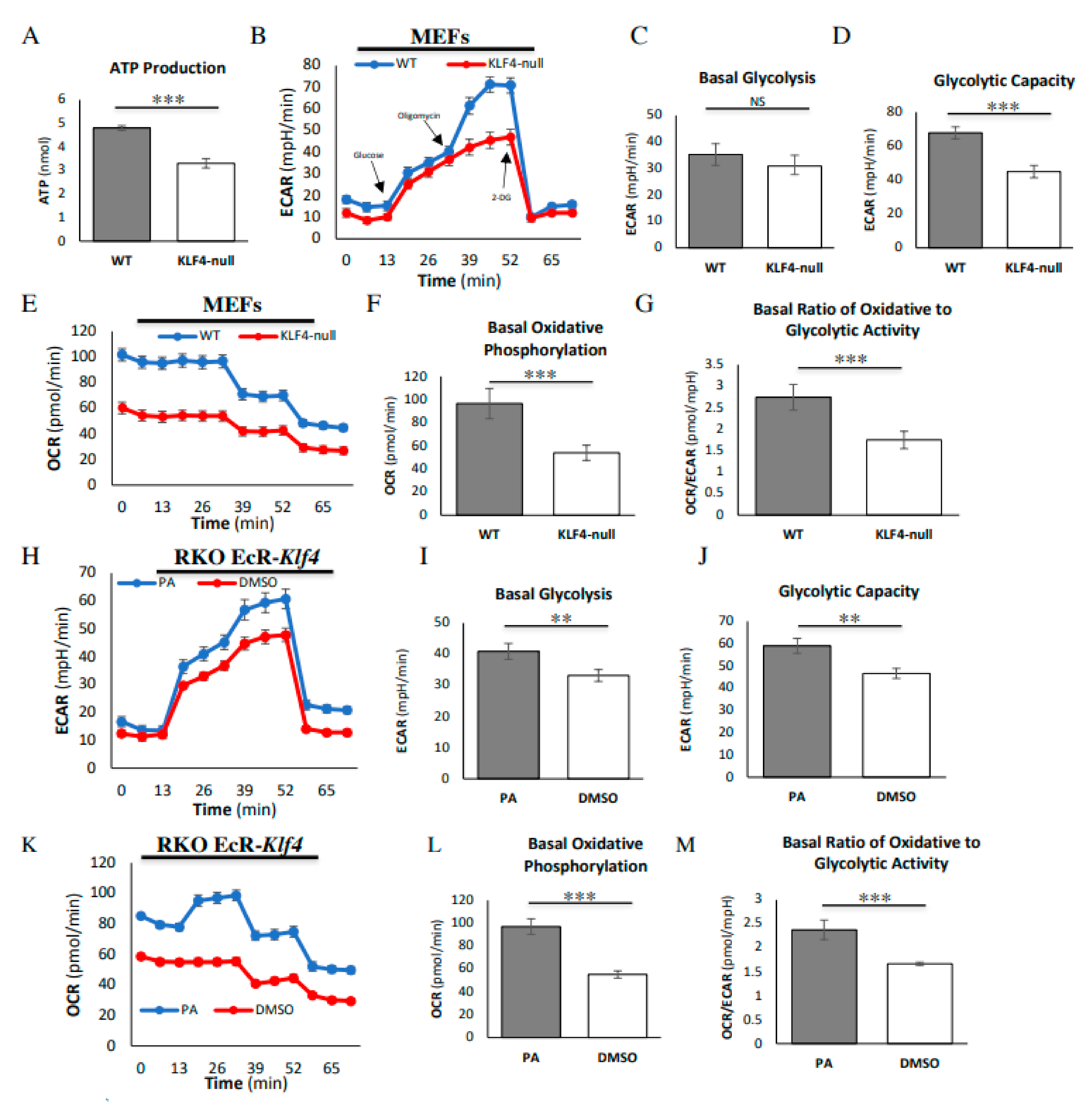
3.1. KLF4 Increases Energy Efficiency and Glycolytic Capacity
3.2. KLF4 Increases Expression of Key Glycolytic Proteins
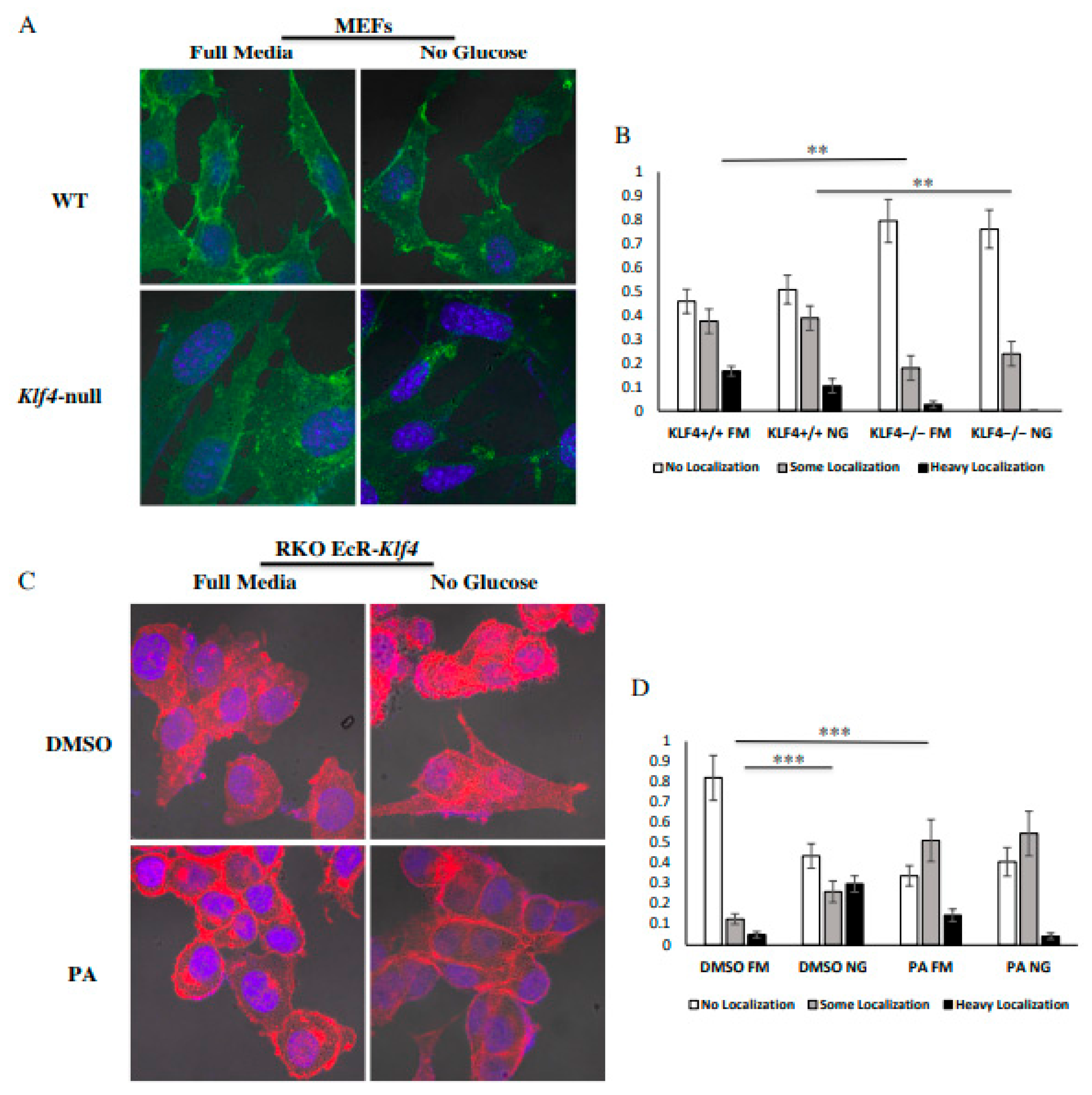
3.3. KLF4 Facilitates Localization of GLUT1 to the Outer Membrane
3.4. KLF4 Aids in Autophagic Cell Death in Response to Glucose Starvation
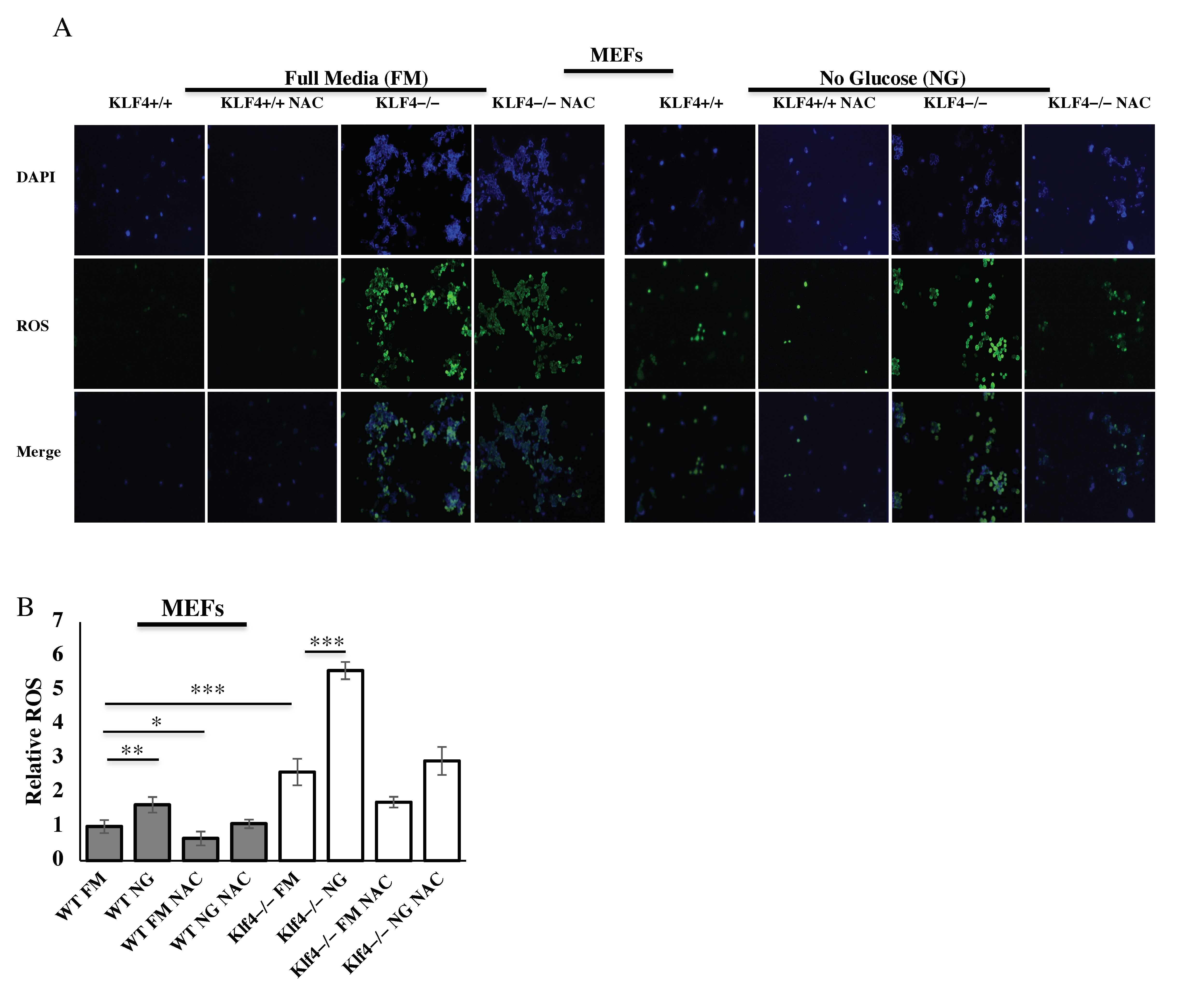
3.5. KLF4 Reduces ROS Induced from Glucose Starvation
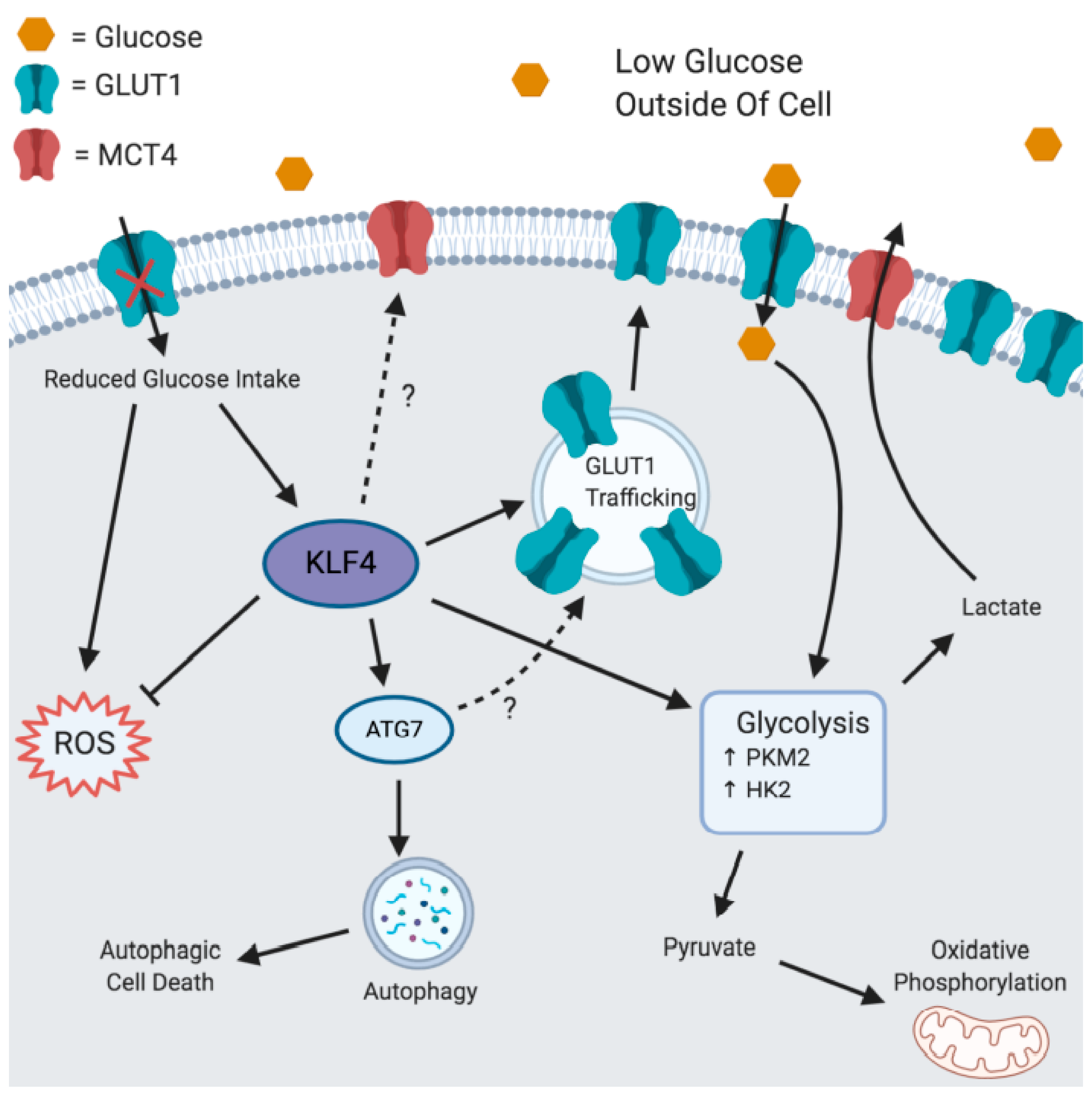
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shields, J.M.; Christy, R.J.; Yang, V.W. Identification and characterization of a gene encoding a gut-enriched Kruppel-like factor expressed during growth arrest. J. Biol. Chem. 1996, 271, 20009–20017. [Google Scholar]
- Garrett-Sinha, L.A.; Eberspaecher, H.; Seldin, M.F.; de Crombrugghe, B.A. Gene for a Novel Zinc-finger Protein Expressed in Differentiated Epithelial Cells and Transiently in Certain Mesenchymal Cells. J. Biol. Chem. 1996, 271, 31384–31390. [Google Scholar]
- Brauer, P.R.; Kim, J.H.; Ochoa, H.J.; Stratton, E.R.; Black, K.M.; Rosencrans, W.; Stacey, E.; Hagos, E.G. Krüppel-like factor 4 mediates cellular migration and invasion by altering RhoA activity. Cell Commun. Adhes. 2018, 24, 1–10. [Google Scholar] [PubMed]
- Liu, C.; DeRoo, E.P.; Stecyk, C.; Wolsey, M.; Szuchnicki, M.; Hagos, E.G. Impaired autophagy in mouse embryonic fibroblasts null for Krüppel-like Factor 4 promotes DNA damage and increases apoptosis upon serum starvation. Mol. Cancer 2015, 14, 101. [Google Scholar] [PubMed]
- Salmon, M.; Spinosa, M.; Zehner, Z.E.; Upchurch, G.R.; Ailawadi, G. Klf4, Klf2, and Zfp148 activate autophagy-related genes in smooth muscle cells during aortic aneurysm formation. Physiol. Rep. 2019, 7, e14058. [Google Scholar] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [PubMed]
- Lee, H.Y.; Ahn, J.B.; Rha, S.Y.; Chung, H.C.; Park, K.H.; Kim, T.S.; Kim, N.K.; Shin, S.J. High KLF4 level in normal tissue predicts poor survival in colorectal cancer patients. World J. Surg. Oncol. 2014, 12, 232. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hu, J.; Li, H.; Wu, C.; Zhao, X.; Liu, C. The Prognostic Value of Decreased KLF4 in Digestive System Cancers: A Meta-Analysis from 17 Studies. Dis. Mark. 2017, 2017, 3064246. [Google Scholar]
- Evans, P.M.; Liu, C. Roles of Krüpel-like factor 4 in normal homeostasis, cancer and stem cells. Acta Biochim. Biophys. Sin. 2008, 40, 554–564. [Google Scholar]
- Ghaleb, A.M.; Yang, V.W. Krüppel-like factor 4 (KLF4): What we currently know. Gene 2017, 611, 27–37. [Google Scholar]
- Ohnishi, S.; Ohnami, S.; Laub, F.; Aoki, K.; Suzuki, K.; Kanai, Y.; Haga, K.; Asaka, M.; Ramirez, F.; Yoshida, T. Downregulation and growth inhibitory effect of epithelial-type Krüppel-like transcription factor KLF4, but not KLF5, in bladder cancer. Biochem. Biophys. Res. Commun. 2003, 308, 251–256. [Google Scholar]
- Hu, W.; Hofstetter, W.L.; Li, H.; Zhou, Y.; He, Y.; Pataer, A.; Wang, L.; Xie, K.; Swisher, S.G.; Fang, B. Putative Tumor-Suppressive Function of Krüppel-Like Factor 4 in Primary Lung Carcinoma. Clin. Cancer Res. 2009, 15, 5688–5695. [Google Scholar]
- Zammarchi, F.; Morelli, M.; Menicagli, M.; Di Cristofano, C.; Zavaglia, K.; Paolucci, A.; Campani, D.; Aretini, P.; Boggi, U.; Mosca, F.; et al. KLF4 is a novel candidate tumor suppressor gene in pancreatic ductal carcinoma. Am. J. Pathol. 2011, 178, 361–372. [Google Scholar]
- Wei, D.; Kanai, M.; Huang, S.; Xie, K. Emerging role of KLF4 in human gastrointestinal cancer. Carcinogenesis 2006, 27, 23–31. [Google Scholar] [PubMed]
- Zhao, W.; Hisamuddin, I.M.; Nandan, M.O.; Babbin, B.A.; Lamb, N.E.; Yang, V.W. Identification of Krüppel-like factor 4 as a potential tumor suppressor gene in colorectal cancer. Oncogene 2004, 23, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Hagos, E.G.; Ghaleb, A.M.; Dalton, W.B.; Bialkowska, A.B.; Yang, V.W. Mouse embryonic fibroblasts null for the Krüppel-like factor 4 gene are genetically unstable. Oncogene 2009, 28, 1197–1205. [Google Scholar] [PubMed]
- El-Karim, E.A.; Hagos, E.G.; Ghaleb, A.M.; Yu, B.; Yang, V.W. Krüppel-like factor 4 regulates genetic stability in mouse embryonic fibroblasts. Mol. Cancer 2013, 12, 1–12. [Google Scholar] [CrossRef]
- Rosencrans, W.; Walsh, Z.; Houerbi, N.; Blum, A.; Belew, A.; Chernak, B.; Liu, C.; Trazo, A.; Olson, A.; Brauer, P.; et al. Cells deficient for Krüppel-Like Factor 4 exhibit mitochondrial dysfunction and impaired mitophagy. Eur. J. Cell Biol. 2020, 99, 151061. [Google Scholar]
- Wang, S.; Shi, X.; Wei, S.; Ma, D.; Oyinlade, O.; Lv, S.Q.; Ying, M.; Zhang, Y.A.; Claypool, S.M.; Watkins, P.; et al. Krüppel-like factor 4 (KLF4) induces mitochondrial fusion and increases spare respiratory capacity of human glioblastoma cells. J. Biol. Chem. 2018, 293, 6544–6555. [Google Scholar]
- Venkatesh, K.V.; Darunte, L.; Bhat, P.J. Warburg Effect. Encycl. Syst. Biol. 2013, 139–141. [Google Scholar] [CrossRef]
- Epstein, T.; Gatenby, R.A.; Brown, J.S. The Warburg effect as an adaptation of cancer cells to rapid fluctuations in energy demand. PLoS ONE 2017, 12, e0185085. [Google Scholar] [CrossRef]
- Zhao, L.; Mao, Y.; Zhao, Y.; Cao, Y.; Chen, X. Role of multifaceted regulators in cancer glucose metabolism and their clinical significance. Oncotarget 2016, 7, 31572. [Google Scholar] [CrossRef]
- La Vecchia, S.; Sebastián, C. Metabolic pathways regulating colorectal cancer initiation and progression. Semin. Cell Dev. Biol. 2020, 98, 63–70. [Google Scholar] [CrossRef]
- Nishimura, K.; Aizawa, S.; Nugroho, F.L.; Shiomitsu, E.; Tran, Y.; Bui, P.L.; Borisova, E.; Sakuragi, Y.; Takada, H.; Kurisaki, A.; et al. A Role for KLF4 in Promoting the Metabolic Shift via TCL1 during Induced Pluripotent Stem Cell Generation. Stem Cell Rep. 2017, 8, 787–801. [Google Scholar] [CrossRef]
- Moon, J.S.; Kim, H.E.; Koh, E.; Park, S.H.; Jin, W.J.; Park, B.W.; Park, S.W.; Kim, K.S. Krüppel-like factor 4 (KLF4) activates the transcription of the gene for the platelet isoform of phosphofructokinase (PFKP) in breast cancer. J. Biol. Chem. 2011, 286, 23808–23816. [Google Scholar] [CrossRef]
- Wood, I.S.; Tryhurn, P. Glucose transporters (GLUT and SGLT): Expanded families of sugar transport proteins. Br. J. Nutr. 2003, 89, 3–9. [Google Scholar] [CrossRef]
- Zierler, K. Whole body glucose metabolism. Am. J. Physiol. 1999, 276, E409–E426. [Google Scholar] [CrossRef]
- Li, Z.Y.; Shi, Y.L.; Liang, G.X.; Yang, J.; Zhuang, S.K.; Lin, J.B.; Ghodbane, A.; Tam, M.; Liang, Z.J.; Zha, Z.G.; et al. Visualization of GLUT1 Trafficking in Live Cancer Cells by the Use of a Dual-Fluorescence Reporter. ACS Omega 2020, 5, 15911–15921. [Google Scholar] [CrossRef]
- Graham, N.A.; Tahmasian, M.; Kohli, B.; Komisopoulou, E.; Zhu, M.; Vivanco, I.; Teitell, M.A.; Wu, H.; Ribas, A.; Lo, R.S.; et al. Glucose deprivation activates a metabolic and signaling amplification loop leading to cell death. Mol. Syst. Biol. 2012, 8, 589. [Google Scholar] [CrossRef]
- El Mjiyad, N.; Caro-Maldonado, A.; Ramirez-Peinado, S.; Munoz-Pinedo, C. Sugar-free approaches to cancer cell killing. Oncogene 2011, 30, 253–264. [Google Scholar] [CrossRef]
- Izuishi, K.; Kato, K.; Ogura, T.; Kinoshita, T.; Esumi, H. Remarkable tolerance of tumor cells to nutrient deprivation: Possible new biochemical target for cancer therapy. Cancer Res. 2000, 60, 6201–6207. [Google Scholar] [PubMed]
- Liu, C.; La Rosa, S.; Hagos, E.G. Oxidative DNA damage causes premature senescence in mouse embryonic fibroblasts deficient for Krüppel-like factor 4. Mol. Carcinog. 2015, 54, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.P.; Perreault, N.; Goldstein, B.G.; Lee, C.S.; Labosky, P.A.; Yang, V.W.; Kaestner, K.H. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development 2002, 129, 2619–2628. [Google Scholar]
- Chen, X.; Johns, D.C.; Geiman, D.E.; Marban, E.; Dang, D.T.; Hamlin, G.; Sun, R.; Yang, V.W. Krüppel-like factor 4 (gut-enriched Krüppel-like factor) inhibits cell proliferation by blocking G1/S progression of the cell cycle. J. Biol. Chem. 2001, 276, 30423–30428. [Google Scholar] [CrossRef]
- Panasyuk, G.; Espeillac, C.; Chauvin, C. PPARγ contributes to PKM2 and HK2 expression in fatty liver. Nat Commun. 2012, 3, 672. [Google Scholar] [CrossRef] [PubMed]
- Bisetto, S.; Wright, M.C.; Nowak, R.A.; Lepore, A.C.; Khurana, T.S.; Loro, E.; Philp, N.J. New Insights into the Lactate Shuttle: Role of MCT4 in the Modulation of the Exercise Capacity. iScience 2019, 22, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Luz, M.C.; Perez, M.M.; Azzalis, L.A.; Sousa, L.V.; Adami, F.; Fonseca, F.L.; Alves, B.D. Evaluation of MCT1, MCT4 and CD147 Genes in Peripheral Blood Cells of Breast Cancer Patients and Their Potential Use as Diagnostic and Prognostic Markers. Int. J. Mol. Sci. 2017, 18, 170. [Google Scholar] [CrossRef]
- Aerni-Flessner, L.; Abi-Jaoude, M.; Koenig, A.; Payne, M.; Hruz, P.W. GLUT4, GLUT1, and GLUT8 are the dominant GLUT transcripts expressed in the murine left ventricle. Cardiovasc. Diabetol. 2012, 11, 63. [Google Scholar] [CrossRef]
- Pyla, R.; Poulose, N.; Jun, J.Y.; Segar, L. Expression of conventional and novel glucose transporters, GLUT1, -9, -10, and -12, in vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2013, 304, C574–C589. [Google Scholar] [CrossRef]
- Kim, J.W.; Dang, C.V. Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res. 2006, 66, 8927–8930. [Google Scholar] [CrossRef]
- Erkkilä, K.; Aito, H.; Aalto, K.; Pentikäinen, V.; Dunkel, L. Lactate inhibits germ cell apoptosis in the human testis. Mol. Hum. Reprod. 2002, 8, 109–117. [Google Scholar] [CrossRef]
- Hirschhaeuser, F.; Sattler, U.G.; Mueller-Klieser, W. Lactate: A metabolic key player in cancer. Cancer Res. 2011, 71, 6921–6925. [Google Scholar] [CrossRef]
- Doherty, J.R.; Cleveland, J.L. Targeting lactate metabolism for cancer therapeutics. J. Clin. Investig. 2013, 123, 3685–3692. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, B.; Zhang, X.H.; Nie, C.J.; Li, Y.H.; Wen, J.K. Localization and function of KLF4 in cytoplasm of vascular smooth muscle cell. Biochem. Biophys. Res. Commun. 2013, 436, 162–168. [Google Scholar] [CrossRef]
- Luo, W.W.; Lian, H.; Zhong, B.; Shu, H.B.; Li, S. Krüppel-like factor 4 negatively regulates cellular antiviral immune response. Cell. Mol. Immunol. 2016, 13, 65–72. [Google Scholar] [CrossRef]
- Villarreal, G., Jr.; Zhang, Y.; Larman, H.B.; Gracia-Sancho, J.; Koo, A.; García-Cardeña, G. Defining the regulation of KLF4 expression and its downstream transcriptional targets in vascular endothelial cells. Biochem. Biophys. Res. Commun. 2010, 391, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.; Meyer-Schaller, N.; Arnold, P.; Antoniadis, H.; Pachkov, M.; van Nimwegen, E.; Christofori, G. Klf4 is a transcriptional regulator of genes critical for EMT, including Jnk1 (Mapk8). PLoS ONE 2013, 8, e57329. [Google Scholar] [CrossRef]
- Anastasiou, D.; Poulogiannis, G.; Asara, J.M.; Boxer, M.B.; Jiang, J.K.; Shen, M.; Bellinger, G.; Sasaki, A.T.; Locasale, J.W.; Auld, D.S.; et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 2011, 334, 1278–1283. [Google Scholar] [CrossRef]
- Zahra, K.; Dey, T.; Ashish, A.; Pandey, U.; Mishra, S.P. Pyruvate Kinase M2 and Cancer: The role of PKM2 in promoting tumorigenesis. Front. Oncol. 2020, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Cui, J.; Du, J.; Wei, D.; Jia, Z.; Zhang, J.; Zhu, Z.; Gao, Y.; Xie, K. A novel KLF4/LDHA signaling pathway regulates aerobic glycolysis in and progression of pancreatic cancer. Clin. Cancer Res. 2014, 20, 4370–4380. [Google Scholar] [CrossRef]
- Wang, Y.; Martins, I.; Ma, Y.; Kepp, O.; Galluzzi, L.; Kroemer, G. Autophagy-dependent ATP release from dying cells via lysosomal exocytosis. Autophagy 2013, 9, 1624–1625. [Google Scholar] [CrossRef]
- Lee, H.J.; Cho, E.D.; Lee, K.W.; Kim, J.H.; Cho, S.G.; Lee, S.J. Autophagic failure promotes the exocytosis and intercellular transfer of α-synuclein. Exp. Mol. Med. 2013, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Von Mollard, G.F.; Stahl, B.; Li, C.; Südhof, T.C.; Jahn, R. Rab proteins in regulated exocytosis. Trends Biochem. Sci. 1994, 19, 164–168. [Google Scholar] [CrossRef]
- Novick, P.; Medkova, M.; Dong, G.; Hutagalung, A.; Reinisch, K.; Grosshans, B. Interactions between Rabs, tethers, SNAREs and their regulators in exocytosis. Biochem. Soc. Trans. 2006, 34, 683–686. [Google Scholar] [CrossRef]
- Hagos, E.G.; Ghaleb, A.M.; Kumar, A.; Neish, A.S.; Yang, V.W. Expression profiling and pathway analysis of Krüppel-like factor 4 in mouse embryonic fibroblasts. Am. J. Cancer Res. 2011, 1, 85–97. [Google Scholar]
- Loeb, L.A. A mutator phenotype in cancer. Cancer Res. 2001, 61, 3230–3239. [Google Scholar]
- Ren, Y.; Shen, H.M. Critical role of AMPK in redox regulation under glucose starvation. Redox Biol. 2019, 25, 101154. [Google Scholar] [CrossRef]
- Thoreen, C.C.; Sabatini, D.M. AMPK and p53 help cells through lean times. Cell Metab. 2005, 1, 287–288. [Google Scholar] [CrossRef]
- Jones, R.G.; Plas, D.R.; Kubek, S.; Buzzai, M.; Mu, J.; Xu, Y.; Birnbaum, M.J.; Thompson, C.B. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol. Cell 2005, 18, 283–293. [Google Scholar] [CrossRef]
- Reid, M.A.; Kong, M. Dealing with hunger: Metabolic stress responses in tumors. J. Carcinog. 2013, 12, 17. [Google Scholar]
- Bonini, M.G.; Gantner, B.N. The multifaceted activities of AMPK in tumor progression--why the "one size fits all" definition does not fit at all? IUBMB Life 2013, 65, 889–896. [Google Scholar] [CrossRef]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Tai, P.; Ascoli, M. Reactive oxygen species (ROS) play a critical role in the cAMP-induced activation of Ras and the phosphorylation of ERK1/2 in Leydig cells. Mol. Endocrinol. 2011, 25, 885–893. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, K.; Liu, Z.; Xu, Q.; You, B.; Li, C.; Cao, J.; Zhou, H.; Li, X.; Chen, J.; et al. VPO1 modulates vascular smooth muscle cell phenotypic switch by activating extracellular signal-regulated kinase 1/2 (ERK 1/2) in abdominal aortic aneurysms. J. Am. Heart Assoc. 2018, 7, e010069. [Google Scholar] [CrossRef]
- Godmann, M.; Kosan, C.; Behr, R. Krüppel-like factor 4 is widely expressed in the mouse male and female reproductive tract and responds as an immediate early gene to activation of the protein kinase A in TM4 Sertoli cells. Reprod. Camb. Engl. 2010, 139, 771–782. [Google Scholar] [CrossRef]
- Cullingford, T.E.; Butler, M.J.; Marshall, A.K.; Tham, E.; Sugden, P.H.; Clerk, A. Differential regulation of Krüppel-like factor family transcription factor expression in neonatal rat cardiac myocytes: Effects of endothelin-1, oxidative stress and cytokines. Biochim. Biophys. Acta 2008, 1783, 1229–1236. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blum, A.; Mostow, K.; Jackett, K.; Kelty, E.; Dakpa, T.; Ryan, C.; Hagos, E. KLF4 Regulates Metabolic Homeostasis in Response to Stress. Cells 2021, 10, 830. https://doi.org/10.3390/cells10040830
Blum A, Mostow K, Jackett K, Kelty E, Dakpa T, Ryan C, Hagos E. KLF4 Regulates Metabolic Homeostasis in Response to Stress. Cells. 2021; 10(4):830. https://doi.org/10.3390/cells10040830
Chicago/Turabian StyleBlum, Andrew, Kate Mostow, Kailey Jackett, Estelle Kelty, Tenzing Dakpa, Carly Ryan, and Engda Hagos. 2021. "KLF4 Regulates Metabolic Homeostasis in Response to Stress" Cells 10, no. 4: 830. https://doi.org/10.3390/cells10040830
APA StyleBlum, A., Mostow, K., Jackett, K., Kelty, E., Dakpa, T., Ryan, C., & Hagos, E. (2021). KLF4 Regulates Metabolic Homeostasis in Response to Stress. Cells, 10(4), 830. https://doi.org/10.3390/cells10040830





