Cells with Many Talents: Lymphatic Endothelial Cells in the Brain Meninges
Abstract
1. Introduction
2. Lymphangiogenesis
3. Fluid and Macromolecule Clearance from the Brain
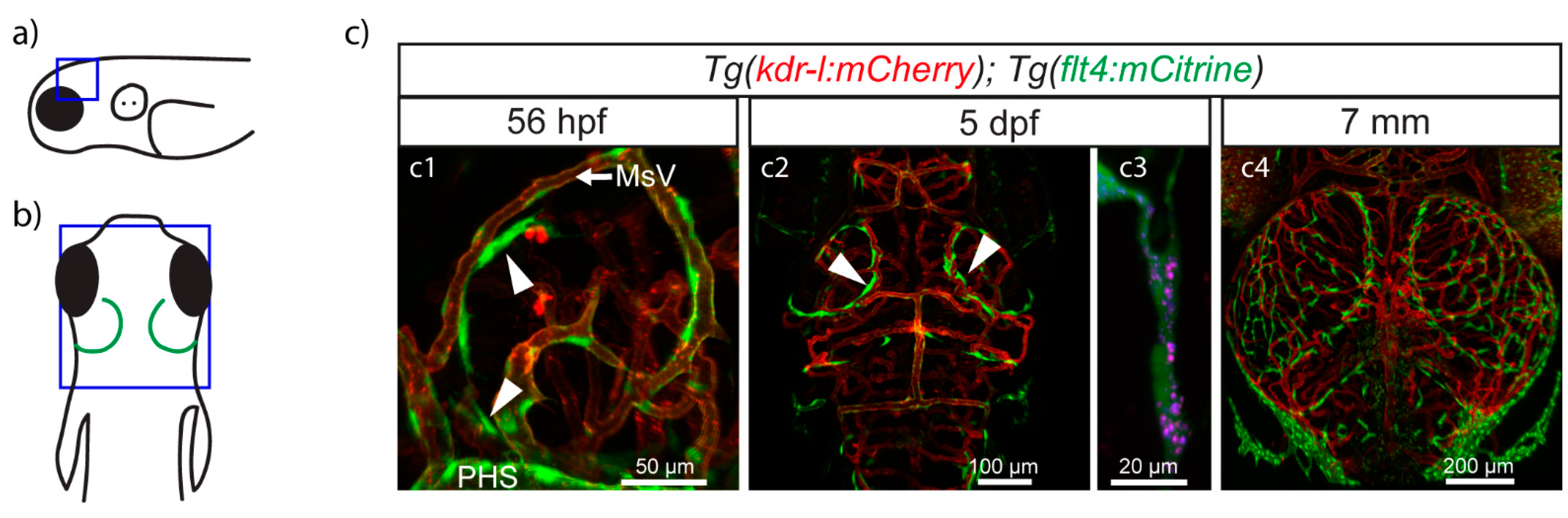
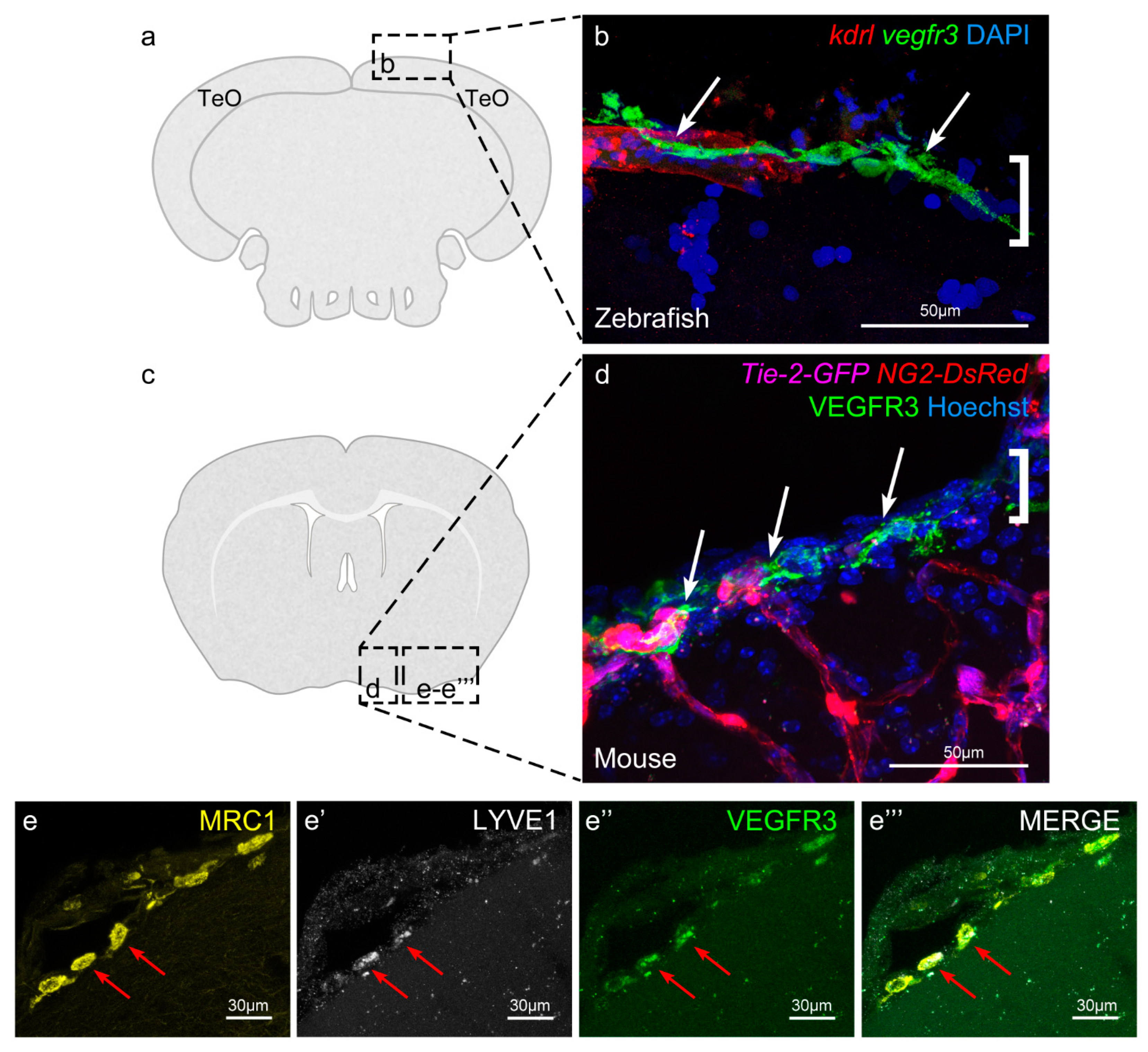
4. BLECs/FGPs/muLECs
5. Meningeal Lymphatic Cells in Mammals
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mauri, C.; Wang, G.; Schulte-Merker, S. From fish embryos to human patients: Lymphangiogenesis in development and disease. Curr. Opin. Immunol. 2018, 53, 167–172. [Google Scholar] [CrossRef]
- Oliver, G.; Kipnis, J.; Randolph, G.J.; Harvey, N.L. The Lymphatic Vasculature in the 21st Century: Novel Functional Roles in Homeostasis and Disease. Cell 2020, 182, 270–296. [Google Scholar] [CrossRef]
- González-Loyola, A.; Petrova, T.V. Development and aging of the lymphatic vascular system. Adv. Drug Deliv. Rev. 2021, 169, 63–78. [Google Scholar] [CrossRef]
- Tammela, T.; Alitalo, K. Lymphangiogenesis: Molecular Mechanisms and Future Promise. Cell 2010, 140, 460–476. [Google Scholar] [CrossRef]
- Küchler, A.M.; Gjini, E.; Peterson-Maduro, J.; Cancilla, B.; Wolburg, H.; Schulte-Merker, S. Development of the Zebrafish Lymphatic System Requires Vegfc Signaling. Curr. Biol. 2006, 16, 1244–1248. [Google Scholar] [CrossRef] [PubMed]
- Yaniv, K.; Isogai, S.; Castranova, D.; Dye, L.; Hitomi, J.; Weinstein, B.M. Live imaging of lymphatic development in the zebrafish. Nat. Med. 2006, 12, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Petrova, T.V.; Koh, G.Y. Organ-specific lymphatic vasculature: From development to pathophysiology. J. Exp. Med. 2018, 215, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Isogai, S.; Lawson, N.D.; Torrealday, S.; Horiguchi, M.; Weinstein, B.M. Angiogenic network formation in the developing vertebrate trunk. Development 2003, 130, 5281–5290. [Google Scholar] [CrossRef]
- Hägerling, R.; Pollmann, C.; Andreas, M.; Schmidt, C.; Nurmi, H.; Adams, R.H.; Alitalo, K.; Andresen, V.; Schulte-Merker, S.; Kiefer, F. A novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultramicroscopy. EMBO J. 2013, 32, 629–644. [Google Scholar] [CrossRef]
- Nicenboim, J.; Malkinson, G.; Lupo, T.; Asaf, L.; Sela, Y.; Mayseless, O.; Gibbs-Bar, L.; Senderovich, N.; Hashimshony, T.; Shin, M.; et al. Lymphatic vessels arise from specialized angioblasts within a venous niche. Nature 2015, 522, 56–61. [Google Scholar] [CrossRef]
- Karkkainen, M.J.; Haiko, P.; Sainio, K.; Partanen, J.; Taipale, J.; Petrova, T.V.; Jeltsch, M.; Jackson, D.G.; Talikka, M.; Rauvala, H.; et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 2004, 5, 74–80. [Google Scholar] [CrossRef]
- Dumont, D.J.; Jussila, L.; Taipale, J.; Lymboussaki, A.; Mustonen, T.; Pajusola, K.; Breitman, M.; Alitalo, K. Cardiovascular Failure in Mouse Embryos Deficient in VEGF Receptor-3. Science 1998, 282, 946–949. [Google Scholar] [CrossRef] [PubMed]
- Bos, F.L.; Caunt, M.; Peterson-Maduro, J.; Planas-Paz, L.; Kowalski, J.; Karpanen, T.; van Impel, A.; Tong, R.; Ernst, J.A.; Korving, J.; et al. CCBE1 Is Essential for Mammalian Lymphatic Vascular Development and Enhances the Lymphangiogenic Effect of Vascular Endothelial Growth Factor-C In Vivo. Circ. Res. 2011, 109, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Essalmani, R.; Hamelin, J.; Marcinkiewicz, J.; Chamberland, A.; Mbikay, M.; Chrétien, M.; Seidah, N.G.; Prat, A. Deletion of the Gene Encoding Proprotein Convertase 5/6 Causes Early Embryonic Lethality in the Mouse. Mol. Cell. Biol. 2006, 26, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Roebroek, A.J.M.; Umans, L.; Pauli, I.G.L.; Robertson, E.J.; van Leuven, F.; Van de Ven, W.J.; Constam, D.B. Failure of ventral closure and axial rotation in embryos lacking the proprotein convertase Furin. Development 1998, 125, 4863–4876. [Google Scholar] [PubMed]
- Janssen, L.; Dupont, L.; Bekhouche, M.; Noel, A.; Leduc, C.; Voz, M.; Peers, B.; Cataldo, D.; Apte, S.S.; Dubail, J.; et al. ADAMTS3 activity is mandatory for embryonic lymphangiogenesis and regulates placental angiogenesis. Angiogenesis 2016, 19, 53–65. [Google Scholar] [CrossRef]
- Roukens, M.G.; Peterson-Maduro, J.; Padberg, Y.; Jeltsch, M.; Leppänen, V.-M.; Bos, F.L.; Alitalo, K.; Schulte-Merker, S.; Schulte, D. Functional Dissection of the CCBE1 Protein. Circ. Res. 2015, 116, 1660–1669. [Google Scholar] [CrossRef]
- Hogan, B.M.; Bos, F.L.; Bussmann, J.; Witte, M.; Chi, N.C.; Duckers, H.J.; Schulte-Merker, S. ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat. Genet. 2009, 41, 396–398. [Google Scholar] [CrossRef]
- Wang, G.; Muhl, L.; Padberg, Y.; Dupont, L.; Peterson-Maduro, J.; Stehling, M.; le Noble, F.; Colige, A.; Betsholtz, C.; Schulte-Merker, S.; et al. Specific fibroblast subpopulations and neuronal structures provide local sources of Vegfc-processing components during zebrafish lymphangiogenesis. Nat. Commun. 2020, 11, 2724–2744. [Google Scholar] [CrossRef]
- Hogan, B.M.; Schulte-Merker, S. How to Plumb a Pisces: Understanding Vascular Development and Disease Using Zebrafish Embryos. Dev. Cell 2017, 42, 567–583. [Google Scholar] [CrossRef]
- Padberg, Y.; Schulte-Merker, S.; van Impel, A. The lymphatic vasculature revisited—new developments in the zebrafish. In Public Health; 2017; Volume 121, pp. 221–238. ISBN 9780128034736. [Google Scholar]
- Vaahtomeri, K.; Karaman, S.; Mäkinen, T.; Alitalo, K. Lymphangiogenesis guidance by paracrine and pericellular factors. Genes Dev. 2017, 31, 1615–1634. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Merker, S.; Sabine, A.; Petrova, T.V. Lymphatic vascular morphogenesis in development, physiology, and disease. J. Cell Biol. 2011, 193, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Kazenwadel, J.; Harvey, N.L. Lymphatic endothelial progenitor cells: Origins and roles in lymphangiogenesis. Curr. Opin. Immunol. 2018, 53, 81–87. [Google Scholar] [CrossRef]
- Martinez-Corral, I.; Ulvmar, M.H.; Stanczuk, L.; Tatin, F.; Kizhatil, K.; John, S.W.M.; Alitalo, K.; Ortega, S.; Makinen, T. Nonvenous Origin of Dermal Lymphatic Vasculature. Circ. Res. 2015, 116, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.; Norman, S.; Vieira, J.M.; Masters, M.; Rohling, M.; Dubé, K.N.; Bollini, S.; Matsuzaki, F.; Carr, C.A.; Riley, P.R. Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature 2015, 522, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Stanczuk, L.; Martinez-Corral, I.; Ulvmar, M.H.; Zhang, Y.; Laviña, B.; Fruttiger, M.; Adams, R.H.; Saur, D.; Betsholtz, C.; Ortega, S.; et al. cKit Lineage Hemogenic Endothelium-Derived Cells Contribute to Mesenteric Lymphatic Vessels. Cell Rep. 2015, 10, 1708–1721. [Google Scholar] [CrossRef]
- Okuda, K.S.; Astin, J.W.; Misa, J.P.; Flores, M.V.; Crosier, K.E.; Crosier, P.S. lyve1 expression reveals novel lymphatic vessels and new mechanisms for lymphatic vessel development in zebrafish. Development 2012, 139, 2381–2391. [Google Scholar] [CrossRef] [PubMed]
- Eng, T.C.; Chen, W.; Okuda, K.S.; Misa, J.P.; Padberg, Y.; Crosier, K.E.; Crosier, P.S.; Hall, C.J.; Schulte-Merker, S.; Hogan, B.M.; et al. Zebrafish facial lymphatics develop through sequential addition of venous and non-venous progenitors. EMBO Rep. 2019, 20, 1–17. [Google Scholar] [CrossRef]
- Flexner, L.B. Some Problems of the Origin, Circulation and Absorption of the Cerebrospinal Fluid. Q. Rev. Biol. 1933, 8, 397–422. [Google Scholar] [CrossRef]
- Rennels, M.L.; Gregory, T.F.; Blaumanis, O.R.; Fujimoto, K.; Grady, P.A. Evidence for a “Paravascular” fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 1985, 326, 47–63. [Google Scholar] [CrossRef]
- Cserr, H.F.; Ostrach, L.H. Bulk flow of interstitial fluid after intracranial injection of Blue Dextran 2000. Exp. Neurol. 1974, 45, 50–60. [Google Scholar] [CrossRef]
- Cserr, H.F.; Cooper, D.N.; Milhorat, T.H. Flow of cerebral interstitial fluid as indicated by the removal of extracellular markers from rat caudate nucleus. Exp. Eye Res. 1977, 25, 461–473. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef]
- Tarasoff-Conway, J.M.; Carare, R.O.; Osorio, R.S.; Glodzik, L.; Butler, T.; Fieremans, E.; Axel, L.; Rusinek, H.; Nicholson, C.; Zlokovic, B.V.; et al. Clearance systems in the brain—implications for Alzheimer disease. Nat. Rev. Neurol. 2015, 11, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Plog, B.A.; Nedergaard, M. The Glymphatic System in Central Nervous System Health and Disease: Past, Present, and Future. Annu. Rev. Pathol. Mech. Dis. 2018, 13, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Proulx, S.T. Cerebrospinal fluid outflow: A review of the historical and contemporary evidence for arachnoid villi, perineural routes, and dural lymphatics. Cell. Mol. Life Sci. 2021. [Google Scholar] [CrossRef]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Aspelund, A.; Antila, S.; Proulx, S.T.; Karlsen, T.V.; Karaman, S.; Detmar, M.; Wiig, H.; Alitalo, K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015, 212, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Hershenhouse, K.S.; Shauly, O.; Gould, D.J.; Patel, K.M. Meningeal Lymphatics: A Review and Future Directions From a Clinical Perspective. Neurosci. Insights 2019, 14, 117906951988902. [Google Scholar] [CrossRef] [PubMed]
- Da Mesquita, S.; Louveau, A.; Vaccari, A.; Smirnov, I.; Cornelison, R.C.; Kingsmore, K.M.; Contarino, C.; Onengut-Gumuscu, S.; Farber, E.; Raper, D.; et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 2018, 560, 185–191. [Google Scholar] [CrossRef]
- Benveniste, H.; Liu, X.; Koundal, S.; Sanggaard, S.; Lee, H.; Wardlaw, J. The Glymphatic System and Waste Clearance with Brain Aging: A Review. Gerontology 2019, 65, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Haorah, J. How does the brain remove its waste metabolites from within? Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 238–249. [Google Scholar] [PubMed]
- Wardlaw, J.M.; Benveniste, H.; Nedergaard, M.; Zlokovic, B.V.; Mestre, H.; Lee, H.; Doubal, F.N.; Brown, R.; Ramirez, J.; MacIntosh, B.J.; et al. Perivascular spaces in the brain: Anatomy, physiology and pathology. Nat. Rev. Neurol. 2020, 16, 137–153. [Google Scholar] [CrossRef]
- Agarwal, N.; Carare, R.O. Cerebral Vessels: An Overview of Anatomy, Physiology, and Role in the Drainage of Fluids and Solutes. Front. Neurol. 2021, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Pizzo, M.E.; Preston, J.E.; Janigro, D.; Thorne, R.G. The role of brain barriers in fluid movement in the CNS: Is there a “glymphatic” system? Acta Neuropathol. 2018, 135, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Ries, M.; Decker, Y.; Müller, A.; Riner, C.; Bücker, A.; Fassbender, K.; Detmar, M.; Proulx, S.T. Rapid lymphatic efflux limits cerebrospinal fluid flow to the brain. Acta Neuropathol. 2019, 137, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Hablitz, L.M.; Vinitsky, H.S.; Sun, Q.; Stæger, F.F.; Sigurdsson, B.; Mortensen, K.N.; Lilius, T.O.; Nedergaard, M. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci. Adv. 2019, 5, eaav5447. [Google Scholar] [CrossRef]
- Castranova, D.; Samasa, B.; Venero Galanternik, M.; Jung, H.M.; Pham, V.N.; Weinstein, B.M. Live Imaging of Intracranial Lymphatics in the Zebrafish. Circ. Res. 2021, 128, 42–58. [Google Scholar] [CrossRef] [PubMed]
- van Lessen, M.; Shibata-Germanos, S.; van Impel, A.; Hawkins, T.A.; Rihel, J.; Schulte-Merker, S. Intracellular uptake of macromolecules by brain lymphatic endothelial cells during zebrafish embryonic development. eLife 2017, 6, 1–24. [Google Scholar] [CrossRef]
- Galanternik, M.V.; Castranova, D.; Gore, A.V.; Blewett, N.H.; Jung, H.M.; Stratman, A.N.; Kirby, M.R.; Iben, J.; Miller, M.F.; Kawakami, K.; et al. A novel perivascular cell population in the zebrafish brain. eLife 2017, 6. [Google Scholar] [CrossRef]
- Bower, N.I.; Koltowska, K.; Pichol-Thievend, C.; Virshup, I.; Paterson, S.; Lagendijk, A.K.; Wang, W.; Lindsey, B.W.; Bent, S.J.; Baek, S.; et al. Mural lymphatic endothelial cells regulate meningeal angiogenesis in the zebrafish. Nat. Neurosci. 2017, 20, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Pomares, L. The mannose receptor. J. Leukoc. Biol. 2012, 92, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Padberg, Y.; Van Impel, A.; Van Lessen, M.; Bussmann, J.; Schulte-Merker, S. Meningeal lymphatic endothelial cells fulfill scavenger endothelial cell function and employ Mrc1a for cargo uptake. bioRxiv 2019. [Google Scholar] [CrossRef]
- Shibata-Germanos, S.; Goodman, J.R.; Grieg, A.; Trivedi, C.A.; Benson, B.C.; Foti, S.C.; Faro, A.; Castellan, R.F.P.; Correra, R.M.; Barber, M.; et al. Structural and functional conservation of non-lumenized lymphatic endothelial cells in the mammalian leptomeninges. Acta Neuropathol. 2020, 139, 383–401. [Google Scholar] [CrossRef]
- Platt, N.; da Silva, R.P.; Gordon, S. Recognizing death: The phagocytosis of apoptotic cells. Trends Cell Biol. 1998, 8, 365–372. [Google Scholar] [CrossRef]
- Campbell, F.; Bos, F.L.; Sieber, S.; Arias-Alpizar, G.; Koch, B.E.; Huwyler, J.; Kros, A.; Bussmann, J. Directing Nanoparticle Biodistribution through Evasion and Exploitation of Stab2-Dependent Nanoparticle Uptake. ACS Nano 2018, 12, 2138–2150. [Google Scholar] [CrossRef]
- Sørensen, K.K.; Simon-Santamaria, J.; McCuskey, R.S.; Smedsrød, B. Liver Sinusoidal Endothelial Cells. Compr. Physiol. 2015, 5, 1751–1774. [Google Scholar] [CrossRef]
- Chen, J.; He, J.; Ni, R.; Yang, Q.; Zhang, Y.; Luo, L. Cerebrovascular Injuries Induce Lymphatic Invasion into Brain Parenchyma to Guide Vascular Regeneration in Zebrafish. Dev. Cell 2019, 49, 697–710.e5. [Google Scholar] [CrossRef]
- Curado, S.; Stainier, D.Y.R.; Anderson, R.M. Nitroreductase-mediated cell/tissue ablation in zebrafish: A spatially and temporally controlled ablation method with applications in developmental and regeneration studies. Nat. Protoc. 2008, 3, 948–954. [Google Scholar] [CrossRef]
- Rutkowski, J.M.; Boardman, K.C.; Swartz, M.A. Characterization of lymphangiogenesis in a model of adult skin regeneration. Am. J. Physiol. Circ. Physiol. 2006, 291, H1402–H1410. [Google Scholar] [CrossRef]
- Liu, C.; Wu, C.; Yang, Q.; Gao, J.; Li, L.; Yang, D.; Luo, L. Macrophages Mediate the Repair of Brain Vascular Rupture through Direct Physical Adhesion and Mechanical Traction. Immunity 2016, 44, 1162–1176. [Google Scholar] [CrossRef] [PubMed]
- Gancz, D.; Raftrey, B.C.; Perlmoter, G.; Marín-Juez, R.; Semo, J.; Matsuoka, R.L.; Karra, R.; Raviv, H.; Moshe, N.; Addadi, Y.; et al. Distinct origins and molecular mechanisms contribute to lymphatic formation during cardiac growth and regeneration. eLife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.M.; Herman, A.; Ikegami, M.; Greenberg, J.M.; Akeson, A.L. Lymphatic ontogeny and effect of hypoplasia in developing lung. Mech. Dev. 2011, 128, 29–40. [Google Scholar] [CrossRef]
- Mato, M.; Ookawara, S. A simple method for observation of capillary nets in rat brain cortex. Experientia 1979, 35, 501–503. [Google Scholar] [CrossRef]
- Mato, M.; Ookawara, S.; Sugamata, M.; Aikawa, E. Evidence for the possible function of the fluorescent granular perithelial cells in brain as scavengers of high-molecular-weight waste products. Experientia 1984, 40, 399–402. [Google Scholar] [CrossRef]
- Mato, M.; Ookawara, S.; Aikawa, E.; Kawasaki, K. Studies on fluorescent granular perithelium (F.G.P.) of rat cerebral cortex—Especially referring to morphological changes in aging. Anat. Anz. 1981, 149, 486–501. [Google Scholar] [PubMed]
- Mato, M.; Ookawara, S.; Sakamoto, A.; Aikawa, E.; Ogawa, T.; Mitsuhashi, U.; Masuzawa, T.; Suzuki, H.; Honda, M.; Yazaki, Y.; et al. Involvement of specific macrophage-lineage cells surrounding arterioles in barrier and scavenger function in brain cortex. Proc. Natl. Acad. Sci. 1996, 93, 3269–3274. [Google Scholar] [CrossRef]
- Mendes-Jorge, L.; Ramos, D.; Luppo, M.; Llombart, C.; Alexandre-Pires, G.; Nacher, V.; Melgarejo, V.; Correia, M.; Navarro, M.; Carretero, A.; et al. Scavenger Function of Resident Autofluorescent Perivascular Macrophages and Their Contribution to the Maintenance of the Blood–Retinal Barrier. Investig. Opthalmology Vis. Sci. 2009, 50, 5997. [Google Scholar] [CrossRef]
- Mato, M.; Mato, T.K. Distribution and number of fluorescent granular perithelial cells in coronal sections of rats cerebrum. Experientia 1983, 39, 1374–1376. [Google Scholar] [CrossRef]
- Mato, M.; Ookawara, S.; Saito-Taki, T. Serological determinants of fluorescent granular perithelial cells along small cerebral blood vessels in rodent. Acta Neuropathol. 1986, 72, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Mato, M.; Ookawara, S.; Kurihara, K. Uptake of exogenous substances and marked infoldings of the fluorescent granular pericyte in cerebral fine vessels. Am. J. Anat. 1980, 157, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Mato, M.; Aikawa, E.; Mato, T.K.; Kurihara, K. Tridimensional observation of fluorescent granular perithelial (FGP) cells in rat cerebral blood vessels. Anat. Rec. 1986, 215, 413–419. [Google Scholar] [CrossRef]
- Mato, M.; Ookawara, S.; Mato, T.K.; Namiki, T. An attempt to differentiate further between microglia and fluorescent granular perithelial (FGP) cells by their capacity to incorporate exogenous protein. Am. J. Anat. 1985, 172, 125–140. [Google Scholar] [CrossRef]
- Ahn, J.H.; Cho, H.; Kim, J.H.; Kim, S.H.; Ham, J.S.; Park, I.; Suh, S.H.; Hong, S.P.; Song, J.H.; Hong, Y.K.; et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 2019, 572, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Ineichen, B.V.; Detmar, M.; Proulx, S.T. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat. Commun. 2017, 8, 1434. [Google Scholar] [CrossRef] [PubMed]
- Kress, B.T.; Iliff, J.J.; Xia, M.; Wang, M.; Wei Bs, H.S.; Zeppenfeld, D.; Xie, L.; Hongyi Kang, B.S.; Xu, Q.; Liew, J.A.; et al. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 2014, 76, 845–861. [Google Scholar] [CrossRef]
- Harrison, M.R.; Feng, X.; Mo, G.; Aguayo, A.; Villafuerte, J.; Yoshida, T.; Pearson, C.A.; Schulte-Merker, S.; Lien, C.-L. Late developing cardiac lymphatic vasculature supports adult zebrafish heart function and regeneration. eLife 2019, 8, 1–21. [Google Scholar] [CrossRef]

| BLECs/FGPs/muLECs | LLECs | Mato Cells | Meningeal Lymphatic Vessels | |
|---|---|---|---|---|
| Organism | Zebrafish [50,51,52] | Mammals [55] | Mammals [65] | Zebrafish and mammals [38,39,49] |
| Localization | Meninges, outside basement membrane of meningeal blood vessels [50,51,52] | Leptomeninges, outside basement membrane of meningeal blood vessels [55] | Brain cortex, in the Virchow-Robin space of arterioles and venules [68,70] | Dura mater in mice, meninges in zebrafish [38,39,49] |
| Marker/gene expression | Lymphatic marker genes (vegfr3, prox1, fli1a, stabilin 1 and 2, mafba, lyve1b nrp2a); mrc1a; pro-lymphangiogenic and angiogenic factors [50,51,52] | Lymphatic marker genes (Prox1, Lyve1, Vegfr3); Mrc1 [55] | Macrophage marker genes (1a antigen, Fcγ2a and 2b) Type I and II Scavenger Receptors [68,71] | Lymphatic marker genes (lyve1b and prox1 in zebrafish, Lyve1, Prox1, Vegfr3, Podoplanin in mice); mrc1a in zebrafish, CD31 in mice [38,39,49] |
| Function | Uptake of exogenous substances; blood vessel regeneration in trauma model [50,51,52,59] | Uptake of Amyloid β and Dextran [55] | Scavenging function [66,68,69,72] | Fluid drainage from the brain, transport of immune cells [38,39,49] |
| Prominent lysosomal vesicles | Large, circular, homogeneous [50,51,52] | Large, circular, homogeneous [55] | Size and shape variable depending on age and diet [73] | None |
| Origin | Venous choroidal vascular plexus. Not of haemopoietic origin [50,51,52] | Unknown, but not of haemopoietic origin [55] | Unknown, but suggested to derive from leptomeningeal cells [74] | Facial lymphatics in fish, at least in part [49]. Unclear in mammals. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suárez, I.; Schulte-Merker, S. Cells with Many Talents: Lymphatic Endothelial Cells in the Brain Meninges. Cells 2021, 10, 799. https://doi.org/10.3390/cells10040799
Suárez I, Schulte-Merker S. Cells with Many Talents: Lymphatic Endothelial Cells in the Brain Meninges. Cells. 2021; 10(4):799. https://doi.org/10.3390/cells10040799
Chicago/Turabian StyleSuárez, Irina, and Stefan Schulte-Merker. 2021. "Cells with Many Talents: Lymphatic Endothelial Cells in the Brain Meninges" Cells 10, no. 4: 799. https://doi.org/10.3390/cells10040799
APA StyleSuárez, I., & Schulte-Merker, S. (2021). Cells with Many Talents: Lymphatic Endothelial Cells in the Brain Meninges. Cells, 10(4), 799. https://doi.org/10.3390/cells10040799






