Direct Generation of Immortalized Erythroid Progenitor Cell Lines from Peripheral Blood Mononuclear Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of Lentiviruses
2.2. Generation of iEPCs from Peripheral Blood Mononuclear Cells (PBMNCs)
2.3. Generation of iEPCs from CD34+ HSPCs
2.4. Differentiation of iEPCs
2.5. Morphology Analysis and Staining
2.6. Flow Cytometry
2.7. Karyotyping
2.8. High-Performance Liquid Chromatography (HPLC) for Globin Chain Analysis
2.9. Knockdown of BCL11A in iEPCs
2.10. CRISPR/Cas9 Based Gene Editing of BCL11A Exon 2 and Enhancer Regions
2.11. Quantitative Real-Time PCR Analysis
2.12. Western Blot Analysis
3. Results
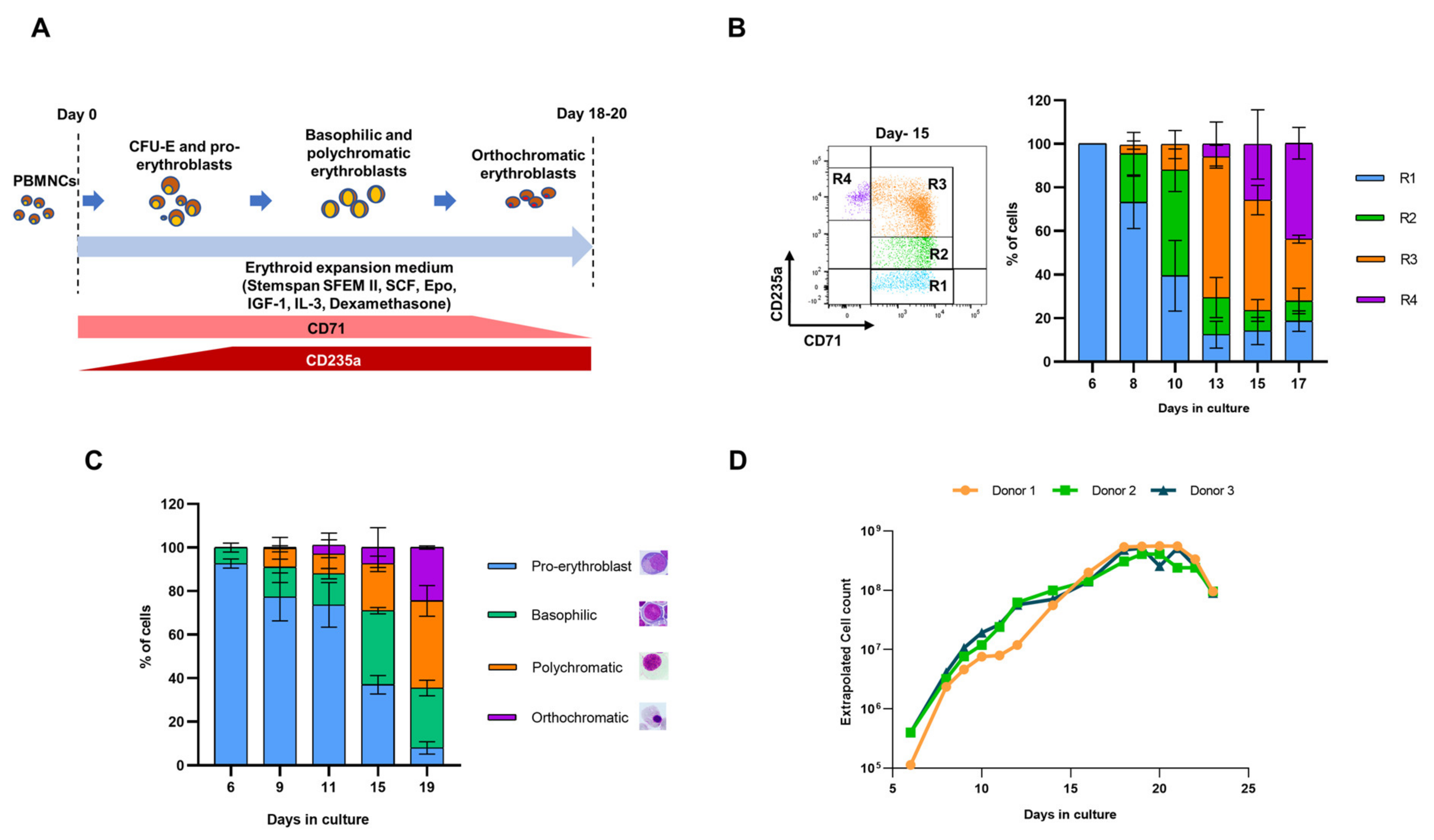
3.1. Generation of iEPCs from PBMNCs
3.2. Variegation in HEE Expression Causes Lag in Immortalization of EPCs
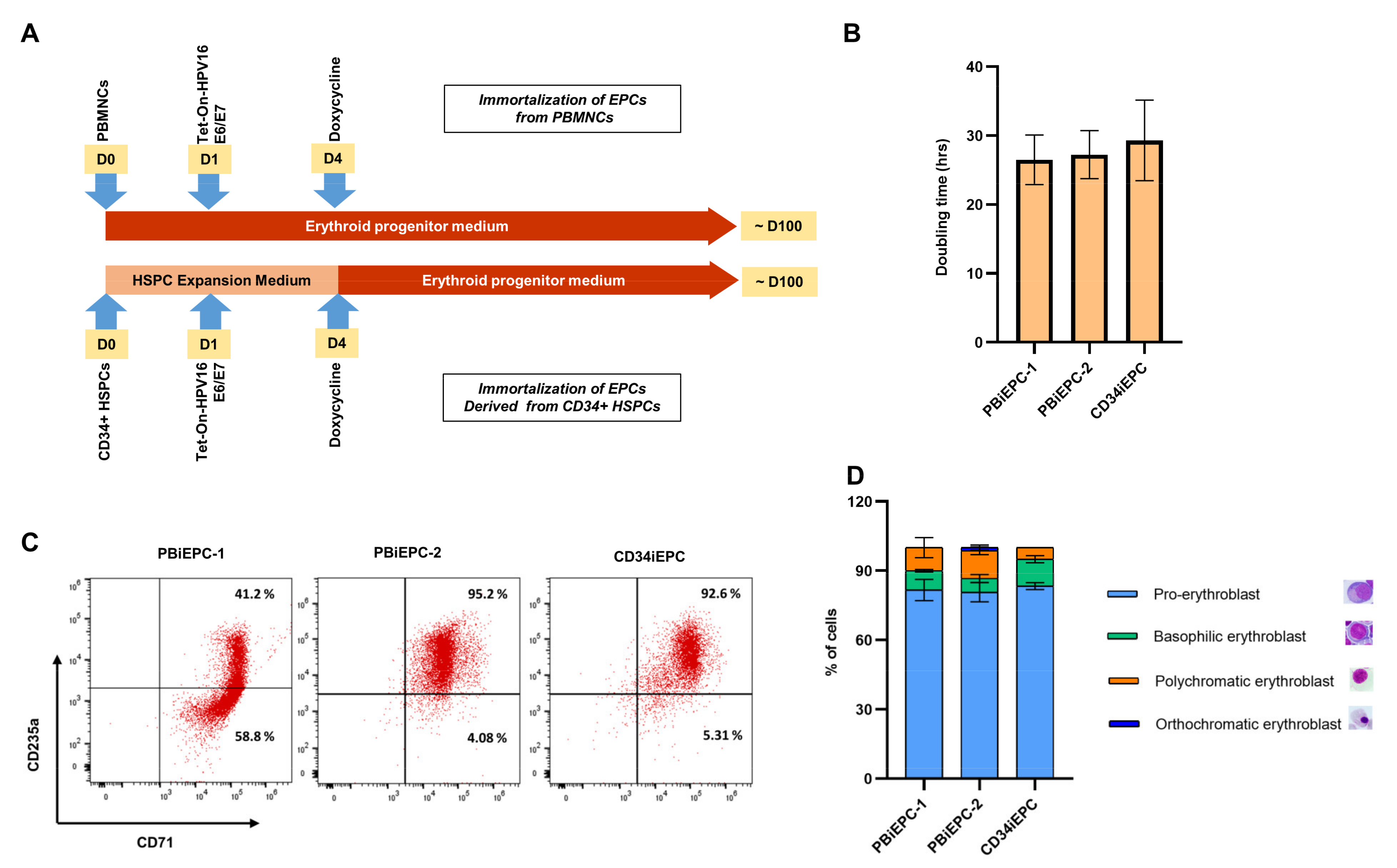
3.3. Generation of an iEPC Line from CD34+ HSPCs
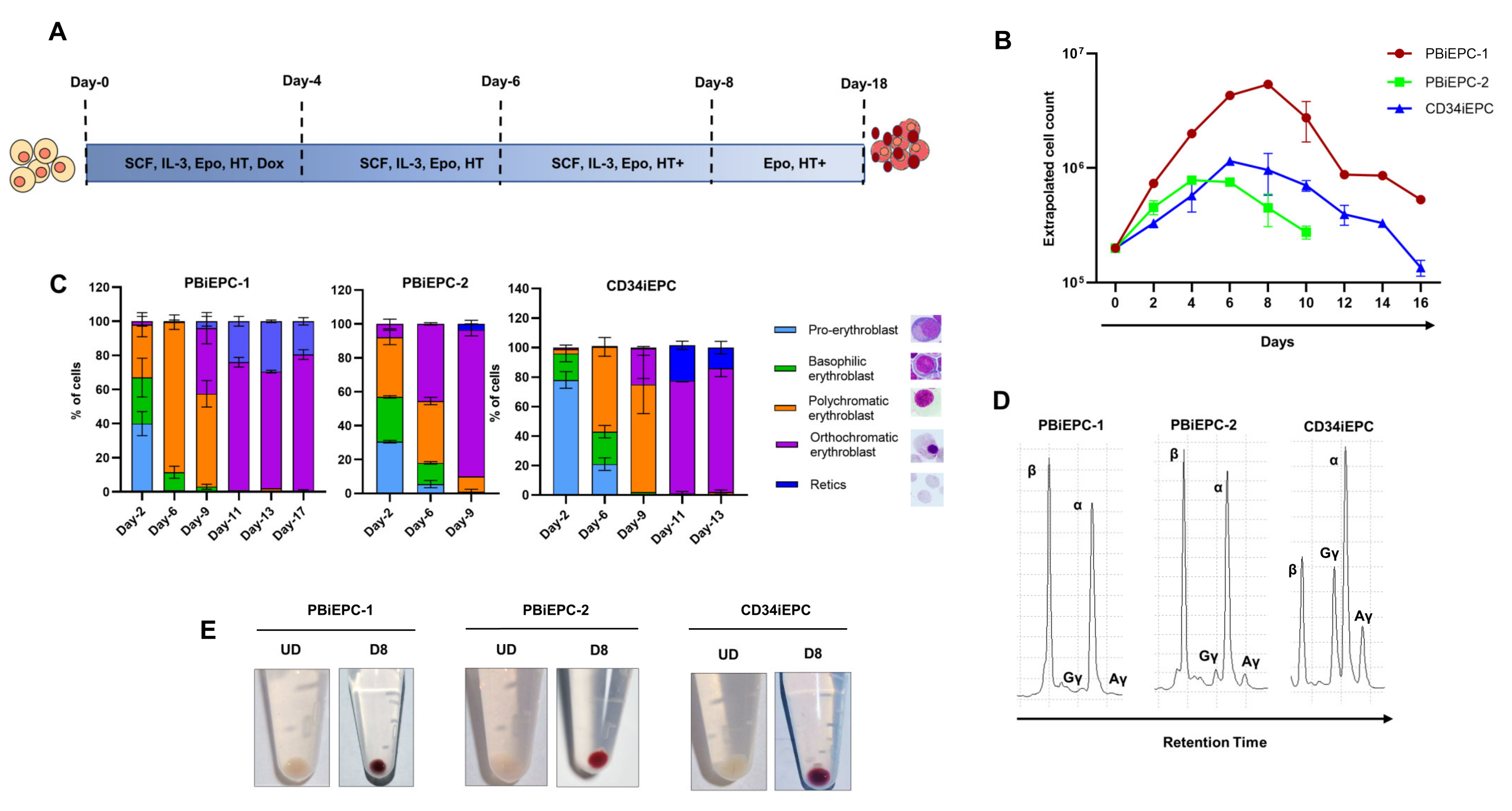
3.4. Differentiation of iEPCs
3.5. Karyotype Analysis of iEPCs
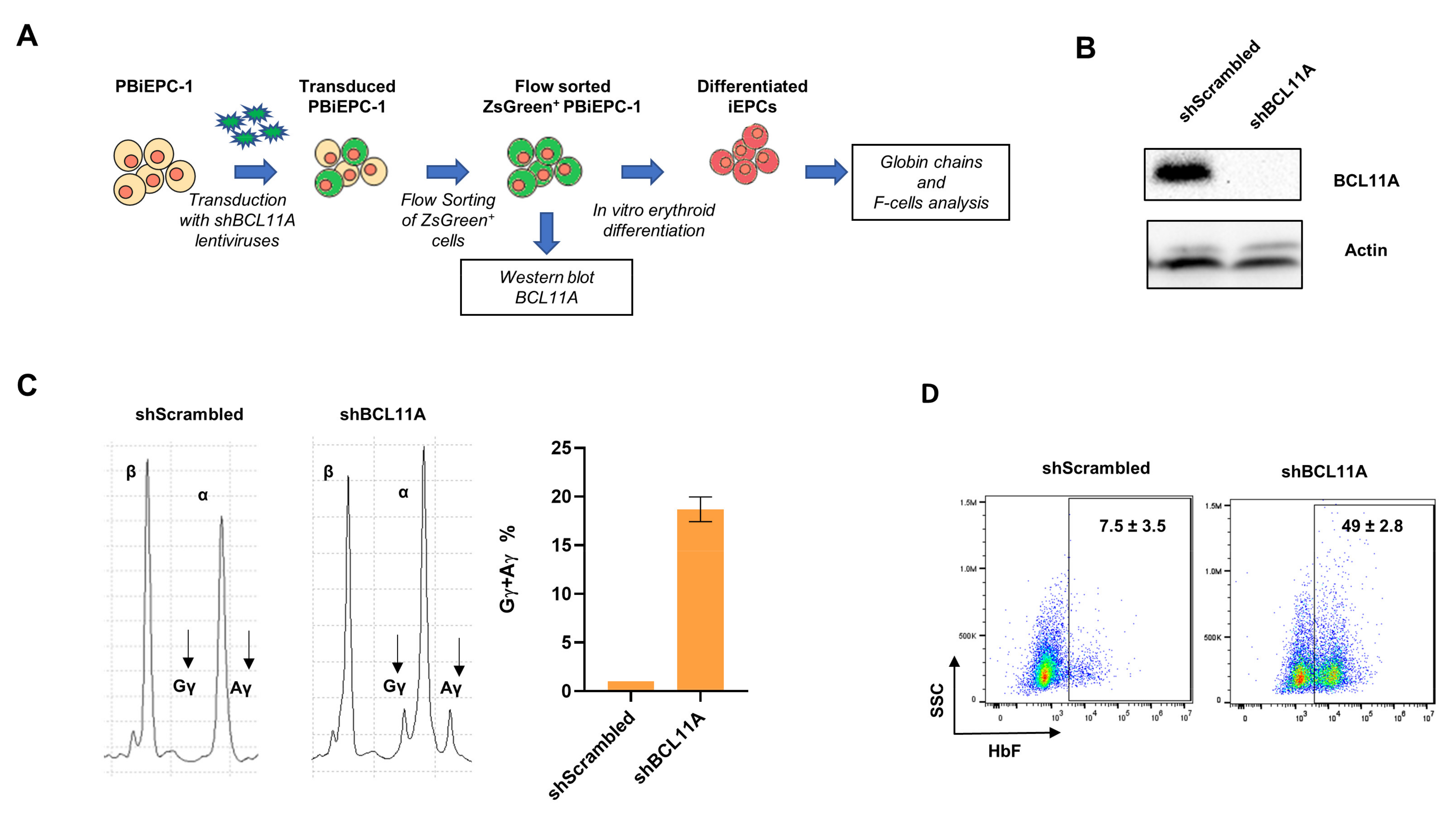
3.6. Gene Manipulation of PBiEPC-1
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weatherall, D.; Akinyanju, O.; Fucharoen, S.; Olivieri, N.; Musgrove, P. Chapter 34. Inherited Disorders of Hemoglobin. In Disease Control Priorities in Developing Countries, 2nd ed.; Jamison, D.T., Breman, J.G., Measham, A.R., Alleyne, G., Claeson, M., Evans, D.B., Jha, P., Mills, A., Musgrove, P., Eds.; World Bank Publications: Washington, DC, USA, 2006; pp. 663–680. [Google Scholar]
- Da Costa, L.; Leblanc, T.; Mohandas, N. Diamond-Blackfan anemia. Blood 2020, 136, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Babbs, C. The pathogenesis, diagnosis and management of CDA type I. Br. J. Haematol. 2019, 185. [Google Scholar] [CrossRef]
- Ashley, R.; Yan, H.; Wang, N.; Hale, J.; Dulmovits, B.M.; Papoin, J.; Olive, M.; Udeshi, N.D.; Carr, S.A.; Vlachos, A.; et al. Glucocorticoids induce the expansion of an immature human CFU-E population. bioRxiv 2019, 722850. [Google Scholar] [CrossRef]
- Qu, X.; Zhang, S.; Wang, S.; Wang, Y.; Li, W.; Huang, Y.; Zhao, H.; Wu, X.; An, C.; Guo, X.; et al. TET2 deficiency leads to stem cell factor–dependent clonal expansion of dysfunctional erythroid progenitors. Blood 2018, 132, 2406–2417. [Google Scholar] [CrossRef] [PubMed]
- Neildez-Nguyen, T.M.A.; Wajcman, H.; Marden, M.C.; Bensidhoum, M.; Moncollin, V.; Giarratana, M.-C.; Kobari, L.; Thierry, D.; Douay, L. Human erythroid cells produced ex vivo at large scale differentiate into red blood cells in vivo. Nat. Biotechnol. 2002, 20, 467–472. [Google Scholar] [CrossRef]
- Scott, C.; Downes, D.J.; Brown, J.M.; Babbs, C.; Olijnik, A.-A.; Gosden, M.; Beagrie, R.; Schwessinger, R.; Fisher, C.A.; Rose, A.; et al. Modelling erythropoiesis in congenital dyserythropoietic anaemia type I (CDA-I). bioRxiv 2019, 744367. [Google Scholar] [CrossRef]
- O’Brien, K.A.; Farrar, J.E.; Vlachos, A.; Anderson, S.M.; Tsujiura, C.A.; Lichtenberg, J.; Blanc, L.; Atsidaftos, E.; Elkahloun, A.; An, X.; et al. Molecular convergence in ex vivo models of Diamond-Blackfan anemia. Blood 2017, 129, 3111–3120. [Google Scholar] [CrossRef]
- Ludwig, L.S.; Gazda, H.T.; Eng, J.C.; Eichhorn, S.W.; Thiru, P.; Ghazvinian, R.; George, T.I.; Gotlib, J.R.; Beggs, A.H.; Sieff, C.A.; et al. Altered translation of GATA1 in Diamond-Blackfan anemia. Nat. Med. 2014, 20, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Paes, B.C.M.F.; Stabeli, L.C.J.R.; Costa, P.N.M.; Orellana, M.D.; Kashima, S.; Covas, D.T.; Picanço-Castro, V. Generation of hematopoietic stem/progenitor cells with sickle cell mutation from induced pluripotent stem cell in serum-free system. Hematol. Transfus. Cell Ther. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lange, L.; Hoffmann, D.; Schwarzer, A.; Ha, T.-C.; Philipp, F.; Lenz, D.; Morgan, M.; Schambach, A. Inducible Forward Programming of Human Pluripotent Stem Cells to Hemato-endothelial Progenitor Cells with Hematopoietic Progenitor Potential. Stem Cell Rep. 2020, 14, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alegria, E.; Menegatti, S.; Fadlullah, M.Z.H.; Menendez, P.; Lacaud, G.; Kouskoff, V. Early Human Hemogenic Endothelium Generates Primitive and Definitive Hematopoiesis In Vitro. Stem Cell Rep. 2018, 11, 1061–1074. [Google Scholar] [CrossRef]
- Sugimura, R.; Jha, D.K.; Han, A.; Soria-Valles, C.; da Rocha, E.L.; Lu, Y.-F.; Goettel, J.A.; Serrao, E.; Rowe, R.G.; Malleshaiah, M.; et al. Haematopoietic stem and progenitor cells from human pluripotent stem cells. Nature 2017, 545, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Lapillonne, H.; Kobari, L.; Mazurier, C.; Tropel, P.; Giarratana, M.-C.; Zanella-Cleon, I.; Kiger, L.; Wattenhofer-Donzé, M.; Puccio, H.; Hebert, N.; et al. Red blood cell generation from human induced pluripotent stem cells: Perspectives for transfusion medicine. Haematologica 2010, 95, 1651–1659. [Google Scholar] [CrossRef]
- Mills, J.A.; Paluru, P.; Weiss, M.J.; Gadue, P.; French, D.L. Hematopoietic Differentiation of Pluripotent Stem Cells in Culture. In Hematopoietic Stem Cell Protocols; Humana Press: New York, NY, USA, 2014; Volume 1185, pp. 181–194. ISBN 9781493911332. [Google Scholar]
- Kaufman, D.S. Toward clinical therapies using hematopoietic cells derived from human pluripotent stem cells. Blood 2009, 114, 3513–3523. [Google Scholar] [CrossRef] [PubMed]
- Doulatov, S.; Vo, L.T.; Chou, S.S.; Kim, P.G.; Arora, N.; Li, H.; Hadland, B.K.; Bernstein, I.D.; Collins, J.J.; Zon, L.I.; et al. Induction of multipotential hematopoietic progenitors from human pluripotent stem cells via respecification of lineage-restricted precursors. Cell Stem Cell 2013, 13, 459–470. [Google Scholar] [CrossRef]
- Khoriaty, R.; Vasievich, M.P.; Jones, M.; Everett, L.; Chase, J.; Tao, J.; Siemieniak, D.; Zhang, B.; Maillard, I.; Ginsburg, D. Absence of a red blood cell phenotype in mice with hematopoietic deficiency of SEC23B. Mol. Cell. Biol. 2014, 34, 3721–3734. [Google Scholar] [CrossRef]
- Kurita, R.; Suda, N.; Sudo, K.; Miharada, K.; Hiroyama, T.; Miyoshi, H.; Tani, K.; Nakamura, Y. Establishment of immortalized human erythroid progenitor cell lines able to produce enucleated red blood cells. PLoS ONE 2013, 8, e59890. [Google Scholar] [CrossRef] [PubMed]
- Trakarnsanga, K.; Griffiths, R.E.; Wilson, M.C.; Blair, A.; Satchwell, T.J.; Meinders, M.; Cogan, N.; Kupzig, S.; Kurita, R.; Nakamura, Y.; et al. An immortalized adult human erythroid line facilitates sustainable and scalable generation of functional red cells. Nat. Commun. 2017, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Canver, M.C.; Smith, E.C.; Sher, F.; Pinello, L.; Sanjana, N.E.; Shalem, O.; Chen, D.D.; Schupp, P.G.; Vinjamur, D.S.; Garcia, S.P.; et al. BCL11A enhancer dissection by Cas9-mediated in situ saturating mutagenesis. Nature 2015, 527, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Grevet, J.D.; Lan, X.; Hamagami, N.; Edwards, C.R.; Sankaranarayanan, L.; Ji, X.; Bhardwaj, S.K.; Face, C.J.; Posocco, D.F.; Abdulmalik, O.; et al. Domain-focused CRISPR screen identifies HRI as a fetal hemoglobin regulator in human erythroid cells. Science 2018, 361, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Hargreaves, V.V.; Zhu, Q.; Kurland, J.V.; Hong, J.; Kim, W.; Sher, F.; Macias-Trevino, C.; Rogers, J.M.; Kurita, R.; et al. Direct Promoter Repression by BCL11A Controls the Fetal to Adult Hemoglobin Switch. Cell 2018, 173, 430–442.e17. [Google Scholar] [CrossRef]
- Martyn, G.E.; Wienert, B.; Yang, L.; Shah, M.; Norton, L.J.; Burdach, J.; Kurita, R.; Nakamura, Y.; Pearson, R.C.M.; Funnell, A.P.W.; et al. Natural regulatory mutations elevate the fetal globin gene via disruption of BCL11A or ZBTB7A binding. Nat. Genet. 2018, 50, 498–503. [Google Scholar] [CrossRef]
- Ludwig, L.S.; Lareau, C.A.; Bao, E.L.; Nandakumar, S.K.; Muus, C.; Ulirsch, J.C.; Chowdhary, K.; Buenrostro, J.D.; Mohandas, N.; An, X.; et al. Transcriptional States and Chromatin Accessibility Underlying Human Erythropoiesis. Cell Rep. 2019, 27, 3228–3240.e7. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.E.; Magis, W.; Vu, J.; Heo, S.J.; Wartiovaara, K.; Walters, M.C.; Kurita, R.; Nakamura, Y.; Boffelli, D.; Martin, D.I.K.; et al. CRISPR-Cas9 interrogation of a putative fetal globin repressor in human erythroid cells. PLoS ONE 2019, 14, e0208237. [Google Scholar] [CrossRef] [PubMed]
- Wienert, B.; Martyn, G.E.; Kurita, R.; Nakamura, Y.; Quinlan, K.G.R.; Crossley, M. KLF1 drives the expression of fetal hemoglobin in British HPFH. Blood 2017, 130, 803–807. [Google Scholar] [CrossRef]
- Canver, M.C.; Bauer, D.E.; Dass, A.; Yien, Y.Y.; Chung, J.; Masuda, T.; Maeda, T.; Paw, B.H.; Orkin, S.H. Characterization of genomic deletion efficiency mediated by clustered regularly interspaced palindromic repeats (CRISPR)/cas9 nuclease system in mammalian cells. J. Biol. Chem. 2014, 289, 21312–21324. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Wang, X.; Maeda, M.; Canver, M.C.; Sher, F.; Funnell, A.P.W.; Fisher, C.; Suciu, M.; Martyn, G.E.; Norton, L.J.; et al. Transcription factors LRF and BCL11A independently repress expression of fetal hemoglobin. Science 2016, 351, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Jearawiriyapaisarn, N.; Lee, M.P.; Hosoya, T.; Wu, Q.; Myers, G.; Lim, K.C.; Kurita, R.; Nakamura, Y.; Vojtek, A.B.; et al. BAP1 regulation of the key adaptor protein NCoR1 is critical for γ-globin gene repression. Genes Dev. 2018, 32, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Moir-Meyer, G.; Cheong, P.L.; Olijnik, A.-A.; Brown, J.; Knight, S.; King, A.; Kurita, R.; Nakamura, Y.; Gibbons, R.J.; Higgs, D.R.; et al. Robust CRISPR/Cas9 Genome Editing of the HUDEP-2 Erythroid Precursor Line Using Plasmids and Single-Stranded Oligonucleotide Donors. Methods Protoc. 2018, 1, 28. [Google Scholar] [CrossRef]
- Satchwell, T.J.; Wright, K.E.; Haydn-Smith, K.L.; Sánchez-Román Terán, F.; Moura, P.L.; Hawksworth, J.; Frayne, J.; Toye, A.M.; Baum, J. Genetic manipulation of cell line derived reticulocytes enables dissection of host malaria invasion requirements. Nat. Commun. 2019, 10, 3806. [Google Scholar] [CrossRef] [PubMed]
- Hawksworth, J.; Satchwell, T.J.; Meinders, M.; Daniels, D.E.; Regan, F.; Thornton, N.M.; Wilson, M.C.; Dobbe, J.G.G.; Streekstra, G.J.; Trakarnsanga, K.; et al. Enhancement of red blood cell transfusion compatibility using CRISPR-mediated erythroblast gene editing. EMBO Mol. Med. 2018, 10, e8454. [Google Scholar] [CrossRef] [PubMed]
- Trakarnsanga, K.; Tipgomut, C.; Metheetrairut, C.; Wattanapanitch, M.; Khuhapinant, A.; Poldee, S.; Kurita, R.; Nakamura, Y.; Srisawat, C.; Frayne, J. Generation of an immortalised erythroid cell line from haematopoietic stem cells of a haemoglobin E/β-thalassemia patient. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Filippone, C.; Franssila, R.; Kumar, A.; Saikko, L.; Kovanen, P.E.; Söderlund-Venermo, M.; Hedman, K. Erythroid Progenitor Cells Expanded from Peripheral Blood without Mobilization or Preselection: Molecular Characteristics and Functional Competence. PLoS ONE 2010, 5, e9496. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B.J.; Housman, D. Characterization of an erythroid precursor cell of high proliferative capacity in normal human peripheral blood. Proc. Natl. Acad. Sci. USA 1977, 74, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Krantz, S.B.; Kans, J.S.; Dessypris, E.N.; Sawyer, S.; Glick, A.D.; Civin, C.I. Purification of human erythroid colony-forming units and demonstration of specific binding of erythropoietin. J. Clin. Investig. 1987, 80, 357–366. [Google Scholar] [CrossRef]
- Heshusius, S.; Heideveld, E.; Burger, P.; Thiel-Valkhof, M.; Sellink, E.; Varga, E.; Ovchynnikova, E.; Visser, A.; Martens, J.H.A.; von Lindern, M.; et al. Large-scale in vitro production of red blood cells from human peripheral blood mononuclear cells. Blood Adv. 2019, 3, 3337–3350. [Google Scholar] [CrossRef]
- Zhang, S.; Olivier, E.N.; Yan, Z.; Suzuka, S.; Roberts, K.; Wang, K.; Bouhassira, E.E. MNC-RED A Chemically-Defined Method to Produce Enucleated Red Blood Cells from Adult Peripheral Blood Mononuclear Cells. bioRxiv 2019, 616755. [Google Scholar] [CrossRef]
- Van Den Akker, E.; Satchwell, T.J.; Pellegrin, S.; Daniels, G.; Toye, A.M. The majority of the in vitro erythroid expansion potential resides in CD34 – cells, outweighing the contribution of CD34 + cells and significantly increasing the erythroblast yield from peripheral blood samples. Haematologica 2010, 95, 1594–1598. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, J.; Xue, F.; Halverson, G.; Reid, M.; Guo, A.; Chen, L.; Raza, A.; Galili, N.; Jaffray, J.; et al. Isolation and functional characterization of human erythroblasts at distinct stages: Implications for understanding of normal and disordered erythropoiesis in vivo. Blood 2019, 121, 3246–3254. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Leach, J.; Reittie, J.E.; Atzberger, A.; Lee-prudhoe, J.; Wood, W.G.; Higgs, D.R.; Iborra, F.J.; Buckle, V.J. Coregulated human globin genes are frequently in spatial proximity when active. J. Cell Biol. 2006, 172, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hale, J.; Bhagia, P.; Xue, F.; Chen, L.; Jaffray, J.; Yan, H.; Lane, J.; Gallagher, P.G.; Mohandas, N.; et al. Isolation and transcriptome analyses of human erythroid progenitors: BFU-E and CFU-E. Blood 2014, 124, 3636–3645. [Google Scholar] [CrossRef] [PubMed]
- Loucari, C.C.; Patsali, P.; van Dijk, T.B.; Stephanou, C.; Papasavva, P.; Zanti, M.; Kurita, R.; Nakamura, Y.; Christou, S.; Sitarou, M.; et al. Rapid and Sensitive Assessment of Globin Chains for Gene and Cell Therapy of Hemoglobinopathies. Hum. Gene Ther. Methods 2018, 29, 60–74. [Google Scholar] [CrossRef]
- Knott, S.R.V.; Maceli, A.R.; Erard, N.; Chang, K.; Marran, K.; Zhou, X.; Gordon, A.; ElDemerdash, O.; Wagenblast, E.; Kim, S.; et al. A Computational Algorithm to Predict shRNA Potency. Mol. Cell 2014, 56, 796–807. [Google Scholar] [CrossRef]
- Corporation Synthego. Knockout Guide Design. Available online: https://design.synthego.com/#/ (accessed on 8 October 2020).
- Montague, T.G.; Cruz, J.M.; Gagnon, J.A.; Church, G.M.; Valen, E. CHOPCHOP: A CRISPR/Cas9 and TALEN web tool for genome editing. Nucleic Acids Res. 2014, 42, W401–W407. [Google Scholar] [CrossRef] [PubMed]
- Hsiau, T.; Conant, D.; Rossi, N.; Maures, T.; Waite, K.; Yang, J.; Joshi, S.; Kelso, R.; Holden, K.; Enzmann, B.L.; et al. Inference of CRISPR Edits from Sanger Trace Data. bioRxiv 2019, 251082. [Google Scholar] [CrossRef]
- Migliaccio, A.R.; Palis, J. Blood in a dish: In vitro synthesis of red blood cells. Drug Discov. Today Dis. Mech. 2011, 8, e3–e8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Migliaccio, A.R.; Masselli, E.; Varricchio, L.; Whitsett, C. Ex-vivo expansion of red blood cells: How real for transfusion in humans? Blood Rev. 2012, 26, 81–95. [Google Scholar] [CrossRef]
- Merryweather-Clarke, S.; Higgs, D.R.; McGowan, S.J.; Soneji, A.T.; Roberts, D.J.; Buckle, V.J.; Waugh, C.; Taylor, S.; Clark, K.; Robson, K.J.H.; et al. Global gene expression analysis of human erythroid progenitors. Blood 2011, 117, e96–e108. [Google Scholar] [CrossRef] [PubMed]
- Zingariello, M.; Bardelli, C.; Sancillo, L.; Ciaffoni, F.; Genova, M.L.; Girelli, G.; Migliaccio, A.R. Dexamethasone Predisposes Human Erythroblasts Toward Impaired Lipid Metabolism and Renders Their ex vivo Expansion Highly Dependent on Plasma Lipoproteins. Front. Physiol. 2019, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Narla, A.; Dutt, S.; McAuley, J.R.; Al-Shahrour, F.; Hurst, S.; McConkey, M.; Neuberg, D.; Ebert, B.L. Dexamethasone and lenalidomide have distinct functional effects on erythropoiesis. Blood 2011, 118, 2296–2304. [Google Scholar] [CrossRef] [PubMed]
- Vinjamur, D.S.; Bauer, D.E. Growing and Genetically Manipulating Human Umbilical Cord Blood-Derived Erythroid Progenitor (HUDEP) Cell Lines. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018; Volume 1698, pp. 275–284. ISBN 978-1-4939-7427-6. [Google Scholar]
- Kurita, R.; Funato, K.; Abe, T.; Watanabe, Y.; Shiba, M.; Tadokoro, K.; Nakamura, Y.; Nagai, T.; Satake, M. Establishment and characterization of immortalized erythroid progenitor cell lines derived from a common cell source. Exp. Hematol. 2019, 69, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.-J.; Wall, E.A.; Zavzavadjian, J.R.; Santat, L.A.; Liu, J.; Hwang, J.-I.; Rebres, R.; Roach, T.; Seaman, W.; Simon, M.I.; et al. A single lentiviral vector platform for microRNA-based conditional RNA interference and coordinated transgene expression. Proc. Natl. Acad. Sci. USA 2006, 103, 13759–13764. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Keyvanfar, K.; Wan, Z.; Kajigaya, S.; Young, N.S.; Zhi, N. Establishment of an erythroid cell line from primary CD36+ erythroid progenitor cells. Exp Hematol. 2010, 17, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Daniels, D.E.; Downes, D.J.; Ferrer-Vicens, I.; Ferguson, D.C.J.; Singleton, B.K.; Wilson, M.C.; Trakarnsanga, K.; Kurita, R.; Nakamura, Y.; Anstee, D.J.; et al. Comparing the two leading erythroid lines BEL-A and HUDEP-2. Haematologica 2020, 105, e389. [Google Scholar] [CrossRef] [PubMed]
- Miharada, K.; Hiroyama, T.; Sudo, K.; Nagasawa, T.; Nakamura, Y. Efficient enucleation of erythroblasts differentiated in vitro from hematopoietic stem and progenitor cells. Nat. Biotechnol. 2006, 24, 1255–1256. [Google Scholar] [CrossRef]
- Sankaran, V.G.; Taher, A.T.; Duca, L.; Musallam, K.M.; Cappellini, M.D.; Nathan, D.G. Fetal hemoglobin levels and morbidity in untransfused patients with -thalassemia intermedia. Blood 2011, 119, 364–367. [Google Scholar] [CrossRef]
- Baek, E.J.; Kim, H.-S.; Kim, J.-H.; Kim, N.J.; Kim, H.O. Stroma-free mass production of clinical-grade red blood cells (RBCs) by using poloxamer 188 as an RBC survival enhancer. Transfusion 2009, 49, 2285–2295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, C.; Wang, L.; Shen, B.; Guan, X.; Tian, J.; Ren, Z.; Ding, X.; Ma, Y.; Dai, W.; et al. Large-Scale Ex Vivo Generation of Human Red Blood Cells from Cord Blood CD34(+) Cells. Stem Cells Transl. Med. 2017, 6, 1698–1709. [Google Scholar] [CrossRef]
- Balogh, P.; Adelman, E.R.; Pluvinage, J.V.; Capaldo, B.J.; Freeman, K.C.; Singh, S.; Elagib, K.E.; Nakamura, Y.; Kurita, R.; Sashida, G.; et al. RUNX3 levels in human hematopoietic progenitors are regulated by aging and dictate erythroid-myeloid balance. Haematologica 2019, 104, 905–913. [Google Scholar] [CrossRef]
- Zhou, Z.; Giles, K.E.; Felsenfeld, G. DNA·RNA triple helix formation can function as a cis-acting regulatory mechanism at the human β-globin locus. Proc. Natl. Acad. Sci. USA 2019, 116, 6130–6139. [Google Scholar] [CrossRef] [PubMed]
- Sher, F.; Hossain, M.; Seruggia, D.; Schoonenberg, V.A.C.; Yao, Q.; Cifani, P.; Dassama, L.M.K.; Cole, M.A.; Ren, C.; Vinjamur, D.S.; et al. Rational targeting of a NuRD subcomplex guided by comprehensive in situ mutagenesis. Nat. Genet. 2019, 51, 1149–1159. [Google Scholar] [CrossRef]
- Traxler, E.A.; Yao, Y.; Wang, Y.D.; Woodard, K.J.; Kurita, R.; Nakamura, Y.; Hughes, J.R.; Hardison, R.C.; Blobel, G.A.; Li, C.; et al. A genome-editing strategy to treat β-hemoglobinopathies that recapitulates a mutation associated with a benign genetic condition. Nat. Med. 2016, 22, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Morrison, T.A.; Wilcox, I.; Luo, H.-Y.; Farrell, J.J.; Kurita, R.; Nakamura, Y.; Murphy, G.J.; Cui, S.; Steinberg, M.H.; Chui, D.H.K. A long noncoding RNA from the HBS1L-MYB intergenic region on chr6q23 regulates human fetal hemoglobin expression. Blood Cells Mol. Dis. 2018, 69, 1–9. [Google Scholar] [CrossRef]
- Yu, X.; Azzo, A.; Bilinovich, S.M.; Li, X.; Dozmorov, M.; Kurita, R.; Nakamura, Y.; Williams, D.C.; Ginder, G.D. Disruption of the MBD2-NuRD complex but not MBD3-NuRD induces high level HbF expression in human erythroid cells. Haematologica 2019, 104. [Google Scholar] [CrossRef] [PubMed]
- Lessard, S.; Gatof, E.S.; Beaudoin, M.; Schupp, P.G.; Sher, F.; Ali, A.; Prehar, S.; Kurita, R.; Nakamura, Y.; Baena, E.; et al. An erythroid-specific ATP2B4 enhancer mediates red blood cell hydration and malaria susceptibility. J. Clin. Investig. 2017, 127, 3065–3074. [Google Scholar] [CrossRef] [PubMed]
- Canver, M.C.; Lessard, S.; Pinello, L.; Wu, Y.; Ilboudo, Y.; Stern, E.N.; Needleman, A.J.; Galactéros, F.; Brugnara, C.; Kutlar, A.; et al. Variant-aware saturating mutagenesis using multiple Cas9 nucleases identifies regulatory elements at trait-associated loci. Nat. Genet. 2017, 49, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Demirci, S.; Gudmundsdottir, B.; Li, Q.; Haro-Mora, J.J.; Nassehi, T.; Drysdale, C.; Yapundich, M.; Gamer, J.; Seifuddin, F.; Tisdale, J.F.; et al. βT87Q-Globin Gene Therapy Reduces Sickle Hemoglobin Production, Allowing for Ex Vivo Anti-sickling Activity in Human Erythroid Cells. Mol. Ther. Methods Clin. Dev. 2020, 17, 912–921. [Google Scholar] [CrossRef]
- Georgomanoli, M.; Papapetrou, E.P. Modeling blood diseases with human induced pluripotent stem cells. Dis. Models Mech. 2019, 12, dmm039321. [Google Scholar] [CrossRef] [PubMed]

| Cell Line | Days | Karyotype |
|---|---|---|
| PBiEPC-1 | 162 | 45,XY,-10,der(10),add(21)(q22)[20] |
| 169 | 45,XY,-10,der(10),add(21)(q22)[18] | |
| PBiEPC-2 | 174 | 48,XY,+8,-18,+19,+21[10]/49,XY,+8,del(18)(p11.2),+19,+21[7] |
| 181 | 44~48,XY,-Y,+8,-18,+19,+21[cp6] | |
| CD34iEPC | 143 | 80~87<4n>,XXX,der(X),-3,add(4)(p13),del(4)(q21),der(4;11)(p13;q23),der(5;11)(q35;q23),-7, add(7)(p14), der(8;15)(p23;p10),-9, add(9)(q34),der(10),-12, der(13;15)(p10;p10),-14,-17, i(17)(q10), add(21)(p10), add(21)(q21), +mar[cp20] |
| 157 | 79~92<4n>,XXX,der(X),-3,add(6)(p23),-7,t(7;10)(q32;q24),add(8)(p27), der(8),-9, -10,-11,-11,-12, der(13), -14,-15,der(15),-16,der(17),i(17)(q10),der(21),+mar,+mar[cp20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagchi, A.; Nath, A.; Thamodaran, V.; Ijee, S.; Palani, D.; Rajendiran, V.; Venkatesan, V.; Datari, P.; Pai, A.A.; Janet, N.B.; et al. Direct Generation of Immortalized Erythroid Progenitor Cell Lines from Peripheral Blood Mononuclear Cells. Cells 2021, 10, 523. https://doi.org/10.3390/cells10030523
Bagchi A, Nath A, Thamodaran V, Ijee S, Palani D, Rajendiran V, Venkatesan V, Datari P, Pai AA, Janet NB, et al. Direct Generation of Immortalized Erythroid Progenitor Cell Lines from Peripheral Blood Mononuclear Cells. Cells. 2021; 10(3):523. https://doi.org/10.3390/cells10030523
Chicago/Turabian StyleBagchi, Abhirup, Aneesha Nath, Vasanth Thamodaran, Smitha Ijee, Dhavapriya Palani, Vignesh Rajendiran, Vigneshwaran Venkatesan, Phaneendra Datari, Aswin Anand Pai, Nancy Beryl Janet, and et al. 2021. "Direct Generation of Immortalized Erythroid Progenitor Cell Lines from Peripheral Blood Mononuclear Cells" Cells 10, no. 3: 523. https://doi.org/10.3390/cells10030523
APA StyleBagchi, A., Nath, A., Thamodaran, V., Ijee, S., Palani, D., Rajendiran, V., Venkatesan, V., Datari, P., Pai, A. A., Janet, N. B., Balasubramanian, P., Nakamura, Y., Srivastava, A., Mohankumar, K. M., Thangavel, S., & Velayudhan, S. R. (2021). Direct Generation of Immortalized Erythroid Progenitor Cell Lines from Peripheral Blood Mononuclear Cells. Cells, 10(3), 523. https://doi.org/10.3390/cells10030523







