Dopamine: The Neuromodulator of Long-Term Synaptic Plasticity, Reward and Movement Control
Abstract
1. Introduction
1.1. What Is Dopamine?
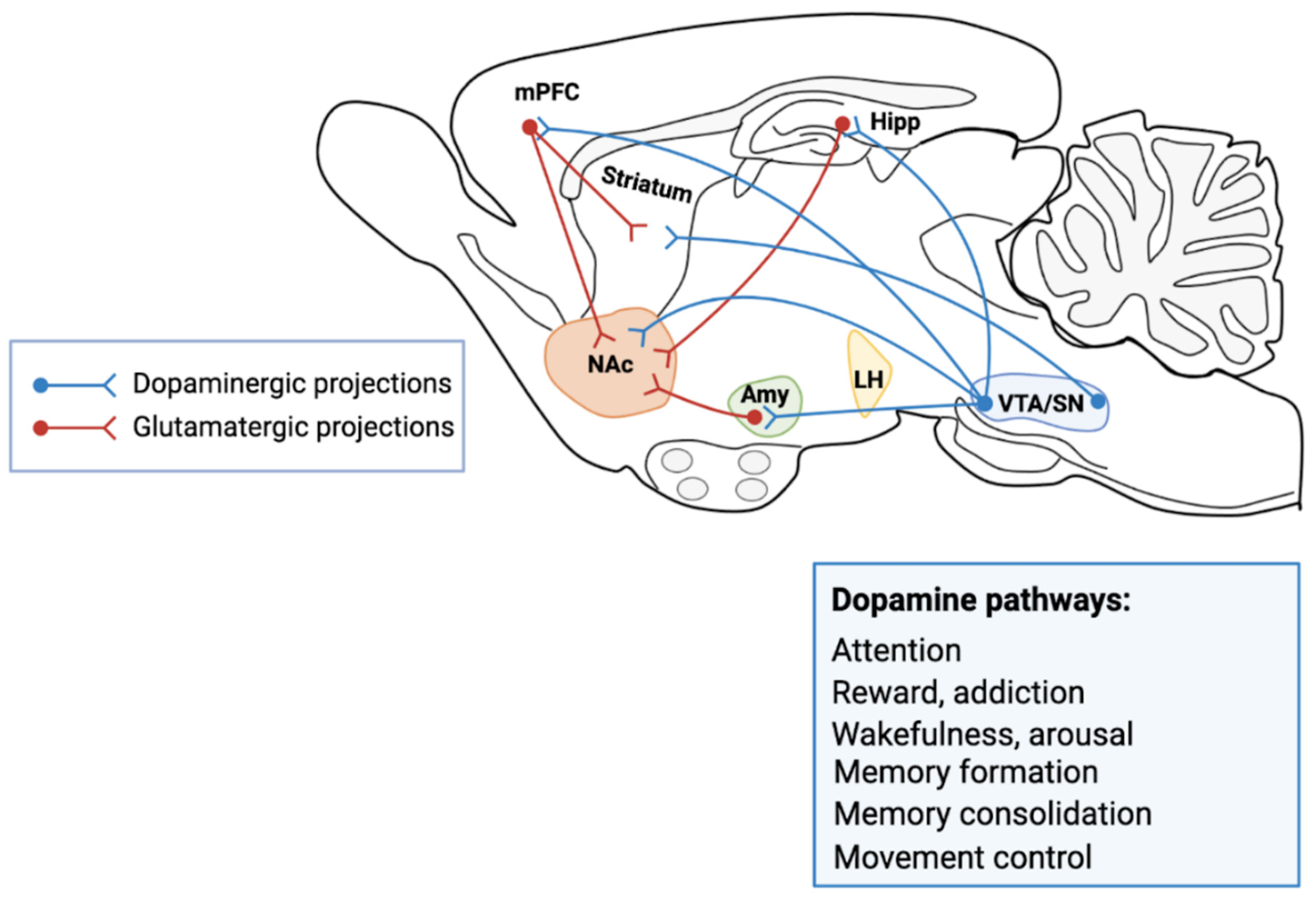
1.2. The Mesencephalic Dopaminergic System
2. Synaptic Plasticity: Homosynaptic and Heterosynaptic Theories
2.1. Dopamine Triggers Heterosynaptic Plasticity in the Hippocampus and Regulates Cognitive Processes
2.2. Dopamine Modulate the Spike Timing Dependent Plasticity (STDP) in Cortical Circuits: A Mechanism of Hebbian Plasticity
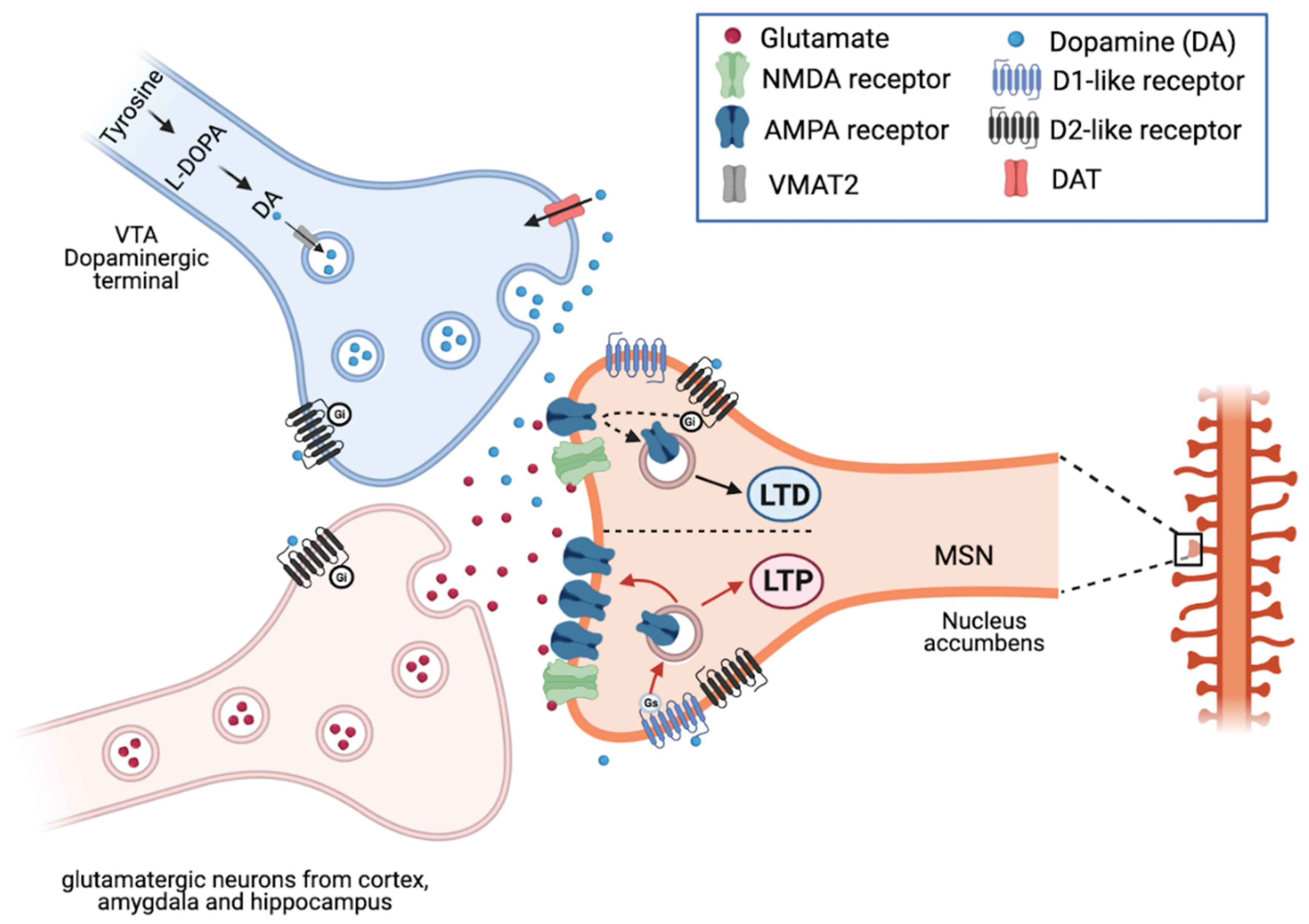
2.3. Dopamine and Reward System
2.4. Dopamine in the Striatum and Movement Control
3. Dopamine System at the Interface between Motor Control and Cognitive Functions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A2A | Adenosine 2A receptor |
| AC | Adenylate Cyclase |
| Ach | Acetylcholine |
| ADHD | Attention Deficit Hyperactivity Disorder |
| Cck | Corticotropin-releasing hormone receptor 1 |
| CREB | c-AMP response element-binding protein |
| DA | Dopamine |
| DAergic | Dopaminergic |
| DAR | Dopamine Receptor |
| DAT | Dopamine Transporter |
| dMSNs | direct Medium Spiny Neurons |
| GABA | γ-aminobutyric acid |
| GIRK | G-protein gated inwardly rectifying K+ |
| GPCR | G protein-coupled receptor family |
| GPe | external segment of the Globus Pallidus |
| GPi | internal segment of Globus Pallidus |
| HD | Huntington’s diseases |
| iMSNs | indirect medium spiny neurons |
| L-DOPA | L-3,4-diidrossifenilalanina |
| LC | Locus Coeruleus |
| LTD | Long-Term Depression |
| LTP | Long-Term Potentiation |
| MAPK | Mitogen Activated Kinase |
| mDAergic | midbrain Dopaminergic neurons |
| nAc | nucleus Accumbens |
| PD | Parkinson’s Disease |
| PFC | Prefrontal Cortex |
| PKA | Protein Kinase A |
| RPE | Reward Prediction Error |
| SN | Substantia Nigra |
| SNpc | Substantia Nigra pars compacta |
| SNpr | Substantia Nigra pars reticulata |
| STDP | Spike-Timing-Dependent Plasticity |
| VMAT2 | Vesicular monoamine transporter 2 |
| VTA | Ventral Tegmental Area |
References
- Halasz, N.; Nowycky, M.; Hokfelt, T.; Shepherd, G.M.; Markey, K.; Goldstein, M. Dopaminergic periglomerular cells in the turtle olfactory bulb. Brain Res. Bull. 1982, 9, 383–389. [Google Scholar] [CrossRef]
- Djamgoz, M.B.; Wagner, H.J. Localization and function of dopamine in the adult vertebrate retina. Neurochem. Int. 1992, 20, 139–191. [Google Scholar] [CrossRef]
- Pilgrim, C. The unexpected promiscuity of steroid hormones. Eur. J. Histochem. EJH 1999, 43, 261–264. [Google Scholar]
- Takada, M. Widespread dopaminergic projections of the subparafascicular thalamic nucleus in the rat. Brain Res. Bull. 1993, 32, 301–309. [Google Scholar] [CrossRef]
- Nevue, A.A.; Felix, R.A., 2nd; Portfors, C.V. Dopaminergic projections of the subparafascicular thalamic nucleus to the auditory brainstem. Hear. Res. 2016, 341, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Consales, C.; Volpicelli, F.; Greco, D.; Leone, L.; Colucci-D’Amato, L.; Perrone-Capano, C.; di Porzio, U. GDNF signaling in embryonic midbrain neurons in vitro. Brain Res. 2007, 1159, 28–39. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, R.; Pulcrano, S.; De Sanctis, C.; Volpicelli, F.; Guatteo, E.; von Oerthel, L.; Latagliata, E.C.; Esposito, R.; Piscitelli, R.M.; Perrone-Capano, C.; et al. miR-34b/c Regulates Wnt1 and Enhances Mesencephalic Dopaminergic Neuron Differentiation. Stem Cell Rep. 2018, 10, 1237–1250. [Google Scholar] [CrossRef] [PubMed]
- Greco, D.; Volpicelli, F.; Di Lieto, A.; Leo, D.; Perrone-Capano, C.; Auvinen, P.; di Porzio, U. Comparison of gene expression profile in embryonic mesencephalon and neuronal primary cultures. PLoS ONE 2009, 4, e4977. [Google Scholar] [CrossRef]
- Perrone-Capano, C.; di Porzio, U. Epigenetic factors and midbrain dopaminergic neurone development. BioEssays News Rev. Mol. Cell. Dev. Biol. 1996, 18, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Suresh, P.; Gnanabharathi, B.; Hirsch, E.C.; Ravindranath, V. Genes critical for development and differentiation of dopaminergic neurons are downregulated in Parkinson’s disease. bioRxiv 2020. [Google Scholar] [CrossRef]
- Volpicelli, F.; Caiazzo, M.; Greco, D.; Consales, C.; Leone, L.; Perrone-Capano, C.; Colucci D’Amato, L.; di Porzio, U. Bdnf gene is a downstream target of Nurr1 transcription factor in rat midbrain neurons in vitro. J. Neurochem. 2007, 102, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, F.; De Gregorio, R.; Pulcrano, S.; Perrone-Capano, C.; di Porzio, U.; Bellenchi, G.C. Direct regulation of Pitx3 expression by Nurr1 in culture and in developing mouse midbrain. PLoS ONE 2012, 7, e30661. [Google Scholar] [CrossRef]
- Volpicelli, F.; Perrone-Capano, C.; Bellenchi, G.C.; Colucci-D’Amato, L.; di Porzio, U. Molecular Regulation in Dopaminergic Neuron Development. Cues to Unveil Molecular Pathogenesis and Pharmacological Targets of Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 3995. [Google Scholar] [CrossRef]
- Volpicelli, F.; Perrone-Capano, C.; Da Pozzo, P.; Colucci-D’Amato, L.; di Porzio, U. Modulation of nurr1 gene expression in mesencephalic dopaminergic neurones. J. Neurochem. 2004, 88, 1283–1294. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, E.V.; Gainetdinov, R.R.; Gurevich, V.V. G protein-coupled receptor kinases as regulators of dopamine receptor functions. Pharmacol. Res. 2016, 111, 1–16. [Google Scholar] [CrossRef]
- Maurice, N.; Tkatch, T.; Meisler, M.; Sprunger, L.K.; Surmeier, D.J. D1/D5 dopamine receptor activation differentially modulates rapidly inactivating and persistent sodium currents in prefrontal cortex pyramidal neurons. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 2268–2277. [Google Scholar] [CrossRef]
- Witkowski, G.; Szulczyk, B.; Rola, R.; Szulczyk, P. D(1) dopaminergic control of G protein-dependent inward rectifier K(+) (GIRK)-like channel current in pyramidal neurons of the medial prefrontal cortex. Neuroscience 2008, 155, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ge, S.N.; Zhang, J.R.; Chen, L.; Yan, Z.Q.; Heng, L.J.; Zhao, T.Z.; Li, W.X.; Jia, D.; Zhu, J.L.; et al. Systemic blockade of dopamine D2-like receptors increases high-voltage spindles in the globus pallidus and motor cortex of freely moving rats. PLoS ONE 2013, 8, e64637. [Google Scholar] [CrossRef]
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G. Dopamine receptors: From structure to function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar] [CrossRef] [PubMed]
- Perreault, M.L.; Hasbi, A.; O’Dowd, B.F.; George, S.R. Heteromeric dopamine receptor signaling complexes: Emerging neurobiology and disease relevance. Neuropsychopharmacology 2014, 39, 156–168. [Google Scholar] [CrossRef]
- Walker, J.K.; Gainetdinov, R.R.; Feldman, D.S.; McFawn, P.K.; Caron, M.G.; Lefkowitz, R.J.; Premont, R.T.; Fisher, J.T. G protein-coupled receptor kinase 5 regulates airway responses induced by muscarinic receptor activation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L312–L319. [Google Scholar] [CrossRef][Green Version]
- Beaulieu, J.M.; Gainetdinov, R.R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Haga, T.; Lameh, J.; Sadee, W. Sequestration of dopamine D2 receptors depends on coexpression of G-protein-coupled receptor kinases 2 or 5. Eur. J. Biochem. 1999, 260, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Tiberi, M.; Nash, S.R.; Bertrand, L.; Lefkowitz, R.J.; Caron, M.G. Differential regulation of dopamine D1A receptor responsiveness by various G protein-coupled receptor kinases. J. Biol. Chem. 1996, 271, 3771–3778. [Google Scholar] [CrossRef]
- Villar, V.A.; Jones, J.E.; Armando, I.; Palmes-Saloma, C.; Yu, P.; Pascua, A.M.; Keever, L.; Arnaldo, F.B.; Wang, Z.; Luo, Y.; et al. G protein-coupled receptor kinase 4 (GRK4) regulates the phosphorylation and function of the dopamine D3 receptor. J. Biol. Chem. 2009, 284, 21425–21434. [Google Scholar] [CrossRef]
- Watanabe, H.; Xu, J.; Bengra, C.; Jose, P.A.; Felder, R.A. Desensitization of human renal D1 dopamine receptors by G protein-coupled receptor kinase 4. Kidney Int. 2002, 62, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.R.; Bychkov, E.; Gurevich, V.V.; Benovic, J.L.; Gurevich, E.V. Altered expression and subcellular distribution of GRK subtypes in the dopamine-depleted rat basal ganglia is not normalized by l-DOPA treatment. J. Neurochem. 2008, 104, 1622–1636. [Google Scholar] [CrossRef]
- Schultz, W. Multiple dopamine functions at different time courses. Annu. Rev. Neurosci. 2007, 30, 259–288. [Google Scholar] [CrossRef] [PubMed]
- Roffman, J.L.; Tanner, A.S.; Eryilmaz, H.; Rodriguez-Thompson, A.; Silverstein, N.J.; Ho, N.F.; Nitenson, A.Z.; Chonde, D.B.; Greve, D.N.; Abi-Dargham, A.; et al. Dopamine D1 signaling organizes network dynamics underlying working memory. Sci. Adv. 2016, 2, e1501672. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Jasanoff, A. Local and global consequences of reward-evoked striatal dopamine release. Nature 2020, 580, 239–244. [Google Scholar] [CrossRef]
- Hofman, M.A. Evolution of the human brain: When bigger is better. Front. Neuroanat. 2014, 8, 15. [Google Scholar] [CrossRef]
- Volpicelli, F.; Speranza, L.; di Porzio, U.; Crispino, M.; Perrone-Capano, C. The serotonin receptor 7 and the structural plasticity of brain circuits. Front. Behav. Neurosci. 2014, 8, 318. [Google Scholar] [CrossRef]
- Chistiakova, M.; Bannon, N.M.; Bazhenov, M.; Volgushev, M. Heterosynaptic plasticity: Multiple mechanisms and multiple roles. Neuroscientist 2014, 20, 483–498. [Google Scholar] [CrossRef]
- Bliss, T.V.; Collingridge, G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef]
- Caya-Bissonnette, L. Heterosynaptic Plasticity in Cortical Interneurons. J. Neurosci. 2020, 40, 1793–1794. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.H.; Giustetto, M.; Huang, Y.Y.; Hawkins, R.D.; Kandel, E.R. Is heterosynaptic modulation essential for stabilizing Hebbian plasticity and memory? Nat. Rev. Neurosci. 2000, 1, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Turrigiano, G.G.; Nelson, S.B. Homeostatic plasticity in the developing nervous system. Nat. Rev. Neurosci. 2004, 5, 97–107. [Google Scholar] [CrossRef]
- Turrigiano, G.G.; Leslie, K.R.; Desai, N.S.; Rutherford, L.C.; Nelson, S.B. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 1998, 391, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Madadi Asl, M.; Vahabie, A.H.; Valizadeh, A. Dopaminergic Modulation of Synaptic Plasticity, Its Role in Neuropsychiatric Disorders, and Its Computational Modeling. Basic Clin. Neurosci. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Navakkode, S.; Chew, K.C.M.; Tay, S.J.N.; Lin, Q.; Behnisch, T.; Soong, T.W. Bidirectional modulation of hippocampal synaptic plasticity by Dopaminergic D4-receptors in the CA1 area of hippocampus. Sci. Rep. 2017, 7, 15571. [Google Scholar] [CrossRef]
- Hammad, H.; Wagner, J.J. Dopamine-mediated disinhibition in the CA1 region of rat hippocampus via D3 receptor activation. J. Pharmacol. Exp. Ther. 2006, 316, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Brzosko, Z.; Schultz, W.; Paulsen, O. Retroactive modulation of spike timing-dependent plasticity by dopamine. eLife 2015, 4. [Google Scholar] [CrossRef]
- Varela, J.A.; Hirsch, S.J.; Chapman, D.; Leverich, L.S.; Greene, R.W. D1/D5 modulation of synaptic NMDA receptor currents. J. Neurosci. 2009, 29, 3109–3119. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Dani, J.A. Dopamine D1 and D5 receptors modulate spike timing-dependent plasticity at medial perforant path to dentate granule cell synapses. J. Neurosci. 2014, 34, 15888–15897. [Google Scholar] [CrossRef]
- Duszkiewicz, A.J.; McNamara, C.G.; Takeuchi, T.; Genzel, L. Novelty and Dopaminergic Modulation of Memory Persistence: A Tale of Two Systems. Trends Neurosci. 2019, 42, 102–114. [Google Scholar] [CrossRef]
- Kempadoo, K.A.; Mosharov, E.V.; Choi, S.J.; Sulzer, D.; Kandel, E.R. Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc. Natl. Acad. Sci. USA 2016, 113, 14835–14840. [Google Scholar] [CrossRef]
- Menezes, J.; Alves, N.; Borges, S.; Roehrs, R.; de Carvalho Myskiw, J.; Furini, C.R.; Izquierdo, I.; Mello-Carpes, P.B. Facilitation of fear extinction by novelty depends on dopamine acting on D1-subtype dopamine receptors in hippocampus. Proc. Natl. Acad. Sci. USA 2015, 112, E1652–E1658. [Google Scholar] [CrossRef]
- Micale, V.; Stepan, J.; Jurik, A.; Pamplona, F.A.; Marsch, R.; Drago, F.; Eder, M.; Wotjak, C.T. Extinction of avoidance behavior by safety learning depends on endocannabinoid signaling in the hippocampus. J. Psychiatr. Res. 2017, 90, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Duszkiewicz, A.J.; Sonneborn, A.; Spooner, P.A.; Yamasaki, M.; Watanabe, M.; Smith, C.C.; Fernandez, G.; Deisseroth, K.; Greene, R.W.; et al. Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 2016, 537, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Gasbarri, A.; Packard, M.G.; Campana, E.; Pacitti, C. Anterograde and retrograde tracing of projections from the ventral tegmental area to the hippocampal formation in the rat. Brain Res. Bull. 1994, 33, 445–452. [Google Scholar] [CrossRef]
- Ghanbarian, E.; Motamedi, F. Ventral tegmental area inactivation suppresses the expression of CA1 long term potentiation in anesthetized rat. PLoS ONE 2013, 8, e58844. [Google Scholar] [CrossRef]
- Scatton, B.; Simon, H.; Le Moal, M.; Bischoff, S. Origin of dopaminergic innervation of the rat hippocampal formation. Neurosci. Lett. 1980, 18, 125–131. [Google Scholar] [CrossRef]
- Broussard, J.I. Co-transmission of dopamine and glutamate. J. Gen. Physiol. 2012, 139, 93–96. [Google Scholar] [CrossRef]
- Smith, C.C.; Greene, R.W. CNS dopamine transmission mediated by noradrenergic innervation. J. Neurosci. 2012, 32, 6072–6080. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Ma, T.; Cheng, Y.; Huang, C.C.Y.; Wang, X.; Lu, J.; Wang, J. Dopamine D1 or D2 receptor-expressing neurons in the central nervous system. Addict. Biol. 2018, 23, 569–584. [Google Scholar] [CrossRef]
- Bagot, R.C.; Parise, E.M.; Pena, C.J.; Zhang, H.X.; Maze, I.; Chaudhury, D.; Persaud, B.; Cachope, R.; Bolanos-Guzman, C.A.; Cheer, J.F.; et al. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat. Commun. 2015, 6, 7062. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, L.A.; Kreitzer, A.C. Cortico-Basal Ganglia Circuit Function in Psychiatric Disease. Annu. Rev. Physiol. 2016, 78, 327–350. [Google Scholar] [CrossRef] [PubMed]
- Frey, U.; Schroeder, H.; Matthies, H. Dopaminergic antagonists prevent long-term maintenance of posttetanic LTP in the CA1 region of rat hippocampal slices. Brain Res. 1990, 522, 69–75. [Google Scholar] [CrossRef]
- Lisman, J.; Grace, A.A.; Duzel, E. A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP. Trends Neurosci. 2011, 34, 536–547. [Google Scholar] [CrossRef]
- Yoshii, A.; Constantine-Paton, M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev. Neurobiol. 2010, 70, 304–322. [Google Scholar] [CrossRef]
- Pezze, M.; Bast, T. Dopaminergic modulation of hippocampus-dependent learning: Blockade of hippocampal D1-class receptors during learning impairs 1-trial place memory at a 30-min retention delay. Neuropharmacology 2012, 63, 710–718. [Google Scholar] [CrossRef]
- Broussard, J.I.; Yang, K.; Levine, A.T.; Tsetsenis, T.; Jenson, D.; Cao, F.; Garcia, I.; Arenkiel, B.R.; Zhou, F.M.; De Biasi, M.; et al. Dopamine Regulates Aversive Contextual Learning and Associated In Vivo Synaptic Plasticity in the Hippocampus. Cell Rep. 2016, 14, 1930–1939. [Google Scholar] [CrossRef] [PubMed]
- Moncada, D.; Viola, H. Induction of long-term memory by exposure to novelty requires protein synthesis: Evidence for a behavioral tagging. J. Neurosci. 2007, 27, 7476–7481. [Google Scholar] [CrossRef] [PubMed]
- O’Carroll, C.M.; Martin, S.J.; Sandin, J.; Frenguelli, B.; Morris, R.G. Dopaminergic modulation of the persistence of one-trial hippocampus-dependent memory. Learn Mem. 2006, 13, 760–769. [Google Scholar] [CrossRef]
- Rossato, J.I.; Bevilaqua, L.R.; Izquierdo, I.; Medina, J.H.; Cammarota, M. Dopamine controls persistence of long-term memory storage. Science 2009, 325, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Redondo, R.L.; Morris, R.G. Relevance of synaptic tagging and capture to the persistence of long-term potentiation and everyday spatial memory. Proc. Natl. Acad. Sci. USA 2010, 107, 19537–19542. [Google Scholar] [CrossRef]
- McNamara, C.G.; Dupret, D. Two sources of dopamine for the hippocampus. Trends Neurosci. 2017, 40, 383–384. [Google Scholar] [CrossRef] [PubMed]
- Wagatsuma, A.; Okuyama, T.; Sun, C.; Smith, L.M.; Abe, K.; Tonegawa, S. Locus coeruleus input to hippocampal CA3 drives single-trial learning of a novel context. Proc. Natl. Acad. Sci. USA 2018, 115, E310–E316. [Google Scholar] [CrossRef]
- Grace, A.A. Dopamine system dysregulation by the hippocampus: Implications for the pathophysiology and treatment of schizophrenia. Neuropharmacology 2012, 62, 1342–1348. [Google Scholar] [CrossRef]
- Cepeda, C.; Murphy, K.P.; Parent, M.; Levine, M.S. The role of dopamine in Huntington’s disease. Prog. Brain Res. 2014, 211, 235–254. [Google Scholar] [CrossRef]
- Calabresi, P.; Ghiglieri, V.; Mazzocchetti, P.; Corbelli, I.; Picconi, B. Levodopa-induced plasticity: A double-edged sword in Parkinson’s disease? Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2015, 370. [Google Scholar] [CrossRef]
- Leo, D.; Sorrentino, E.; Volpicelli, F.; Eyman, M.; Greco, D.; Viggiano, D.; di Porzio, U.; Perrone-Capano, C. Altered midbrain dopaminergic neurotransmission during development in an animal model of ADHD. Neurosci. Biobehav. Rev. 2003, 27, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xiao, H.; Sun, H.; Zou, L.; Zhu, L.Q. Role of dopamine receptors in ADHD: A systematic meta-analysis. Mol. Neurobiol. 2012, 45, 605–620. [Google Scholar] [CrossRef]
- Xiang, Q.; Bi, R.; Xu, M.; Zhang, D.F.; Tan, L.; Zhang, C.; Fang, Y.; Yao, Y.G. Rare Genetic Variants of the Transthyretin Gene Are Associated with Alzheimer’s Disease in Han Chinese. Mol. Neurobiol. 2017, 54, 5192–5200. [Google Scholar] [CrossRef]
- Arnsten, A.F. Catecholamine influences on dorsolateral prefrontal cortical networks. Biol. Psychiatry 2011, 69, e89–e99. [Google Scholar] [CrossRef] [PubMed]
- Arnsten, A.F.; Wang, M.J.; Paspalas, C.D. Neuromodulation of thought: Flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron 2012, 76, 223–239. [Google Scholar] [CrossRef]
- Ott, T.; Nieder, A. Dopamine and Cognitive Control in Prefrontal Cortex. Trends Cogn. Sci. 2019, 23, 213–234. [Google Scholar] [CrossRef]
- Clark, K.L.; Noudoost, B. The role of prefrontal catecholamines in attention and working memory. Front. Neural Circuits 2014, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Thiele, A.; Bellgrove, M.A. Neuromodulation of Attention. Neuron 2018, 97, 769–785. [Google Scholar] [CrossRef] [PubMed]
- Louth, E.L.; Jørgensen, R.L.; Korshøj, A.R.; Sørensen, J.C.H.; Capogna, M. Dopaminergic neuromodulation of spike timing dependent plasticity in mature adult rodent and human cortical neurons. bioRxiv 2020. [Google Scholar] [CrossRef]
- Ruan, H.; Saur, T.; Yao, W.D. Dopamine-enabled anti-Hebbian timing-dependent plasticity in prefrontal circuitry. Front. Neural Circuits 2014, 8, 38. [Google Scholar] [CrossRef]
- Williams, S.M.; Goldman-Rakic, P.S. Widespread origin of the primate mesofrontal dopamine system. Cereb. Cortex 1998, 8, 321–345. [Google Scholar] [CrossRef] [PubMed]
- Petrides, M. Lateral prefrontal cortex: Architectonic and functional organization. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2005, 360, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Wise, S.P. Forward frontal fields: Phylogeny and fundamental function. Trends Neurosci. 2008, 31, 599–608. [Google Scholar] [CrossRef]
- Wise, R.A. Catecholamine theories of reward: A critical review. Brain Res. 1978, 152, 215–247. [Google Scholar] [CrossRef]
- Schultz, W. Responses of midbrain dopamine neurons to behavioral trigger stimuli in the monkey. J. Neurophysiol. 1986, 56, 1439–1461. [Google Scholar] [CrossRef]
- Schultz, W. Predictive reward signal of dopamine neurons. J. Neurophysiol. 1998, 80, 1–27. [Google Scholar] [CrossRef]
- Schultz, W. Dopamine reward prediction error coding. Dialogues Clin. Neurosci. 2016, 18, 23–32. [Google Scholar]
- Schultz, W.; Romo, R. Dopamine neurons of the monkey midbrain: Contingencies of responses to stimuli eliciting immediate behavioral reactions. J. Neurophysiol. 1990, 63, 607–624. [Google Scholar] [CrossRef] [PubMed]
- Montague, P.R.; Dayan, P.; Sejnowski, T.J. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J. Neurosci. 1996, 16, 1936–1947. [Google Scholar] [CrossRef]
- Hamid, A.A.; Pettibone, J.R.; Mabrouk, O.S.; Hetrick, V.L.; Schmidt, R.; Vander Weele, C.M.; Kennedy, R.T.; Aragona, B.J.; Berke, J.D. Mesolimbic dopamine signals the value of work. Nat. Neurosci. 2016, 19, 117–126. [Google Scholar] [CrossRef]
- Steinberg, E.E.; Keiflin, R.; Boivin, J.R.; Witten, I.B.; Deisseroth, K.; Janak, P.H. A causal link between prediction errors, dopamine neurons and learning. Nat. Neurosci. 2013, 16, 966–973. [Google Scholar] [CrossRef]
- Maes, E.J.P.; Sharpe, M.J.; Usypchuk, A.A.; Lozzi, M.; Chang, C.Y.; Gardner, M.P.H.; Schoenbaum, G.; Iordanova, M.D. Causal evidence supporting the proposal that dopamine transients function as temporal difference prediction errors. Nat. Neurosci. 2020, 23, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, M.J.; Batchelor, H.M.; Mueller, L.E.; Yun Chang, C.; Maes, E.J.P.; Niv, Y.; Schoenbaum, G. Dopamine transients do not act as model-free prediction errors during associative learning. Nat. Commun. 2020, 11, 106. [Google Scholar] [CrossRef]
- Morrens, J.; Aydin, C.; Janse van Rensburg, A.; Esquivelzeta Rabell, J.; Haesler, S. Cue-Evoked Dopamine Promotes Conditioned Responding during Learning. Neuron 2020, 106, 142–153.e147. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.P.H.; Schoenbaum, G.; Gershman, S.J. Rethinking dopamine as generalized prediction error. Proc. Biol. Sci. 2018, 285. [Google Scholar] [CrossRef]
- Stalnaker, T.A.; Howard, J.D.; Takahashi, Y.K.; Gershman, S.J.; Kahnt, T.; Schoenbaum, G. Dopamine neuron ensembles signal the content of sensory prediction errors. eLife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.K.; Batchelor, H.M.; Liu, B.; Khanna, A.; Morales, M.; Schoenbaum, G. Dopamine Neurons Respond to Errors in the Prediction of Sensory Features of Expected Rewards. Neuron 2017, 95, 1395–1405. [Google Scholar] [CrossRef]
- Keiflin, R.; Pribut, H.J.; Shah, N.B.; Janak, P.H. Ventral Tegmental Dopamine Neurons Participate in Reward Identity Predictions. Curr. Biol. 2019, 29, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Menegas, W.; Akiti, K.; Amo, R.; Uchida, N.; Watabe-Uchida, M. Dopamine neurons projecting to the posterior striatum reinforce avoidance of threatening stimuli. Nat. Neurosci. 2018, 21, 1421–1430. [Google Scholar] [CrossRef]
- Menegas, W.; Babayan, B.M.; Uchida, N.; Watabe-Uchida, M. Opposite initialization to novel cues in dopamine signaling in ventral and posterior striatum in mice. eLife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, E.E.; Gore, F.; Heifets, B.D.; Taylor, M.D.; Norville, Z.C.; Beier, K.T.; Foldy, C.; Lerner, T.N.; Luo, L.; Deisseroth, K.; et al. Amygdala-Midbrain Connections Modulate Appetitive and Aversive Learning. Neuron 2020, 106, 1026–1043. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.X.; Pizano, K.; Gundersen, G.W.; Hayes, C.L.; Fleming, W.T.; Holt, S.; Cox, J.M.; Witten, I.B. Distinct signals in medial and lateral VTA dopamine neurons modulate fear extinction at different times. eLife 2020, 9. [Google Scholar] [CrossRef]
- Engelhard, B.; Finkelstein, J.; Cox, J.; Fleming, W.; Jang, H.J.; Ornelas, S.; Koay, S.A.; Thiberge, S.Y.; Daw, N.D.; Tank, D.W.; et al. Specialized coding of sensory, motor and cognitive variables in VTA dopamine neurons. Nature 2019, 570, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Ecker, J.R.; Geschwind, D.H.; Kriegstein, A.R.; Ngai, J.; Osten, P.; Polioudakis, D.; Regev, A.; Sestan, N.; Wickersham, I.R.; Zeng, H. The BRAIN Initiative Cell Census Consortium: Lessons Learned toward Generating a Comprehensive Brain Cell Atlas. Neuron 2017, 96, 542–557. [Google Scholar] [CrossRef]
- Poulin, J.F.; Gaertner, Z.; Moreno-Ramos, O.A.; Awatramani, R. Classification of Midbrain Dopamine Neurons Using Single-Cell Gene Expression Profiling Approaches. Trends Neurosci. 2020, 43, 155–169. [Google Scholar] [CrossRef]
- Kramer, D.J.; Risso, D.; Kosillo, P.; Ngai, J.; Bateup, H.S. Combinatorial Expression of Grp and Neurod6 Defines Dopamine Neuron Populations with Distinct Projection Patterns and Disease Vulnerability. eNeuro 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Heymann, G.; Jo, Y.S.; Reichard, K.L.; McFarland, N.; Chavkin, C.; Palmiter, R.D.; Soden, M.E.; Zweifel, L.S. Synergy of Distinct Dopamine Projection Populations in Behavioral Reinforcement. Neuron 2020, 105, 909–920e5. [Google Scholar] [CrossRef]
- Morales, M.; Margolis, E.B. Ventral tegmental area: Cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci. 2017, 18, 73–85. [Google Scholar] [CrossRef]
- Yoo, J.H.; Zell, V.; Gutierrez-Reed, N.; Wu, J.; Ressler, R.; Shenasa, M.A.; Johnson, A.B.; Fife, K.H.; Faget, L.; Hnasko, T.S. Ventral tegmental area glutamate neurons co-release GABA and promote positive reinforcement. Nat. Commun. 2016, 7, 13697. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, S.; Wang, H.L.; Barker, D.J.; Miranda-Barrientos, J.; Morales, M. VTA glutamatergic inputs to nucleus accumbens drive aversion by acting on GABAergic interneurons. Nat. Neurosci. 2016, 19, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.T.; Tan, K.R.; O’Connor, E.C.; Nikonenko, I.; Muller, D.; Luscher, C. Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature 2012, 492, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Pickel, V.M.; Johnson, E.; Carson, M.; Chan, J. Ultrastructure of spared dopamine terminals in caudate-putamen nuclei of adult rats neonatally treated with intranigral 6-hydroxydopamine. Brain Res. Dev. Brain Res. 1992, 70, 75–86. [Google Scholar] [CrossRef]
- Lapper, S.R.; Smith, Y.; Sadikot, A.F.; Parent, A.; Bolam, J.P. Cortical input to parvalbumin-immunoreactive neurones in the putamen of the squirrel monkey. Brain Res. 1992, 580, 215–224. [Google Scholar] [CrossRef]
- Gerfen, C.R.; Surmeier, D.J. Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci. 2011, 34, 441–466. [Google Scholar] [CrossRef]
- Tepper, J.M.; Abercrombie, E.D.; Bolam, J.P. Basal ganglia macrocircuits. Prog. Brain Res. 2007, 160, 3–7. [Google Scholar] [CrossRef]
- Cazorla, M.; de Carvalho, F.D.; Chohan, M.O.; Shegda, M.; Chuhma, N.; Rayport, S.; Ahmari, S.E.; Moore, H.; Kellendonk, C. Dopamine D2 receptors regulate the anatomical and functional balance of basal ganglia circuitry. Neuron 2014, 81, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, P.; Picconi, B.; Tozzi, A.; Ghiglieri, V.; Di Filippo, M. Direct and indirect pathways of basal ganglia: A critical reappraisal. Nat. Neurosci. 2014, 17, 1022–1030. [Google Scholar] [CrossRef]
- Beaulieu, J.M.; Espinoza, S.; Gainetdinov, R.R. Dopamine receptors—IUPHAR Review 13. Br. J. Pharmacol. 2015, 172, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Gainetdinov, R.R.; Premont, R.T.; Bohn, L.M.; Lefkowitz, R.J.; Caron, M.G. Desensitization of G protein-coupled receptors and neuronal functions. Annu. Rev. Neurosci. 2004, 27, 107–144. [Google Scholar] [CrossRef]
- Augustin, S.M.; Beeler, J.A.; McGehee, D.S.; Zhuang, X. Cyclic AMP and afferent activity govern bidirectional synaptic plasticity in striatopallidal neurons. J. Neurosci. 2014, 34, 6692–6699. [Google Scholar] [CrossRef][Green Version]
- Bagetta, V.; Picconi, B.; Marinucci, S.; Sgobio, C.; Pendolino, V.; Ghiglieri, V.; Fusco, F.R.; Giampa, C.; Calabresi, P. Dopamine-dependent long-term depression is expressed in striatal spiny neurons of both direct and indirect pathways: Implications for Parkinson’s disease. J. Neurosci. 2011, 31, 12513–12522. [Google Scholar] [CrossRef]
- Augustin, S.M.; Chancey, J.H.; Lovinger, D.M. Dual Dopaminergic Regulation of Corticostriatal Plasticity by Cholinergic Interneurons and Indirect Pathway Medium Spiny Neurons. Cell Rep. 2018, 24, 2883–2893. [Google Scholar] [CrossRef]
- Kharkwal, G.; Radl, D.; Lewis, R.; Borrelli, E. Dopamine D2 receptors in striatal output neurons enable the psychomotor effects of cocaine. Proc. Natl. Acad. Sci. USA 2016, 113, 11609–11614. [Google Scholar] [CrossRef]
- Daigle, T.L.; Ferris, M.J.; Gainetdinov, R.R.; Sotnikova, T.D.; Urs, N.M.; Jones, S.R.; Caron, M.G. Selective deletion of GRK2 alters psychostimulant-induced behaviors and dopamine neurotransmission. Neuropsychopharmacology 2014, 39, 2450–2462. [Google Scholar] [CrossRef]
- Nadjar, A.; Brotchie, J.M.; Guigoni, C.; Li, Q.; Zhou, S.B.; Wang, G.J.; Ravenscroft, P.; Georges, F.; Crossman, A.R.; Bezard, E. Phenotype of striatofugal medium spiny neurons in parkinsonian and dyskinetic nonhuman primates: A call for a reappraisal of the functional organization of the basal ganglia. J. Neurosci. 2006, 26, 8653–8661. [Google Scholar] [CrossRef] [PubMed]
- Viggiano, D.; Speranza, L.; Crispino, M.; Bellenchi, G.C.; di Porzio, U.; Iemolo, A.; De Leonibus, E.; Volpicelli, F.; Perrone-Capano, C. Information content of dendritic spines after motor learning. Behav. Brain Res. 2018, 336, 256–260. [Google Scholar] [CrossRef]
- Syed, E.C.; Grima, L.L.; Magill, P.J.; Bogacz, R.; Brown, P.; Walton, M.E. Action initiation shapes mesolimbic dopamine encoding of future rewards. Nat. Neurosci. 2016, 19, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.N.; Bakhurin, K.I.; Petter, E.A.; Watson, G.D.R.; Kim, N.; Friedman, A.D.; Yin, H.H. Ventral Tegmental Dopamine Neurons Control the Impulse Vector during Motivated Behavior. Curr. Biol. 2020, 30, 2681–2694.e2685. [Google Scholar] [CrossRef]
- Pillon, B.; Czernecki, V.; Dubois, B. Dopamine and cognitive function. Curr. Opin. Neurol. 2003, 16, S17–S22. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Pillon, B. Cognitive deficits in Parkinson’s disease. J. Neurol. 1997, 244, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Kataoka, H.; Ueno, S. Can levodopa prevent cognitive decline in patients with Parkinson’s disease? Am. J. Neurodegener. Dis. 2017, 6, 9–14. [Google Scholar]
- Asanuma, M.; Miyazaki, I.; Ogawa, N. Dopamine- or L-DOPA-induced neurotoxicity: The role of dopamine quinone formation and tyrosinase in a model of Parkinson’s disease. Neurotox. Res. 2003, 5, 165–176. [Google Scholar] [CrossRef]
- Nishijima, H.; Ueno, T.; Funamizu, Y.; Ueno, S.; Tomiyama, M. Levodopa treatment and dendritic spine pathology. Mov. Disord. Off. J. Mov. Disord. Soc. 2018, 33, 877–888. [Google Scholar] [CrossRef]
- Li, H.; Hirano, S.; Furukawa, S.; Nakano, Y.; Kojima, K.; Ishikawa, A.; Tai, H.; Horikoshi, T.; Iimori, T.; Uno, T.; et al. The Relationship Between the Striatal Dopaminergic Neuronal and Cognitive Function With Aging. Front. Aging Neurosci. 2020, 12, 41. [Google Scholar] [CrossRef]
- Eusebio, A.; Azulay, J.P.; Ceccaldi, M.; Girard, N.; Mundler, O.; Guedj, E. Voxel-based analysis of whole-brain effects of age and gender on dopamine transporter SPECT imaging in healthy subjects. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1778–1783. [Google Scholar] [CrossRef]
- Kazumata, K.; Dhawan, V.; Chaly, T.; Antonini, A.; Margouleff, C.; Belakhlef, A.; Neumeyer, J.; Eidelberg, D. Dopamine transporter imaging with fluorine-18-FPCIT and PET. J. Nucl. Med. 1998, 39, 1521–1530. [Google Scholar]
- Matsuda, H.; Murata, M.; Mukai, Y.; Sako, K.; Ono, H.; Toyama, H.; Inui, Y.; Taki, Y.; Shimomura, H.; Nagayama, H.; et al. Japanese multicenter database of healthy controls for [(123)I]FP-CIT SPECT. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- Varrone, A.; Dickson, J.C.; Tossici-Bolt, L.; Sera, T.; Asenbaum, S.; Booij, J.; Kapucu, O.L.; Kluge, A.; Knudsen, G.M.; Koulibaly, P.M.; et al. European multicentre database of healthy controls for [123I]FP-CIT SPECT (ENC-DAT): Age-related effects, gender differences and evaluation of different methods of analysis. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Costa, K.M. The effects of aging on substantia nigra dopamine neurons. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 15133–15134. [Google Scholar] [CrossRef]
- Stamford, J.A. Development and ageing of the rat nigrostriatal dopamine system studied with fast cyclic voltammetry. J. Neurochem. 1989, 52, 1582–1589. [Google Scholar] [CrossRef]
- Stanford, J.A.; Giardina, K.; Gerhardt, G.A. In vivo microdialysis studies of age-related alterations in potassium-evoked overflow of dopamine in the dorsal striatum of Fischer 344 rats. Int. J. Dev. Neurosci. 2000, 18, 411–416. [Google Scholar] [CrossRef]
- Levin, H.; Troyanskaya, M.; Petrie, J.; Wilde, E.A.; Hunter, J.V.; Abildskov, T.J.; Scheibel, R.S. Methylphenidate Treatment of Cognitive Dysfunction in Adults After Mild to Moderate Traumatic Brain Injury: Rationale, Efficacy, and Neural Mechanisms. Front. Neurol. 2019, 10, 925. [Google Scholar] [CrossRef]
- Sassi, K.L.M.; Rocha, N.P.; Colpo, G.D.; John, V.; Teixeira, A.L. Amphetamine Use in the Elderly: A Systematic Review of the Literature. Curr. Neuropharmacol. 2020, 18, 126–135. [Google Scholar] [CrossRef]
- Leijenaar, J.F.; Groeneveld, G.J.; Klaassen, E.S.; Leeuwis, A.E.; Scheltens, P.; Weinstein, H.C.; van Gerven, J.M.A.; Barkhof, F.; van der Flier, W.M.; Prins, N.D. Methylphenidate and galantamine in patients with vascular cognitive impairment-the proof-of-principle study STREAM-VCI. Alzheimer’s Res. Ther. 2020, 12, 10. [Google Scholar] [CrossRef]
- Winton-Brown, T.T.; Fusar-Poli, P.; Ungless, M.A.; Howes, O.D. Dopaminergic basis of salience dysregulation in psychosis. Trends Neurosci. 2014, 37, 85–94. [Google Scholar] [CrossRef]
- Miller, R.; Chouinard, G. Loss of striatal cholinergic neurons as a basis for tardive and L-dopa-induced dyskinesias, neuroleptic-induced supersensitivity psychosis and refractory schizophrenia. Biol. Psychiatry 1993, 34, 713–738. [Google Scholar] [CrossRef]
- Wang, H.D.; Deutch, A.Y. Dopamine depletion of the prefrontal cortex induces dendritic spine loss: Reversal by atypical antipsychotic drug treatment. Neuropsychopharmacology 2008, 33, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, J.; Mengod, G. D2 and D4 dopamine receptor mRNA distribution in pyramidal neurons and GABAergic subpopulations in monkey prefrontal cortex: Implications for schizophrenia treatment. Neuroscience 2010, 170, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Wedzony, K.; Chocyk, A.; Mackowiak, M.; Fijal, K.; Czyrak, A. Cortical localization of dopamine D4 receptors in the rat brain--immunocytochemical study. J. Physiol. Pharmacol. 2000, 51, 205–221. [Google Scholar] [PubMed]
- Furth, K.E.; Mastwal, S.; Wang, K.H.; Buonanno, A.; Vullhorst, D. Dopamine, cognitive function, and gamma oscillations: Role of D4 receptors. Front. Cell. Neurosci. 2013, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Viggiano, D. The hyperactive syndrome: Metanalysis of genetic alterations, pharmacological treatments and brain lesions which increase locomotor activity. Behav. Brain Res. 2008, 194, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Viggiano, A.; Cacciola, G.; Widmer, D.A.; Viggiano, D. Anxiety as a neurodevelopmental disorder in a neuronal subpopulation: Evidence from gene expression data. Psychiatry Res. 2015, 228, 729–740. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, C.; Bellenchi, G.C.; Viggiano, D. A meta-analytic approach to genes that are associated with impaired and elevated spatial memory performance. Psychiatry Res. 2018, 261, 508–516. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Speranza, L.; di Porzio, U.; Viggiano, D.; de Donato, A.; Volpicelli, F. Dopamine: The Neuromodulator of Long-Term Synaptic Plasticity, Reward and Movement Control. Cells 2021, 10, 735. https://doi.org/10.3390/cells10040735
Speranza L, di Porzio U, Viggiano D, de Donato A, Volpicelli F. Dopamine: The Neuromodulator of Long-Term Synaptic Plasticity, Reward and Movement Control. Cells. 2021; 10(4):735. https://doi.org/10.3390/cells10040735
Chicago/Turabian StyleSperanza, Luisa, Umberto di Porzio, Davide Viggiano, Antonio de Donato, and Floriana Volpicelli. 2021. "Dopamine: The Neuromodulator of Long-Term Synaptic Plasticity, Reward and Movement Control" Cells 10, no. 4: 735. https://doi.org/10.3390/cells10040735
APA StyleSperanza, L., di Porzio, U., Viggiano, D., de Donato, A., & Volpicelli, F. (2021). Dopamine: The Neuromodulator of Long-Term Synaptic Plasticity, Reward and Movement Control. Cells, 10(4), 735. https://doi.org/10.3390/cells10040735






