Effect of Agroecological Conditions on Biologically Active Compounds and Metabolome in Carrot
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Production Systems
2.3. Spontaneous Infection
2.4. Chemical Analyses
2.4.1. AA Determination
2.4.2. Carotene Determination
2.5. Metabolomic Fingerprinting
2.5.1. DART-OrbitrapMS Method
2.5.2. Chemometric Analysis
3. Results and Discussion
3.1. Effect of Farming System on AA Content of Carrot Cultivars
3.2. Effect of Farming System on the Content of Carotenes in Carrot Cultivars
3.3. Effect of Locality on AA and Carotenes Content of Carrot Cultivars
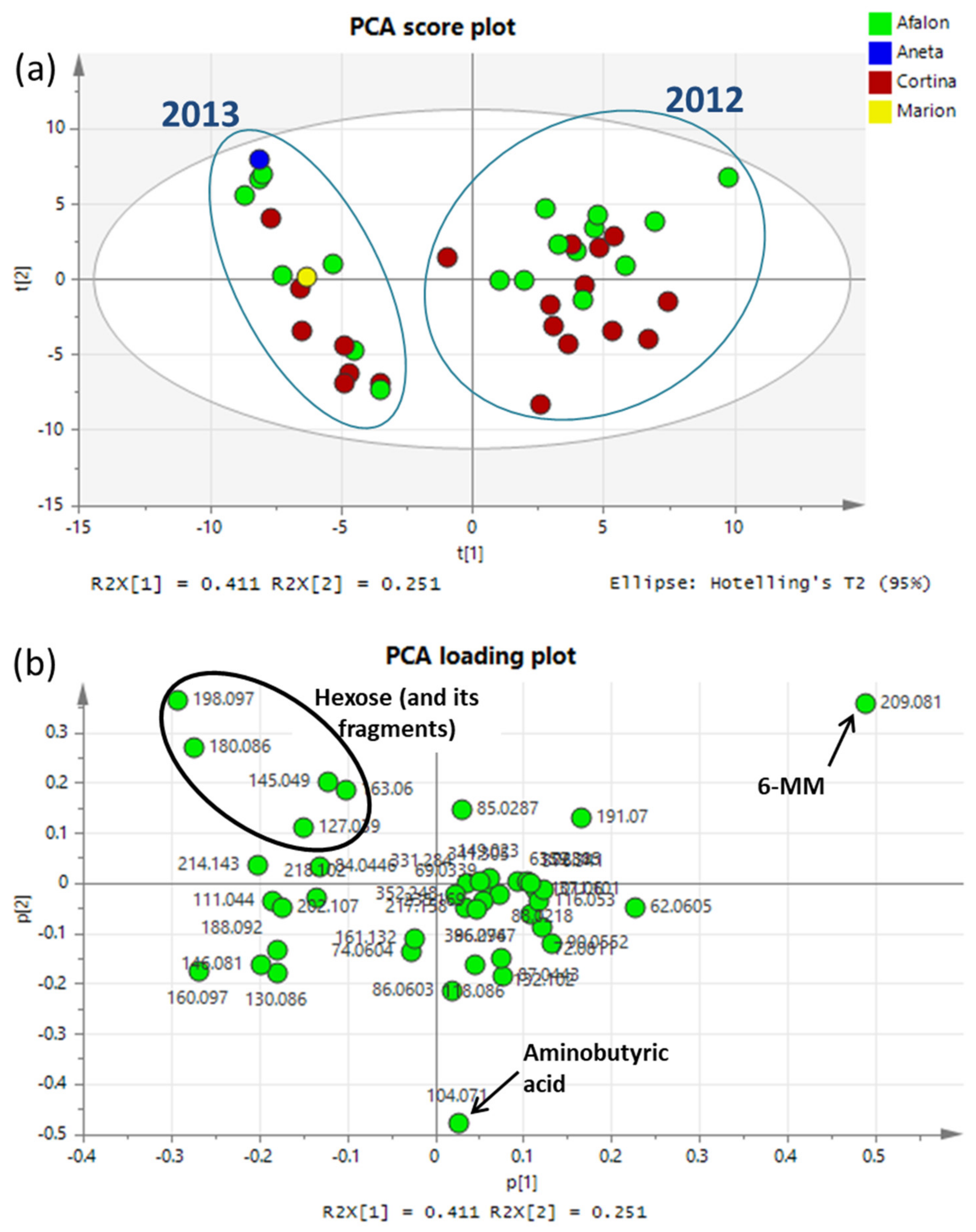
3.4. Metabolomic Profiling
Marker Identification
3.5. Effect of Farming System on Spontaneous Infection of Carrot
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, A.; Cawood, M.; Iqbal, Q.; Arino, A.; Batool, A.; Tariq, R.M.S.; Azam, M.; Akhtar, S. Phytochemicals in Daucus carota and their health benefits–review article. Foods 2019, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Buchtová, I. Situational and Development Review: Vegetables; Ministry of Agriculture of the Czech Republic, Institute of Agricultural Economics and Information: Prague, Czech Republic, 2019; Available online: http://eagri.cz/public/web/file/644345/SVZ_Zelenina_12_2019.pdf (accessed on 30 September 2020). (In Czech)
- Bozalan, N.K.; Karadeniz, F. Carotenoid profile, total phenolic content, and antioxidant activity of carrots. Int. J. Food Propert. 2011, 14, 1060–1068. [Google Scholar] [CrossRef]
- Cwalina-Ambroziak, B.; Amarowicz, R.; Glosek, M.; Janiak, M. Changes in the concentrations of phenolic acids in carrot plants inoculated with Alternaria radicina Meier, Drechsler & Eddy. Acta Sci. Pol. Hortorum Cultus 2014, 13, 97–108. [Google Scholar]
- Seljasen, R.; Vogt, G.; Olsen, E.; Lea, P.; Hogetveit, L.A.; Tajet, T.; Meadow, R.; Bengtsson, G.B. Influence of field attack by carrot psyllid (Trioza apicalis Förster) on sensory quality, antioxidant capacity and content of terpenes, falcarindiol and 6-methoxymellein of carrots (Daucus carrota L.). J. Agric. Food Chem. 2013, 61, 2831–2838. [Google Scholar] [CrossRef][Green Version]
- Lachman, J.; Orsák, M.; Pivec, V. Antioxidant contents and composition in some vegetables and their role in human nutrition. Zahrad. Horticul. Sci. 2000, 27, 65–78. [Google Scholar]
- Smirnoff, N.; Wheeler, G.L. Ascorbic acid in plants: Biosynthesis and function. Crit. Rev. Biochem. Mol. Biol. 2000, 35, 291–314. [Google Scholar] [CrossRef]
- Nicolle, C.; Simon, G.; Rock, E.; Amouroux, P.; Remesy, C. Genetic variability influences carotenoid, vitamin, phenolic, and mineral content in white, yellow, purple, orange, and dark-orange carrot cultivars. J. Soc. Hortic. Sci. 2004, 129, 523–529. [Google Scholar] [CrossRef]
- Kim, J.E.; Rensing, K.H.; Douglas, C.J.; Cheng, K.M. Chromoplasts ultrastructure and estimated carotene content in root secondary phloem of different carrot varieties. Planta 2010, 231, 549–558. [Google Scholar] [CrossRef]
- Mustafa, A.; Trevino, L.M.; Turner, C. Pressurized hot ethanol extraction of carotenoids from carrot by-products. Molecules 2012, 17, 1809. [Google Scholar] [CrossRef]
- Alasalvar, C.; Grigor, M.; Zhang, D.; Quantick, P.C.; Shadidi, F. Comparison of volatiles, phenolics, sugars, antioxidant vitamins and sensory quality of different colored carrot varieties. J. Agric. Food Chem. 2001, 49, 410–416. [Google Scholar] [CrossRef]
- Metzger, B.T.; Barnes, D.M.; Reed, J.D. Purple carrot (Daucus carota L. ) polyacetylenes decrease lipopolysacharide-induced expression of inflammatory proteins in macrophage and endothelial cells. J. Agric. Food Chem. 2008, 56, 3554–3560. [Google Scholar] [CrossRef] [PubMed]
- Seljasen, R.; Bengtsson, G.B.; Hoftun, H.; Vogt, G. Sensory and chemical changes in five varieties of carrot (Daucus carota L.) in response to mechanical stress at harvest and post-harvest. J. Sci. Food Agric. 2001, 81, 436–447. [Google Scholar] [CrossRef]
- Kidmose, U.; Hansen, S.L.; Christensen, L.P.; Edelenbos, M.; Larsen, E.; Norbaek, R. Effects of genotype, root size, storage, and processing on bioactive compounds in organically grown carrots (Daucus carota L.). J. Food Sci. 2004, 69, S388–S394. [Google Scholar] [CrossRef]
- Rico, D.; Martín-Diana, A.B.; Barat, J.M.; Barry-Ryan, C. Extending and measuring the quality of fresh-cut fruit and vegetables: A review. Trends Food Sci. Technol. 2007, 18, 373–386. [Google Scholar] [CrossRef]
- Matějková, J.; Petříková, K. Variation in content of carotenoids and vitamin C in carrots. Not. Sci. Biol. 2010, 2, 88–91. [Google Scholar] [CrossRef]
- Stan, S.D.; Kar, S.; Stoner, G.D. Bioactive food components and cancer risk reduction. J. Cell. Biochem. 2008, 104, 339–356. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, C.; Al-Farsi, M.; Quantick, P.; Shahidi, F.; Wiktorowicz, R. Effect of chill storage and modified atmosphere packaging (MAP) on antioxidant activity, anthocyanins, carotenoids, phenolics and sensory quality of ready-to-eat shredded orange and purple carrots. Food Chem. 2005, 89, 69–76. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Soltoft, M.; Nielsen, J.; Holst Laursen, K.; Husted, S.; Halekoh, U.; Knuthsen, P. Effects of organic and conventional growth systems on the content of flavonoids in onions and phenolic acids in carrots and potatoes. J. Agric. Food Chem. 2010, 58, 10323–10329. [Google Scholar] [CrossRef]
- Sliwinska, A.; Naliwajski, M.R.; Pietrosiuk, A.; Syklowska-Baranek, K. In vitro response of Polyscias filicifolia (Araliaceae) shoots to elicitation with alarmone-diadenosine triphosphate, methyl jasmonate, and salicylic acid. Cells 2021, 10, 419. [Google Scholar] [CrossRef]
- Ceglie, F.G.; Amodio, M.L.; Colelli, G. Effect of organic production systems on quality and postharvest performance of horticultural produce. Horticulturae 2016, 2, 4. [Google Scholar] [CrossRef]
- Faligowska, A.; Panasiewicz, K.; Szymanska, G.; Ratajczak, K.; Sulewska, H.; Pszczolkowska, A.; Kocira, L.A. Influence of farming system on weed infestation and on productivity of narrow-leaved lupin (Lupinus angustifolius L.). Agriculture 2020, 10, 459. [Google Scholar] [CrossRef]
- Rembialkowska, E. Organic farming as a system to provide better vegetable quality. In Proceedings of the International Conference on Quality in Chains, Vols 1 and 2: An Integrated View on Fruit and Vegetable Quality; Tijskens, L.M.M., Vollebregt, H.M., Eds.; Acta Hortic: Leuven, Belgium, 2003; Volume 604, pp. 473–479. [Google Scholar]
- Khakbazan, M.; Henry, R.; Haung, J.; Mohr, R.; Peters, R.; Fillmore, S.; Rodd, V.; Mills, A. Economics of organically managed and conventional potato production systems in Atlantic Canada. Can. J. Plant Sci. 2015, 95, 161–174. [Google Scholar] [CrossRef]
- Mitchell, A.E.; Hong, Y.J.; Koh, E.; Barret, D.M.; Bryant, D.E.; Denison, R.F.; Kaffka, S. Ten-year comparison of the influence of organic and conventional crop management practices on the content of flavonoids in tomatoes. J. Agric. Food Chem. 2007, 55, 6154–6159. [Google Scholar] [CrossRef] [PubMed]
- Sink, N.; Mikulic-Petrovsek, M.; Veberic, R.; Kacjan Marsic, N. Chemical composition and morphometric traits and yield of carrots grown in organic and integrated farming systems. Turk. J. Agric. For. 2017, 41, 452–462. [Google Scholar] [CrossRef]
- Soltoft, M.; Bysted, A.; Madsen, K.H.; Mark, A.B.; Bűgel, S.G.; Nielsen, J.; Knuthsen, P. Effects of organic and conventional growth on the content of carotenoids in carrot roots, and on intake and plasma status of carotenoids in humans. J. Sci. Food Agric. 2011, 91, 767–775. [Google Scholar] [CrossRef]
- Pacifico, D.; Onofri, C.; Parisi, B.; Ostano, P.; Mandolino, G. Influence of organic farming on the potato transcriptome. Sustainability 2017, 9, 779. [Google Scholar] [CrossRef]
- Ierna, A.; Parisi, B. Crop growth and tuber yield of “early” potato crop under organic and conventional farming. Scient. Horticult. 2014, 165, 260–265. [Google Scholar] [CrossRef]
- Brazinskiene, V.; Asakaviciute, R.; Miezeliene, A.; Alencikiene, G.; Ivanauskas, L.; Jakstas, V.; Viskelis, P.; Razukas, A. Effect of farming systems on the yield, quality parameters and sensory properties of conventionally and organically grown potato (Solanum tuberosum L.) tubers. Food Chem. 2014, 145, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Tein, B.; Kauer, K.; Runno-Paurson, E.; Eremeev, V.; Luik, A.; Selge, A.; Loit, E. The potato tuber disease occurrence as affected by conventional and organic farming systems. Am. J. Potato Res. 2015, 92, 662–672. [Google Scholar] [CrossRef]
- Lazzaro, I.; Moretti, A.; Giorni, P.; Brera, C.; Battilani, P. Organic vs. conventional farming: Differences in infection by mycotoxin-producing fungi on maize and wheat in Northern and Central Italy. Crop Prot. 2015, 72, 22–30. [Google Scholar] [CrossRef]
- Farrar, J.J.; Pryor, B.M.; Davis, R.M. Alternaria diseases of carrot. Plant Dis. 2004, 88, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Gugino, B.K.; Carroll, J.; Chen, J.; Ludwig, J.; Abawi, G. Carrot Leaf Blight Diseases and Their Management in New York. In NYS Integrated Pest Management Program; New York State IPM Program: Geneva, NY, USA, 2004; Available online: www.nysipm.cornell.edu/factsheets/vegetables/misc/clb.pdf (accessed on 1 April 2021).
- Harding, V.K.; Heale, J.B. Isolation and identification of the antifungal compounds accumulating in the induced resistence response of carrot root slices to Botrytis cinerea. Physiol. Plant Pathol. 1980, 17, 277–289. [Google Scholar]
- Mercier, J.; Kuc, J. Elicitation of 6-methoxymellein in carrot leaves by Cercospora carotae. J. Sci. Food Agric. 1997, 73, 60–62. [Google Scholar] [CrossRef]
- Lecomte, M.; Berruyer, R.; Hamama, L.; Boedo, C.; Hudhomme, P.; Bersihand, S.; Arul, J.; N’Guyen, G.; Gatto, J.; Guilet, D.; et al. Inhibitory effects of the carrot metabolites 6-methoxymellein and falcarindiol on development of the fungal leaf blight pathogen Alternaria dauci. Physiol. Mol. Plant Pathol. 2012, 80, 58–67. [Google Scholar] [CrossRef]
- Louarn, S.; Nawrocki, A.; Edelenbos, M.; Jensen, D.F.; Jensen, O.N.; Collinge, D.B.; Jensen, B. The influence of the fungal pathogen Mycocentrospora acerina on the proteome and polyacetylenes and 6-methoxymellein in organic and conventionally cultivated carrots (Daucus carota) during postharvest storage. J. Proteom. 2012, 75, 962–977. [Google Scholar] [CrossRef]
- Mercier, J.; Roussel, D.; Charles, M.T.; Arul, J. Systemic and local responses associated with UV and pathogen induced resistance to Botrytis cinerea in stored carrot. Phytopathology 2000, 90, 981–986. [Google Scholar] [CrossRef]
- Kouassi, N.; Corcuff, R.; Arul, J.; Tweddell, R.J. Effect of storage temperature and age after harvest on the accumulation of the phytoalexin 6-methoxymellein in UV-C treated carrots. Acta Hortic. 2012, 945, 135–138. [Google Scholar] [CrossRef]
- Fan, X.; Mattheis, J.P.; Roberts, R.G. Biosynthesis of phytoalexin in carrot root requires ethylene action. Physiol. Plant. 2000, 110, 450–454. [Google Scholar] [CrossRef]
- Kramer, M.; Bufler, G.; Ulrich, D.; Leitenberger, M.; Conrad, J.; Carle, R.; Kammerer, D.R. Effect of ethylene and 1-methylcyclopropene on bitter compounds in carrots (Daucus carota L.). Postharvest. Biol. Technol. 2012, 73, 28–36. [Google Scholar] [CrossRef]
- Eisebai, M.F.; Ghabbour, H.A.; Legrave, N.; Fontaine-Vive, F.; Mehiri, M. New bioactive chlorinated cyclopentene derivatives from the marine-derived fungus Phoma sp. Med. Chem. Res. 2018, 27, 1885–1892. [Google Scholar] [CrossRef]
- Medical, T. Isolation and antimicrobial activity of the phytoalexin 6-methoxymellein from cultured carrot cells. Phytochemistry 1983, 22, 669–672. [Google Scholar]
- Mercier, J.; Arul, J.; Julien, C. Effect of food preparation on the isocoumarin, 6-methoxymellein content of UV-treated carrots. Food Res. Int. 1994, 27, 401–404. [Google Scholar] [CrossRef]
- Jayaraj, J.; Rahman, M.; Wan, A.; Punja, Z.K. Enhanced resistance to foliar fungal pathogens in carrot by application of elicitors. Ann. Appl. Biol. 2009, 155, 71–80. [Google Scholar] [CrossRef]
- Amin, M.; Kurosaki, F.; Nishi, A. Extracellular pectinolytic enzymes of fungi elicit phytoalexin accumulation in carrot suspension culture. J. Gen. Microbiol. 1986, 132, 771–777. [Google Scholar]
- De Girolamo, A.; Solfrizzo, M.; Vitti, C.; Visconti, A. Occurrence of 6-methoxymellein in fresh and processed carrots and relevant effect of storage and processing. J. Agric. Food Chem. 2004, 52, 6478–6484. [Google Scholar] [CrossRef]
- Liu, R.; Choi, H.S.; Kim, S.L.; Kim, J.H.; Yun, B.S.; Lee, B.S. 6-Methoxymellein isolated from carrot (Daucus carota L.) targets breast cancer stem cells by regulating NF-κB signaling. Molecules 2020, 25, 4374. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, A.; Dubourg, C.; Briard, M. Evaluation of carrot resistance to alternaria leaf blight in controlled environments. Plant Pathol. 2006, 55, 68–72. [Google Scholar] [CrossRef]
- Lundegardh, B.; Botek, P.; Schulzová, V.; Hajšlová, J.; Stromnerg, A.; Andersson, C. Impact of different green manures on the content of S-alk(en)yl-L-cysteine sulfoxides and L-ascorbic acid in leek (Allium porrum). J. Agric. Food Chem. 2008, 56, 2102–2111. [Google Scholar] [CrossRef]
- Bhave, A.; Schulzova, V.; Chmelarova, H.; Mrnka, L.; Hajslova, J. Assessment of the rosehips based on their biologically active compound content. J. Food Drug Anal. 2017, 25, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.D.; Karki, S.; Thakur, N.S.; Attri, S. Chemical composition, functional properties and processing of carrot–A review. J. Food Sci. Technol. 2012, 49, 22–32. [Google Scholar] [CrossRef]
- Bratu, M.; Avram, D.; Buruleanu, L. The minerals and vitamin content variation from different vegetables raw materials. Appl. Sci. Res. Coll. Lith. 2006, 4, 86–88. [Google Scholar]
- Faisal, N.A.; Chatha, S.A.S.; Hussain, A.I.; Ikram, M.; Bukhari, S.A. Liaison of phenolic acid and biological activity of escalating cultivars of Daucus carota. Int. J. Food Prop. 2017, 20, 2782–2792. [Google Scholar] [CrossRef][Green Version]
- Seljasen, R.; Lea, P.; Torp, T.; Riley, H.; Berentsen, E.; Thomsen, M.; Bengtsson, G.B. Effect of genotype, soil, type, year and fertilization on sensory and morphological attributes of carrots (Daucus carrota L.). J. Sci. Food. Agric. 2012, 92, 1786–1799. [Google Scholar] [CrossRef]
- Perrin, F.; Dubois-Laurent, C.; Gibon, Y.; Citerne, S.; Huet, S.; Suel, A.; Le Clerc, V.; Briard, M.; Hamama, L.; Peltier, D.; et al. Combined Alternaria dauci infection and water stresses impact carotenoid content of carrot leaves and roots. Environ. Exp. Bot. 2017, 142, 125–134. [Google Scholar] [CrossRef]
- Novotný, Č.; Schulzová, V.; Krmela, A.; Hajšlová, J.; Svobodová, K.; Koudela, M. Ascorbic acid and glucosinolate levels in new Czech cabbage cultivars: Effect of production system and fungal infection. Molecules 2018, 23, 1855. [Google Scholar] [CrossRef] [PubMed]
- Fjelkner-Modig, S.; Bengtsson, H.; Stegmark, R.; Nystrom, S. The influence of organic and integrated production on nutritional, sensory and agricultural aspects of vegetable raw materials for food production. Acta Agric. Scand. Sect. B Soil Plant Sci. 2001, 50, 102–113. [Google Scholar] [CrossRef]
- Middleton, E.M.; Teramura, A.H. The role of flavonol glycosides and carotenoids in protecting soybean from ultraviolet-B damage. Plant Physiol. 1993, 103, 741–752. [Google Scholar] [CrossRef]
- Evers, A.M.; Tuuri, H.; Hagg, M.; Plaami, S.; Hakkinen, U.; Talvitie, H. Soil forming and plant density effects on carrot yield and internal quality. Plant Foods Hum. Nutr. 1997, 51, 283–294. [Google Scholar] [CrossRef]
- Heinonen, M.I.; Ollilainen, V.; Linkola, E.K.; Varo, P.T.; Koivistoinen, P.E. Carotenoids in Finnish foods: Vegetables, fruits, and berries. J. Agric. Food Chem. 1989, 37, 655–659. [Google Scholar] [CrossRef]
- Simon, P.W.; Wolff, X.Y. Carotenes in typical and dark orange carrots. J. Agric. Food Chem. 1987, 35, 1017–1022. [Google Scholar] [CrossRef]
- Brandt, K.; Leifert, C.; Sanderson, R.; Seal, J.S. Agroecosystem management and nutritional quality of plant foods: The case of organic fruits and vegetables. Crit. Rev. Plan Sci. 2011, 30, 177–197. [Google Scholar] [CrossRef]
- Novotná, H.; Kmiecik, O.; Galazka, M.; Krtková, V.; Hurajová, A.; Schulzová, V.; Hallmann, E.; Rembialkowska, E.; Hajšlová, J. Metabolomic fingerprinting employing DART-TOFMS for authentication of tomatoes and peppers from organic and conventional farming. Food Add. Contam. Part A 2012, 29, 1335–1346. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.J.; Scott, K.J. Development and evaluation of an HPLC method for the analysis of carotenoids in foods, and the measurement of the carotenoid content of vegetables and fruits commonly consumed in the UK. Food Chem. 1995, 54, 101–111. [Google Scholar] [CrossRef]
- Maiani, G.; Periago Caston, M.J.; Catasta, G.; Toti, E.; Goni, I.; Cambrodon, A.B.; Granado-Lorencio, F.; Olmedilla-Alonso, B.; Knuthsen, P.; Valoti, M.; et al. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Molec. Nutr. Food Res. 2009, 53, S194–S218. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, F.; Raffo, A.; Kristensen, H.L.; Thorup-Kristensen, A.; Ploeger, A.; Kahl, J. Multi-method comparison of carrot quality from a conventional and three organic cropping systems with increasing levels of nutrient recycling. J. Sci. Food Agric. 2012, 92, 2855–2869. [Google Scholar] [CrossRef]
- Kotíková, Z.; Hejtmánková, A.; Lachman, J.; Hamouz, K.; Trnková, E.; Dvořák, P. Effect of selected factors on total carotenoid content in potato tubers (Solanum tuberosum L.). Plant Soil Environ. 2007, 53, 355–360. [Google Scholar] [CrossRef]
- Brdar-Jokanovič, M.; Koren, A.; Ljevnaič-Mašič, B.; Kiprovski, B.; Sikora, V. Yield and quality parameters of Hokkaido type pumpkins grown in Serbia. Genetika 2019, 51, 377–387. [Google Scholar] [CrossRef]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid metabolism in plants. Molec. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef]
- Diretto, G.; Tavazza, R.; Welsch, R.; Pizzichini, D.; Mourgues, F.; Papacchioli, V.; Beyer, P.; Giuliano, G. Metabolic engineering of potato tuber carotenoids through tuber-specific silencing of lycopene epsilon cyclase. BMC Plant Biol. 2006, 6, 13. [Google Scholar] [CrossRef]
- Yu, B.; Lydiate, D.; Young, L.; Schäfer, U.; Hannoufa, A. Enhancing the carotenoid content of Brassica napus seeds by downregulating lycopene epsilon cyclase. Transgen. Res. 2008, 17, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, Y.H.; Ahn, Y.O.; Jeong, J.C.; Lee, H.S.; Kwak, S.S. Downregulation of the lycopene ε-cyclase gene increases carotenoid synthesis via the β-branch-specific pathway and enhances salt-stress tolerance in sweet potato transgenic calli. Physiol. Plant. 2013, 147, 432–442. [Google Scholar] [CrossRef]
- Hajslova, J.; Cajka, T.; Vaclavik, L. Challenging applications offered by direct analysis in real time (DART) in food-quality and safety analysis. TrAC Trends Analyt. Chem. 2011, 30, 204–218. [Google Scholar] [CrossRef]
- Cody, R.B.; Laramée, J.A.; Durst, H.D. Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal. Chem. 2005, 77, 2297–2302. [Google Scholar] [CrossRef]
- Guilherme, R.; Aires, A.; Rodrigues, N.; Peres, A.M.; Pereira, J.A. Phenolics and antioxidant activity of green and red sweet peppers from organic and conventional agriculture: A comparative study. Agriculture 2020, 10, 652. [Google Scholar] [CrossRef]
- Rotem, J. The Genus Alternaria: Biology, Epidemiology and Pathogenicity; American Phytopathological Society: St. Paul, MN, USA, 1994. [Google Scholar]
- Carvalho, A.M.G.; Junqueira, A.M.; Vieira, J.V.; Reis, A.; Silva, J.B.C. Produtividade, florescimento premature, e queima-das-folhas em cenou-ra cultivada em Sistema organico e convencional. Hortic. Bras. 2005, 23, 250–254. [Google Scholar] [CrossRef]
- Gugino, T.L.; Caroll, J.E.; Widmer, T.L.; Chen, P.; Abawi, G.S. Field evaluation of carrot cultivars susceptibility to fungal leaf blight diseases in New York. Crop Prot. 2007, 26, 709–714. [Google Scholar] [CrossRef]
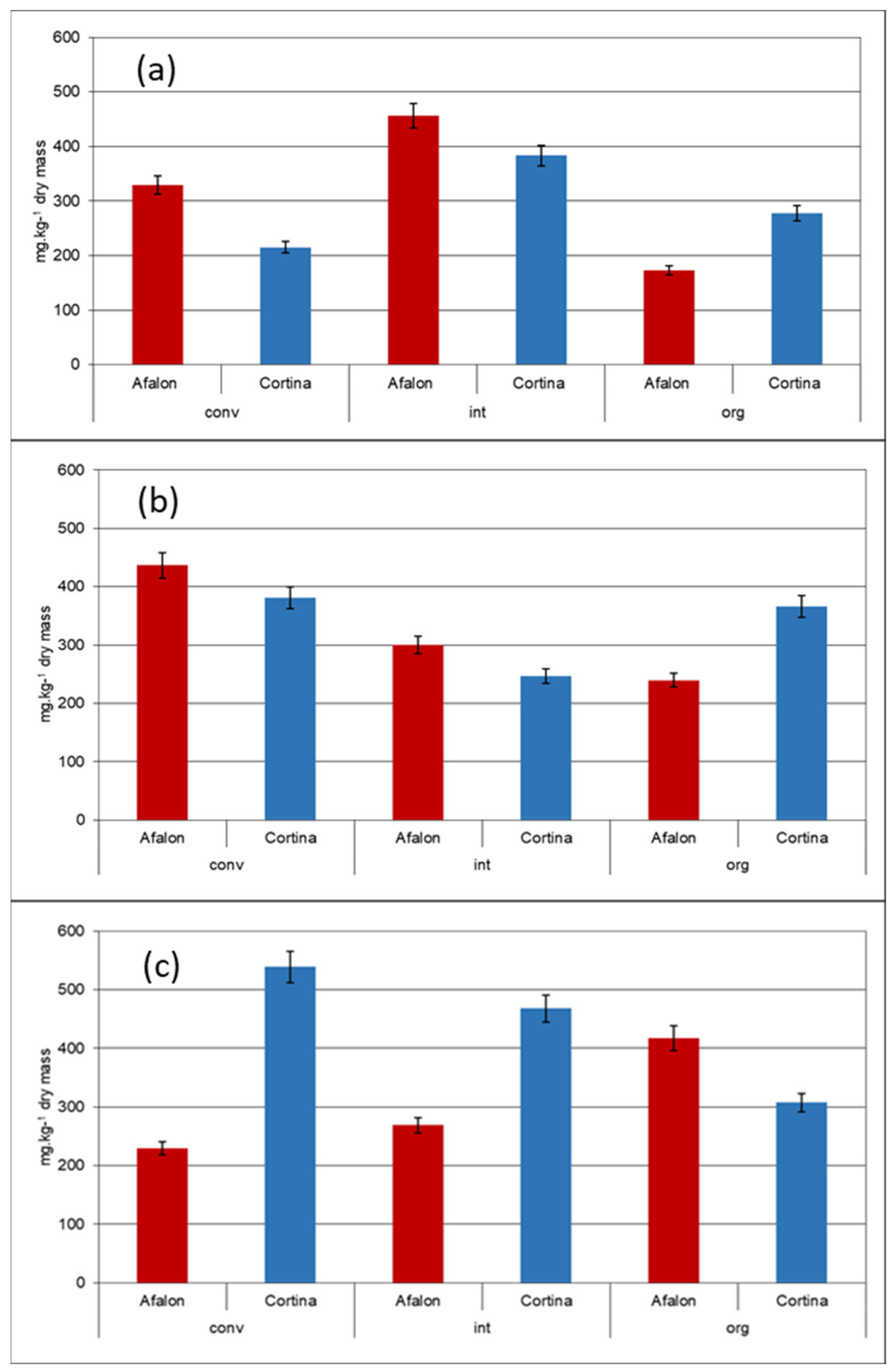
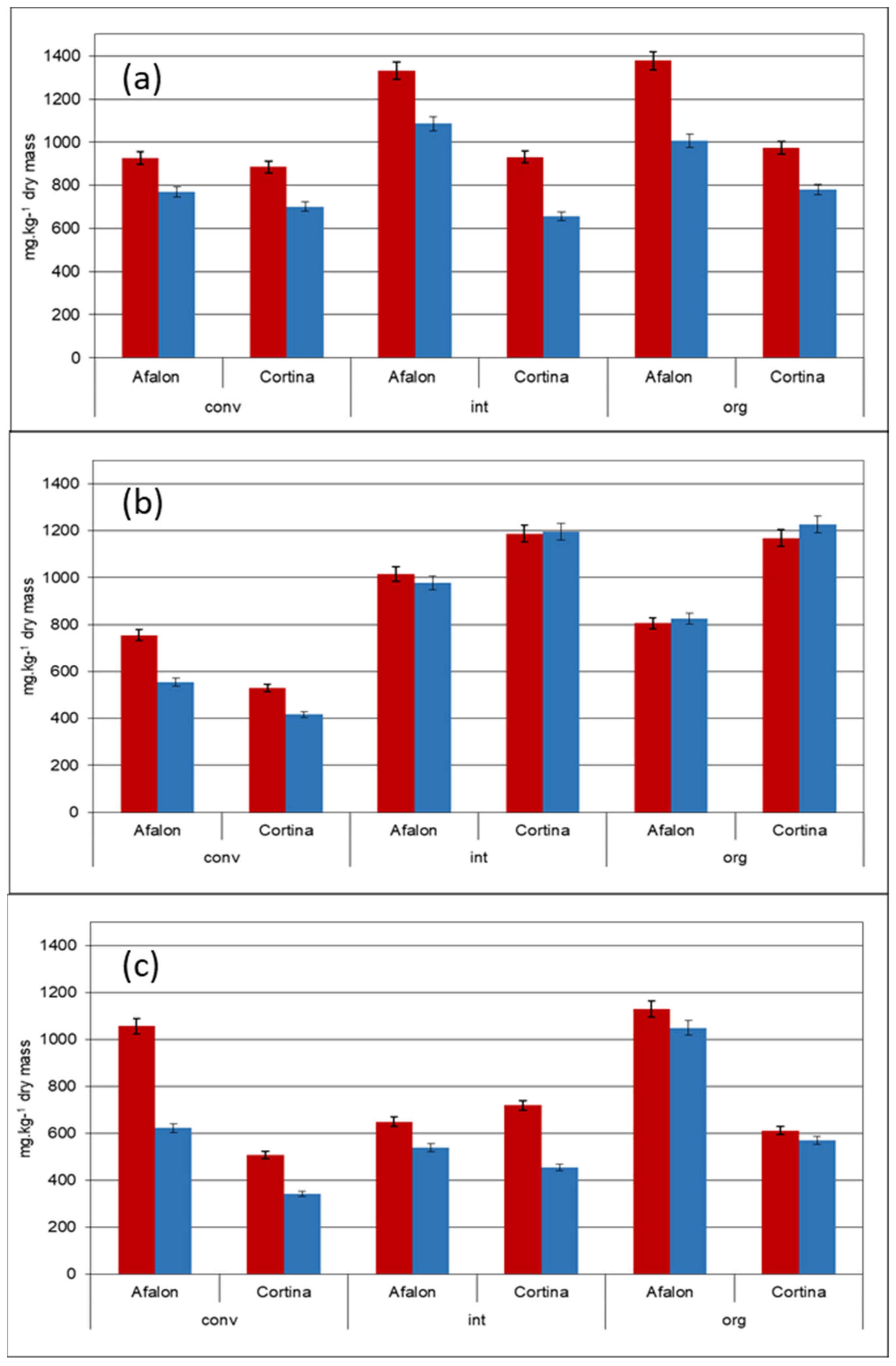
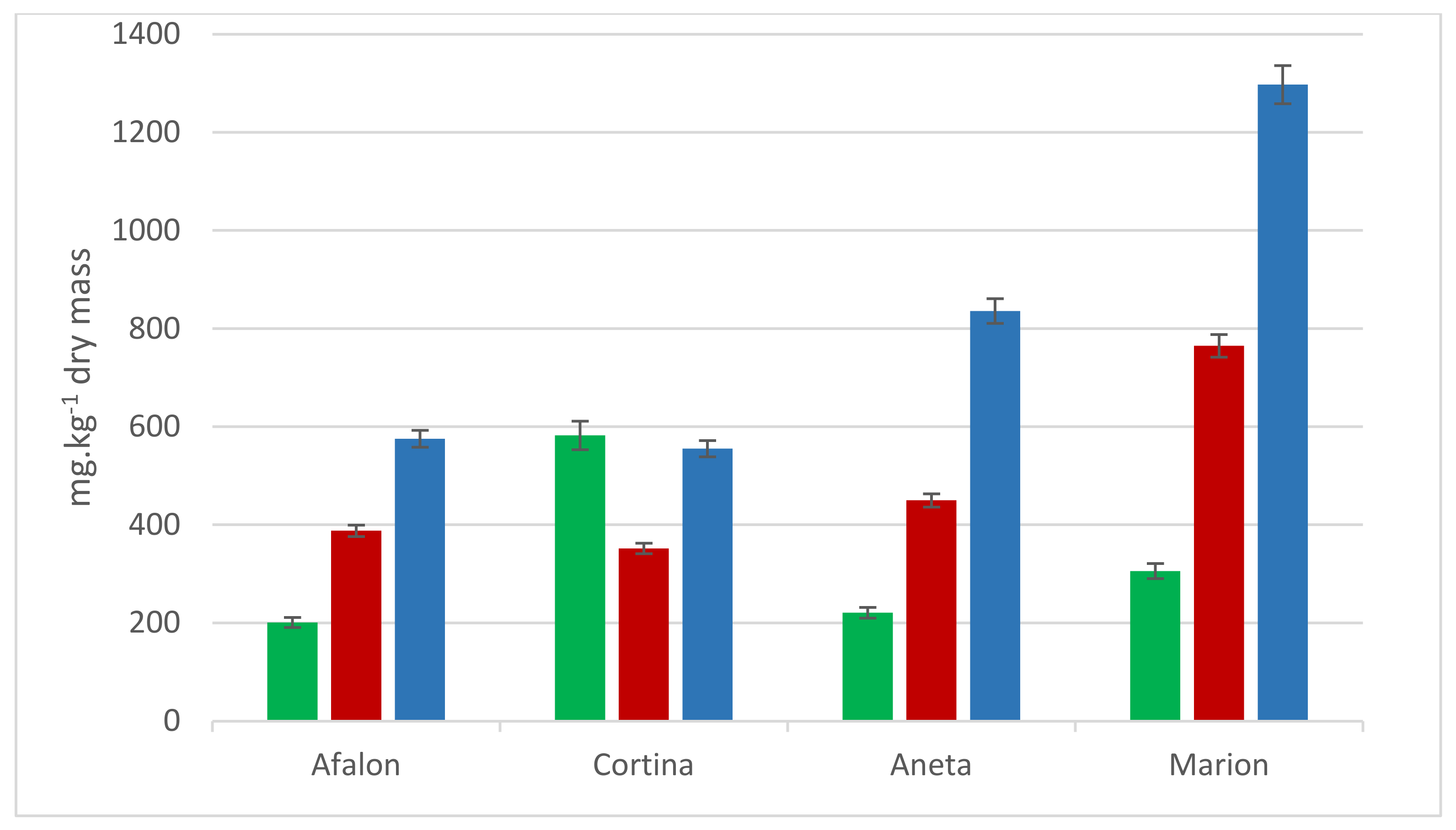

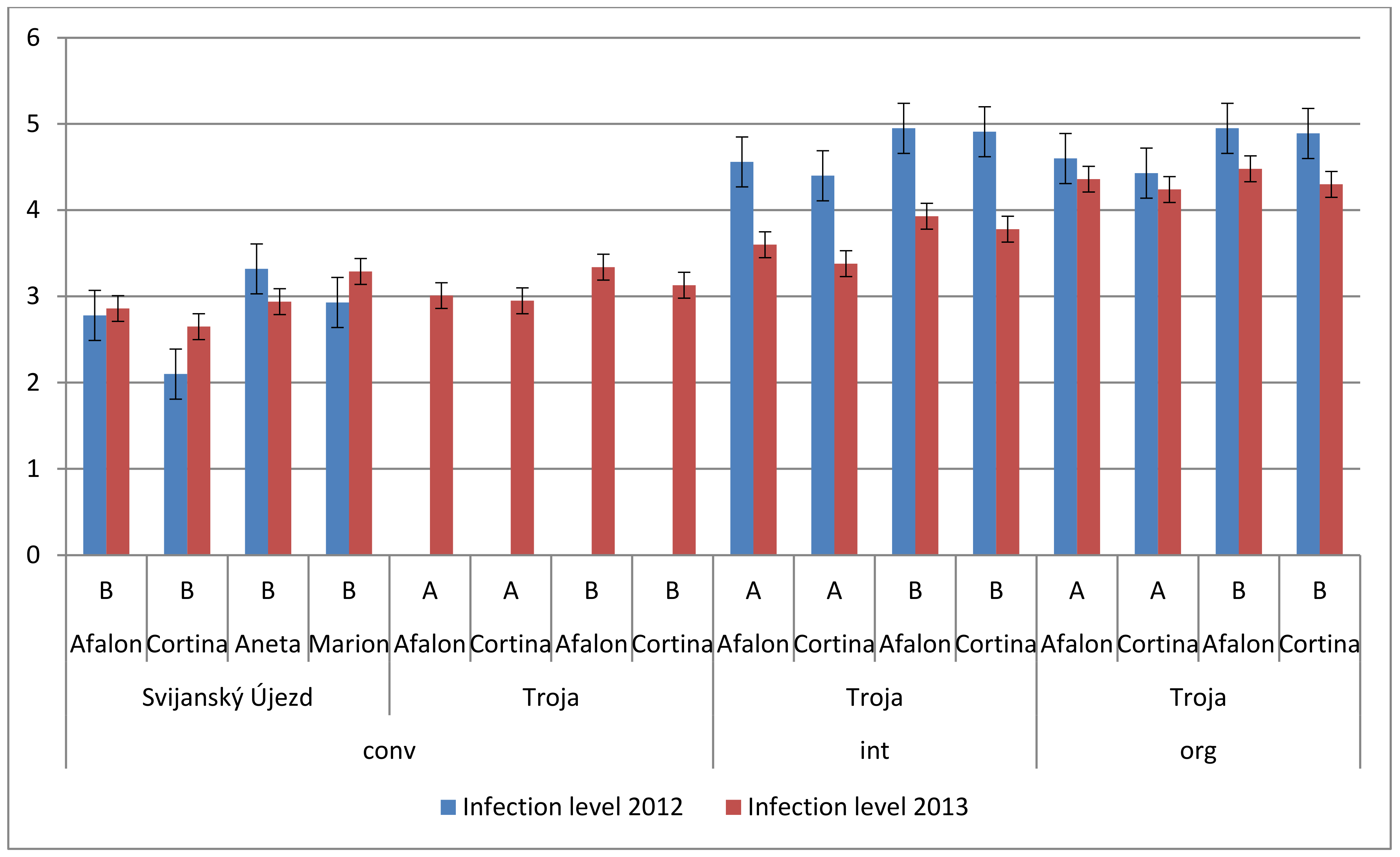
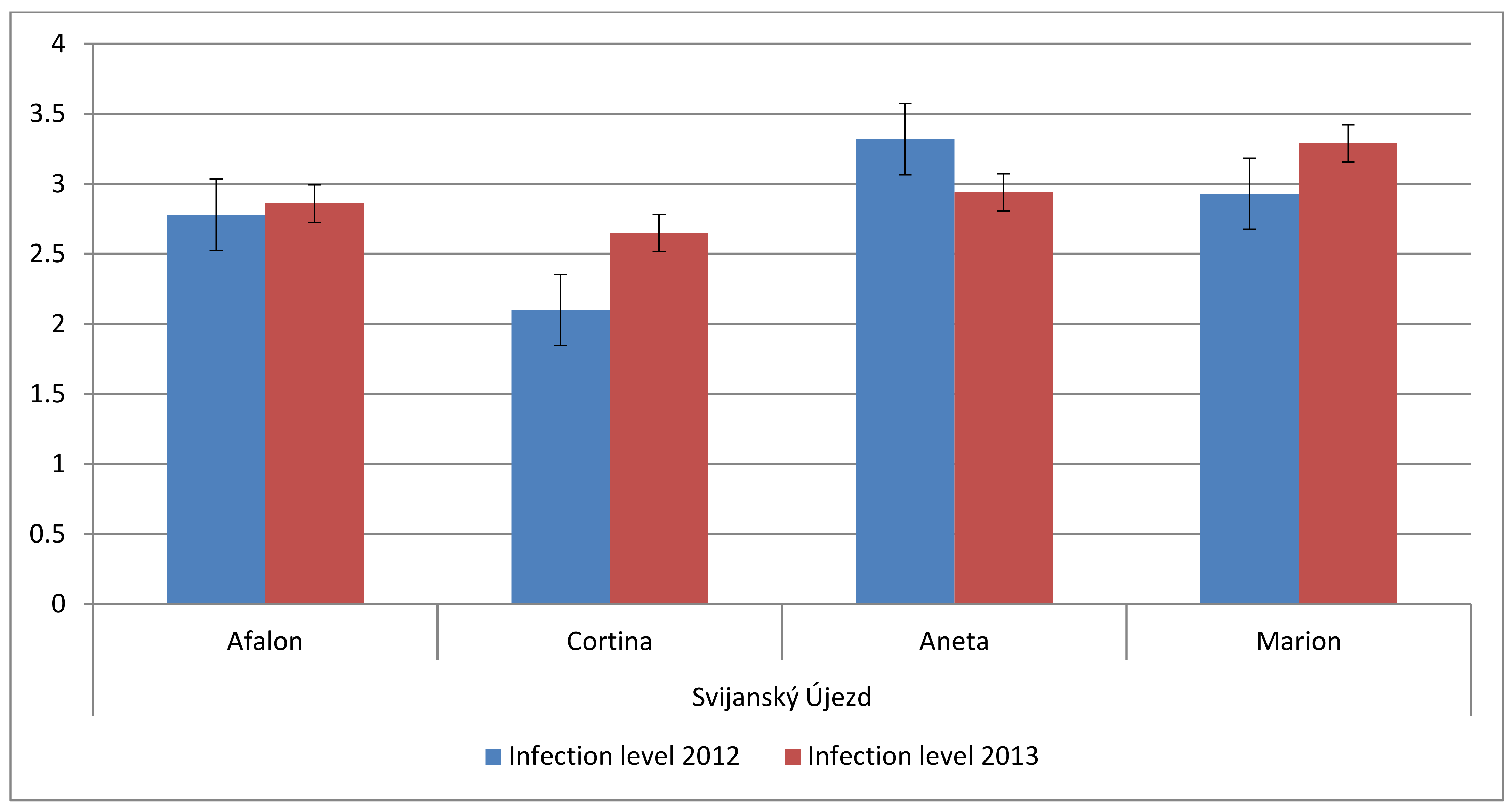
| Factor | Trends in Carotenes Content | Degrees of Freedom | p-Value |
|---|---|---|---|
| Year | Significantly higher in 2012; a higher ratio of β- to α-carotene in 2013 | 20 | 0.0327 * |
| Cultivar (Cortina vs. Afalon) in 2012 | Significantly higher in Afalon | 4 | 0.0467 * |
| Cultivar (Cortina vs. Afalon) in 2013 | Higher in Afalon in some cases, but statistically inconclusive | 16 | 0.1640 |
| Farming system (org. + int. vs. conv) | significantly lower in conventionally grown carrot; significantly higher β- to α-carotene ratio | 20 | 0.00783 * |
| Seeding density | Higher in 6 × 105 seeds per ha, in particular α-carotene | 14 | 0.0864 |
| Locality (Troja vs. S. Újezd) | No significant differences | 6 | 0.3761 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koudela, M.; Schulzova, V.; Krmela, A.; Chmelarova, H.; Hajslova, J.; Novotny, C. Effect of Agroecological Conditions on Biologically Active Compounds and Metabolome in Carrot. Cells 2021, 10, 784. https://doi.org/10.3390/cells10040784
Koudela M, Schulzova V, Krmela A, Chmelarova H, Hajslova J, Novotny C. Effect of Agroecological Conditions on Biologically Active Compounds and Metabolome in Carrot. Cells. 2021; 10(4):784. https://doi.org/10.3390/cells10040784
Chicago/Turabian StyleKoudela, Martin, Vera Schulzova, Ales Krmela, Hana Chmelarova, Jana Hajslova, and Cenek Novotny. 2021. "Effect of Agroecological Conditions on Biologically Active Compounds and Metabolome in Carrot" Cells 10, no. 4: 784. https://doi.org/10.3390/cells10040784
APA StyleKoudela, M., Schulzova, V., Krmela, A., Chmelarova, H., Hajslova, J., & Novotny, C. (2021). Effect of Agroecological Conditions on Biologically Active Compounds and Metabolome in Carrot. Cells, 10(4), 784. https://doi.org/10.3390/cells10040784







