Abstract
Intracellular organelles enwrapped in membranes along with a complex network of vesicles trafficking in, out and inside the cellular environment are one of the main features of eukaryotic cells. Given their central role in cell life, compartmentalization and mechanisms allowing their maintenance despite continuous crosstalk among different organelles have been deeply investigated over the past years. Here, we review the multiple functions exerted by the endosomal sorting complex required for transport (ESCRT) machinery in driving membrane remodeling and fission, as well as in repairing physiological and pathological membrane damages. In this way, ESCRT machinery enables different fundamental cellular processes, such as cell cytokinesis, biogenesis of organelles and vesicles, maintenance of nuclear–cytoplasmic compartmentalization, endolysosomal activity. Furthermore, we discuss some examples of how viruses, as obligate intracellular parasites, have evolved to hijack the ESCRT machinery or part of it to execute/optimize their replication cycle/infection. A special emphasis is given to the herpes simplex virus type 1 (HSV-1) interaction with the ESCRT proteins, considering the peculiarities of this interplay and the need for HSV-1 to cross both the nuclear-cytoplasmic and the cytoplasmic-extracellular environment compartmentalization to egress from infected cells.
1. Introduction
Membrane-surrounded organelles characterize eukaryotic cells and guarantee the compartmentalization of distinctive processes and functions. Intracellular membranes not only maintain the integrity of these compartments but, thanks to finely tuned vesicle trafficking, also play a pivotal role in the crosstalk between organelles themselves. Dynamic, constant and controlled remodeling processes enable the exchange of signals, information and materials between membranes that is crucial for the functioning of biological systems [1]. Two main types of membrane involutions can be formed. The first type of vesicles excludes the cytosolic environment and occurs during “classical” budding events, such as endocytosis. The second type of vesicles, instead, originates from a “reverse-topology” membrane scission which includes the cytosol and is mediated by the endosomal sorting complex required for transport (ESCRT) machinery [2]. This network of proteins is involved in different essential cellular processes, such as cytokinesis, autophagy, multivesicular body (MVB) and extracellular vesicle (EV) biogenesis, plasma, nuclear and endolysosomal membrane repair [2,3]. This list is far from being exhaustive, but represents the pathways involving ESCRTs that are better characterized. Viruses, being obligate intracellular parasites, have evolved to hijack highly conserved cellular pathways throughout their replication cycle [4]. Thus, and not surprisingly, viruses exploit the host intracellular membrane trafficking machinery to execute crucial steps of infection, such as (i) entering the target cell; (ii) transporting their genomic materials to the site of replication; (iii) if enveloped, acquiring their external lipid coating; (iv) exiting from infected cells. At the same time, several enveloped and non-enveloped viruses induce profound membrane remodeling/proliferation in infected cells to create specialized compartments where viral genome replication and/or new virion assembly occurs [5,6,7]. Interestingly, some insect and plant viruses are able to modify mitochondrial and peroxisomal membranes for their replication [7]. Finally, EVs play a broad-spectrum role in the pathogenesis of viral infection. Indeed, viruses not only adopt exosomes to accomplish specific steps of their life cycle, but also exploit these EVs to transfer both viral and cellular factors, such as proteins and non-coding RNAs, outside the infected cells to promote infection and to escape from the immune system [8].
Here, we review the similarities and differences of various ESCRT-dependent cellular processes, including EV biogenesis, emphasizing mechanisms of ESCRT recruitment by viruses. Furthermore, we focus on how the herpes simplex virus type 1 (HSV-1), a complex DNA-enveloped virus, interacts with ESCRT proteins to cross the nuclear envelope and egress from infected cells.
2. The ESCRT Machinery: An Overview
The ESCRT machinery and its associated factors include a network of different proteins (roughly 20 in yeast and 30 in mammals) that are sequentially recruited to the inner surface of the membrane necks of vesicles, mostly budding away from the cytosol (the so-called “reverse topology” budding event). ESCRT proteins were originally identified in budding yeasts in studies aimed at the identification of factors involved in the biogenesis of the MVBs [9,10]. MVBs contain intraluminal vesicles (ILVs) that arise from the budding of the limiting endosomal membrane into the lumen of the organelle. When MVBs fuse to the lysosomes, the content of those ILVs is degraded [11]. This mechanism of degradation has been well described in the case of both misfolded cell surface proteins and of G-coupled proteins and tyrosine kinase receptors, channels and transporters, which need to be downregulated after responding to specific stimuli [12]. The nascent ILVs are connected to the limiting membrane by a narrow membrane neck, which must be cut to release them into the lumen. In MVB biogenesis, the ESCRT machinery drives both budding and scission of ILVs. In different pathways, proteins other than the ESCRTs are responsible for the formation of the vesicle/membrane neck, while ESCRTs and associated factors drive membrane scission. Although ESCRT machinery is the master player of reverse topology budding events, some exceptions to this rule are reported in the literature. For instance, in the peroxisome biogenesis of budding yeasts, one of the ESCRTs, namely ESCRT-III, which is described in the next paragraph, is involved in a classical topology membrane scission event. Indeed, it allows the scission of pre-peroxisomal vesicles from the endoplasmic reticulum (ER) membrane into the cytosol [13].
The core of the ESCRT machinery consists of three complexes (ESCRT-I, ESCRT-II, ESCRT-III), as well as of the associated protein Alix (BRO1 in yeast) and the AAA-type ATP-ase, the vacuolar protein sorting (VPS) 4 [2,3,10]. ESCRT-I is a stalk-shaped heterotetrameric complex of proteins encompassing tumor suppressor gene 101, TSG101 (Vps23 in yeast), VPS28, VPS37 (from A to D), and UBAP1, MVB12A, or MVB12B [14,15,16]. ESCRT-II is a Y-shaped complex constituted by VPS22 (also known as EAP30), VPS36 (also known as EAP45), and two copies of VPS25 (also known as EAP20) [17]. ESCRT-III is formed by the so-called charged multivesicular body (CHMP) proteins 1 to 7, i.e., CHMP1A/B (Did2 in yeast); CHMP2A/B (Vps2 in yeast); CHMP3, (Vps24 in yeast); CHMP4A/B/C (Snf7 in yeast); CHMP5 (Vps60 in yeast); CHMP6 (Vps20 in yeast) and CHMP7 (Chm7 in yeast). Finally, increased sodium tolerance-1 (IST1) is also part of this complex [18]. While ESCRT-III is directly involved in remodeling and severing membranes, ESCRT-I and ESCRT-II often function together as assembly factors for ESCRT-III. Indeed, those two complexes cooperate to bring ESCRT-III to the site of the membranes where budding events are going to take place [19]. On the other hand, Alix [20,21] can work as an alternative way for the recruitment and activation of ESCRT-III. Indeed, Alix, via its Bro1 domain [22], can directly bind CHMP4. In some cases, the His domain-containing protein tyrosine phosphatase HD-PTP, an Alix-like protein also bearing a Bro1 domain, can replace Alix itself in recruiting CHMP4 [23,24]. An additional Bro1 containing protein, Bro1 domain/CAAX motif containing-protein (BROX), has been suggested to function in an Alix-like manner [25]. ESCRT-III assembly at the side of vesicle budding and membrane pinching off are dynamic processes. The AAA+ ATPase VPS4 [26] has a crucial role in recycling ESCRT-III subunits by extracting ESCRT-III monomers from the assembly. VPS4 functions as a hexamer and is characterized by an N-terminal microtubule interaction and transport (MIT) domain [27], along with a catalytic domain [28]. The MIT domain can bind to the MIT-interaction motifs (MIMs) that are present within ESCRT-III components. In particular, VPS4 has been shown to strongly bind to the MIM containing ESCRT-III proteins CHMP1, CHMP2, CHMP6, and IST1 [29,30]. ESCRT-III and VPS4 are universally involved in ESCRT-dependent membrane dynamic processes [31] that are reviewed in the following sections. Thus, it is not surprising that both ESCRT-III and VPS4 have homologs in archaea [32,33,34,35,36]. At the basis of most of the reverse topology fission events, indeed, are sequential steps involving ESCRT-III (Figure 1).
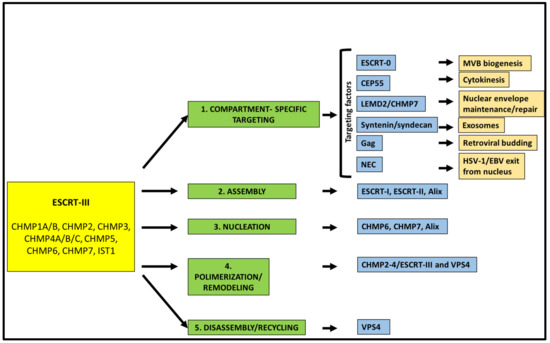
Figure 1.
Schematic representation of the sequential steps leading to ESCRT-III polymerization and functioning. The main factors involved in each step are reported, along with the biological processes in which ESCRT-III plays a role based on its compartment-specific recruitment (light orange rectangles).
First, ESCRT-III is targeted to the correct membrane/cell compartment. In most cases, this occurs thanks to proteins that act as compartment-specific targeting factors by forming molecular bridges for the recruitment of the ESCRT machinery (Figure 1). Several of these factors have been characterized over the years, including the cellular and viral proteins ESCRT-0, the centrosomal protein 55 (CEP55), syndecan/syntenin, Gag, LEMD2 (LEM Domain 2 protein)–CHMP7, BFRF1 and UL31/34. Each of these proteins contributes to the targeting of the ESCRT machinery towards a certain cellular compartment and facilitates a specific function of this complex. For instance, and as better detailed below, syndecan/syntenin are involved in the biogenesis of exosomes, CEP55 in the ESCRT-III mediated cell abscission during cytokinesis, BFRF1 and UL31/34 enable the egress of two herpesviruses, Epstein–Barr virus (EBV) and the herpes simplex virus type 1 (HSV-1), respectively, from the inner nuclear membrane (INM) of infected cells. Usually, ESCRT-III does not directly interact with these proteins, but it needs the involvement of assembly factors as the above-mentioned ESCRT-I/ESCRT-II, Alix and HD-PTP. In particular, Alix and HD-PTP, by binding both ESCRT-III and ESCRT-I [37,38], function as ESCRT-II alternatives. After assembly, nucleation and polymerization of ESCRT-III take place. In these steps, ESCRT-III components, and in particular CHMP4, need to be activated, a crucial process that can be mediated by both ESCRT-I/ESCRT-II or ESCRT-0/Alix [39,40]. ESCRT-III function relies on a finely tuned balance between the upstream factors that allow filament nucleation and the downstream proteins, including VPS4, that control filament polymerization rate [10]. If this balance is altered, the consequences for the cell can be detrimental. CHMP4-containing ESCRT filaments, as well as filaments composed of other ESCRT-III proteins (i.e., CHMP-2 and CHMP-3), are highly dynamic, a feature essential to determine membrane remodeling and scission. Furthermore, CHMP4 interacts with CHMP3 leading to the sliding of polymers that contribute to the ability of ESCRT-III filaments to change their architecture to adapt to the different phases of the membrane fission [41]. Interestingly, there is evidence that also VPS4 plays a role during the ESCRT-III remodeling process, in addition to functioning as a recycling factor for the proteins composing this complex [42].
3. ESCRT Machinery Functions in Fundamental Cellular Pathways
Membrane remodeling followed by reverse topology budding events and/or fission enables different fundamental cellular processes, including biogenesis of MVBs; cytokinesis; plasma and intracellular membrane maintenance/repair/reformation; micro- and macroautophagy. The involvement of the ESCRT machinery in these pathways, with a focus on results obtained in mammalian cells, is discussed in the next paragraphs and summarized in Figure 2.
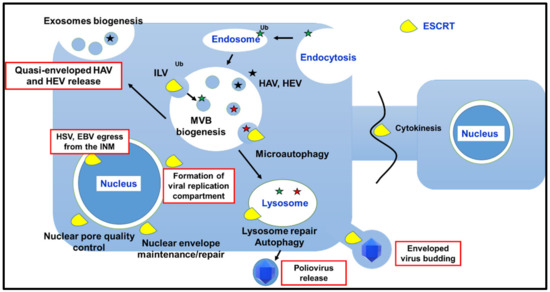
Figure 2.
Schematic representation of the better-characterized cellular and viral processes (highlighted by red rectangles) that involve ESCRT-machinery or specific ESCRT factors. HAV stands for hepatitis A virus; HEV for hepatitis E virus; HSV for herpes simplex virus; EBV for Epstein–Barr virus; INM for inner nuclear membrane. The involvement of ESCRT machinery in the viral replication cycles is discussed in the next paragraphs.
3.1. ESCRTs Involvement in MVB Biogenesis
Historically, one of the first studied functions of ESCRTs was their central role in the biogenesis of MVBs. Typically, MVBs are involved in protein trafficking throughout the endosomal-lysosomal pathway [43]. Transmembrane proteins endocytosed from the plasma membrane face two main fates: (i) they can be recycled back to the plasma membrane or the Golgi apparatus (GA) or can be retained to the limiting membrane of the MVB itself; (ii) they can be targeted to the ILVs and thus degraded, once mature MVBs fuse with the lysosomes. The ESCRT machinery is involved in both protein sorting as well as in ILV biogenesis and budding. MVBs display other important biological functions mostly related to the release of EVs, which can significantly influence intercellular signaling as well as the extracellular microenvironment [44,45,46]. Indeed, ILVs can give rise to exosomes upon a fusion of MVBs with the plasma membrane [43,47]. In conclusion, intracellular trafficking of MVBs, which is mainly regulated by Rab proteins, follows at least three different pathways: degradation (fusion with lysosomes), back-fusion (recycling of proteins) and secretion (release of exosomes). Central to all is the biogenesis of ILVs, which begins in early endosomes [48,49]. Proteins intended for degradation are usually marked by ubiquitin. Ubiquitin is a highly conserved protein of 76 amino acids that can be covalently linked to target proteins through a multistep process known as ubiquitylation [50]. Ubiquitin plays a central role in the MVB biogenesis pathway. Indeed, mono-ubiquitylation is necessary and sufficient to trigger the ESCRT-dependent endosomal sorting of membrane proteins and their degradation through the MVB/lysosomal pathway [51,52]. Moreover, ubiquitin plays a role in the recruitment and function of ESCRT components during the ILVs formation. Endosomal proteins, typically marked by lysine-63-linked ubiquitin, enter into the nascent ILVs by interacting with a ubiquitin-binding complex, known as ESCRT-0, constituted by HRS and STAM (Vps27 and Hse1 in budding yeast, respectively) [2,53]. ESCRT-0 is recruited to the early endosome limiting membrane by the interaction of an HRS FYVE domain with phosphatidylinositol 3-phosphate [54,55], a lipid that enriches this type of membrane. Once assembled as a heterotetrameric complex (two HRS and two STAM subunits) [56], ESCRT-0 can efficiently bind ubiquitylated cargoes since it displays several ubiquitin-binding domains (UBDs). Clathrin, along with ESCRT-0, concentrates ubiquitylated proteins in specific patches where sorted cargoes are handed over to ESCRT-I, once again thanks to ubiquitin recognition. Indeed, TSG101 contains a ubiquitin E2 variant (UEV) domain that binds ubiquitin. Furthermore, the mutually exclusive ESCRT-I components MVB12 or UBAP1 can bind ubiquitin through the ubiquitin associated (UBA) or the solenoid of overlapping UBA (SOUBA) domains, respectively. ESCRT-I then binds ESCRT-II, which contains a GRAM-like ubiquitin-binding in Eap45 (GLUE) domain. Finally, ESCRT-III and VPS4 are recruited to the sites of the endosomal membranes where cargo proteins have been concentrated, leading to membrane deformation, ILVs formation and budding [42,57,58,59]. As protein ubiquitylation is crucial for ESCRT-mediated cargo sorting into ILVs, and ESCRT components themselves (i.e., HRS, TSG101 and Alix) can be ubiquitylated, it is not surprising that both ubiquitin ligases and de-ubiquitylating enzymes are associated with the ESCRT machinery, with a key regulative role [60]. Of note, ESCRT-III has no known UBDs, and cargo deubiquitylation, at least in budding yeast, takes place prior to ILVs sorting, a process that contributes to maintaining the correct cytosolic levels of free ubiquitin (Figure 2). Finally, in mammalian cells, ILVs biogenesis can occur independently from ESCRT-0 and ESCRT-I. In this alternative pathway, Alix binds and sorts cargo proteins irrespectively of their ubiquitylation state [61,62,63]. Interestingly, the proteoglycan syndecan can recruit the ESCRT machinery via Alix along with syntenin-1, which functions as a bridge between the first two factors [64]. The syndecan-syntenin-Alix complex is, then, involved in the engagement of ESCRT-III during the biogenesis of exosomes. Exosomes are the smallest subclass of EVs with a diameter ranging from 30 to 120 nm. In addition to the Alix-mediated mechanisms, also classical ESCRT-I/ESCRT-II-dependent engagement of ESCRT-III has been described in exosome formation, as well as ESCRT machinery-independent processes [8]. Exosomes play a key role in cell-to-cell signaling and are emerging as important tools for diagnosis/treatment of diseases, cancer included, and as prognostics markers. Indeed, their cargo, which encompasses not only proteins but also lipids and nucleic acids, such as micro-ribonucleic acids (miRNAs) and long non-coding RNAs (lncRNAs), as well as functional messenger RNAs (mRNAs), is protected and can be delivered to target cells or quantified upon vesicle harvesting [8].
3.2. Role of ESCRT Machinery in Autophagy
Taking into account its role in the endolysosomal pathway, the involvement of the ESCRT machinery in autophagy is not unexpected. Indeed, autophagy is a process central for cell survival, as, among the best characterized of its functions, it mediates degradation of large intracellular materials (as organelles) by lysosomes. The autophagosome is a double-membrane structure that can originate from a variety of cellular membranes, including the ER, mitochondria and plasma membrane [65]. Autophagy is divided into macroautophagy and microautophagy. In the first case, cytoplasmic content is surrounded by a double membrane phagosome that can then fuse with lysosomes to degrade intraluminal substances. Macroautophagy plays a crucial role not only for cell catabolism, but also as a defense against intracellular pathogens and for removing damaged organelles. Microautophagy, instead, is based on the budding of ILVs from the endosome/lysosome membranes for degradation of specific cytosolic cargoes. In this sense, although the process closely resembles the MVB biogenesis, microautophagy is a distinct pathway with respect to MVB formation, as it does not involve endocytosed proteins but cytosolic molecules. ESCRT machinery plays a role in both types of autophagy [66,67,68]. This is not surprising in the case of microautophagy due to the aforementioned similarities. However, ESCRT-III and VPS4 are also recruited to the forming autophagosome, where they participate in the sealing of the double membrane coating. Furthermore, when ESCRT functions are blocked, cells accumulate autophagosomes, thus suggesting that ESCRTs are also part of the machinery allowing the fusion of autophagosomes with lysosomes [69,70].
3.3. Role of the ESCRT Machinery in Cytokinesis
Over the years, different studies carried on both in vitro and in model organisms (i.e., the fruit fly Drosophila melanogaster and the nematode Caenorhabditis elegans) have revealed the involvement of specific ESCRT factors also in the final phases of cell cytokinesis, the process is known as abscission [71,72,73,74,75]. Specifically, TSG101 and Alix play a pivotal role in this context, as they are directly engaged to the midbody ring by interactions with the centrosomal protein of 55 kDa (CEP55) [71,72,74,76,77]. Furthermore, TSG101 binds to septin 9, one of the septin ring proteins [78]. TSG101 and Alix work in parallel, allowing ESCRT-III nucleation and assembly at the midbody ring [79,80]. ESCRT-III is bound to the membrane via the microtubule interacting and trafficking domain containing 1 (MITD1) protein [81,82] and polymerizes in filaments that stretch out like arms from each side of the midbody ring. VPS4 functions by continuously remodeling these arms [42,58,83]. In the late phases of abscission, part of ESCRT-III and VPS4 localizes at the level of a secondary and thinner intracellular bridge between daughter cells that is generated by actin cytoskeleton remodeling along with microtubule reorganization and fusion of endocytic vesicles to the plasma membrane. ESCRT-III recruits the AAA-ATPase spastin that determines microtubule severing, followed by cell scission [75,84,85,86]. Interestingly, ESCRT-III and VPS4 homologs have also been characterized in archaea [35,87], where they work in concert with the cell division machinery (Cdv) to regulate abscission. Thus, it is likely that ESCRT-III/VPS4 involvement in the late phases of cytokinesis represents the ancestral role of the ESCRT machinery. Finally, the ESCRT-III function is modulated by abscission checkpoint regulators through different mechanisms [73,88,89]. In particular, CHMP4C plays a crucial role in this context. Indeed, it has been recently shown that a CHMP4C polymorphism (CHMP4CA232T), which disrupts the abscission checkpoint, leads to genome instability and is associated with different types of cancer [90]. On the other hand, and not surprisingly, failures in general of the process of abscission are connected with tumorigenesis [91]. This finding suggests that ESCRT-III components may be connected to oncogenesis via the induction of non-checkpointed aneuploidy. On the other hand, and not surprisingly, failures in the process of abscission, by leading to cells with an unstable tetraploid content, are, in general, connected with tumorigenesis [91]. Under this respect, different roles in cancer development are emerging for CEP55 [92]. Furthermore, it has been reported that when full chromosomes or part of them do not properly segregate, they can recruit their own nuclear membrane and form micronuclei (MN). Importantly, due to protein distribution defects within the inner membrane, during interphase, a large fraction of MN collapses. In this context, ruptures of the membrane are not repaired and trigger MN disruption followed by massive DNA damage and genomic instability [93]. Recently, Vietri and coworkers have elegantly linked this process, known as catastrophic nuclear envelope collapse, to the MN inability of limiting CHMP7-LEMD2 accumulation to the site of lesions. The role of this complex in recruiting ESCRT-III/VP4 to the site of nuclear envelope ruptures is discussed in more detail in Section 3.4.2. As a consequence, ESCRT-III over-accumulates, causing dramatic membrane distortion, DNA stress, and, in the end, chromosome fragmentation [94]. This work, on one hand, further supports the notion that ESCRT-III nucleation and polymerization need to be finely regulated in physiological processes to keep cells healthy and alive. On the other hand, it clearly indicates a role for ESCRT-III in genome instability and in tumorigenesis. Interestingly, different ESCRT components have been linked to cancer development by mechanisms other than defective cytokinesis. Among these mechanisms, which have been extensively reviewed elsewhere [95,96,97], is the control of downregulation of tumor-related receptors by their correct sorting into MVBs, as well as some of the functions displayed by ESCRT proteins in the nucleus that are discussed later in this review.
3.4. Involvement of the ESCRT Machinery in Damage Repair of Cellular Membranes
3.4.1. Plasma Membrane Repair
Plasma membrane damages can occur in response to different pathophysiological insults, pathogen-mediated ones included. These lesions compromise cell life, thus need to be rapidly repaired. Recruitment of lysosomes to the plasma membrane triggered by Ca2+ influxes is one of the main repair mechanisms for lesions ranging from 200 to 500 nm in size [98,99]. ESCRT machinery, by contrast, appears to be involved in repairing smaller lesions (<100 nm) of the cell surface. In this case, Ca2+ influx determines a rapid localization of CHMP4B to the sites of damages. Additional ESCRT-III components along with VPS4 are then recruited to the sites of the lesions, although CHMP4B seems to play a major role in injury resealing [99,100,101]. Interestingly, Alix and TSG101 are the main ESCRT-III assembly factors in this context, while ESCRT-0 and ESCRT-II, as well as additional known assembly cofactors (CHMP6), seem to be dispensable [101]. In particular, Alix interacts with the plasma membrane where it binds TSG101 in a Ca2+ dependent manner [102]. Thus, ESCRT-III plays a crucial protective role in contrasting membrane lesion-mediated types of cell death [103,104]. As an example, it has been shown that ESCRT-III can rescue cells from early-stages of necroptosis, at least temporarily, by acting on the membrane microdomains that are permeabilized by mixed lineage kinase domain-like pseudokinase (MLKL), one of the necroptotic proteins functioning at the plasma membrane [104].
3.4.2. Nuclear Envelope Maintenance and Repair
ESCRT-III is a key player also in the safeguard of nuclear membrane integrity. The nuclear envelope is responsible for the compartmentalization of the cell genome and, thus, of all the nuclear activities. Therefore, its rupture is associated with diseases [105]. At the structural level, the nuclear envelope is a double membrane encompassing the INM and the outer nuclear membrane (ONM), as well as nuclear pore complexes (NPCs) that are involved in controlling the trafficking of macromolecules in and out of the nucleus. Nuclear envelope ruptures can be either a physiological or a pathological event. Indeed, during a normal cell cycle, the nuclear membrane breaks down to allow the interaction between the mitotic spindle and chromosomes. Next, the nuclear envelope reassembles to form the nuclei of the daughter cells [106]. ESCRT-III and VPS4 are involved in the late steps of the reassembly process by executing membrane fission required for sealing the nuclear envelope [107,108]. As seen in the cytokinesis abscission, ESCRT-III recruits the AAA-ATPase spastin to accomplish microtubule severing, a step that is crucial for nuclear compartmentalization [107,108,109]. Interestingly, in this case, recruitment of ESCRT-III does not occur through the canonical ESCRT targeting/bridging factors. By contrast, it is mediated by CHMP7, an accessory ESCRT-III protein [107] that works in concert with the nuclear envelope protein LEMD2 and with additional regulator factors [109,110]. This fundamental function of ESCRT-III appears to be evolutionarily conserved, thus further supporting the notion that ESCRT-III is the central complex of ESCRT machinery [111]. In addition to the involvement in nuclear membrane reconstitution upon mitosis, ESCRT-III core subunits and VPS4 have been described to play a role in sealing the usually transient nuclear envelope damages that occur during the interphase and under pathological conditions [105,112,113,114]. Interestingly it has been recently reported that also the barrier to autoantigen factor (BAF) functions in nuclear envelope repair, most likely upstream of ESCRT-III [115]. Thus, BAF may be involved in an alternative pathway to maintain/reconstitute nucleocytoplasmic compartmentalization in cells lacking ESCRT-III. Finally, ESCRT-III appears to act also in the NPC quality surveillance [116].
3.4.3. Endolysosomal Membrane Repair
Injuries in endolysosomal membranes can be caused by different chemical and biological stresses, as well as by pathogens. Recent evidence indicates that damages in these membranes lead to the Ca2+ and ESCRT-I-mediated recruitment of ESCRT-III. By contrast, ESCRT-0 is not required [117,118]. Interestingly, both TSG101 and Alix are engaged by damaged lysosomal membranes and seem to cooperate in early events after injuries [117,118,119]. Data suggest that ESCRT-III represents the first line of intervention in lysosome repair. Indeed, it does not require large ruptures to be activated in its sealing function [117]. The role played by the ESCRT machinery in the repair of lysosome damages has been better analyzed in the context of injuries caused by pathogens, e.g., intracellular bacteria that exploit phagosomes for their survival. For instance, it has been reported that ESCRT-III is localized to the phagolysosomes that contain replicating Coxiella burnetii, most likely to repair membrane lesions induced by bacterial factors/proliferation [120]. Importantly, replication of the bacterium is impaired by TSG101 depletion [118], indicating that ESCRT machinery is crucial to maintain the integrity of the bacterium-colonized phagolysosomes and, thus, to sustain bacterial replication. ESCRT-III is also engaged by Mycobacterium tuberculosis-damaged phagolysosomes. In this way, bacterial virulence factors are prevented from being released in the cytosol [121]. Overall, these findings point to the ESCRT machinery as a potential target for antimicrobial drug design.
In conclusion, ESCRT machinery is involved in many fundamental cellular processes, and when it is not correctly working or it is not finely regulated, it is linked to diseases, cancer included [97]. The importance of ESCRT machinery is further highlighted by the result of knockout studies carried on in mice. Indeed, the loss of TSG101, VPS25, and CHMP5, components of ESCRT-I, ESCRT-II, and ESCRT-III, respectively, is embryonically lethal in mice [122,123,124]. By contrast, deletions of core ESCRT genes in the yeast Saccharomyces cerevisiae are not lethal and enabled the study of ESCRTs in MVB biogenesis. In addition to yeast, Drosophila [125,126] and Arabidopsis thaliana [127] have also been employed as models to analyze the effects of the removal of specific ESCRTs on different cellular pathways. Finally, works based on strategies (mainly small interfering RNAs and more recently CRISPR-Cas9 editing) aimed at silencing the expression of ESCRT factors/cofactors in mammalian cells have been crucial to elucidate the key players in each process and their interplay. Some of these studies, focused on the main cellular processes addressed in this review, are summarized in Table 1.

Table 1.
Table reports the key players for the major processes in mammalian cells involving ESCRTs. The results of silencing studies supporting the role played by these key players are also displayed.
4. ESCRT Machinery and Viral Replication Cycle
Being obligate intracellular parasites, viruses have evolved to hijack fundamental and highly conserved cellular pathways/machineries to execute each step of their replication. This concept is true without any exception, and it is a feature shared among viruses infecting both prokaryotic and eukaryotic cells. Taking into account the crucial functions played by ESCRT proteins, and especially by ESCRT-III and VPS4, in cell life, it does not come as a surprise that viruses can interfere with ESCRT-mediated processes or directly exploit the ESCRT machinery to maximize their chances to establish a successful infection in the host. The main steps of viral replication involving the entire ESCRT machinery or some selected components are reported in Figure 2.
First, viruses have evolved to either antagonize or take advantage of ESCRT-driven processes as autophagy and EV secretion. The interplay between autophagy and viruses has been excellently reviewed elsewhere [132]. We mention the involvement of autophagy in viral transmission later. As far as EVs are concerned, it is interesting to note that they can act both as promoters or inhibitors of viral spreading and infection [8,133]. This dual effect is well exemplified by EVs released from human immunodeficiency virus type 1 (HIV-1)-infected cells. On one hand, EVs can be loaded with HIV proteins that, once transferred to neighboring cells, increase their susceptibility to viral infection. Among these proteins, the accessory Nef can be recruited into EVs [134] and, upon delivery to latently infected cells, it can activate viral replication [135]. Furthermore, Nef-containing EVs have been described to exert several effects on CD4+ T cells. For instance, it has been reported to induce senescence and death or to suppress their cytotoxic activity [136,137]. At the same time, Nef-positive EVs affect humoral immune response by inhibiting the production of IgA and IgG by B-lymphocytes [138]. Interestingly, it has been reported that EVs released from HIV-1 infected cells are capable of transferring viral co-receptors to recipient cells, thus increasing their susceptibility to viral infection [139,140]. Finally, EVs have also been found to carry the HIV transactivation response element (TAR) [141]. TAR is a stem-loop-shaped sequence present at the 5′ end of HIV transcripts that interacts with Tat, the main viral transactivator protein, and upregulates viral RNA production. The TAR molecule delivered by EVs could be processed into miRNAs that target a Bcl-2 interacting protein, resulting in a block of apoptosis and thus supporting virus production [141]. On the other hand, it has been shown that EVs produced by infected cells can contain APOBEC3G, one of the main host anti-HIV proteins, while the viral protein Vif, which antagonizes APOBEC3G, is not recruited into EVs [142]. APOBEC3G-loaded EVs would then render recipient cells more resistant to infection. Another example of EVs carrying anti-HIV factors is the vesicles containing the antiviral soluble host molecule cGAMP that acts through interferon and innate immune responses [143]. In conclusion, the interplay between EVs and HIV appears to be extremely complex and not fully disclosed yet [144]. Additional examples of viruses adopting exosomes to facilitate their spreading are the hepatitis B virus [145], HTLV-1 [146] as well as rhinovirus. In the latter case, virus infected cells secrete EVs that induce the upregulation of viral receptors in monocytes, thus increasing the spectrum of cells that can be infected by the virus [147]. Interestingly, it has been recently reported that also cells infected with viruses belonging to the Coronaviridae family, coronaviruses, may produce exosomes with different functions in the replication cycle, pathogenesis and cell response to infection [148]. A coronavirus, the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), is the virus causing the current pandemic. In this context, it has been shown that exosomes can transfer the angiotensin-converting enzyme 2 (ACE2), the main receptor that allows SARS-CoV-2 entry, to other cells rendering them susceptible to viral infection [149]. However, the same EVs might also work as a decoy for circulating SARS-CoV-2 [150], thus suggesting a potential role as therapeutic agents. The idea of using exosomes to interfere with viral infections is not novel [151], as well as the possibility to develop vaccination strategies based on EVs loaded with viral antigens/nucleic acids to stimulate antiviral immune responses, as it naturally occurs from infected cells. Finally, it must be mentioned that EVs can also transfer different miRNAs and even long non-coding RNAs that can affect viral spreading/pathogenesis as well as the host immune system [152,153,154]. The role of EVs in viral transmission is further discussed in Section 4.4 below.
On the other hand, viruses are able to exploit the ESCRT machinery to execute their life cycle. Viral replication encompasses different steps that can be summarized as follows: entry into target cells, uncoating of the viral genome, replication and transcription of the viral genome, assembly of new viral progeny, egress from infected cells (Figure 3).
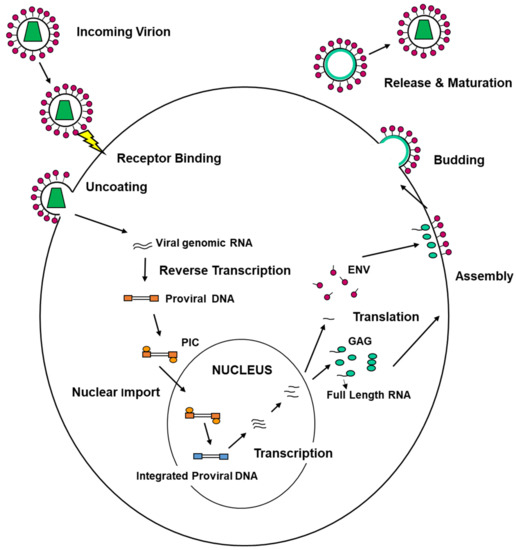
Figure 3.
Schematic representation of the main steps in a typical viral life cycle. The figure reports the replication of HIV-1. Upon entry into target cells, mediated by fusion of the viral envelope with the cell surface, the viral genome, which is single-stranded positive RNA (two molecules per viral particle), is partially uncoated from the virion structural proteins, although it is shielded by viral and cellular factors, not entirely known yet (pre-integration complex, PIC). PIC travels to the nucleus and, at the same time, the viral RNA is retrotranscribed by one of the viral enzymes into double-stranded DNA (provirus). Once entered the nucleus, the proviral DNA is integrated into the cellular genome and becomes part of it. Indeed, it is the cellular RNA polymerase II that transcribes the integrated provirus in mRNAs that are translated into the cytosol to give rise to the structural proteins Gag-Pol and Env. The first one drives—in concert with cellular proteins—the assembly and egress of the progeny virions. The latter enriches the viral envelope and is essential to bind the cellular receptors during the next replication round. Regulatory and accessory proteins are also translated. Furthermore, full-length genomic RNAs are also produced to be packaged into the novel budding particles. Once released in the extracellular environment, the new virions terminate their maturation, and they become infectious. Different viruses, due to the specific biological features (in a particular type of genome and presence or absence of an envelope), might differently execute the steps of their life cycle. However, the main steps remain the same: entry, uncoating, genome replication and transcription, protein translation, assembly of progeny virions, egress from infected cells. These stages may present certain differences in the different viral families, also due to the different biological features that characterize each family itself (with the type of genomic nucleic acids and presence or absence of a lipid envelope enwrapping the particle as the main ones in this context). What remains a common trait is the necessity for the virus to interact with the cellular apparatus throughout its replication. This is also the main reason why viruses can alter cell functions, thus causing a disease, and are all pathogenic.
Seminal studies aimed at dissecting the mechanisms allowing the egress of HIV-1 from infected cells discovered the role played by the ESCRT machinery in the viral life cycle. Some of these studies have also contributed, over the years, to our understanding of the functioning of the machinery in fundamental cellular processes, despite its involvement in viral replication. It is now clear that different viruses can exploit ESCRT proteins or part of them to accomplish virtually all phases of their replication cycle. Interestingly, the interaction between ESCRTs and viruses is of ancient origin, as it has been shown that in Archaea, ESCRT-III components and VPS4 homologs support the replication of the Sulfolobus-turreted icosahedral virus [2]. We summarize below some of the functions of the ESCRT machinery in the viral life cycle with a special accent on the late steps of HSV-1 replication.
Finally, supporting a pivotal role of the ESCRT machinery in the viral life cycle, the host immune system evolved to target this pathway to control infections. In this context, the interferon-stimulated gene 15 (ISG15) represents a key player as it targets the ESCRT proteins in budding complexes to block the release of viruses [155,156]. Furthermore, specific polymorphisms within the 5’ sequence of TSG101 encoding gene (between the −183 and +181 nucleotides with respect to the translation start codon) have been associated with the acquired immunodeficiency syndrome (AIDS) progression, likely due to an effect on plasma HIV-1 load [157]. Overall, these findings strongly suggest that ESCRT proteins and TSG101, in particular, may be interesting targets for the development of antiviral drugs. In this context, pump inhibitors of the prazole family, which interact with TSG101, have been already tested and proved to efficiently inhibit a number of enveloped viruses [158,159], as we further discuss later.
4.1. ESCRT Machinery and Viral Entry
To enter into animal cells, viruses must cross the plasma membrane. This non-trivial process can be executed by two main routes: (1) fusion of the viral envelope (if present) with the cellular membrane, a strategy that does not cause membrane injuries [160]; (2) receptor-mediated endocytosis of viral particles that occurs for both enveloped and naked viruses. Members belonging to Picornaviridae, a family encompassing small naked RNA viruses, in most cases enter host cells by creating a pore within the endosomal membrane to translocate their genome in the cytosol where it is replicated [161]. Viruses that exploit the endolysosomal pathway need to escape from it to gain access to the cytosol before undergoing degradation or recycling back to the plasma membrane [162]. In the case of enveloped viruses, this step is carried out by fusion of their envelope with the endosomal membrane, a process that, as mentioned above, should not determine ruptures of the phospholipid bilayer. On the other hand, naked viruses usually cause lesions while crossing the endosomal membranes. This process would result in the exposure of the organelle content in the cytosol with toxic effects and triggering of inflammation. Although most of the mechanisms facilitating this step and the network of cell/viral proteins playing a role are still to be fully elucidated, evidence indicates that ESCRT machinery may play a role in this context, especially when small injuries are involved. It must be mentioned that, at least to our knowledge, this aspect has not been investigated yet. However, it is interesting to note that several viruses display, within virion structural proteins, conserved and short proline-rich motifs, known as late domains (L-domains) that are better described later and that interact with ESCRT and ESCRT related factors [163]. These domains, when exposed in entering viral particles, could act to recruit the ESCRT machinery, for instance, to control membrane damage. Interestingly one of these L-domains, the PPxY motif, is present in the adenoviral membrane lytic protein VI [164]. Adenoviruses are naked DNA viruses that have been extensively studied also with respect to the mechanism of entry into target cells. Upon receptor-mediated endocytosis, adenoviral particles undergo capsid perturbation with the release of the protein VI. Protein VI displays membrane lytic activity [165], and it is crucial for viral infectivity [166]. Membrane damages create pores large enough for enabling viral particle access to the cytosol [167,168]. Protein VI is also known to counteract cellular autophagy, which is activated to remove damaged endosomes [169]. Currently, it is not clear whether this process relies on the ESCRT machinery, although protein VI can interact through a PPxY motif with the Nedd4 ubiquitin ligase, one of the E3 enzymes playing a role in certain ESCRT-mediated processes, as better detailed below. It would be of importance to investigate whether protein VI can also engage the ESCRT machinery prior to exerting its control on autophagy by preventing the cell from repairing small endosomal lesions. Indeed, this could represent a novel and additional mechanism of cell-intrinsic response to a viral infection that may even go beyond the adenoviral entry.
As additional hints on the role that ESCRT components play in viral entry, it has been demonstrated that Kaposi’s sarcoma herpesvirus (KHSV) hijacks the ESCRT machinery for efficient entry in endothelial cells [170]. HRS and TSG101 are key factors in this context. Interestingly, the involvement of TSG101 is common among viruses besides KHSV that enter target cells by micropinocytosis. Indeed, this ESCRT-I component has been shown to contribute to the entry of the Crimean-Congo hemorrhagic fever virus (CCHFV), human papillomaviruses, and echovirus-1 [171,172,173]. Furthermore, in the case of KHSV, TSG101 is also important for the correct trafficking of viral particles through the endosomal pathway [170]. In the case of CCHFV, a tick-borne bunyavirus that can cause a severe clinical manifestation in humans, not only silencing of TSG101 but also depletion of Vps24, VPS4 and Alix inhibits infection. Moreover, virions are trapped in the MVBs when cells are treated with bafilomycin A. This finding suggests that these organelles are the site of virus-endosome membrane fusion. In addition, the highly pathogenic Old World arenavirus Lassa virus (LASV) and the prototypic arenavirus lymphocytic choriomeningitis virus (LCMV) have been described to exploit HRS, Tsg101, ESCRT-III factors, as well as VPS4 to enter into cells [174]. Finally, a study adopting a genome-wide RNA-interference screening has suggested a role for ESCRT components necessary for ILV formation in rotavirus cell entry [175]
4.2. ESCRT Machinery Involvement in the Formation of Viral Replication/Assembly Compartments
Once they get access to the cell, several RNA and DNA viruses remodel cellular membranes and exploit host proteins to build specialized compartments where viral genome replication takes place. In these virus-induced “factories”, viral and cellular factors involved in the process are concentrated and preserved from innate immune response mechanisms. Replication compartments originate from different cell membranes, including the plasma membrane, ER, GA, peroxisomes, mitochondria and endosomes [7]. Their biogenesis resembles the ILVs formation process. However, in this case, a membrane fission event does not take place. In addition to genome replication, these compartments can also facilitate the assembly of viral progeny. Working as a shield towards antiviral defenses, the replication compartments are especially useful for most RNA viruses. Indeed, during replication of their genome, high copies of double-stranded RNAs are produced that are the main trigger of the interferon response. Interestingly, ESCRT components have been shown to play a role in the formation of replication compartments of different plus-stranded RNA plant viruses [176,177,178,179]. Flaviviridae, a family of single-stranded positive-sense RNA viruses that include some human pathogens as dengue virus (DENV) and Japanese encephalitis virus (JEV), induce remodeling of the ER membranes to form compartments for genome replication and assembly of viral particles. An elegant study performed by adopting a mass spectrometry approach has shown that several ESCRT factors are re-localized to these compartments. In agreement with the functional relevance of this finding, RNAi screening production of both DENV and JEV particles was significantly affected by lack of TSG101 or of specific ESCRT-III components. However, it was unaffected by depletion of VPS4, thus suggesting that a unique and specific set of ESCRT factors contributes to the biogenesis of the flavivirus-induced replication compartment [180]. It must be mentioned that, while in the case of plant viruses, the role of the ESCRT-machinery is clearly linked to the formation of the replication compartments, in the case of other viruses, flaviviruses included, it is very difficult to distinguish between a role in this process or purely in particle assembly. This is particularly true for certain large DNA viruses, as the ones belonging to the Herpesviridae family, the herpesviruses, whose genome replication and particle assembly occur in the nucleus and in the cytosol, respectively. For instance, both VPS4 and CHMP1 have been reported to localize in the proximity of the human cytomegalovirus (HCMV)-induced cytoplasmic assembly compartments [181]. However, it is very difficult to understand whether these proteins participate in the biogenesis of these specialized sites of viral maturation, to the process of viral morphogenesis or to both.
4.3. ESCRT Machinery and Viral Egress from Infected Cells
Enveloped viruses are enwrapped by host membranes and usually egress from infected cells without necessarily causing cell death. Examples of enveloped viruses are HIV, herpesviruses, influenza virus, flaviviruses and coronaviruses (SARS-CoV-2 included). Naked viruses are not surrounded by host membranes and typically are released upon cell lysis [65,182]. Examples of un-enveloped viruses are adenovirus, poliovirus, norovirus, HAV. Enveloped viruses leave the cells through a complex process known as budding that requires two main membrane modifications, (i) a deformation around the assembling virions and (ii) fission, resulting in the detachment of the viral particles from the cellular surface. Budding takes place not only at the plasma membrane but also into intracellular organelles that then fuse with the plasma membrane, releasing viral particles in the external environment [183]. Assembly of virions and budding is usually strictly linked processes. As mentioned above, studies aimed at the dissection of the molecular mechanisms enabling HIV-1 budding have been instrumental for understanding how enveloped viruses are released from infected cells and for unrevealing the viral/cellular protein network involved in the process. In 1991, Göttlinger and coworkers identified in the C-terminal p6 domain of HIV-1 protein Gag, which encodes for the main structural components of the virion, i.e., matrix, nucleocapsid and capsid, a highly conserved PT/SAP motif playing a crucial role in the detachment of budded virions from the cell surface [184]. Starting from this seminal finding, additional research has led to the identification in the Gag protein of all known retroviruses of short proline-rich sequences, named late assembly or L-domains [185], functionally equivalent to the HIV-1 PT/SAP motif. To date, in addition to the PT/SAP motif, typical of most lentiviruses, two further L-domains have been well characterized: the PPXY-type L-domain present in the Gag proteins of oncoretroviruses, first, described in [185], and the YPXnL-type motif, identified in the Gag protein of the equine infectious anemia virus (EIAV) [186]. Besides retroviruses, L-domains and L-domain-like sequences have been identified in the structural proteins of most RNA-enveloped viruses, such as rhabdoviruses, filoviruses, arenaviruses, and paramyxoviruses [186], and in some DNA-enveloped viruses [187,188,189,190,191]. Interesting exceptions have been reported. For instance, neither the hepatitis C virus nor the influenza virus appears to have canonical L-domains or L-domain-like sequences [192,193], while the Bluetongue virus (BTV) presents both a PSAP and a PPXY motif, but at the level of the nonstructural protein NS3 [194]. Early studies indicated that L-domains act independently from their position in the viral protein and can be exchanged between unrelated viruses without losing their ability to mediate budding [195,196,197]. Furthermore, different types of L-domains often occur in combination; for instance, the HIV-1 p6 contains, in addition to its primary PTAP-type L-domain, an auxiliary L-domain of the YPXnL type [20,197,198], and the Ebola virus has a PTAPPEY domain which combines the PTAP and the PPXY types of L-domains [199]. Overall, these features suggested that the L-domains may represent docking sites for a set of cellular proteins belonging to a specific pathway exploited by retroviruses to execute budding efficiently. The first indication on this pathway came from previous observations that cellular proteins, such as the epithelial Na+ channel, contained sequences overlapping with the L-domains and that their endocytosis was dependent on these motifs [199,200] and on ubiquitylation [201]. At the same time, studies were supporting the hypothesis on the role of ubiquitin in the viral budding process [50]. In particular, we were able to show that ubiquitin residues involved in retroviral budding were the ones known to play a role in endocytosis [202]. Importantly, in 2001, the Carter and Sundquist laboratories discovered that the PT/SAP motif of HIV-1 p6 binds to TSG101 and that this interaction is crucial for viral budding [203,204]. Later, we show that Alix also plays a role in this context, and it is recruited to the site of budding through a YPXnL domain mapping in the Gag-p6 [20]. Finally, the PPXY type of L-domains was found to bind Nedd4 like ubiquitin ligases that facilitate ESCRT-machinery engagement [50,205]. Over the last two decades, several studies aimed at dissecting the complex viral/cellular protein network that enables most of the RNA-enveloped viruses to hijack the ESCRT-machinery. An updated review on this topic focused on retroviruses was recently published by Rose and coworkers [206], and several excellent and more general ones are available [12,18]. Thus, we do not further describe these aspects here. However, taking inspiration mostly from studies focused on retroviruses, we would like to point out some clear common traits among mechanisms adopted by enveloped viruses to usurp ESCRT-machinery during egress from infected cells: (1) ESCRT-III and VPS4 represent the master players in this process; (2) early data suggested that, while Alix and ESCRT-I were sufficient to recruit ESCRT-III to the side of HIV-1 budding, ESCRT-II was dispensable. By contrast, ESCRT-II is crucial for the exit of the avian sarcoma leukemia virus (ASLV). ASLV Gag displays a PPxY type L-domain [207], while, as already mentioned, HIV-1 Gag contains both a PT/SAP and a YPXnL L-domain. Overall, these findings support the notion that different L domains connect retroviral structural proteins with a different array of ESCRT proteins to execute budding; (3) on the other hand, it has been shown that mutations affecting Alix engagement do not entirely inhibit HIV-1 budding, suggesting a compensatory role for ESCRT-II [208,209] or for additional BRO1 containing proteins in this process. Importantly, most retroviruses have evolved redundant mechanisms to hijack ESCRT-III/VPS4 to the site of budding, as exemplified by HIV [210], and as we reported, for instance, in the case of the closely related feline immunodeficiency virus (FIV) [211]. Strikingly, budding of HIV-1 and FIV devoid of functional L-domains can be efficiently rescued by overexpression of Nedd4-like ubiquitin ligases, although both lentiviruses lack a PPxY motif within their Gag [212,213,214,215]. Finally, retroviruses have been shown to engage ESCRT components also through Gag ubiquitylation [50,204,216], as well as through the Gag Nucleocapsid region [53,211,217,218,219]; (4) RNA-enveloped viruses that seem to egress independently from the ESCRT-machinery, e.g., the influenza virus [220], encode proteins that might functionally replace ESCRT-III [193]; (5) large DNA-enveloped viruses can adopt ESCRT factors to egress from infected cells, but with some clear peculiarities that are discussed later in the case of HSV-1.
4.4. EVs and Autophagy in Viral Transmission
As the healthy counterpart, virus infected cells can secrete EVs that can exert different functions as described above. These EVs can shed viral proteins, and nucleic acids and either trigger immune responses or affect recipient cells, favoring viral spreading [8]. Interestingly, EVs and enveloped viral particles share common biogenesis machinery, and in some cases, it is difficult to isolate EVs containing viral molecules from forms of defective viral particles as they display similar density, size, and composition [8]. As mentioned above, viruses are distinguished in enveloped and naked viruses. However, in 2013, this concept was challenged by the finding of both types of particles in the supernatant of liver cells infected with the hepatitis A virus (HAV), as well as in vivo [221,222]. A follow-up study demonstrated that HAV enveloped particles could be found inside MVBs and ESCRT depletion inhibited their release from infected cells, thus suggesting exosomes as an egress pathway. Furthermore, these findings also indicate that the envelopment of HAV relies on the same cellular machinery exploited by most of the known enveloped viruses [65]. Importantly, HAV-containing exosomes, named “quasi-enveloped” HAV (eHAV), are able to protect viral particles from antibody-mediated neutralization. In addition to HAV, also hepatitis E virus (HEV), another naked virus, could be found in MVB. The release of eHEV particles is likely to occur via the exosomal pathway [223,224].
Interestingly, exosomes seem to play a role also in the egress of HIV. Indeed, Gould and coworkers demonstrated that EVs secreted by dendritic cells infected with HIV were able to transfer the infectious virus to CD4+ T lymphocytes [225]. Similar results were reported for hepatitis C virus (HCV). Exosomes loaded with HCV were indeed isolated from infected liver cell lines [226]. In addition, hepatic exosomes allowed transmission of infectious HCV particles in vitro and were resistant to antibody neutralization, at least partially. In agreement, HCV release was found to follow both an exosome-driven and an exosome-free route, as in the case of HAV. Importantly, exosome-associated HCV particles were infectious and resistant to neutralizing antibodies [227]. Furthermore, it was also demonstrated that EVs could deliver HCV replication-competent subgenomic RNAs allowing infection of recipient cells, even in the absence of infectious particles and independently from known viral receptors [228]. This latter aspect is particularly relevant as it would shield the virus from neutralization, an additional immune evasion strategy that may be exploited by other viruses. In this respect, also EVs secreted from Zika virus (ZIKV) or foot and mouth disease virus (FMDV) infected cells contain viral RNAs and proteins and can support viral transmission as well as function as an escape strategy from neutralization [229,230]. Furthermore, infectious particles of severe fever with thrombocytopenia syndrome (SFTS) virus, a tick-borne bunyavirus associated with hemorrhagic fever, were found in exosomes and were shown to infect cells, bypassing the need of classical viral receptors [231]. Finally, DENV-infected insect cells produce EVs loaded with virus-like particles that are able to infect other cells [232].
EVs can contribute to the spreading of infection also by increasing the spectrum of cells susceptible to viral entry and/or viral infectivity. One of the mechanisms allowing this EV-mediated activity is the delivery of viral receptors from the producing cell to the recipient one, as described above. Moreover, in the case of HIV-1, it has been shown that resting CD4+ T lymphocytes, which are usually refractory to infection, become permissive to viral replication by the combined function of the TNF-converting enzyme ADAM17 and Nef, delivered by exosomes [136]. Additionally, Nef-containing EVs can alter lipid rafts on the cell surface, facilitating HIV entry by fusion and thus increasing infectivity [233]. It must be noticed that certain viruses that acquire their envelope by budding into cellular organelles, e.g., the HSV-1, can hijack the exosomes to facilitate their transmission, at least from certain cell types [234,235]. We discuss HSV-1 egress from infected cells later in this review.
Surprisingly, studies carried on with cells infected by certain members of the Picornaviridae family, e.g., poliovirus, revealed that also these viruses could egress from host cells in vesicles before cell lysis [236]. However, these vesicles did not derive from MVBs, but rather from autophagosomes [236,237]. As already mentioned, autophagosomes by fusing with lysosomes display a pivotal role in different aspects of cell biology. However, it is known that these structures can also fuse with the plasma membrane to release molecules and organelles in the extracellular environment, a process known as “secretory autophagy” [237]. Thus, the above-mentioned viruses hijack this specialized type of autophagy to egress from infected cells. Indeed, virus-containing autophagosomes, which in the case of the poliovirus originate from the ER, do not fuse with lysosomes but with the plasma membrane and release vesicles enwrapped in a single membrane and loaded with viral particles [236]. By contrast, to exosome-mediated uptake of viruses, autophagosome-derived vesicles drive viral entry into recipient cells via the respective physiological receptors. This finding suggests that vesicles are disrupted upon binding with the new host cell, releasing particles that need to bind to the surface receptors [236]. Furthermore, lipid recognition seems to play a role as well. Indeed, proteins able to bind the phosphatidylserine, a lipid enriching the autophagosome-derived vesicles, inhibited viral infection [236].
Overall, these findings indicate that vesicles can contribute to cell egress and transmission of both naked and enveloped viruses. The emerging picture is complex, and the impacts on the host immune response and viral pathogenicity urge further studies.
5. The Travel of Herpes Simplex Virus Type 1 from the Nucleus to the Extracellular Environment: Is There a Role for the ESCRT Machinery?
The Herpesviridae are large, enveloped DNA viruses that not only replicate their genome within the nucleus, but also start the process of assembling progeny particles in the same cellular compartment. Newly assembled nucleocapsids need to cross the nuclear membranes to translocate into the cytoplasm. In the cytosol, nucleocapsids are enwrapped in proteins that constitute what is known as tegument, an amorphous coat that is typical of herpes viral particles. Next, tegument surrounded nucleocapsids bud into vesicles derived from host organelles to acquire their envelope. Finally, these vesicles fuse with the plasma membrane to release the mature viral progeny in the extracellular environment. Therefore, the biogenesis of herpesviral particles is a multistep process involving at least two budding and fusion events and complex traffic of nascent virions from inside the nucleus to outside the cell. Notably, ESCRT components play a role along this complex process, although with peculiar and distinct features with respect to the better-clarified strategies evolved by retroviruses and several other families of enveloped RNA viruses to hijack the ESCRT machinery during budding. One of the most striking peculiarities in the assembly and maturation of herpesviral particles is the first budding from the nuclear envelope. If ESCRTs are involved in this process, they need to be available within the nuclear environment. This is indeed the case. When TSG101 was described as a key component of the cellular machinery involved in HIV-1 budding, this finding came as a surprise as this factor was mainly known for its nuclear functions, as also suggested by its name (tumor suppressor gene 101). Indeed, TSG101 was initially characterized for its ability to inhibit not only the transcriptional activity of the nuclear hormone receptor superfamily [238,239], but also for its ability to suppress the activity of certain viral promoters and transcriptional activators [239]. As an example, TSG01 can interact with Rta, a transactivator of EBV lytic genes, and, as a consequence, it can be recruited to the viral promoters [240]. TSG101 improves binding of Rta to these promoters and its transactivating activity. Furthermore, TSG101 is known to play a role in the p53 pathway [241]. Indeed, TSG101 inhibits ubiquitylation and degradation of the mouse double minute 2 homolog (MDM2) protein. MDM2 is an E3 ubiquitin ligase, which targets p53 for degradation by recognizing its N-terminal transactivation domain (TAD). Furthermore, MDM2 can also inhibit p53 transcriptional activation. The effect of TSG101 on MDM2 stability results in p53 downregulation accompanied by a stimulation of the cell cycle [242]. As in the case of TSG101, also ESCRT-II components were first described as nuclear proteins. Indeed, the yeast orthologues of ESCRT-II subunits are named ELL-associated proteins (EAPs). ELL binds to the RNA polymerase II, functioning as an elongation factor. Interestingly, also in rats, ELL interacts with three factors belonging to ESCRT-II (EAP20/Vps25, EAP30/Vps22, and EAP45/hVps36), giving rise to a heterotetrameric complex that enhances transcription elongation, at least in vitro [243,244]. Overall, ESCRT-II appears to interfere with the expression of specific genes by regulating the rate of promoter initiation of transcription. Finally, mammalian ESCRT-III subunits (CHMPs) were initially studied for their functions in the nucleus as suggested by their initial name, i.e. chromatin-modifying proteins, that was then changed into charged multivesicular body proteins upon discovery of their cytoplasmic functions. These two names are currently used interchangeably. In the context of CHMP nuclear activities, several studies focused on CHMP1. This protein is characterized by a predicted bipartite nuclear localization signal, and it is found both in the cytosol and in the nuclear matrix. Interestingly, nuclear CHMP1 appears to be post-translationally modified with respect to the cytosolic counterpart. This modification may contribute to the regulation of CHMP1 compartmentalization or to the reason for a different activity of the protein in the two cellular spaces. In the nucleus, CHMP1 interacts with polycomb-like proteins that, along with the other components of the polycomb group (PcG), enable chromatin condensation and gene silencing during development. Furthermore, it has been suggested that CHMP1 might be involved in chromosome condensation during mitosis [245]. Interestingly, in a screening aimed at the identification of human CHMP interactors, 19 out of 45 novel-fished-out factors were nucleus-related proteins [246]. Finally, several CHMPs take part in nuclear sumoylation pathways. These distinct functions of the nucleoplasmic ESCRT components with respect to the activity displayed by the cytoplasmic counterpart are a striking example of the importance/role of cell compartmentalization.
Interestingly, however, herpesviruses, at least HSV-1 and EBV that have been studied under this respect exploit nucleoplasmic ESCRTs to facilitate nuclear inner membrane remodeling and fission in the first envelopment step, as better explained below. Thus, in this context, nuclear ESCRTs function as their cytoplasmic counterpart. HSV-1 belongs to the Alphaherpesvirinae subfamily of the Herpesviridae, and it is a neurotropic virus ubiquitous in the human population. HSV-1 usually causes localized mucocutaneous lesions in the facial region. Primary infection often occurs after direct interpersonal contact and precedes the retrograde transport of the virus to the sensory nerve ganglia, where it establishes a lifelong latent infection with recurrent infections [247]. In fragile patients, HSV-1 can systemically disseminate and cause fatal infections. In particular, HSV-1 can cause acute encephalitis and severe neonatal infections. The process by which HSV-1 virions exit the nuclear and then the cytoplasmic membranes of infected cells is very complex and not fully elucidated yet. Viral DNA replication and gene transcription take place in the nucleus where, during productive (lytic) infection, nucleocapsids are also assembled. Althoug a sufficient dilatation of nuclear pores to allow egress of viral capsids was described [248], the most widely accepted and supported model postulates that HSV-1 capsids by budding from the INM acquire a lipid envelope, which then fuses with the ONM [249]. A central role is played by the herpesviral nuclear egress complex (NEC) that is composed of viral proteins encoded by the UL31 and UL34 genes. NEC forms a hexagonal lattice on the inner surface of the nuclear membrane, and it is sufficient to induce perinuclear vesicles in uninfected cells expressing alphaherpesviral NEC proteins alone. Furthermore, HSV-1 NEC can mediate the budding of membrane vesicles in the absence of endogenous cellular proteins and ATP [249]. The UL34 protein is likely the real effector, but in the absence of the UL31 protein, it fails to localize to the nucleus, as it lacks a nuclear import signal. It is noteworthy that HSV-1s bearing deletions or dominant-negative mutations within the UL34 gene display highly reduced viral infectious titers with a corresponding accumulation of intranuclear viral capsids; however, some infectious virions can still be recovered. Different mechanisms may be activated in this case, including nuclear pore enlargement (see above) or nuclear envelope breakdown, but the relevance of such alternative pathways under physiological conditions is unclear. NEC-mediated budding is also likely to have a quality control function, selecting mature, DNA-filled C-capsids rather than type A and B capsids [250]. Conversely, budding is probably negatively regulated to reduce the number of capsid-less vesicles, for example, by phosphorylation of UL31 by the US3 viral kinase. Regulation of budding is even more complex and could involve tegument proteins that localize to the nucleus, such as UL36, UL47, UL16, UL11 and UL21, along with nonstructural proteins like UL24 [251] and the neurovirulence factor ICP34.5 [252]. De-envelopment of perinuclear enveloped virions (PEVs) is not as well characterized as envelopment: NEC alone is not able to perform it efficiently, and viral glycoproteins gB and gH play a role in the process; however, both proteins are dispensable for it [249].
It has been recently shown that HSV-1 employs nucleoplasmic ESCRT proteins to bud across the INM. In particular, UL34 can interact with Alix in infected cells, and UL31/UL34 leads to the localization of CHMP4B to the nuclear envelope. At the same time, virions accumulate in the perinuclear space of cells depleted of CHMP4A/B/C or Alix, likely because scission at the INM is inhibited [253]. Overall, these data indicate that HSV-1 NEC via Alix can recruit CHMP4 to the site of budding at the INM. However, a dominant-negative version of VPS4 did not abrogate transit of nascent nucleocapsids to the cytosol [253,254,255,256], suggesting no major nuclear egress defects. On the other hand, it has been demonstrated that NEC alone is sufficient to drive membrane scission, at least in vitro [257]. Furthermore, Alix does not appear to be essential for HSV-1 replication [258]. Thus, CHMP4/VPS4 would be necessary to optimize the rate of HSV-1 budding into the perinuclear space, but not strictly required. On the other hand, HSV-1 infection has been shown to trigger autophagy at the nuclear membranes [259,260], leading to degradation of lamins that constitute a barrier for HSV-1 nuclear egress [260,261]. Under this respect, ESCRT-III/VPS4 could facilitate HSV-1 budding from the INM by functioning in the biogenesis of the autophagosome and thus in laminin destruction [66,262]. Finally, ESCRT-III may be recruited to help the nuclear envelope stressed by HSV-1 budding nucleocapsid to maintain its integrity, although, as described above, CHMP-7 and not Alix is the factor recruiting CHMP4B at the INM during this process [263]. Of note, as discussed below, TSG101 has been recently implicated in HSV-1 ability to cross the nuclear envelope. Interestingly also the NEC of EBV, constituted by BFLF2 and BFRF1 proteins, has been described to interact with ESCRT components during EBV capsid nuclear envelopment [264]. In this case, not only Alix, CHMP4B and VPS4 play a role, but also the ubiquitin ligase Itch/AIP4 that belongs to the Nedd4 family of E3 enzymes. As in the case of retroviral Gag, BFRF1, which interacts with Itch, is ubiquitylated at several lysine residues [265]. Mutations of these residues affect the release of enveloped particles from infected cells, likely due to an impairment of the EBV particle trafficking from the nucleus to the cytosol [265]. Although some viral tegument proteins localize to the nucleus, as mentioned above, most of them are acquired in the cytosol: subsequently, virions undergo secondary envelopment by budding into cytoplasmic membranes. The exact nature of this membrane is still under debate. HSV-1 capsids have been observed enveloping at structures morphologically resembling the trans-Golgi network (TGN) [266]. This finding would be consistent with the lipid composition of the final HSV-1 envelope [267]. An alternative model identifies endocytic tubules deriving from the plasma membrane and containing viral envelope proteins at the site of cytoplasmic envelopment [268]. Following secondary envelopment, HSV-1 hijacks secretory pathways to exit the cell by exocytosis, though at late-stages of infection, some virions are released following cell death and lysis. Among tegument proteins, UL36 and UL37 are crucial for envelopment [269] as they mediate both interactions with cytoskeleton proteins to transport virions towards the Golgi apparatus and with envelope glycoproteins. Other tegument proteins are essential (UL48) or auxiliary (e.g., UL51 and UL7) for secondary envelopment [262]. The precise identity of the compartment in which secondary envelopment takes place is still unknown, though it surely involves post-Golgi membranes, which may include TGN or endosomes, or possibly membranes of heterogeneous origin [262]. Viral glycoproteins are trafficked to the same post-Golgi membranes via different mechanisms. In particular, while some viral surface proteins have clear localization signals, others do not [188]. Knockdown of Rab6A, involved in intracellular trafficking, inhibits viral secondary envelopment [268]. Enveloped virions then are translocated to the plasma membrane by a mechanism, which is associated with proteins directly involved in the secretory pathway, such as Rab GTPases, GAP-43, kinesin-1 and SNAP-2, as well as upstream regulators like protein kinase [247]. Since the discovery that the dominant-negative version of VPS4 (VPS-EQ) affects HSV-1 cytoplasmic envelopment and replication [254], several studies have connected ESCRT machinery also to this step of viral egress from infected cells. For instance, it has been shown that dominant forms of different CHMPs can also inhibit HSV-1 replication [258]. In particular, electron microscopy studies suggested that in cells overexpressing VPS-EQ, partially enveloped nucleocapsids of HSV-1 accumulate in the cytosol as the final step of membrane fission into the lumen of a cytoplasmic organelle did not occur [256]. This phenotype is reminiscent of the one found for HIV-1 in cells expressing the same dominant-negative protein or in the case of mutation of the PT/SAP L-domain [20,184]. Importantly, a number of HSV-1 proteins contain binding motifs for Alix as well as for TSG101 [258]. However, neither the dominant-negative version of these proteins nor their depletion affects HSV-1 yield [258]. Interestingly, it has been reported that proton pump inhibitors of the prazole family, by interfering with TSG101, affect not only the budding of HIV-1 but also of different enveloped viruses, EBV included [158,159], and, more recently, in a pre-print manuscript by Leis and coworkers, of HSV-1 and HSV-2 [270]. It must be noted that the drugs appear to inhibit HSV-1 transit through the nuclear membrane and not the final envelopment in the cytosol. In any case, these studies, on one hand, further connect TSG101 to the egress of different viruses, including HSV-1, from infected cells. On the other hand, they support the possibility of adopting ESCRT proteins as a target for antiviral therapy. Despite the presence of a PPxY domain in HSV-1 UL56 protein, there is no evidence that interaction with Nedd4 is involved in ubiquitin-mediated recruitment of the ESCRT machinery to execute viral envelopment. In agreement, the viral RING finger E3 ubiquitin ligase ICP0 does not seem to play a role in ESCRT engagement.
In conclusion, although TSG101 may play a role as suggested by the prazole-based data, the mechanism adopted by HSV-1 to recruit ESCRT-III to the side of the final envelopment is still under evaluation. Interestingly, different studies have focused on the viral tegument and envelope proteins as potential ESCRT-III recruiting factors/cofactors. Under this respect, we were able to show that glycoprotein B (gB), one of the most highly conserved glycoproteins across the Herpesviridae family, was sorted into MVB membranes [188]. In cells expressing VPS4-EQ, the site of intracellular gB accumulation and maturation was altered, indicating that gB traffic was dependent on a functional MVB pathway. Interestingly, gB was ubiquitylated in both infected and transfected cells. A partial deletion of the gB cytoplasmic tail resulted in a dramatic reduction of ubiquitination, as well as of progeny virus assembly and release to the extracellular compartment. Our data support the view that the sorting of gB to MVB membranes may represent a critical step in HSV envelopment and egress [188]. On the other hand, the major and highly conserved tegument protein UL36 appears to be a very promising candidate due to its essential role in the final envelopment of HSV-1 particles. Supporting this hypothesis, UL36 encompasses an amino-terminal functional deubiquitylase (DUB) domain [271,272] that would fit with the role of ubiquitin in ESCRT-mediated viral budding described above. We were able to show that HSV-1 UL36 regulates the ubiquitination of TSG101 [189]. However, as UL36 DUB activity is not essential for HSV-1 replication [258,273], we could not conclude that this finding is relevant in the context of viral envelopment. Under this respect, an elegant study carried on by adopting light and scanning electron microscopy strongly suggested a role for UL36 in the recruitment of capsids to the site of ESCRT-III/Vps4 localization [256], although this effect may be indirect. Finally, a recent study has demonstrated that the tegument protein UL51 has an impressive structural similarity to the ESCRT-III subunit CHMP4B, and can give rise to long ESCRT-III-like polymers in vitro [274]. Thus, UL51 could facilitate CHMP polymerization or may even functionally replace CHMPs originating ESCRT-III-like filaments. Conceivably, any envelope and tegument proteins involved in cytoplasmic envelopment could facilitate the recruitment of ESCRT components as well as the docking of nucleocapsid-containing vesicles at the assembly sites. Studies may also be complicated by the redundant functions of different viral factors.
Overall, although ESCRT components are clearly involved in HSV-1 final envelopment and egress from infected cells, there are still several open questions to address before fully understanding all the peculiar aspects of these complex late steps of viral replication.
6. Conclusions and Outlook
The ESCRT machinery is involved in many fundamental cellular pathways. In eukaryotic cells, ESCRTs contribute to both maintenance of cell compartmentalization, as well as to the biogenesis of vesicles and thus to the crosstalk among organelles and between the cell and the extracellular environment. Over the past years, efforts have been made to gain further insights into the molecular mechanisms enabling most of the ESCRT-mediated pathways. However, there are still unmet questions that need to be addressed, including a better understanding of the precise role of the individual interactions and steps allowing the recruitment of the same set of proteins to different locations to facilitate or play key roles in a variety of processes. Viruses interact with the ESCRT components in different steps of their replication cycle and/or influence ESCRT-mediated actions to their advantage. Thus, the study of viral/ESCRT protein interplay might contribute to unraveling aspects of the ESCRT machinery functions as well as of its regulation that are still under debate. In this context, HSV-1 and herpesviruses, in general, might be of great interest as they exploit ESCRT components in peculiar ways that differ from the ones that have been well characterized for HIV-1 and most RNA-enveloped viruses. Particularly interesting is the fact that HSV-1 uses both nuclear and cytoplasmic ESCRT factors to cross the nuclear and plasma membrane barriers. Furthermore, nascent virions engage cytoskeleton elements as well as secretory pathways to travel throughout the cell and exit in the extracellular environment. Thus, studying the missing links of this travel may contribute to our knowledge not only on the ESCRT machinery functions, but also on the molecular exchanges between intracellular organelles and compartments.
Author Contributions
Conceptualization, A.C., A.R., G.P. and C.P.; writing—original draft preparation, A.C., A.R., G.P. and C.P.; writing—review and editing, A.C., A.R., G.P. and C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rajwar, A.; Morya, V.; Kharbanda, S.; Bhatia, D. DNA Nanodevices to Probe and Program Membrane Organization, Dynamics, and Applications. J. Membr. Biol. 2020, 253, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Vietri, M.; Radulovic, M.; Stenmark, H. The many functions of ESCRTs. Nat. Rev. Mol. Cell Biol. 2020, 21, 25–42. [Google Scholar] [CrossRef]
- Campsteijn, C.; Vietri, M.; Stenmark, H. Novel ESCRT functions in cell biology: Spiraling out of control? Curr. Opin. Cell Biol. 2016, 41, 1–8. [Google Scholar] [CrossRef]
- Robinson, M.; Schor, S.; Barouch-Bentov, R.; Einav, S. Viral journeys on the intracellular highways. Cell Mol. Life Sci. 2018, 75, 3693–3714. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zhou, S.; Gao, S.; Deng, H. Remodeling of host membranes during herpesvirus assembly and egress. Protein Cell 2019, 10, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lan, Y.; Sanyal, S. Membrane heist: Coronavirus host membrane remodeling during replication. Biochimie 2020, 179, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Krijnse-Locker, J. Modification of intracellular membrane structures for virus replication. Nat. Rev. Microbiol. 2008, 6, 363–374. [Google Scholar] [CrossRef]
- Martins, S.T.; Alves, L.R. Extracellular Vesicles in Viral Infections: Two Sides of the Same Coin? Front. Cell Infect. Microbiol. 2020, 10, 593170. [Google Scholar] [CrossRef]
- Katzmann, D.J.; Babst, M.; Emr, S.D. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 2001, 106, 145–155. [Google Scholar] [CrossRef]
- Schoneberg, J.; Lee, I.H.; Iwasa, J.H.; Hurley, J.H. Reverse-topology membrane scission by the ESCRT proteins. Nat. Rev. Mol. Cell Biol. 2017, 18, 5–17. [Google Scholar] [CrossRef]
- Hanson, P.I.; Cashikar, A. Multivesicular body morphogenesis. Annu. Rev. Cell Dev. Biol. 2012, 28, 337–362. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Akram, Z.; Iqbal, H.M.N.; Munn, A.L. The regulation of Endosomal Sorting Complex Required for Transport and accessory proteins in multivesicular body sorting and enveloped viral budding—An overview. Int. J. Biol. Macromol. 2019, 127, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mast, F.D.; Herricks, T.; Strehler, K.M.; Miller, L.R.; Saleem, R.A.; Rachubinski, R.A.; Aitchison, J.D. ESCRT-III is required for scissioning new peroxisomes from the endoplasmic reticulum. J. Cell Biol. 2018, 217, 2087–2102. [Google Scholar] [CrossRef] [PubMed]
- Kostelansky, M.S.; Sun, J.; Lee, S.; Kim, J.; Ghirlando, R.; Hierro, A.; Emr, S.D.; Hurley, J.H. Structural and functional organization of the ESCRT-I trafficking complex. Cell 2006, 125, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Kostelansky, M.S.; Schluter, C.; Tam, Y.Y.; Lee, S.; Ghirlando, R.; Beach, B.; Conibear, E.; Hurley, J.H. Molecular architecture and functional model of the complete yeast ESCRT-I heterotetramer. Cell 2007, 129, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Weissenhorn, W.; Gottlinger, H. ESCRT-I part II: Forming the real ESCRT-I complex. Cell Host Microbe 2007, 2, 1–2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hierro, A.; Sun, J.; Rusnak, A.S.; Kim, J.; Prag, G.; Emr, S.D.; Hurley, J.H. Structure of the ESCRT-II endosomal trafficking complex. Nature 2004, 431, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Horvath, P.; Muller-Reichert, T. A Structural View on ESCRT-Mediated Abscission. Front. Cell Dev. Biol. 2020, 8, 586880. [Google Scholar] [CrossRef] [PubMed]
- Gill, D.J.; Teo, H.; Sun, J.; Perisic, O.; Veprintsev, D.B.; Emr, S.D.; Williams, R.L. Structural insight into the ESCRT-I/-II link and its role in MVB trafficking. EMBO J. 2007, 26, 600–612. [Google Scholar] [CrossRef]
- Strack, B.; Calistri, A.; Craig, S.; Popova, E.; Gottlinger, H.G. AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell 2003, 114, 689–699. [Google Scholar] [CrossRef]
- von Schwedler, U.K.; Stuchell, M.; Muller, B.; Ward, D.M.; Chung, H.Y.; Morita, E.; Wang, H.E.; Davis, T.; He, G.P.; Cimbora, D.M.; et al. The protein network of HIV budding. Cell 2003, 114, 701–713. [Google Scholar] [CrossRef]
- Kim, J.; Sitaraman, S.; Hierro, A.; Beach, B.M.; Odorizzi, G.; Hurley, J.H. Structural basis for endosomal targeting by the Bro1 domain. Dev. Cell 2005, 8, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Zhang, L.; Taylor, S.; Mironov, A.; Urbe, S.; Woodman, P. Recruitment of UBPY and ESCRT exchange drive HD-PTP-dependent sorting of EGFR to the MVB. Curr. Biol. 2013, 23, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, M.D.; Piper, S.C.; Bright, N.A.; Evans, J.L.; Boname, J.M.; Bowers, K.; Lehner, P.J.; Luzio, J.P. A non-canonical ESCRT pathway, including histidine domain phosphotyrosine phosphatase (HD-PTP), is used for down-regulation of virally ubiquitinated MHC class I. Biochem. J. 2015, 471, 79–88. [Google Scholar] [CrossRef]
- Zhai, Q.; Landesman, M.B.; Robinson, H.; Sundquist, W.I.; Hill, C.P. Structure of the Bro1 domain protein BROX and functional analyses of the ALIX Bro1 domain in HIV-1 budding. PLoS ONE 2011, 6, e27466. [Google Scholar] [CrossRef] [PubMed]
- Monroe, N.; Hill, C.P. Meiotic Clade AAA ATPases: Protein Polymer Disassembly Machines. J. Mol. Biol. 2016, 428, 1897–1911. [Google Scholar] [CrossRef]
- Hurley, J.H.; Yang, D. MIT domainia. Dev. Cell 2008, 14, 6–8. [Google Scholar] [CrossRef]
- Monroe, N.; Han, H.; Gonciarz, M.D.; Eckert, D.M.; Karren, M.A.; Whitby, F.G.; Sundquist, W.I.; Hill, C.P. The oligomeric state of the active Vps4 AAA ATPase. J. Mol. Biol. 2014, 426, 510–525. [Google Scholar] [CrossRef] [PubMed]
- Obita, T.; Saksena, S.; Ghazi-Tabatabai, S.; Gill, D.J.; Perisic, O.; Emr, S.D.; Williams, R.L. Structural basis for selective recognition of ESCRT-III by the AAA ATPase Vps4. Nature 2007, 449, 735–739. [Google Scholar] [CrossRef]
- Stuchell-Brereton, M.D.; Skalicky, J.J.; Kieffer, C.; Karren, M.A.; Ghaffarian, S.; Sundquist, W.I. ESCRT-III recognition by VPS4 ATPases. Nature 2007, 449, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Christ, L.; Raiborg, C.; Wenzel, E.M.; Campsteijn, C.; Stenmark, H. Cellular Functions and Molecular Mechanisms of the ESCRT Membrane-Scission Machinery. Trends Biochem. Sci. 2017, 42, 42–56. [Google Scholar] [CrossRef]
- Samson, R.Y.; Dobro, M.J.; Jensen, G.J.; Bell, S.D. The Structure, Function and Roles of the Archaeal ESCRT Apparatus. Subcell Biochem. 2017, 84, 357–377. [Google Scholar] [CrossRef]
- Ghazi-Tabatabai, S.; Obita, T.; Pobbati, A.V.; Perisic, O.; Samson, R.Y.; Bell, S.D.; Williams, R.L. Evolution and assembly of ESCRTs. Biochem Soc. Trans. 2009, 37, 151–155. [Google Scholar] [CrossRef]
- Samson, R.Y.; Bell, S.D. Ancient ESCRTs and the evolution of binary fission. Trends Microbiol. 2009, 17, 507–513. [Google Scholar] [CrossRef]
- Samson, R.Y.; Obita, T.; Hodgson, B.; Shaw, M.K.; Chong, P.L.; Williams, R.L.; Bell, S.D. Molecular and structural basis of ESCRT-III recruitment to membranes during archaeal cell division. Mol. Cell 2011, 41, 186–196. [Google Scholar] [CrossRef]
- Samson, R.Y.; Obita, T.; Freund, S.M.; Williams, R.L.; Bell, S.D. A role for the ESCRT system in cell division in archaea. Science 2008, 322, 1710–1713. [Google Scholar] [CrossRef]
- Bissig, C.; Gruenberg, J. ALIX and the multivesicular endosome: ALIX in Wonderland. Trends Cell Biol. 2014, 24, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, L.; Woodman, P. Dissecting the role of His domain protein tyrosine phosphatase/PTPN23 and ESCRTs in sorting activated epidermal growth factor receptor to the multivesicular body. Biochem. Soc. Trans. 2018, 46, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Henne, W.M.; Borbat, P.P.; Buchkovich, N.J.; Freed, J.H.; Mao, Y.; Fromme, J.C.; Emr, S.D. Structural basis for activation, assembly and membrane binding of ESCRT-III Snf7 filaments. Elife 2015, 4. [Google Scholar] [CrossRef]
- Tang, S.; Buchkovich, N.J.; Henne, W.M.; Banjade, S.; Kim, Y.J.; Emr, S.D. ESCRT-III activation by parallel action of ESCRT-I/II and ESCRT-0/Bro1 during MVB biogenesis. Elife 2016, 5. [Google Scholar] [CrossRef]
- Banjade, S.; Tang, S.; Shah, Y.H.; Emr, S.D. Electrostatic lateral interactions drive ESCRT-III heteropolymer assembly. Elife 2019, 8. [Google Scholar] [CrossRef]
- Schoneberg, J.; Pavlin, M.R.; Yan, S.; Righini, M.; Lee, I.H.; Carlson, L.A.; Bahrami, A.H.; Goldman, D.H.; Ren, X.; Hummer, G.; et al. ATP-dependent force generation and membrane scission by ESCRT-III and Vps4. Science 2018, 362, 1423–1428. [Google Scholar] [CrossRef]
- Peng, X.; Yang, L.; Ma, Y.; Li, Y.; Li, H. Focus on the morphogenesis, fate and the role in tumor progression of multivesicular bodies. Cell Commun. Signal. 2020, 18, 122. [Google Scholar] [CrossRef]
- Eitan, E.; Suire, C.; Zhang, S.; Mattson, M.P. Impact of lysosome status on extracellular vesicle content and release. Ageing Res. Rev. 2016, 32, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Latifkar, A.; Ling, L.; Hingorani, A.; Johansen, E.; Clement, A.; Zhang, X.; Hartman, J.; Fischbach, C.; Lin, H.; Cerione, R.A.; et al. Loss of Sirtuin 1 Alters the Secretome of Breast Cancer Cells by Impairing Lysosomal Integrity. Dev. Cell 2019, 49, 393–408.e397. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Kovalenko, A.; Bogdanov, K.; Wallach, D. MLKL, the Protein that Mediates Necroptosis, Also Regulates Endosomal Trafficking and Extracellular Vesicle Generation. Immunity 2017, 47, 51–65.e57. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e418. [Google Scholar] [CrossRef]
- Raiborg, C.; Rusten, T.E.; Stenmark, H. Protein sorting into multivesicular endosomes. Curr. Opin. Cell Biol. 2003, 15, 446–455. [Google Scholar] [CrossRef]
- Raiborg, C.; Stenmark, H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature 2009, 458, 445–452. [Google Scholar] [CrossRef]
- Calistri, A.; Munegato, D.; Carli, I.; Parolin, C.; Palu, G. The ubiquitin-conjugating system: Multiple roles in viral replication and infection. Cells 2014, 3, 386–417. [Google Scholar] [CrossRef]
- Mukhopadhyay, D.; Riezman, H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science 2007, 315, 201–205. [Google Scholar] [CrossRef]
- Piper, R.C.; Luzio, J.P. Ubiquitin-dependent sorting of integral membrane proteins for degradation in lysosomes. Curr. Opin. Cell Biol. 2007, 19, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Bache, K.G.; Brech, A.; Mehlum, A.; Stenmark, H. HRS regulates multivesicular body formation via ESCRT recruitment to endosomes. J. Cell Biol. 2003, 162, 435–442. [Google Scholar] [CrossRef]
- Raiborg, C.; Bache, K.G.; Gillooly, D.J.; Madshus, I.H.; Stang, E.; Stenmark, H. HRS sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat. Cell Biol. 2002, 4, 394–398. [Google Scholar] [CrossRef]
- Gillooly, D.J.; Morrow, I.C.; Lindsay, M.; Gould, R.; Bryant, N.J.; Gaullier, J.M.; Parton, R.G.; Stenmark, H. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 2000, 19, 4577–4588. [Google Scholar] [CrossRef]
- Mayers, J.R.; Fyfe, I.; Schuh, A.L.; Chapman, E.R.; Edwardson, J.M.; Audhya, A. ESCRT-0 assembles as a heterotetrameric complex on membranes and binds multiple ubiquitinylated cargoes simultaneously. J. Biol. Chem. 2011, 286, 9636–9645. [Google Scholar] [CrossRef] [PubMed]
- Henne, W.M.; Buchkovich, N.J.; Zhao, Y.; Emr, S.D. The endosomal sorting complex ESCRT-II mediates the assembly and architecture of ESCRT-III helices. Cell 2012, 151, 356–371. [Google Scholar] [CrossRef] [PubMed]
- Mierzwa, B.E.; Chiaruttini, N.; Redondo-Morata, L.; von Filseck, J.M.; Konig, J.; Larios, J.; Poser, I.; Muller-Reichert, T.; Scheuring, S.; Roux, A.; et al. Dynamic subunit turnover in ESCRT-III assemblies is regulated by Vps4 to mediate membrane remodelling during cytokinesis. Nat. Cell Biol. 2017, 19, 787–798. [Google Scholar] [CrossRef]
- Adell, M.A.; Vogel, G.F.; Pakdel, M.; Muller, M.; Lindner, H.; Hess, M.W.; Teis, D. Coordinated binding of Vps4 to ESCRT-III drives membrane neck constriction during MVB vesicle formation. J. Cell Biol. 2014, 205, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Clague, M.J.; Liu, H.; Urbe, S. Governance of endocytic trafficking and signaling by reversible ubiquitylation. Dev. Cell 2012, 23, 457–467. [Google Scholar] [CrossRef]
- Dores, M.R.; Paing, M.M.; Lin, H.; Montagne, W.A.; Marchese, A.; Trejo, J. AP-3 regulates PAR1 ubiquitin-independent MVB/lysosomal sorting via an ALIX-mediated pathway. Mol. Biol. Cell 2012, 23, 3612–3623. [Google Scholar] [CrossRef]
- Dores, M.R.; Lin, H.; Neil, J.G.; Mendez, F.; Trejo, J. The alpha-arrestin ARRDC3 mediates ALIX ubiquitination and G protein-coupled receptor lysosomal sorting. Mol. Biol. Cell 2015, 26, 4660–4673. [Google Scholar] [CrossRef] [PubMed]
- Dores, M.R.; Grimsey, N.J.; Mendez, F.; Trejo, J. ALIX Regulates the Ubiquitin-Independent Lysosomal Sorting of the P2Y1 Purinergic Receptor via a YPX3L Motif. PLoS ONE 2016, 11, e0157587. [Google Scholar] [CrossRef]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef]
- Altan-Bonnet, N. Extracellular vesicles are the Trojan horses of viral infection. Curr. Opin. Microbiol. 2016, 32, 77–81. [Google Scholar] [CrossRef]
- Takahashi, Y.; He, H.; Tang, Z.; Hattori, T.; Liu, Y.; Young, M.M.; Serfass, J.M.; Chen, L.; Gebru, M.; Chen, C.; et al. An autophagy assay reveals the ESCRT-III component CHMP2A as a regulator of phagophore closure. Nat. Commun. 2018, 9, 2855. [Google Scholar] [CrossRef]
- Zhen, Y.; Spangenberg, H.; Munson, M.J.; Brech, A.; Schink, K.O.; Tan, K.W.; Sorensen, V.; Wenzel, E.M.; Radulovic, M.; Engedal, N.; et al. ESCRT-mediated phagophore sealing during mitophagy. Autophagy 2020, 16, 826–841. [Google Scholar] [CrossRef]
- Sahu, R.; Kaushik, S.; Clement, C.C.; Cannizzo, E.S.; Scharf, B.; Follenzi, A.; Potolicchio, I.; Nieves, E.; Cuervo, A.M.; Santambrogio, L. Microautophagy of cytosolic proteins by late endosomes. Dev. Cell 2011, 20, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Filimonenko, M.; Stuffers, S.; Raiborg, C.; Yamamoto, A.; Malerod, L.; Fisher, E.M.; Isaacs, A.; Brech, A.; Stenmark, H.; Simonsen, A. Functional multivesicular bodies are required for autophagic clearance of protein aggregates associated with neurodegenerative disease. J. Cell Biol. 2007, 179, 485–500. [Google Scholar] [CrossRef]
- Rusten, T.E.; Simonsen, A. ESCRT functions in autophagy and associated disease. Cell Cycle 2008, 7, 1166–1172. [Google Scholar] [CrossRef]
- Carlton, J.G.; Martin-Serrano, J. Parallels between cytokinesis and retroviral budding: A role for the ESCRT machinery. Science 2007, 316, 1908–1912. [Google Scholar] [CrossRef] [PubMed]
- Carlton, J.G.; Agromayor, M.; Martin-Serrano, J. Differential requirements for Alix and ESCRT-III in cytokinesis and HIV-1 release. Proc. Natl. Acad. Sci. USA 2008, 105, 10541–10546. [Google Scholar] [CrossRef] [PubMed]
- Carlton, J.G.; Caballe, A.; Agromayor, M.; Kloc, M.; Martin-Serrano, J. ESCRT-III governs the Aurora B-mediated abscission checkpoint through CHMP4C. Science 2012, 336, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Morita, E.; Sandrin, V.; Chung, H.Y.; Morham, S.G.; Gygi, S.P.; Rodesch, C.K.; Sundquist, W.I. Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J. 2007, 26, 4215–4227. [Google Scholar] [CrossRef]
- Guizetti, J.; Schermelleh, L.; Mantler, J.; Maar, S.; Poser, I.; Leonhardt, H.; Muller-Reichert, T.; Gerlich, D.W. Cortical constriction during abscission involves helices of ESCRT-III-dependent filaments. Science 2011, 331, 1616–1620. [Google Scholar] [CrossRef]
- Lee, H.H.; Elia, N.; Ghirlando, R.; Lippincott-Schwartz, J.; Hurley, J.H. Midbody targeting of the ESCRT machinery by a noncanonical coiled coil in CEP55. Science 2008, 322, 576–580. [Google Scholar] [CrossRef]
- Mierzwa, B.; Gerlich, D.W. Cytokinetic abscission: Molecular mechanisms and temporal control. Dev. Cell 2014, 31, 525–538. [Google Scholar] [CrossRef]
- Karasmanis, E.P.; Hwang, D.; Nakos, K.; Bowen, J.R.; Angelis, D.; Spiliotis, E.T. A Septin Double Ring Controls the Spatiotemporal Organization of the ESCRT Machinery in Cytokinetic Abscission. Curr. Biol. 2019, 29, 2174–2182.e2177. [Google Scholar] [CrossRef] [PubMed]
- Goliand, I.; Nachmias, D.; Gershony, O.; Elia, N. Inhibition of ESCRT-II-CHMP6 interactions impedes cytokinetic abscission and leads to cell death. Mol. Biol. Cell 2014, 25, 3740–3748. [Google Scholar] [CrossRef]
- Christ, L.; Wenzel, E.M.; Liestol, K.; Raiborg, C.; Campsteijn, C.; Stenmark, H. ALIX and ESCRT-I/II function as parallel ESCRT-III recruiters in cytokinetic abscission. J. Cell Biol. 2016, 212, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Hadders, M.A.; Agromayor, M.; Obita, T.; Perisic, O.; Caballe, A.; Kloc, M.; Lamers, M.H.; Williams, R.L.; Martin-Serrano, J. ESCRT-III binding protein MITD1 is involved in cytokinesis and has an unanticipated PLD fold that binds membranes. Proc. Natl. Acad. Sci. USA 2012, 109, 17424–17429. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Chang, J.; Renvoise, B.; Tipirneni, A.; Yang, S.; Blackstone, C. MITD1 is recruited to midbodies by ESCRT-III and participates in cytokinesis. Mol. Biol. Cell. 2012, 23, 4347–4361. [Google Scholar] [CrossRef] [PubMed]
- Goliand, I.; Adar-Levor, S.; Segal, I.; Nachmias, D.; Dadosh, T.; Kozlov, M.M.; Elia, N. Resolving ESCRT-III Spirals at the Intercellular Bridge of Dividing Cells Using 3D STORM. Cell Rep. 2018, 24, 1756–1764. [Google Scholar] [CrossRef] [PubMed]
- Elia, N.; Sougrat, R.; Spurlin, T.A.; Hurley, J.H.; Lippincott-Schwartz, J. Dynamics of endosomal sorting complex required for transport (ESCRT) machinery during cytokinesis and its role in abscission. Proc. Natl. Acad. Sci. USA 2011, 108, 4846–4851. [Google Scholar] [CrossRef]
- Reid, E.; Connell, J.; Edwards, T.L.; Duley, S.; Brown, S.E.; Sanderson, C.M. The hereditary spastic paraplegia protein spastin interacts with the ESCRT-III complex-associated endosomal protein CHMP1B. Hum. Mol. Genet. 2005, 14, 19–38. [Google Scholar] [CrossRef]
- Yang, D.; Rismanchi, N.; Renvoise, B.; Lippincott-Schwartz, J.; Blackstone, C.; Hurley, J.H. Structural basis for midbody targeting of spastin by the ESCRT-III protein CHMP1B. Nat. Struct. Mol. Biol. 2008, 15, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Lindas, A.C.; Karlsson, E.A.; Lindgren, M.T.; Ettema, T.J.; Bernander, R. A unique cell division machinery in the Archaea. Proc. Natl. Acad. Sci. USA 2008, 105, 18942–18946. [Google Scholar] [CrossRef]
- Thoresen, S.B.; Campsteijn, C.; Vietri, M.; Schink, K.O.; Liestol, K.; Andersen, J.S.; Raiborg, C.; Stenmark, H. ANCHR mediates Aurora-B-dependent abscission checkpoint control through retention of VPS4. Nat. Cell Biol. 2014, 16, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Caballe, A.; Wenzel, D.M.; Agromayor, M.; Alam, S.L.; Skalicky, J.J.; Kloc, M.; Carlton, J.G.; Labrador, L.; Sundquist, W.I.; Martin-Serrano, J. ULK3 regulates cytokinetic abscission by phosphorylating ESCRT-III proteins. Elife 2015, 4, e06547. [Google Scholar] [CrossRef]
- Sadler, J.B.A.; Wenzel, D.M.; Williams, L.K.; Guindo-Martinez, M.; Alam, S.L.; Mercader, J.M.; Torrents, D.; Ullman, K.S.; Sundquist, W.I.; Martin-Serrano, J. A cancer-associated polymorphism in ESCRT-III disrupts the abscission checkpoint and promotes genome instability. Proc. Natl. Acad. Sci. USA 2018, 115, E8900–E8908. [Google Scholar] [CrossRef]
- Lens, S.M.A.; Medema, R.H. Cytokinesis defects and cancer. Nat. Rev. Cancer 2019, 19, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Tandon, D.; Banerjee, M. Centrosomal protein 55: A new paradigm in tumorigenesis. Eur. J. Cell Biol. 2020, 99, 151086. [Google Scholar] [CrossRef] [PubMed]
- Hatch, E.M.; Fischer, A.H.; Deerinck, T.J.; Hetzer, M.W. Catastrophic nuclear envelope collapse in cancer cell micronuclei. Cell 2013, 154, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Vietri, M.; Schultz, S.W.; Bellanger, A.; Jones, C.M.; Petersen, L.I.; Raiborg, C.; Skarpen, E.; Pedurupillay, C.R.J.; Kjos, I.; Kip, E.; et al. Unrestrained ESCRT-III drives micronuclear catastrophe and chromosome fragmentation. Nat. Cell Biol. 2020, 22, 856–867. [Google Scholar] [CrossRef]
- Tanaka, N.; Kyuuma, M.; Sugamura, K. Endosomal sorting complex required for transport proteins in cancer pathogenesis, vesicular transport, and non-endosomal functions. Cancer Sci. 2008, 99, 1293–1303. [Google Scholar] [CrossRef]
- Mattissek, C.; Teis, D. The role of the endosomal sorting complexes required for transport (ESCRT) in tumorigenesis. Mol. Membr. Biol. 2014, 31, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Alfred, V.; Vaccari, T. When membranes need an ESCRT: Endosomal sorting and membrane remodelling in health and disease. Swiss Med. Wkly. 2016, 146, w14347. [Google Scholar] [CrossRef]
- Andrews, N.W.; Almeida, P.E.; Corrotte, M. Damage control: Cellular mechanisms of plasma membrane repair. Trends Cell Biol. 2014, 24, 734–742. [Google Scholar] [CrossRef]
- Jimenez, A.J.; Maiuri, P.; Lafaurie-Janvore, J.; Divoux, S.; Piel, M.; Perez, F. ESCRT machinery is required for plasma membrane repair. Science 2014, 343, 1247136. [Google Scholar] [CrossRef] [PubMed]
- Grassel, L.; Fast, L.A.; Scheffer, K.D.; Boukhallouk, F.; Spoden, G.A.; Tenzer, S.; Boller, K.; Bago, R.; Rajesh, S.; Overduin, M.; et al. The CD63-Syntenin-1 Complex Controls Post-Endocytic Trafficking of Oncogenic Human Papillomaviruses. Sci. Rep. 2016, 6, 32337. [Google Scholar] [CrossRef]
- Scheffer, L.L.; Sreetama, S.C.; Sharma, N.; Medikayala, S.; Brown, K.J.; Defour, A.; Jaiswal, J.K. Mechanism of Ca(2)(+)-triggered ESCRT assembly and regulation of cell membrane repair. Nat. Commun. 2014, 5, 5646. [Google Scholar] [CrossRef]
- Sun, S.; Zhou, X.; Corvera, J.; Gallick, G.E.; Lin, S.H.; Kuang, J. ALG-2 activates the MVB sorting function of ALIX through relieving its intramolecular interaction. Cell Discov. 2015, 1, 15018. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, S.; Shkarina, K.; Demarco, B.; Heilig, R.; Santos, J.C.; Broz, P. ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science 2018, 362, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.N.; Guy, C.; Olauson, H.; Becker, J.U.; Yang, M.; Fitzgerald, P.; Linkermann, A.; Green, D.R. ESCRT-III Acts Downstream of MLKL to Regulate Necroptotic Cell Death and Its Consequences. Cell 2017, 169, 286–300.e216. [Google Scholar] [CrossRef]
- Robijns, J.; Houthaeve, G.; Braeckmans, K.; De Vos, W.H. Loss of Nuclear Envelope Integrity in Aging and Disease. Int Rev. Cell Mol. Biol. 2018, 336, 205–222. [Google Scholar] [CrossRef]
- Guttinger, S.; Laurell, E.; Kutay, U. Orchestrating nuclear envelope disassembly and reassembly during mitosis. Nat. Rev. Mol. Cell Biol. 2009, 10, 178–191. [Google Scholar] [CrossRef]
- Vietri, M.; Schink, K.O.; Campsteijn, C.; Wegner, C.S.; Schultz, S.W.; Christ, L.; Thoresen, S.B.; Brech, A.; Raiborg, C.; Stenmark, H. Spastin and ESCRT-III coordinate mitotic spindle disassembly and nuclear envelope sealing. Nature 2015, 522, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Olmos, Y.; Hodgson, L.; Mantell, J.; Verkade, P.; Carlton, J.G. ESCRT-III controls nuclear envelope reformation. Nature 2015, 522, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Ventimiglia, L.N.; Cuesta-Geijo, M.A.; Martinelli, N.; Caballe, A.; Macheboeuf, P.; Miguet, N.; Parnham, I.M.; Olmos, Y.; Carlton, J.G.; Weissenhorn, W.; et al. CC2D1B Coordinates ESCRT-III Activity during the Mitotic Reformation of the Nuclear Envelope. Dev. Cell 2018, 47, 547–563.e546. [Google Scholar] [CrossRef]
- Martinelli, N.; Hartlieb, B.; Usami, Y.; Sabin, C.; Dordor, A.; Miguet, N.; Avilov, S.V.; Ribeiro, E.A., Jr.; Gottlinger, H.; Weissenhorn, W. CC2D1A is a regulator of ESCRT-III CHMP4B. J. Mol. Biol. 2012, 419, 75–88. [Google Scholar] [CrossRef]
- Pieper, G.H.; Sprenger, S.; Teis, D.; Oliferenko, S. ESCRT-III/Vps4 Controls Heterochromatin-Nuclear Envelope Attachments. Dev. Cell 2020, 53, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Maciejowski, J.; Hatch, E.M. Nuclear Membrane Rupture and Its Consequences. Annu. Rev. Cell Dev. Biol. 2020, 36, 85–114. [Google Scholar] [CrossRef] [PubMed]
- Denais, C.M.; Gilbert, R.M.; Isermann, P.; McGregor, A.L.; te Lindert, M.; Weigelin, B.; Davidson, P.M.; Friedl, P.; Wolf, K.; Lammerding, J. Nuclear envelope rupture and repair during cancer cell migration. Science 2016, 352, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Raab, M.; Gentili, M.; de Belly, H.; Thiam, H.R.; Vargas, P.; Jimenez, A.J.; Lautenschlaeger, F.; Voituriez, R.; Lennon-Dumenil, A.M.; Manel, N.; et al. ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science 2016, 352, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Halfmann, C.T.; Sears, R.M.; Katiyar, A.; Busselman, B.W.; Aman, L.K.; Zhang, Q.; O’Bryan, C.S.; Angelini, T.E.; Lele, T.P.; Roux, K.J. Repair of nuclear ruptures requires barrier-to-autointegration factor. J. Cell Biol. 2019, 218, 2136–2149. [Google Scholar] [CrossRef] [PubMed]
- Webster, B.M.; Colombi, P.; Jager, J.; Lusk, C.P. Surveillance of nuclear pore complex assembly by ESCRT-III/Vps4. Cell 2014, 159, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Skowyra, M.L.; Schlesinger, P.H.; Naismith, T.V.; Hanson, P.I. Triggered recruitment of ESCRT machinery promotes endolysosomal repair. Science 2018, 360. [Google Scholar] [CrossRef]
- Radulovic, M.; Schink, K.O.; Wenzel, E.M.; Nahse, V.; Bongiovanni, A.; Lafont, F.; Stenmark, H. ESCRT-mediated lysosome repair precedes lysophagy and promotes cell survival. EMBO J. 2018, 37. [Google Scholar] [CrossRef] [PubMed]
- Radulovic, M.; Stenmark, H. ESCRTs in membrane sealing. Biochem. Soc. Trans. 2018, 46, 773–778. [Google Scholar] [CrossRef]
- Mansilla Pareja, M.E.; Bongiovanni, A.; Lafont, F.; Colombo, M.I. Alterations of the Coxiella burnetii Replicative Vacuole Membrane Integrity and Interplay with the Autophagy Pathway. Front. Cell Infect. Microbiol. 2017, 7, 112. [Google Scholar] [CrossRef]
- Mittal, E.; Skowyra, M.L.; Uwase, G.; Tinaztepe, E.; Mehra, A.; Koster, S.; Hanson, P.I.; Philips, J.A. Mycobacterium tuberculosis Type VII Secretion System Effectors Differentially Impact the ESCRT Endomembrane Damage Response. mBio 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.U.; Krempler, A.; Qi, Y.; Park, K.; Henry, M.D.; Triplett, A.A.; Riedlinger, G.; Rucker, I.E.; Hennighausen, L. Tsg101 is essential for cell growth, proliferation, and cell survival of embryonic and adult tissues. Mol. Cell Biol. 2003, 23, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Handschuh, K.; Feenstra, J.; Koss, M.; Ferretti, E.; Risolino, M.; Zewdu, R.; Sahai, M.A.; Benazet, J.D.; Peng, X.P.; Depew, M.J.; et al. ESCRT-II/Vps25 constrains digit number by endosome-mediated selective modulation of FGF-SHH signaling. Cell Rep. 2014, 9, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H.; Xiao, C.; Hayden, M.S.; Lee, K.Y.; Trombetta, E.S.; Pypaert, M.; Nara, A.; Yoshimori, T.; Wilm, B.; Erdjument-Bromage, H.; et al. CHMP5 is essential for late endosome function and down-regulation of receptor signaling during mouse embryogenesis. J. Cell Biol. 2006, 172, 1045–1056. [Google Scholar] [CrossRef]
- Thompson, B.J.; Mathieu, J.; Sung, H.H.; Loeser, E.; Rorth, P.; Cohen, S.M. Tumor suppressor properties of the ESCRT-II complex component Vps25 in Drosophila. Dev. Cell 2005, 9, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Vaccari, T.; Rusten, T.E.; Menut, L.; Nezis, I.P.; Brech, A.; Stenmark, H.; Bilder, D. Comparative analysis of ESCRT-I, ESCRT-II and ESCRT-III function in Drosophila by efficient isolation of ESCRT mutants. J. Cell Sci. 2009, 122, 2413–2423. [Google Scholar] [CrossRef]
- Spitzer, C.; Schellmann, S.; Sabovljevic, A.; Shahriari, M.; Keshavaiah, C.; Bechtold, N.; Herzog, M.; Muller, S.; Hanisch, F.G.; Hulskamp, M. The Arabidopsis elch mutant reveals functions of an ESCRT component in cytokinesis. Development 2006, 133, 4679–4689. [Google Scholar] [CrossRef]
- Razi, M.; Futter, C.E. Distinct roles for Tsg101 and HRS in multivesicular body formation and inward vesiculation. Mol. Biol. Cell 2006, 17, 3469–3483. [Google Scholar] [CrossRef] [PubMed]
- Bache, K.G.; Stuffers, S.; Malerod, L.; Slagsvold, T.; Raiborg, C.; Lechardeur, D.; Walchli, S.; Lukacs, G.L.; Brech, A.; Stenmark, H. The ESCRT-III subunit hVps24 is required for degradation but not silencing of the epidermal growth factor receptor. Mol. Biol. Cell 2006, 17, 2513–2523. [Google Scholar] [CrossRef] [PubMed]
- Stuffers, S.; Sem Wegner, C.; Stenmark, H.; Brech, A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 2009, 10, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Capalbo, L.; Montembault, E.; Takeda, T.; Bassi, Z.I.; Glover, D.M.; D’Avino, P.P. The chromosomal passenger complex controls the function of endosomal sorting complex required for transport-III Snf7 proteins during cytokinesis. Open Biol. 2012, 2, 120070. [Google Scholar] [CrossRef]
- Xiao, Y.; Cai, W. Autophagy and Viral Infection. Adv. Exp. Med. Biol. 2020, 1207, 425–432. [Google Scholar] [CrossRef]
- Purvinsh, L.; Gorshkov, A.; Brodskaia, A.; Vasin, A. Extracellular Vesicles in Viral Pathogenesis: A Case of Dr. Jekyll and Mr. Hyde. Life 2021, 11, 45. [Google Scholar] [CrossRef]
- Raymond, A.D.; Diaz, P.; Chevelon, S.; Agudelo, M.; Yndart-Arias, A.; Ding, H.; Kaushik, A.; Jayant, R.D.; Nikkhah-Moshaie, R.; Roy, U.; et al. Microglia-derived HIV Nef+ exosome impairment of the blood-brain barrier is treatable by nanomedicine-based delivery of Nef peptides. J. Neurovirol. 2016, 22, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Arenaccio, C.; Anticoli, S.; Manfredi, F.; Chiozzini, C.; Olivetta, E.; Federico, M. Latent HIV-1 is activated by exosomes from cells infected with either replication-competent or defective HIV-1. Retrovirology 2015, 12, 87. [Google Scholar] [CrossRef]
- Arenaccio, C.; Chiozzini, C.; Columba-Cabezas, S.; Manfredi, F.; Affabris, E.; Baur, A.; Federico, M. Exosomes from human immunodeficiency virus type 1 (HIV-1)-infected cells license quiescent CD4+ T lymphocytes to replicate HIV-1 through a Nef- and ADAM17-dependent mechanism. J. Virol. 2014, 88, 11529–11539. [Google Scholar] [CrossRef]
- Lenassi, M.; Cagney, G.; Liao, M.; Vaupotic, T.; Bartholomeeusen, K.; Cheng, Y.; Krogan, N.J.; Plemenitas, A.; Peterlin, B.M. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic 2010, 11, 110–122. [Google Scholar] [CrossRef]
- Xu, W.; Santini, P.A.; Sullivan, J.S.; He, B.; Shan, M.; Ball, S.C.; Dyer, W.B.; Ketas, T.J.; Chadburn, A.; Cohen-Gould, L.; et al. HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat. Immunol. 2009, 10, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.; Kleinschmidt, A.; Bruhl, H.; Klier, C.; Nelson, P.J.; Cihak, J.; Plachy, J.; Stangassinger, M.; Erfle, V.; Schlondorff, D. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: A mechanism for cellular human immunodeficiency virus 1 infection. Nat. Med. 2000, 6, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Rozmyslowicz, T.; Majka, M.; Kijowski, J.; Murphy, S.L.; Conover, D.O.; Poncz, M.; Ratajczak, J.; Gaulton, G.N.; Ratajczak, M.Z. Platelet- and megakaryocyte-derived microparticles transfer CXCR4 receptor to CXCR4-null cells and make them susceptible to infection by X4-HIV. AIDS 2003, 17, 33–42. [Google Scholar] [CrossRef]
- Narayanan, A.; Iordanskiy, S.; Das, R.; Van Duyne, R.; Santos, S.; Jaworski, E.; Guendel, I.; Sampey, G.; Dalby, E.; Iglesias-Ussel, M.; et al. Exosomes derived from HIV-1-infected cells contain trans-activation response element RNA. J. Biol. Chem. 2013, 288, 20014–20033. [Google Scholar] [CrossRef] [PubMed]
- Khatua, A.K.; Taylor, H.E.; Hildreth, J.E.; Popik, W. Exosomes packaging APOBEC3G confer human immunodeficiency virus resistance to recipient cells. J. Virol. 2009, 83, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Bridgeman, A.; Maelfait, J.; Davenne, T.; Partridge, T.; Peng, Y.; Mayer, A.; Dong, T.; Kaever, V.; Borrow, P.; Rehwinkel, J. Viruses transfer the antiviral second messenger cGAMP between cells. Science 2015, 349, 1228–1232. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.V.S.; Costa, C.S.; da Silva, L.L.P. The Ambiguous Roles of Extracellular Vesicles in HIV Replication and Pathogenesis. Front. Microbiol. 2018, 9, 2411. [Google Scholar] [CrossRef]
- Li, S.; Li, S.; Wu, S.; Chen, L. Exosomes Modulate the Viral Replication and Host Immune Responses in HBV Infection. Biomed. Res. Int. 2019, 2019, 2103943. [Google Scholar] [CrossRef]
- Jaworski, E.; Narayanan, A.; Van Duyne, R.; Shabbeer-Meyering, S.; Iordanskiy, S.; Saifuddin, M.; Das, R.; Afonso, P.V.; Sampey, G.C.; Chung, M.; et al. Human T-lymphotropic virus type 1-infected cells secrete exosomes that contain Tax protein. J. Biol. Chem. 2014, 289, 22284–22305. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.A. Respiratory epithelial cells as master communicators during viral infections. Curr. Clin. Microbiol. Rep. 2019, 6, 10–17. [Google Scholar] [CrossRef]
- Hassanpour, M.; Rezaie, J.; Nouri, M.; Panahi, Y. The role of extracellular vesicles in COVID-19 virus infection. Infect. Genet. Evol 2020, 85, 104422. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Cheng, C.; Liu, S. Angiotensin-converting enzyme 2 augments the effects of endothelial progenitor cells-exosomes on vascular smooth muscle cell phenotype transition. Cell Tissue Res. 2020, 382, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Cocozza, F.; Nevo, N.; Piovesana, E.; Lahaye, X.; Buchrieser, J.; Schwartz, O.; Manel, N.; Tkach, M.; Thery, C.; Martin-Jaular, L. Extracellular vesicles containing ACE2 efficiently prevent infection by SARS-CoV-2 Spike protein-containing virus. J. Extracell Vesicles 2020, 10, e12050. [Google Scholar] [CrossRef]
- Ipinmoroti, A.O.; Matthews, Q.L. Extracellular Vesicles: Roles in Human Viral Infections, Immune-Diagnostic, and Therapeutic Applications. Pathogens 2020, 9, 1056. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Li, C.Y.; Chang, W.T.; Cheng, W.C.; Yen, C.H.; Tu, W.Y.; Lin, Z.Y.; Lin, C.C.; Yeh, M.L.; Huang, C.F.; et al. Exosome-derived differentiation antagonizing non-protein coding RNA with risk of hepatitis C virus-related hepatocellular carcinoma recurrence. Liver Int. 2020. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Hu, J.; Cao, P.; Yan, Q.; Zhang, S.; Dang, W.; Lu, J. Long noncoding RNAs involvement in Epstein-Barr virus infection and tumorigenesis. Virol. J. 2020, 17, 51. [Google Scholar] [CrossRef]
- Yang, X.; Li, H.; Sun, H.; Fan, H.; Hu, Y.; Liu, M.; Li, X.; Tang, H. Hepatitis B Virus-Encoded MicroRNA Controls Viral Replication. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Pincetic, A.; Kuang, Z.; Seo, E.J.; Leis, J. The interferon-induced gene ISG15 blocks retrovirus release from cells late in the budding process. J. Virol. 2010, 84, 4725–4736. [Google Scholar] [CrossRef]
- Kuang, Z.; Seo, E.J.; Leis, J. Mechanism of inhibition of retrovirus release from cells by interferon-induced gene ISG15. J. Virol. 2011, 85, 7153–7161. [Google Scholar] [CrossRef]
- Bashirova, A.A.; Bleiber, G.; Qi, Y.; Hutcheson, H.; Yamashita, T.; Johnson, R.C.; Cheng, J.; Alter, G.; Goedert, J.J.; Buchbinder, S.; et al. Consistent effects of TSG101 genetic variability on multiple outcomes of exposure to human immunodeficiency virus type 1. J. Virol. 2006, 80, 6757–6763. [Google Scholar] [CrossRef] [PubMed]
- Strickland, M.; Ehrlich, L.S.; Watanabe, S.; Khan, M.; Strub, M.P.; Luan, C.H.; Powell, M.D.; Leis, J.; Tjandra, N.; Carter, C.A. Tsg101 chaperone function revealed by HIV-1 assembly inhibitors. Nat. Commun. 2017, 8, 1391. [Google Scholar] [CrossRef]
- Watanabe, S.M.; Ehrlich, L.S.; Strickland, M.; Li, X.; Soloveva, V.; Goff, A.J.; Stauft, C.B.; Bhaduri-McIntosh, S.; Tjandra, N.; Carter, C. Selective Targeting of Virus Replication by Proton Pump Inhibitors. Sci. Rep. 2020, 10, 4003. [Google Scholar] [CrossRef]
- Ketter, E.; Randall, G. Virus Impact on Lipids and Membranes. Annu. Rev. Virol. 2019, 6, 319–340. [Google Scholar] [CrossRef] [PubMed]
- Tuthill, T.J.; Bubeck, D.; Rowlands, D.J.; Hogle, J.M. Characterization of early steps in the poliovirus infection process: Receptor-decorated liposomes induce conversion of the virus to membrane-anchored entry-intermediate particles. J. Virol. 2006, 80, 172–180. [Google Scholar] [CrossRef]
- Lozach, P.Y.; Huotari, J.; Helenius, A. Late-penetrating viruses. Curr. Opin. Virol 2011, 1, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Votteler, J.; Sundquist, W.I. Virus budding and the ESCRT pathway. Cell Host Microbe 2013, 14, 232–241. [Google Scholar] [CrossRef]
- Wodrich, H.; Henaff, D.; Jammart, B.; Segura-Morales, C.; Seelmeir, S.; Coux, O.; Ruzsics, Z.; Wiethoff, C.M.; Kremer, E.J. A capsid-encoded PPxY-motif facilitates adenovirus entry. PLoS Pathog. 2010, 6, e1000808. [Google Scholar] [CrossRef] [PubMed]
- Wiethoff, C.M.; Wodrich, H.; Gerace, L.; Nemerow, G.R. Adenovirus protein VI mediates membrane disruption following capsid disassembly. J. Virol. 2005, 79, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Moyer, C.L.; Wiethoff, C.M.; Maier, O.; Smith, J.G.; Nemerow, G.R. Functional genetic and biophysical analyses of membrane disruption by human adenovirus. J. Virol. 2011, 85, 2631–2641. [Google Scholar] [CrossRef]
- Prchla, E.; Plank, C.; Wagner, E.; Blaas, D.; Fuchs, R. Virus-mediated release of endosomal content in vitro: Different behavior of adenovirus and rhinovirus serotype 2. J. Cell Biol. 1995, 131, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Farr, G.A.; Zhang, L.G.; Tattersall, P. Parvoviral virions deploy a capsid-tethered lipolytic enzyme to breach the endosomal membrane during cell entry. Proc. Natl. Acad. Sci. USA 2005, 102, 17148–17153. [Google Scholar] [CrossRef]
- Montespan, C.; Marvin, S.A.; Austin, S.; Burrage, A.M.; Roger, B.; Rayne, F.; Faure, M.; Campell, E.M.; Schneider, C.; Reimer, R.; et al. Multi-layered control of Galectin-8 mediated autophagy during adenovirus cell entry through a conserved PPxY motif in the viral capsid. PLoS Pathog. 2017, 13, e1006217. [Google Scholar] [CrossRef]
- Kumar, B.; Roy, A.; Veettil, M.V.; Chandran, B. Insight into the Roles of E3 Ubiquitin Ligase c-Cbl, ESCRT Machinery, and Host Cell Signaling in Kaposi’s Sarcoma-Associated Herpesvirus Entry and Trafficking. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Shtanko, O.; Nikitina, R.A.; Altuntas, C.Z.; Chepurnov, A.A.; Davey, R.A. Crimean-Congo hemorrhagic fever virus entry into host cells occurs through the multivesicular body and requires ESCRT regulators. PLoS Pathog. 2014, 10, e1004390. [Google Scholar] [CrossRef]
- Broniarczyk, J.; Bergant, M.; Gozdzicka-Jozefiak, A.; Banks, L. Human papillomavirus infection requires the TSG101 component of the ESCRT machinery. Virology 2014, 460–461, 83–90. [Google Scholar] [CrossRef]
- Karjalainen, M.; Rintanen, N.; Lehkonen, M.; Kallio, K.; Maki, A.; Hellstrom, K.; Siljamaki, V.; Upla, P.; Marjomaki, V. Echovirus 1 infection depends on biogenesis of novel multivesicular bodies. Cell Microbiol. 2011, 13, 1975–1995. [Google Scholar] [CrossRef] [PubMed]
- Pasqual, G.; Rojek, J.M.; Masin, M.; Chatton, J.Y.; Kunz, S. Old world arenaviruses enter the host cell via the multivesicular body and depend on the endosomal sorting complex required for transport. PLoS Pathog. 2011, 7, e1002232. [Google Scholar] [CrossRef]
- Silva-Ayala, D.; Lopez, T.; Gutierrez, M.; Perrimon, N.; Lopez, S.; Arias, C.F. Genome-wide RNAi screen reveals a role for the ESCRT complex in rotavirus cell entry. Proc. Natl. Acad. Sci. USA 2013, 110, 10270–10275. [Google Scholar] [CrossRef]
- Barajas, D.; Jiang, Y.; Nagy, P.D. A unique role for the host ESCRT proteins in replication of Tomato bushy stunt virus. PLoS Pathog. 2009, 5, e1000705. [Google Scholar] [CrossRef]
- Kovalev, N.; de Castro Martin, I.F.; Pogany, J.; Barajas, D.; Pathak, K.; Risco, C.; Nagy, P.D. Role of Viral RNA and Co-opted Cellular ESCRT-I and ESCRT-III Factors in Formation of Tombusvirus Spherules Harboring the Tombusvirus Replicase. J. Virol. 2016, 90, 3611–3626. [Google Scholar] [CrossRef] [PubMed]
- Richardson, L.G.; Clendening, E.A.; Sheen, H.; Gidda, S.K.; White, K.A.; Mullen, R.T. A unique N-terminal sequence in the Carnation Italian ringspot virus p36 replicase-associated protein interacts with the host cell ESCRT-I component Vps23. J. Virol. 2014, 88, 6329–6344. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.; Zhang, J.; Ollwerther, A.; Wang, X.; Ahlquist, P. Host ESCRT proteins are required for bromovirus RNA replication compartment assembly and function. PLoS Pathog. 2015, 11, e1004742. [Google Scholar] [CrossRef] [PubMed]
- Tabata, K.; Arimoto, M.; Arakawa, M.; Nara, A.; Saito, K.; Omori, H.; Arai, A.; Ishikawa, T.; Konishi, E.; Suzuki, R.; et al. Unique Requirement for ESCRT Factors in Flavivirus Particle Formation on the Endoplasmic Reticulum. Cell Rep. 2016, 16, 2339–2347. [Google Scholar] [CrossRef]
- Tandon, R.; AuCoin, D.P.; Mocarski, E.S. Human cytomegalovirus exploits ESCRT machinery in the process of virion maturation. J. Virol. 2009, 83, 10797–10807. [Google Scholar] [CrossRef]
- Lindenbach, B.D. Virion assembly and release. Curr. Top. Microbiol. Immunol. 2013, 369, 199–218. [Google Scholar] [CrossRef]
- Rossman, J.S.; Lamb, R.A. Viral membrane scission. Ann. Rev. Cell Dev. Biol. 2013, 29, 551–569. [Google Scholar] [CrossRef] [PubMed]
- Gottlinger, H.G.; Dorfman, T.; Sodroski, J.G.; Haseltine, W.A. Effect of mutations affecting the p6 gag protein on human immunodeficiency virus particle release. Proc. Natl. Acad. Sci. USA 1991, 88, 3195–3199. [Google Scholar] [CrossRef] [PubMed]
- Wills, J.W.; Cameron, C.E.; Wilson, C.B.; Xiang, Y.; Bennett, R.P.; Leis, J. An assembly domain of the Rous sarcoma virus Gag protein required late in budding. J. Virol. 1994, 68, 6605–6618. [Google Scholar] [CrossRef]
- Calistri, A.; Salata, C.; Parolin, C.; Palu, G. Role of multivesicular bodies and their components in the egress of enveloped RNA viruses. Rev. Med. Virol. 2009, 19, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Crump, C. Virus Assembly and Egress of HSV. Adv. Exp. Med. Biol. 2018, 1045, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Calistri, A.; Sette, P.; Salata, C.; Cancellotti, E.; Forghieri, C.; Comin, A.; Gottlinger, H.; Campadelli-Fiume, G.; Palu, G.; Parolin, C. Intracellular trafficking and maturation of herpes simplex virus type 1 gB and virus egress require functional biogenesis of multivesicular bodies. J. Virol. 2007, 81, 11468–11478. [Google Scholar] [CrossRef]
- Calistri, A.; Munegato, D.; Toffoletto, M.; Celestino, M.; Franchin, E.; Comin, A.; Sartori, E.; Salata, C.; Parolin, C.; Palu, G. Functional Interaction Between the ESCRT-I Component TSG101 and the HSV-1 Tegument Ubiquitin Specific Protease. J. Cell Physiol. 2015, 230, 1794–1806. [Google Scholar] [CrossRef]
- Garcia, M.L.; Reynolds, T.D.; Mothes, W.; Robek, M.D. Functional characterization of the putative hepatitis B virus core protein late domain using retrovirus chimeras. PLoS ONE 2013, 8, e72845. [Google Scholar] [CrossRef]
- Hurley, J.H.; Hanson, P.I. Membrane budding and scission by the ESCRT machinery: it’s all in the neck. Nat. Rev. Mol. Cell Biol. 2010, 11, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Ariumi, Y.; Kuroki, M.; Maki, M.; Ikeda, M.; Dansako, H.; Wakita, T.; Kato, N. The ESCRT system is required for hepatitis C virus production. PLoS ONE 2011, 6, e14517. [Google Scholar] [CrossRef]
- Rossman, J.S.; Jing, X.; Leser, G.P.; Lamb, R.A. Influenza virus M2 protein mediates ESCRT-independent membrane scission. Cell 2010, 142, 902–913. [Google Scholar] [CrossRef]
- Wirblich, C.; Bhattacharya, B.; Roy, P. Nonstructural protein 3 of bluetongue virus assists virus release by recruiting ESCRT-I protein Tsg101. J. Virol. 2006, 80, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Parent, L.J.; Bennett, R.P.; Craven, R.C.; Nelle, T.D.; Krishna, N.K.; Bowzard, J.B.; Wilson, C.B.; Puffer, B.A.; Montelaro, R.C.; Wills, J.W. Positionally independent and exchangeable late budding functions of the Rous sarcoma virus and human immunodeficiency virus Gag proteins. J. Virol. 1995, 69, 5455–5460. [Google Scholar] [CrossRef]
- Yuan, B.; Campbell, S.; Bacharach, E.; Rein, A.; Goff, S.P. Infectivity of Moloney murine leukemia virus defective in late assembly events is restored by late assembly domains of other retroviruses. J. Virol. 2000, 74, 7250–7260. [Google Scholar] [CrossRef] [PubMed]
- Martin-Serrano, J.; Yarovoy, A.; Perez-Caballero, D.; Bieniasz, P.D. Divergent retroviral late-budding domains recruit vacuolar protein sorting factors by using alternative adaptor proteins. Proc. Natl. Acad. Sci. USA 2003, 100, 12414–12419. [Google Scholar] [CrossRef]
- Martin-Serrano, J.; Bieniasz, P.D. A bipartite late-budding domain in human immunodeficiency virus type 1. J. Virol. 2003, 77, 12373–12377. [Google Scholar] [CrossRef]
- Strack, B.; Calistri, A.; Accola, M.A.; Palu, G.; Gottlinger, H.G. A role for ubiquitin ligase recruitment in retrovirus release. Proc. Natl. Acad. Sci. USA 2000, 97, 13063–13068. [Google Scholar] [CrossRef]
- Staub, O.; Dho, S.; Henry, P.; Correa, J.; Ishikawa, T.; McGlade, J.; Rotin, D. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle’s syndrome. EMBO J. 1996, 15, 2371–2380. [Google Scholar] [CrossRef]
- Staub, O.; Gautschi, I.; Ishikawa, T.; Breitschopf, K.; Ciechanover, A.; Schild, L.; Rotin, D. Regulation of stability and function of the epithelial Na+ channel (ENaC) by ubiquitination. EMBO J. 1997, 16, 6325–6336. [Google Scholar] [CrossRef] [PubMed]
- Strack, B.; Calistri, A.; Gottlinger, H.G. Late assembly domain function can exhibit context dependence and involves ubiquitin residues implicated in endocytosis. J. Virol. 2002, 76, 5472–5479. [Google Scholar] [CrossRef] [PubMed]
- Garrus, J.E.; von Schwedler, U.K.; Pornillos, O.W.; Morham, S.G.; Zavitz, K.H.; Wang, H.E.; Wettstein, D.A.; Stray, K.M.; Cote, M.; Rich, R.L.; et al. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell 2001, 107, 55–65. [Google Scholar] [CrossRef]
- VerPlank, L.; Bouamr, F.; LaGrassa, T.J.; Agresta, B.; Kikonyogo, A.; Leis, J.; Carter, C.A. Tsg101, a homologue of ubiquitin-conjugating (E2) enzymes, binds the L domain in HIV type 1 Pr55(Gag). Proc. Natl. Acad. Sci. USA 2001, 98, 7724–7729. [Google Scholar] [CrossRef] [PubMed]
- Kikonyogo, A.; Bouamr, F.; Vana, M.L.; Xiang, Y.; Aiyar, A.; Carter, C.; Leis, J. Proteins related to the Nedd4 family of ubiquitin protein ligases interact with the L domain of Rous sarcoma virus and are required for gag budding from cells. Proc. Natl. Acad. Sci. USA 2001, 98, 11199–11204. [Google Scholar] [CrossRef] [PubMed]
- Rose, K.M.; Hirsch, V.M.; Bouamr, F. Budding of a Retrovirus: Some Assemblies Required. Viruses 2020, 12, 1188. [Google Scholar] [CrossRef]
- Pincetic, A.; Medina, G.; Carter, C.; Leis, J. Avian sarcoma virus and human immunodeficiency virus, type 1 use different subsets of ESCRT proteins to facilitate the budding process. J. Biol. Chem. 2008, 283, 29822–29830. [Google Scholar] [CrossRef] [PubMed]
- Langelier, C.; von Schwedler, U.K.; Fisher, R.D.; De Domenico, I.; White, P.L.; Hill, C.P.; Kaplan, J.; Ward, D.; Sundquist, W.I. Human ESCRT-II complex and its role in human immunodeficiency virus type 1 release. J. Virol. 2006, 80, 9465–9480. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.; Ip, N.C.Y.; Abbink, T.E.M.; Kenyon, J.C.; Lever, A.M.L. ESCRT-II functions by linking to ESCRT-I in human immunodeficiency virus-1 budding. Cell Microbiol. 2020, 22, e13161. [Google Scholar] [CrossRef]
- Hurley, J.H.; Cada, A.K. Inside job: How the ESCRTs release HIV-1 from infected cells. Biochem. Soc. Trans. 2018, 46, 1029–1036. [Google Scholar] [CrossRef]
- Del Vecchio, C.; Celestino, M.; Celegato, M.; Palu, G.; Parolin, C.; Bouamr, F.; Calistri, A. Alix-Mediated Rescue of Feline Immunodeficiency Virus Budding Differs from That Observed with Human Immunodeficiency Virus. J. Virol. 2020, 94. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.R.; Popova, E.; Yamanaka, H.; Kim, H.C.; Huibregtse, J.M.; Gottlinger, H. Rescue of HIV-1 release by targeting widely divergent NEDD4-type ubiquitin ligases and isolated catalytic HECT domains to Gag. PLoS Pathog. 2010, 6, e1001107. [Google Scholar] [CrossRef]
- Usami, Y.; Popov, S.; Popova, E.; Gottlinger, H.G. Efficient and specific rescue of human immunodeficiency virus type 1 budding defects by a Nedd4-like ubiquitin ligase. J. Virol. 2008, 82, 4898–4907. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Morita, E.; von Schwedler, U.; Muller, B.; Krausslich, H.G.; Sundquist, W.I. NEDD4L overexpression rescues the release and infectivity of human immunodeficiency virus type 1 constructs lacking PTAP and YPXL late domains. J. Virol. 2008, 82, 4884–4897. [Google Scholar] [CrossRef]
- Calistri, A.; Del Vecchio, C.; Salata, C.; Celestino, M.; Celegato, M.; Gottlinger, H.; Palu, G.; Parolin, C. Role of the feline immunodeficiency virus L-domain in the presence or absence of Gag processing: Involvement of ubiquitin and Nedd4-2s ligase in viral egress. J. Cell Physiol. 2009, 218, 175–182. [Google Scholar] [CrossRef]
- Sette, P.; Nagashima, K.; Piper, R.C.; Bouamr, F. Ubiquitin conjugation to Gag is essential for ESCRT-mediated HIV-1 budding. Retrovirology 2013, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Popov, S.; Popova, E.; Inoue, M.; Gottlinger, H.G. Divergent Bro1 domains share the capacity to bind human immunodeficiency virus type 1 nucleocapsid and to enhance virus-like particle production. J. Virol. 2009, 83, 7185–7193. [Google Scholar] [CrossRef] [PubMed]
- Dussupt, V.; Javid, M.P.; Abou-Jaoude, G.; Jadwin, J.A.; de La Cruz, J.; Nagashima, K.; Bouamr, F. The nucleocapsid region of HIV-1 Gag cooperates with the PTAP and LYPXnL late domains to recruit the cellular machinery necessary for viral budding. PLoS Pathog. 2009, 5, e1000339. [Google Scholar] [CrossRef]
- Bello, N.F.; Dussupt, V.; Sette, P.; Rudd, V.; Nagashima, K.; Bibollet-Ruche, F.; Chen, C.; Montelaro, R.C.; Hahn, B.H.; Bouamr, F. Budding of retroviruses utilizing divergent L domains requires nucleocapsid. J. Virol. 2012, 86, 4182–4193. [Google Scholar] [CrossRef] [PubMed]
- Gottlinger, H.G. Influenza exits the cell without an ESCRT. Cell 2010, 142, 839–841. [Google Scholar] [CrossRef]
- Feng, Z.; Hirai-Yuki, A.; McKnight, K.L.; Lemon, S.M. Naked Viruses That Aren’t Always Naked: Quasi-Enveloped Agents of Acute Hepatitis. Ann. Rev. Virol. 2014, 1, 539–560. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Hensley, L.; McKnight, K.L.; Hu, F.; Madden, V.; Ping, L.; Jeong, S.H.; Walker, C.; Lanford, R.E.; Lemon, S.M. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature 2013, 496, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, S.; Takahashi, M.; Jirintai, S.; Tanggis; Kobayashi, T.; Nishizawa, T.; Okamoto, H. The membrane on the surface of hepatitis E virus particles is derived from the intracellular membrane and contains trans-Golgi network protein 2. Arch. Virol 2014, 159, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, S.; Takahashi, M.; Kobayashi, T.; Tanggis; Nishizawa, T.; Nishiyama, T.; Primadharsini, P.P.; Okamoto, H. Characterization of the Quasi-Enveloped Hepatitis E Virus Particles Released by the Cellular Exosomal Pathway. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Useros, N.; Naranjo-Gomez, M.; Erkizia, I.; Puertas, M.C.; Borras, F.E.; Blanco, J.; Martinez-Picado, J. HIV and mature dendritic cells: Trojan exosomes riding the Trojan horse? PLoS Pathog. 2010, 6, e1000740. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnaiah, V.; Thumann, C.; Fofana, I.; Habersetzer, F.; Pan, Q.; de Ruiter, P.E.; Willemsen, R.; Demmers, J.A.; Stalin Raj, V.; Jenster, G.; et al. Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proc. Natl. Acad. Sci. USA 2013, 110, 13109–13113. [Google Scholar] [CrossRef]
- Giannessi, F.; Aiello, A.; Franchi, F.; Percario, Z.A.; Affabris, E. The Role of Extracellular Vesicles as Allies of HIV, HCV and SARS Viruses. Viruses 2020, 12, 571. [Google Scholar] [CrossRef]
- Longatti, A. The Dual Role of Exosomes in Hepatitis A and C Virus Transmission and Viral Immune Activation. Viruses 2015, 7, 6707–6715. [Google Scholar] [CrossRef]
- Zhou, W.; Woodson, M.; Sherman, M.B.; Neelakanta, G.; Sultana, H. Exosomes mediate Zika virus transmission through SMPD3 neutral Sphingomyelinase in cortical neurons. Emerg Microbes Infect. 2019, 8, 307–326. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, S.; Shi, X.; Xu, G.; Shen, C.; Liu, X.; Zheng, H. Exosomes-mediated transmission of foot-and-mouth disease virus in vivo and in vitro. Vet. Microbiol. 2019, 233, 164–173. [Google Scholar] [CrossRef]
- Silvas, J.A.; Popov, V.L.; Paulucci-Holthauzen, A.; Aguilar, P.V. Extracellular Vesicles Mediate Receptor-Independent Transmission of Novel Tick-Borne Bunyavirus. J. Virol. 2016, 90, 873–886. [Google Scholar] [CrossRef]
- Reyes-Ruiz, J.M.; Osuna-Ramos, J.F.; De Jesus-Gonzalez, L.A.; Hurtado-Monzon, A.M.; Farfan-Morales, C.N.; Cervantes-Salazar, M.; Bolanos, J.; Cigarroa-Mayorga, O.E.; Martin-Martinez, E.S.; Medina, F.; et al. Isolation and characterization of exosomes released from mosquito cells infected with dengue virus. Virus Res. 2019, 266, 1–14. [Google Scholar] [CrossRef]
- Dubrovsky, L.; Ward, A.; Choi, S.H.; Pushkarsky, T.; Brichacek, B.; Vanpouille, C.; Adzhubei, A.A.; Mukhamedova, N.; Sviridov, D.; Margolis, L.; et al. Inhibition of HIV Replication by Apolipoprotein A-I Binding Protein Targeting the Lipid Rafts. mBio 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Bello-Morales, R.; Lopez-Guerrero, J.A. Extracellular Vesicles in Herpes Viral Spread and Immune Evasion. Front. Microbiol. 2018, 9, 2572. [Google Scholar] [CrossRef] [PubMed]
- Bello-Morales, R.; Praena, B.; de la Nuez, C.; Rejas, M.T.; Guerra, M.; Galan-Ganga, M.; Izquierdo, M.; Calvo, V.; Krummenacher, C.; Lopez-Guerrero, J.A. Role of Microvesicles in the Spread of Herpes Simplex Virus 1 in Oligodendrocytic Cells. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Mutsafi, Y.; Altan-Bonnet, N. Enterovirus Transmission by Secretory Autophagy. Viruses 2018, 10, 139. [Google Scholar] [CrossRef]
- Teo, Q.W.; van Leur, S.W.; Sanyal, S. Escaping the Lion’s Den: Redirecting autophagy for unconventional release and spread of viruses. FEBS J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hittelman, A.B.; Burakov, D.; Iniguez-Lluhi, J.A.; Freedman, L.P.; Garabedian, M.J. Differential regulation of glucocorticoid receptor transcriptional activation via AF-1-associated proteins. EMBO J. 1999, 18, 5380–5388. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Yanagi, Y.; Masuhiro, Y.; Yano, T.; Yoshikawa, H.; Yanagisawa, J.; Kato, S. A putative tumor suppressor, TSG101, acts as a transcriptional suppressor through its coiled-coil domain. Biochem. Biophys. Res. Commun. 1998, 245, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Chua, H.H.; Lee, H.H.; Chang, S.S.; Lu, C.C.; Yeh, T.H.; Hsu, T.Y.; Cheng, T.H.; Cheng, J.T.; Chen, M.R.; Tsai, C.H. Role of the TSG101 gene in Epstein-Barr virus late gene transcription. J. Virol. 2007, 81, 2459–2471. [Google Scholar] [CrossRef]
- Ruland, J.; Sirard, C.; Elia, A.; MacPherson, D.; Wakeham, A.; Li, L.; de la Pompa, J.L.; Cohen, S.N.; Mak, T.W. p53 accumulation, defective cell proliferation, and early embryonic lethality in mice lacking tsg101. Proc. Natl. Acad. Sci. USA 2001, 98, 1859–1864. [Google Scholar] [CrossRef]
- Li, L.; Liao, J.; Ruland, J.; Mak, T.W.; Cohen, S.N. A TSG101/MDM2 regulatory loop modulates MDM2 degradation and MDM2/p53 feedback control. Proc. Natl. Acad. Sci. USA 2001, 98, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.E.; Miller, T.; Schmidt, S.L.; Shiekhattar, R.; Shilatifard, A. Cloning and characterization of the EAP30 subunit of the ELL complex that confers derepression of transcription by RNA polymerase II. J. Biol. Chem. 1999, 274, 21981–21985. [Google Scholar] [CrossRef] [PubMed]
- Kamura, T.; Burian, D.; Khalili, H.; Schmidt, S.L.; Sato, S.; Liu, W.J.; Conrad, M.N.; Conaway, R.C.; Conaway, J.W.; Shilatifard, A. Cloning and characterization of ELL-associated proteins EAP45 and EAP20. a role for yeast EAP-like proteins in regulation of gene expression by glucose. J. Biol. Chem. 2001, 276, 16528–16533. [Google Scholar] [CrossRef]
- Stauffer, D.R.; Howard, T.L.; Nyun, T.; Hollenberg, S.M. CHMP1 is a novel nuclear matrix protein affecting chromatin structure and cell-cycle progression. J. Cell Sci. 2001, 114, 2383–2393. [Google Scholar] [PubMed]
- Tsang, H.T.; Connell, J.W.; Brown, S.E.; Thompson, A.; Reid, E.; Sanderson, C.M. A systematic analysis of human CHMP protein interactions: Additional MIT domain-containing proteins bind to multiple components of the human ESCRT III complex. Genomics 2006, 88, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Saksena, M.; Denes, C.E.; Diefenbach, R.J.; Cunningham, A.L. Infection and Transport of Herpes Simplex Virus Type 1 in Neurons: Role of the Cytoskeleton. Viruses 2018, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Leuzinger, H.; Ziegler, U.; Schraner, E.M.; Fraefel, C.; Glauser, D.L.; Heid, I.; Ackermann, M.; Mueller, M.; Wild, P. Herpes simplex virus 1 envelopment follows two diverse pathways. J. Virol. 2005, 79, 13047–13059. [Google Scholar] [CrossRef]
- Draganova, E.B.; Zhang, J.; Zhou, Z.H.; Heldwein, E.E. Structural basis for capsid recruitment and coat formation during HSV-1 nuclear egress. Elife 2020, 9, e56627. [Google Scholar] [CrossRef]
- Klupp, B.G.; Granzow, H.; Mettenleiter, T.C. Nuclear envelope breakdown can substitute for primary envelopment-mediated nuclear egress of herpesviruses. J. Virol. 2011, 85, 8285–8292. [Google Scholar] [CrossRef] [PubMed]
- Lymberopoulos, M.H.; Bourget, A.; Ben Abdeljelil, N.; Pearson, A. Involvement of the UL24 protein in herpes simplex virus 1-induced dispersal of B23 and in nuclear egress. Virology 2011, 412, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Pan, S.; Zhang, L.; Baines, J.; Roller, R.; Ames, J.; Yang, M.; Wang, J.; Chen, D.; Liu, Y.; et al. Herpes Simplex Virus 1 Induces Phosphorylation and Reorganization of Lamin A/C through the gamma134.5 Protein That Facilitates Nuclear Egress. J. Virol. 2016, 90, 10414–10422. [Google Scholar] [CrossRef] [PubMed]
- Arii, J.; Watanabe, M.; Maeda, F.; Tokai-Nishizumi, N.; Chihara, T.; Miura, M.; Maruzuru, Y.; Koyanagi, N.; Kato, A.; Kawaguchi, Y. ESCRT-III mediates budding across the inner nuclear membrane and regulates its integrity. Nat. Commun. 2018, 9, 3379. [Google Scholar] [CrossRef]
- Crump, C.M.; Yates, C.; Minson, T. Herpes simplex virus type 1 cytoplasmic envelopment requires functional Vps4. J. Virol. 2007, 81, 7380–7387. [Google Scholar] [CrossRef]
- Kharkwal, H.; Smith, C.G.; Wilson, D.W. Blocking ESCRT-mediated envelopment inhibits microtubule-dependent trafficking of alphaherpesviruses in vitro. J. Virol. 2014, 88, 14467–14478. [Google Scholar] [CrossRef]
- Kharkwal, H.; Smith, C.G.; Wilson, D.W. Herpes Simplex Virus Capsid Localization to ESCRT-VPS4 Complexes in the Presence and Absence of the Large Tegument Protein UL36p. J. Virol. 2016, 90, 7257–7267. [Google Scholar] [CrossRef]
- Reynolds, A.E.; Ryckman, B.J.; Baines, J.D.; Zhou, Y.; Liang, L.; Roller, R.J. U(L)31 and U(L)34 proteins of herpes simplex virus type 1 form a complex that accumulates at the nuclear rim and is required for envelopment of nucleocapsids. J. Virol. 2001, 75, 8803–8817. [Google Scholar] [CrossRef] [PubMed]
- Pawliczek, T.; Crump, C.M. Herpes simplex virus type 1 production requires a functional ESCRT-III complex but is independent of TSG101 and ALIX expression. J. Virol. 2009, 83, 11254–11264. [Google Scholar] [CrossRef] [PubMed]
- English, L.; Chemali, M.; Duron, J.; Rondeau, C.; Laplante, A.; Gingras, D.; Alexander, D.; Leib, D.; Norbury, C.; Lippe, R.; et al. Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat. Immunol. 2009, 10, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Radtke, K.; English, L.; Rondeau, C.; Leib, D.; Lippe, R.; Desjardins, M. Inhibition of the host translation shutoff response by herpes simplex virus 1 triggers nuclear envelope-derived autophagy. J. Virol. 2013, 87, 3990–3997. [Google Scholar] [CrossRef]
- Turan, A.; Grosche, L.; Krawczyk, A.; Muhl-Zurbes, P.; Drassner, C.; Duthorn, A.; Kummer, M.; Hasenberg, M.; Voortmann, S.; Jastrow, H.; et al. Autophagic degradation of lamins facilitates the nuclear egress of herpes simplex virus type 1. J. Cell Biol. 2019, 218, 508–523. [Google Scholar] [CrossRef]
- Owen, D.J.; Crump, C.M.; Graham, S.C. Tegument Assembly and Secondary Envelopment of Alphaherpesviruses. Viruses 2015, 7, 5084–5114. [Google Scholar] [CrossRef] [PubMed]
- Ventimiglia, L.N.; Martin-Serrano, J. ESCRT machinery: Damage control at the nuclear membrane. Cell Res. 2016, 26, 641–642. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, C.P.; Liu, P.T.; Kung, H.N.; Su, M.T.; Chua, H.H.; Chang, Y.H.; Chang, C.W.; Tsai, C.H.; Liu, F.T.; Chen, M.R. The ESCRT machinery is recruited by the viral BFRF1 protein to the nucleus-associated membrane for the maturation of Epstein-Barr Virus. PLoS Pathog. 2012, 8, e1002904. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.P.; Liu, G.T.; Kung, H.N.; Liu, P.T.; Liao, Y.T.; Chow, L.P.; Chang, L.S.; Chang, Y.H.; Chang, C.W.; Shu, W.C.; et al. The Ubiquitin Ligase Itch and Ubiquitination Regulate BFRF1-Mediated Nuclear Envelope Modification for Epstein-Barr Virus Maturation. J. Virol. 2016, 90, 8994–9007. [Google Scholar] [CrossRef]
- Granzow, H.; Klupp, B.G.; Fuchs, W.; Veits, J.; Osterrieder, N.; Mettenleiter, T.C. Egress of alphaherpesviruses: Comparative ultrastructural study. J. Virol. 2001, 75, 3675–3684. [Google Scholar] [CrossRef]
- van Genderen, I.L.; Brandimarti, R.; Torrisi, M.R.; Campadelli, G.; van Meer, G. The phospholipid composition of extracellular herpes simplex virions differs from that of host cell nuclei. Virology 1994, 200, 831–836. [Google Scholar] [CrossRef]
- Hollinshead, M.; Johns, H.L.; Sayers, C.L.; Gonzalez-Lopez, C.; Smith, G.L.; Elliott, G. Endocytic tubules regulated by Rab GTPases 5 and 11 are used for envelopment of herpes simplex virus. EMBO J. 2012, 31, 4204–4220. [Google Scholar] [CrossRef] [PubMed]
- Schipke, J.; Pohlmann, A.; Diestel, R.; Binz, A.; Rudolph, K.; Nagel, C.H.; Bauerfeind, R.; Sodeik, B. The C terminus of the large tegument protein pUL36 contains multiple capsid binding sites that function differently during assembly and cell entry of herpes simplex virus. J. Virol. 2012, 86, 3682–3700. [Google Scholar] [CrossRef]
- Leis, J.; Luan, C.H.; Audia, J.E.; Dunne, S.F.; Heath, C.M. Ilaprazole and other novel prazole-based compounds that bind Tsg101 inhibit viral budding of HSV-1/2 and HIV from cells. BioRxiv 2020. [Google Scholar] [CrossRef]
- Kattenhorn, L.M.; Korbel, G.A.; Kessler, B.M.; Spooner, E.; Ploegh, H.L. A deubiquitinating enzyme encoded by HSV-1 belongs to a family of cysteine proteases that is conserved across the family Herpesviridae. Mol. Cell 2005, 19, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Schlieker, C.; Korbel, G.A.; Kattenhorn, L.M.; Ploegh, H.L. A deubiquitinating activity is conserved in the large tegument protein of the herpesviridae. J. Virol. 2005, 79, 15582–15585. [Google Scholar] [CrossRef]
- Bolstad, M.; Abaitua, F.; Crump, C.M.; O’Hare, P. Autocatalytic activity of the ubiquitin-specific protease domain of herpes simplex virus 1 VP1-2. J. Virol. 2011, 85, 8738–8751. [Google Scholar] [CrossRef] [PubMed]
- Butt, B.G.; Owen, D.J.; Jeffries, C.M.; Ivanova, L.; Hill, C.H.; Houghton, J.W.; Ahmed, M.F.; Antrobus, R.; Svergun, D.I.; Welch, J.J.; et al. Insights into herpesvirus assembly from the structure of the pUL7:pUL51 complex. Elife 2020, 9, e53789. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).