Establishment of Human Leukocyte Antigen-Mismatched Immune Responses after Transplantation of Human Liver Bud in Humanized Mouse Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiment
2.2. NOG-HLA-A2Tg Generation
2.3. Transplant Human Hematopoietic Stem Cell (HSC) to NOG-HLA-A2Tg
2.4. Human-Induced Pluripotent Stem Cell Culture and Liver Bud Generation
2.5. Immuofluorescence
2.6. Tacrolimus Administrating
2.7. Mouse Transplantation Procedure
2.8. Human Albumin Concentration in Mice Blood
2.9. Histological Analysis
2.10. Analysis
3. Results
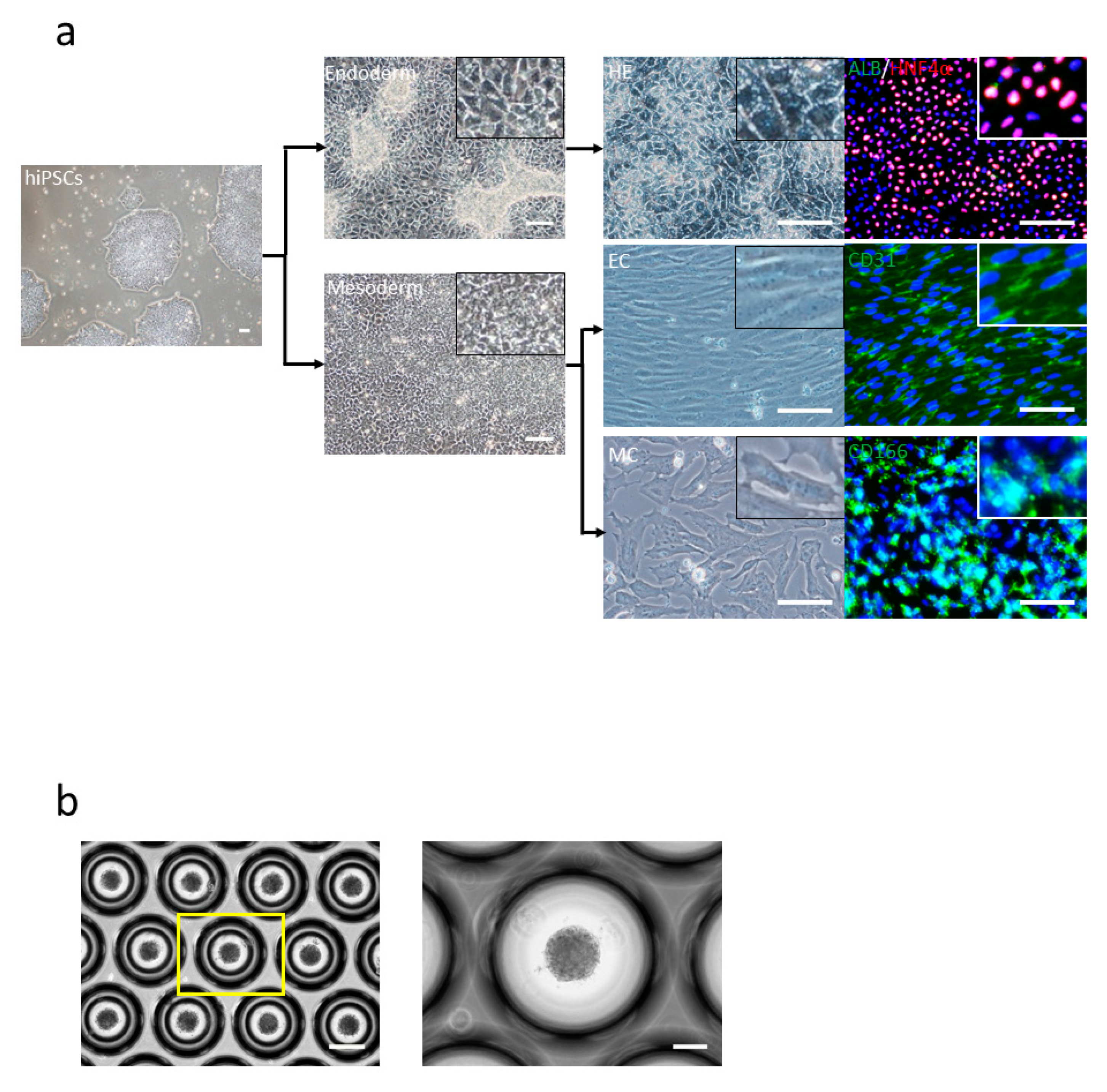
3.1. hiPSC-Derived Liver Bud Generation
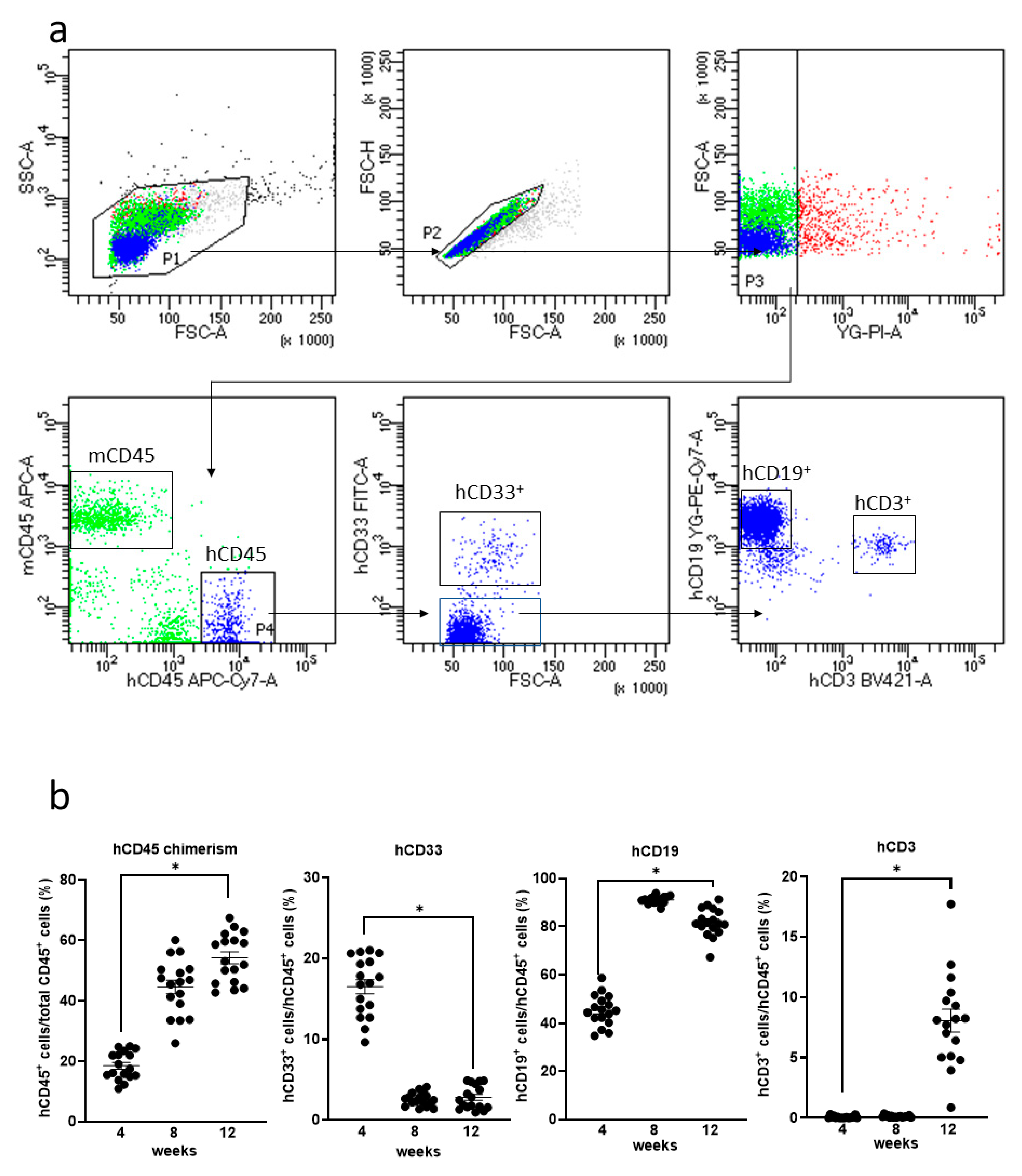
3.2. Generation of Humanized Mice
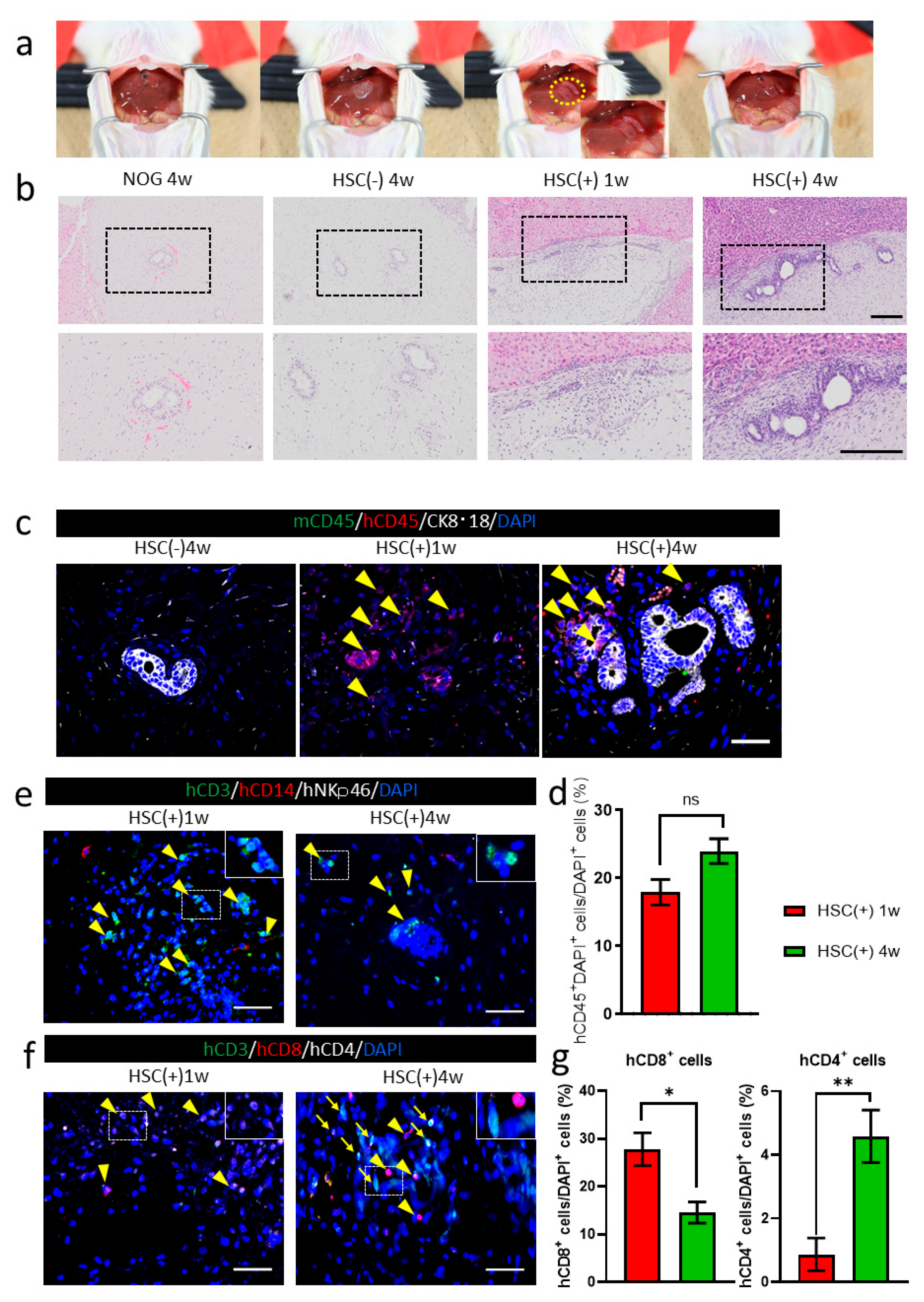
3.3. Allograft Rejection after hiPSC-Derived Liver Bud Transplantation to the NOG-HLA-A2Tg Mice
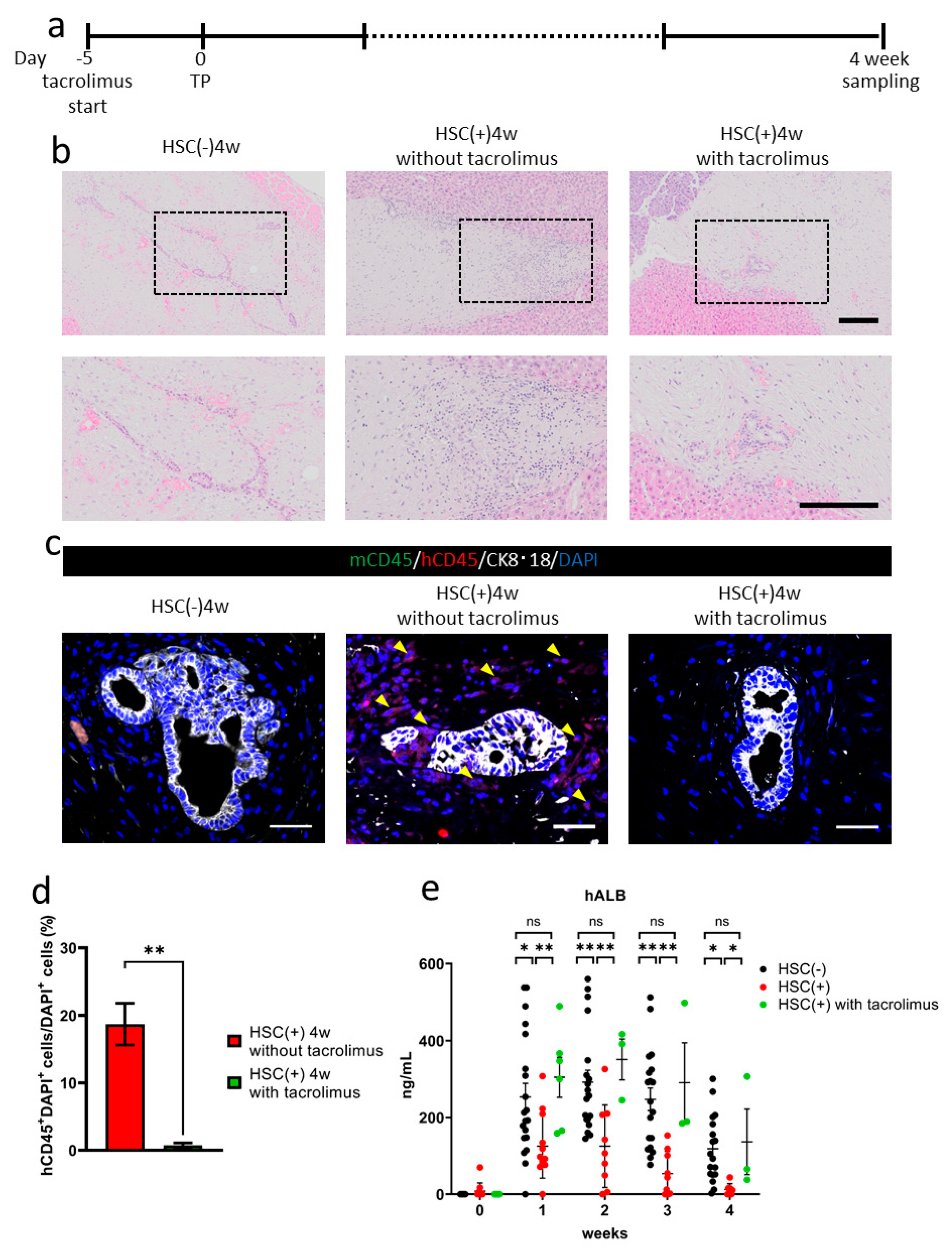
3.4. Suppression of Allograft Rejection by Administrating Tacrolimus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nadalin, S.; Capobianco, I.; Panaro, F.; Di Francesco, F.; Troisi, R.; Sainz-Barriga, M.; Muiesan, P.; Königsrainer, A.; Testa, G. Living Donor Liver Transplantation in Europe. Hepatobiliary Surg. Nutr. 2016, 5, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Alwahsh, S.M.; Rashidi, H.; Hay, D.C. Liver Cell Therapy: Is This the End of the Beginning? Cell. Mol. Life Sci. 2018, 75, 1307–1324. [Google Scholar] [CrossRef] [PubMed]
- Fox, I.J.; Chowdhury, J.R. Hepatocyte Transplantation. Am. J. Transplant. 2004, 4, 7–13. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Souied, E.; Pulido, J.; Staurenghi, G. Autologous Induced Stem-Cell–Derived Retinal Cells for Macular Degeneration. N. Engl. J. Med. 2017, 792. [Google Scholar] [CrossRef]
- Kawamura, T.; Miyagawa, S.; Fukushima, S.; Maeda, A.; Kashiyama, N.; Kawamura, A.; Miki, K.; Okita, K.; Yoshida, Y.; Shiina, T.; et al. Cardiomyocytes Derived from MHC-Homozygous Induced Pluripotent Stem Cells Exhibit Reduced Allogeneic Immunogenicity in MHC-Matched Non-Human Primates. Stem Cell Rep. 2016, 6, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Hermanto, Y.; Maki, T.; Takagi, Y.; Miyamoto, S.; Takahashi, J. Xeno-Free Culture for Generation of Forebrain Oligodendrocyte Precursor Cells from Human Pluripotent Stem Cells. J. Neurosci. Res. 2019, 97, 828–845. [Google Scholar] [CrossRef]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.J.; Van Es, J.H.; Van Den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-Term Expansion of Epithelial Organoids from Human Colon, Adenoma, Adenocarcinoma, and Barrett’s Epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef]
- Quadrato, G.; Nguyen, T.; Macosko, E.Z.; Sherwood, J.L.; Yang, S.M.; Berger, D.R.; Maria, N.; Scholvin, J.; Goldman, M.; Kinney, J.P.; et al. Cell Diversity and Network Dynamics in Photosensitive Human Brain Organoids. Nature 2017, 545, 48–53. [Google Scholar] [CrossRef]
- Lee, J.; Rabbani, C.; Gao, H.; Steinhart, M.; Woodruff, B.M.; Pflum, Z.; Kim, A.; Heller, S.; Liu, Y.; Shipchandler, T.Z.; et al. Hair-Bearing Human Skin Generated Entirely from Pluripotent Stem Cells. bioRxiv 2019, 684282. [Google Scholar] [CrossRef]
- Kajiwara, M.; Aoi, T.; Okita, K.; Takahashi, R.; Inoue, H.; Takayama, N. Correction for Kajiwara et al., Donor-Dependent Variations in Hepatic Differentiation from Human-Induced Pluripotent Stem Cells. Proc. Natl. Acad. Sci. USA 2012, 109, 14716. [Google Scholar] [CrossRef] [PubMed]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.R.; Ueno, Y.; Zheng, Y.W.; Koike, N.; et al. Vascularized and Functional Human Liver from an IPSC-Derived Organ Bud Transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef]
- Takebe, T.; Sekine, K.; Kimura, M.; Yoshizawa, E.; Ayano, S.; Koido, M.; Funayama, S.; Nakanishi, N.; Hisai, T.; Kobayashi, T.; et al. Massive and Reproducible Production of Liver Buds Entirely from Human Pluripotent Stem Cells. Cell Rep. 2017, 21, 2661–2670. [Google Scholar] [CrossRef] [PubMed]
- Camp, J.G.; Sekine, K.; Gerber, T.; Loeffler-Wirth, H.; Binder, H.; Gac, M.; Kanton, S.; Kageyama, J.; Damm, G.; Seehofer, D.; et al. Multilineage Communication Regulates Human Liver Bud Development from Pluripotency. Nature 2017, 546, 533–538. [Google Scholar] [CrossRef]
- Valujskikh, A.; Matesic, D.; Gilliam, A.; Anthony, D.; Haqqi, T.M.; Heeger, P.S. T Cells Reactive to a Single Immunodominant Self-Restricted Allopeptide Induce Skin Graft Rejection in Mice. J. Clin. Investig. 1998, 101, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Morizane, A.; Kikuchi, T.; Hayashi, T.; Mizuma, H.; Takara, S.; Doi, H.; Mawatari, A.; Glasser, M.F.; Shiina, T.; Ishigaki, H.; et al. MHC Matching Improves Engraftment of IPSC-Derived Neurons in Non-Human Primates. Nat. Commun. 2017, 8, 385. [Google Scholar] [CrossRef]
- Ichise, H.; Nagano, S.; Maeda, T.; Miyazaki, M.; Miyazaki, Y.; Kojima, H.; Yawata, N.; Yawata, M.; Tanaka, H.; Saji, H.; et al. NK Cell Alloreactivity against KIR-Ligand-Mismatched HLA-Haploidentical Tissue Derived from HLA Haplotype-Homozygous IPSCs. Stem Cell Rep. 2017, 9, 853–867. [Google Scholar] [CrossRef]
- Xu, H.; Wang, B.; Ono, M.; Kagita, A.; Fujii, K.; Sasakawa, N.; Ueda, T.; Gee, P.; Nishikawa, M.; Nomura, M.; et al. Targeted Disruption of HLA Genes via CRISPR-Cas9 Generates IPSCs with Enhanced Immune Compatibility. Cell Stem Cell 2019, 24, 566–578.e7. [Google Scholar] [CrossRef] [PubMed]
- Kenney, L.L.; Shultz, L.D.; Greiner, D.L.; Brehm, M.A. Humanized Mouse Models for Transplant Immunology. Am. J. Transplant. 2016, 16, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.C.; Kenney, L.L.; Jangalwe, S.; Aryee, K.E.; Greiner, D.L.; Brehm, M.A. Humanized Mouse Models of Clinical Disease. Annu. Rev. Pathol. 2017, 12, 187–215. [Google Scholar] [CrossRef]
- Okita, K.; Matsumura, Y.; Sato, Y.; Okada, A.; Morizane, A.; Okamoto, S.; Hong, H.; Nakagawa, M.; Tanabe, K.; Tezuka, K.I.; et al. A More Efficient Method to Generate Integration-Free Human IPS Cells. Nat. Methods 2011, 8, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Mehler, V.J.; Burns, C.; Moore, M.L. Concise Review: Exploring Immunomodulatory Features of Mesenchymal Stromal Cells in Humanized Mouse Models. Stem Cells 2019, 37, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Shultz, L.D.; Saito, Y.; Najima, Y.; Tanaka, S.; Ochi, T.; Tomizawa, M.; Doi, T.; Sone, A.; Suzuki, N.; Fujiwara, H.; et al. Generation of Functional Human T-Cell Subsets with HLA-Restricted Immune Responses in HLA Class I Expressing NOD/SCID/IL2rγnull Humanized Mice. Proc. Natl. Acad. Sci. USA 2010, 107, 13022–13027. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, A.; Marchesini, D.; Furze, J.; Sherman, L.A.; Chesnut, R.W. Analysis of the HLA-Restricted Influenza-Specific Cytotoxic T Lymphocyte Response in Transgenic Mice Carrying a Chimeric Human-Mouse Class I Major Histocompatibility Complex. J. Exp. Med. 1991, 173, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Murata, S.; Matsuki, K.; Mori, A. The Regenerative Effect of Portal Vein Injection of Liver Organoids by Retrorsine/Partial Hepatectomy in Rats. Int. J. Mol. Sci. 2019, 21, 178. [Google Scholar] [CrossRef]
- Qiu, R.; Murata, S.; Oshiro, K.; Hatada, Y.; Taniguchi, H. Transplantation of Fetal Liver Tissue Coated by Ultra-Purified Alginate Gel over Liver Improves Hepatic Function in the Cirrhosis Rat Model. Sci. Rep. 2020, 10, 4–6. [Google Scholar] [CrossRef]
| Antibodies | Source | Product Number |
|---|---|---|
| APC/Cyanine7 anti-human CD45 Antibody | Biolegend, San Diego, CA, USA | 304014 |
| APC anti-mouse CD45 Antibody | Biolegend, San Diego, CA, USA | 103112 |
| PE anti-human CD3 Antibody | Biolegend, San Diego, CA, USA | 300308 |
| APC anti-human CD19 Antibody FITC anti-human CD33 Antibody Albumin Antibody | Biolegend, San Diego, CA, USA Biolegend, San Diego, CA, USA Novus, E Briarwood Ave, Centennial, CO, USA | 363006 303304 NBP1-32458 |
| Anti-CD3 antibody (ab828) | Abcam, Cambridge, UK | ab828 |
| Human CD4 Antibody | R&D, Minneapolis, MN, USA | AF-379 |
| Human CD8 alpha Antibody | R&D, Minneapolis, MN, USA | MAB3801 |
| CD14 Monoclonal Antibody(5A3) | Invitrogen, Waltham, MA, USA | MA5-14773 |
| Anti-Human CD31, Endothelial Cell | Dako, Santa Clara, CA, USA | M0823 |
| Human CD45 Antibody | R&D, Minneapolis, MN, USA | MAB1430 |
| Anti-CD45 antibody [I3/2.3] | Abcam, Cambridge, UK | ab25386 |
| Anti-CD166 antibody [ERP2759(2)] ab109215 | Abcam, Cambridge, UK | ab109215 |
| HumanNKp46/NCR1 Antibody | R&D, Minneapolis, MN, USA | MAB1850 |
| Pab to Keratins K8/K18 | Progen, Heidelberg, Germany | GP11 |
| HNF4A Monoclonal Antibody | Thermo Fisher, Waltham, MA, USA | MA1-199 |
| Donkey anti-Rat IgG (H + L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 | Invitrogen, Waltham, MA, USA | A-21208 |
| Donkey anti-Rabbit IgG (H + L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 | Invitrogen, Waltham, MA, USA | A-21206 |
| Goat anti-Mouse IgG2a Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 | Invitrogen, Waltham, MA, USA | A-21131 |
| Donkey anti-Mouse IgG (H + L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 555 | Invitrogen, Waltham, MA, USA | A-31570 |
| Goat anti-Mouse IgG1 Cross-Adsorbed Secondary Antibody, Alexa Fluor 555 | Invitrogen, Waltham, MA, USA | A-21127 |
| Donkey anti-Goat IgG (H + L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 647 | Invitrogen, Waltham, MA, USA | A-21447 |
| Donkey anti-Rabbit IgG (H + L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 647 | Invitrogen, Waltham, MA, USA | A-31573 |
| Goat anti-Mouse IgG2b Cross-Adsorbed Secondary Antibody, Alexa Fluor 647 | Invitrogen, Waltham, MA, USA | A-21242 |
| Goat anti-Guinea Pig IgG (H + L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 647 | Invitrogen, Waltham, MA, USA | A-21450 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mori, A.; Murata, S.; Tashiro, N.; Tadokoro, T.; Okamoto, S.; Otsuka, R.; Wada, H.; Murata, T.; Takahashi, T.; Seino, K.-i.; et al. Establishment of Human Leukocyte Antigen-Mismatched Immune Responses after Transplantation of Human Liver Bud in Humanized Mouse Models. Cells 2021, 10, 476. https://doi.org/10.3390/cells10020476
Mori A, Murata S, Tashiro N, Tadokoro T, Okamoto S, Otsuka R, Wada H, Murata T, Takahashi T, Seino K-i, et al. Establishment of Human Leukocyte Antigen-Mismatched Immune Responses after Transplantation of Human Liver Bud in Humanized Mouse Models. Cells. 2021; 10(2):476. https://doi.org/10.3390/cells10020476
Chicago/Turabian StyleMori, Akihiro, Soichiro Murata, Nao Tashiro, Tomomi Tadokoro, Satoshi Okamoto, Ryo Otsuka, Haruka Wada, Tomoki Murata, Takeshi Takahashi, Ken-ichiro Seino, and et al. 2021. "Establishment of Human Leukocyte Antigen-Mismatched Immune Responses after Transplantation of Human Liver Bud in Humanized Mouse Models" Cells 10, no. 2: 476. https://doi.org/10.3390/cells10020476
APA StyleMori, A., Murata, S., Tashiro, N., Tadokoro, T., Okamoto, S., Otsuka, R., Wada, H., Murata, T., Takahashi, T., Seino, K.-i., & Taniguchi, H. (2021). Establishment of Human Leukocyte Antigen-Mismatched Immune Responses after Transplantation of Human Liver Bud in Humanized Mouse Models. Cells, 10(2), 476. https://doi.org/10.3390/cells10020476






