Immune-Mediated Retinal Vasculitis in Posterior Uveitis and Experimental Models: The Leukotriene (LT)B4-VEGF Axis
Abstract
1. Introduction
2. Retinal Vasculitis in Non-Infectious Intraocular Inflammatory Disease: Uveitis
3. Inflammatory Mediators Involved in Retinal Vasculitis
4. Cytokines in Retinal Vasculitis
5. Neovascularisation in Inflammatory Conditions
6. Retinal Damage Associated with Disease Severity
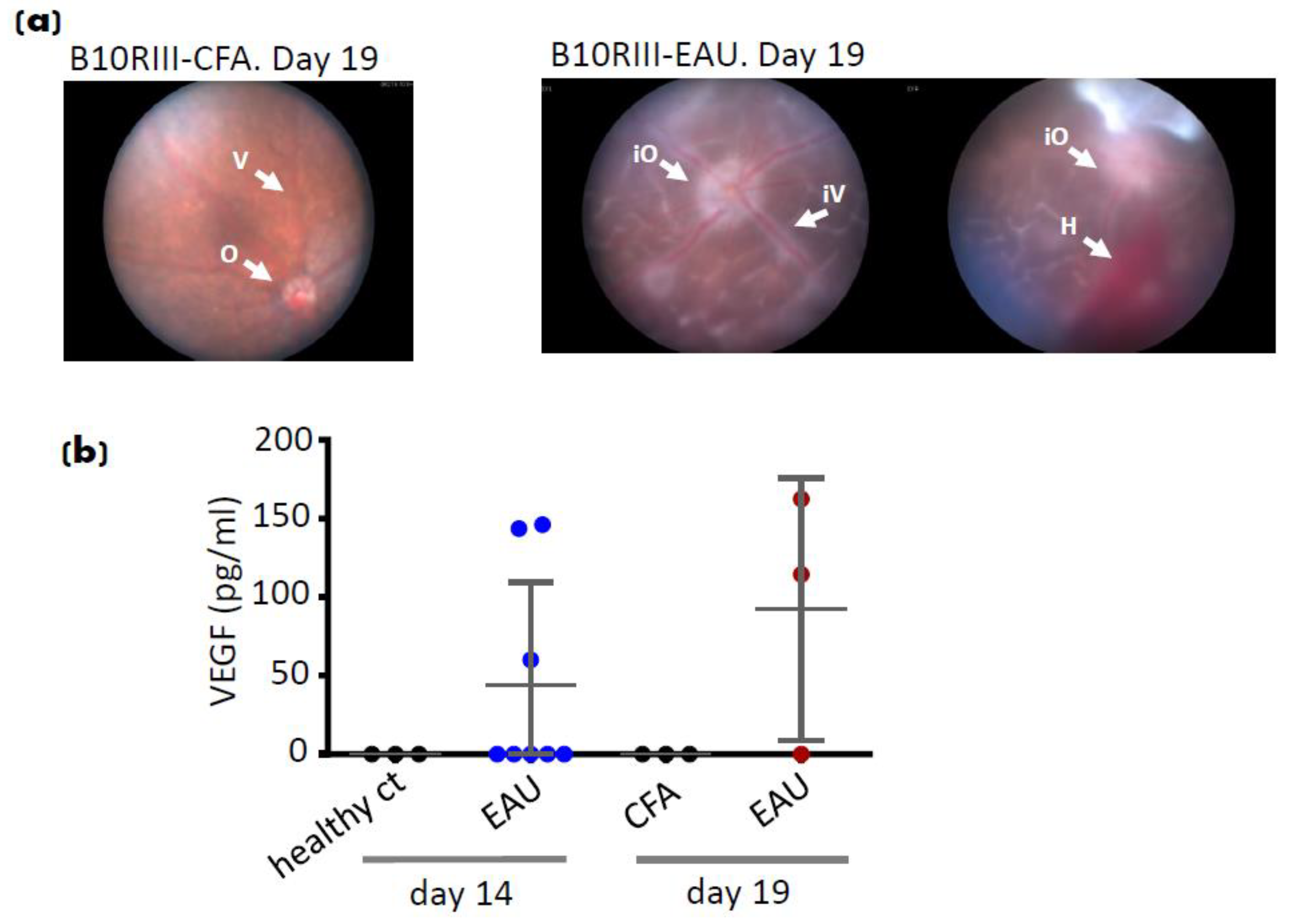
7. EAU Angiogenesis
8. Potential Treatments for Retinal Vasculitis by Targeting Angiogenic Pathways
9. Lipid Mediators in Retinal Vasculitis
10. Leukotriene–Cytokine Associations in Retinal Vasculitis
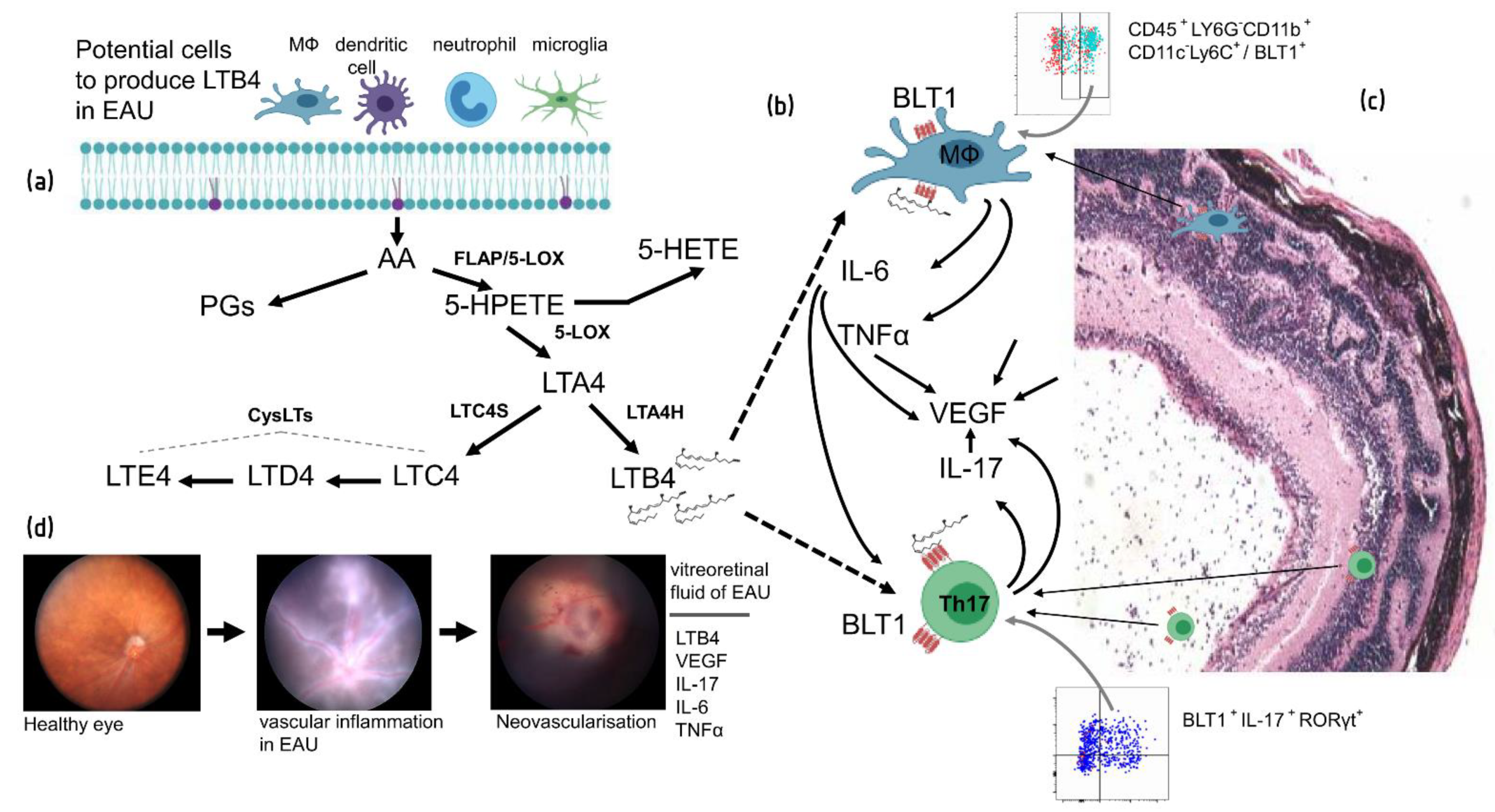
11. Role of LTB4 Pathway in EAU and Uveitis
12. Potential Treatment Approaches in Targeting LT Pathways in Retinal Vasculitis
13. Downregulation of VEGF by an LTB4 Inhibitor (Nomacopan)
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| AA | arachidonic acid |
| ABIN2 | A20 binding inhibitor of NF-κB 2 |
| AMD | age-related macular degradation |
| Ang | Angiopoietin |
| BD | Behçet’s disease |
| CNV | choroidal neovascularisation |
| COPD | chronic obstructive pulmonary disease |
| CXCL8 | c-x-c motif chemokine ligand 8 |
| CysLT | cysteinyl LT |
| EAU | Experimental Autoimmune Uveitis |
| FLAP | 5-LOX activating protein |
| JAK | Janus kinase |
| LM | Lipid mediator |
| LT | Leukotriene |
| MAPK | mitogen-activated protein kinase |
| MCP-1 | monocyte chemoattractant protein-1 |
| NIU | non-infectious uveitis |
| NOX2 | NADPH oxidase 2 |
| OIR | oxyen-induced retinopathy |
| PGE | prostaglandin |
| RGD | Arg-Gly-Asp |
| RPE | retinal pigment epithelium |
| SAg | retinal soluble (S) antigen |
| SPM | specialised pro-resolving mediator |
| STAT | signal transducer and activator of transcription |
| Tie | Tyrosine kinase with immunoglobulin-like and EGF-like domains |
| TNFα | tumour necrosis factor-alpha |
| VCAM | vascular adhesion molecule |
| VEGF | vascular endothelial growth factor |
References
- Lee, R.W.; Nicholson, L.B.; Sen, H.N.; Chan, C.C.; Wei, L.; Nussenblatt, R.B.; Dick, A.D. Autoimmune and autoinflammatory mechanisms in uveitis. Semin. Immunopathol. 2014, 36, 581–594. [Google Scholar] [CrossRef]
- Dick, A.D. Doyne lecture 2016: Intraocular health and the many faces of inflammation. Eye 2017, 31, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Merida, S.; Palacios, E.; Navea, A.; Bosch-Morell, F. New Immunosuppressive Therapies in Uveitis Treatment. Int. J. Mol. Sci. 2015, 16, 18778–18795. [Google Scholar] [CrossRef]
- LaMattina, K.C.; Goldstein, D.A. Adalimumab for the treatment of uveitis. Expert Rev. Clin. Immunol. 2017, 13, 181–188. [Google Scholar] [CrossRef]
- Agarwal, R.K.; Silver, P.B.; Caspi, R.R. Rodent models of experimental autoimmune uveitis. Methods Mol. Biol. 2012, 900, 443–469. [Google Scholar] [CrossRef]
- Caspi, R.R.; Silver, P.B.; Luger, D.; Tang, J.; Cortes, L.M.; Pennesi, G.; Mattapallil, M.J.; Chan, C.C. Mouse models of experimental autoimmune uveitis. Ophthalmic Res. 2008, 40, 169–174. [Google Scholar] [CrossRef]
- Caspi, R.R.; Roberge, F.G.; Chan, C.C.; Wiggert, B.; Chader, G.J.; Rozenszajn, L.A.; Lando, Z.; Nussenblatt, R.B. A new model of autoimmune disease. Experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. J. Immunol. 1988, 140, 1490–1495. [Google Scholar]
- Patel, A.K.; Newcomb, C.W.; Liesegang, T.L.; Pujari, S.S.; Suhler, E.B.; Thorne, J.E.; Foster, C.S.; Jabs, D.A.; Levy-Clarke, G.A.; Nussenblatt, R.B.; et al. Risk of Retinal Neovascularization in Cases of Uveitis. Ophthalmology 2016, 123, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Graham, E.M.; Stanford, M.R.; Shilling, J.S.; Sanders, M.D. Neovascularisation associated with posterior uveitis. Br. J. Ophthalmol. 1987, 71, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Storkebaum, E.; Carmeliet, P. VEGF: A critical player in neurodegeneration. J. Clin. Investig. 2004, 113, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Weiss, K.; Steinbrugger, I.; Weger, M.; Ardjomand, N.; Maier, R.; Wegscheider, B.J.; Wedrich, A.; El-Shabrawi, Y. Intravitreal VEGF levels in uveitis patients and treatment of uveitic macular oedema with intravitreal bevacizumab. Eye 2009, 23, 1812–1818. [Google Scholar] [CrossRef]
- Vinores, S.A.; Chan, C.C.; Vinores, M.A.; Matteson, D.M.; Chen, Y.S.; Klein, D.A.; Shi, A.; Ozaki, H.; Campochiaro, P.A. Increased vascular endothelial growth factor (VEGF) and transforming growth factor beta (TGFbeta) in experimental autoimmune uveoretinitis: Upregulation of VEGF without neovascularization. J. Neuroimmunol. 1998, 89, 43–50. [Google Scholar] [CrossRef]
- Fine, H.F.; Baffi, J.; Reed, G.F.; Csaky, K.G.; Nussenblatt, R.B. Aqueous humor and plasma vascular endothelial growth factor in uveitis-associated cystoid macular edema. Am. J. Ophthalmol. 2001, 132, 794–796. [Google Scholar] [CrossRef]
- Mackensen, F.; Heinz, C.; Becker, M.D.; Heiligenhaus, A. Intravitreal bevacizumab (avastin) as a treatment for refractory macular edema in patients with uveitis: A pilot study. Retina 2008, 28, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Acharya, N.R.; Hong, K.C.; Lee, S.M. Ranibizumab for refractory uveitis-related macular edema. Am. J. Ophthalmol. 2009, 148, 303–309.e302. [Google Scholar] [CrossRef]
- Vinores, S.A.; Kuchle, M.; Mahlow, J.; Chiu, C.; Green, W.R.; Campochiaro, P.A. Blood-ocular barrier breakdown in eyes with ocular melanoma. A potential role for vascular endothelial growth factor/vascular permeability factor. Am. J. Pathol. 1995, 147, 1289–1297. [Google Scholar]
- Duah, E.; Teegala, L.R.; Kondeti, V.; Adapala, R.K.; Keshamouni, V.G.; Kanaoka, Y.; Austen, K.F.; Thodeti, C.K.; Paruchuri, S. Cysteinyl leukotriene 2 receptor promotes endothelial permeability, tumor angiogenesis, and metastasis. Proc. Natl. Acad. Sci. USA 2019, 116, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.J.; Park, J.; Um, J.Y.; Lee, S.S.; Kwak, H.J. Zileuton, a 5-Lipoxygenase Inhibitor, Exerts Anti-Angiogenic Effect by Inducing Apoptosis of HUVEC via BK Channel Activation. Cells 2019, 8, 1182. [Google Scholar] [CrossRef]
- Sasaki, F.; Yokomizo, T. The leukotriene receptors as therapeutic targets of inflammatory diseases. Int. Immunol. 2019, 31, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.; Ke, Y.; Shao, W.H.; Haribabu, B.; Kaplan, H.J.; Sun, D.; Shao, H. Blockade of the interaction of leukotriene b4 with its receptor prevents development of autoimmune uveitis. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Eskandarpour, M.; Chen, Y.H.; Nunn, M.A.; Coupland, S.E.; Weston-Davies, W.; Calder, V.L. Leukotriene B4 and Its Receptor in Experimental Autoimmune Uveitis and in Human Retinal Tissues: Clinical Severity and LTB4 Dependence of Retinal Th17 Cells. Am. J. Pathol. 2020. [Google Scholar] [CrossRef]
- Zhang, H.; Watanabe, R.; Berry, G.J.; Vaglio, A.; Liao, Y.J.; Warrington, K.J.; Goronzy, J.J.; Weyand, C.M. Immunoinhibitory checkpoint deficiency in medium and large vessel vasculitis. Proc. Natl. Acad. Sci. USA 2017, 114, E970–E979. [Google Scholar] [CrossRef] [PubMed]
- Peleg, H.; Ben-Chetrit, E. Vasculitis in the autoinflammatory diseases. Curr. Opin. Rheumatol. 2017, 29, 4–11. [Google Scholar] [CrossRef]
- Chiu, H.H.; Wang, S.S.; Wu, M.H.; Wang, J.K. Aortitis with severe aortic regurgitation in Behcet’s disease: A case report. J. Formos. Med. Assoc. 2010, 109, 82–84. [Google Scholar] [CrossRef][Green Version]
- Rosenbaum, J.T.; Sibley, C.H.; Lin, P. Retinal vasculitis. Curr. Opin. Rheumatol. 2016, 28, 228–235. [Google Scholar] [CrossRef]
- Harmanci, K.; Akan, O.Y.; Pirildar, T.; Ortan, P.; Ulman, C. The Evaluation of the Relationship between sTREM-1, VEGF-B, and VEGF Gene Expression Levels with Disease Activity of Behcet’s Patients. Dis. Markers 2018, 2018, 2649392. [Google Scholar] [CrossRef]
- Cekmen, M.; Evereklioglu, C.; Er, H.; Inaloz, H.S.; Doganay, S.; Turkoz, Y.; Ozerol, I.H. Vascular endothelial growth factor levels are increased and associated with disease activity in patients with Behcet’s syndrome. Int. J. Dermatol. 2003, 42, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Abu El-Asrar, A.M.; Herbort, C.P.; Tabbara, K.F. Differential diagnosis of retinal vasculitis. Middle East. Afr. J. Ophthalmol. 2009, 16, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ku, J.H.; Suhler, E.B.; Choi, D.; Rosenbaum, J.T. The course of retinal vasculitis. Br. J. Ophthalmol. 2014, 98, 785–789. [Google Scholar] [CrossRef]
- Talat, L.; Lightman, S.; Tomkins-Netzer, O. Ischemic retinal vasculitis and its management. J. Ophthalmol. 2014, 2014, 197675. [Google Scholar] [CrossRef]
- Ekinci, N.S.; Alpsoy, E.; Karakas, A.A.; Yilmaz, S.B.; Yegin, O. IL-17A has an important role in the acute attacks of Behcet’s disease. J. Investig. Dermatol. 2010, 130, 2136–2138. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, J.A.; Lee, E.Y.; Lee, Y.J.; Song, Y.W.; Lee, E.B. Imbalance of Th17 to Th1 cells in Behcet’s disease. Clin. Exp. Rheumatol. 2010, 28, S16–S19. [Google Scholar] [PubMed]
- Chi, W.; Zhu, X.; Yang, P.; Liu, X.; Lin, X.; Zhou, H.; Huang, X.; Kijlstra, A. Upregulated IL-23 and IL-17 in Behcet patients with active uveitis. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3058–3064. [Google Scholar] [CrossRef] [PubMed]
- Ooi, K.G.; Galatowicz, G.; Calder, V.L.; Lightman, S.L. Cytokines and chemokines in uveitis: Is there a correlation with clinical phenotype? Clin. Med. Res. 2006, 4, 294–309. [Google Scholar] [CrossRef]
- Penn, J.S.; Madan, A.; Caldwell, R.B.; Bartoli, M.; Caldwell, R.W.; Hartnett, M.E. Vascular endothelial growth factor in eye disease. Prog. Retin. Eye Res. 2008, 27, 331–371. [Google Scholar] [CrossRef]
- Forrester, J.V.; Kuffova, L.; Dick, A.D. Autoimmunity, Autoinflammation, and Infection in Uveitis. Am. J. Ophthalmol. 2018, 189, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, A.G.; Iruela-Arispe, M.L. Extracellular matrix, inflammation, and the angiogenic response. Cardiovasc Res. 2010, 86, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Chen, D.; Yu, L.; Dai, X.; Yang, Y.; Tian, W.; Cheng, X.; Xu, H.; Weng, X.; Fang, M.; et al. Proinflammatory stimuli engage Brahma related gene 1 and Brahma in endothelial injury. Circ. Res. 2013, 113, 986–996. [Google Scholar] [CrossRef]
- Eskandarpour, M.; Alexander, R.; Adamson, P.; Calder, V.L. Pharmacological Inhibition of Bromodomain Proteins Suppresses Retinal Inflammatory Disease and Downregulates Retinal Th17 Cells. J. Immunol. 2017, 198, 1093–1103. [Google Scholar] [CrossRef]
- Gilbert, R.M.; Zhang, X.; Sampson, R.D.; Ehrenstein, M.R.; Nguyen, D.X.; Chaudhry, M.; Mein, C.; Mahmud, N.; Galatowicz, G.; Tomkins-Netzer, O.; et al. Clinical Remission of Sight-Threatening Non-Infectious Uveitis Is Characterized by an Upregulation of Peripheral T-Regulatory Cell Polarized Towards T-bet and TIGIT. Front. Immunol. 2018, 9, 907. [Google Scholar] [CrossRef]
- Nicosia, S.; Capra, V.; Rovati, G.E. Leukotrienes as mediators of asthma. Pulm. Pharm. Ther. 2001, 14, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Sutton, C.E.; Mielke, L.A.; Mills, K.H. IL-17-producing gammadelta T cells and innate lymphoid cells. Eur. J. Immunol. 2012, 42, 2221–2231. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y. Interleukin-17: The Role for Pathological Angiogenesis in Ocular Neovascular Diseases. Tohoku J. Exp. Med. 2019, 247, 87–98. [Google Scholar] [CrossRef]
- Rodrigues, M.; Xin, X.; Jee, K.; Babapoor-Farrokhran, S.; Kashiwabuchi, F.; Ma, T.; Bhutto, I.; Hassan, S.J.; Daoud, Y.; Baranano, D.; et al. VEGF secreted by hypoxic Muller cells induces MMP-2 expression and activity in endothelial cells to promote retinal neovascularization in proliferative diabetic retinopathy. Diabetes 2013, 62, 3863–3873. [Google Scholar] [CrossRef]
- Zhu, Y.; Tan, W.; Demetriades, A.M.; Cai, Y.; Gao, Y.; Sui, A.; Lu, Q.; Shen, X.; Jiang, C.; Xie, B.; et al. Interleukin-17A neutralization alleviated ocular neovascularization by promoting M2 and mitigating M1 macrophage polarization. Immunology 2016, 147, 414–428. [Google Scholar] [CrossRef]
- Yin, H.; Fang, X.; Ma, J.; Chen, M.; Yang, Y.; Guo, S.; Chen, Z.; Su, Z.; Feng, L.; Ye, P.; et al. Idiopathic Choroidal Neovascularization: Intraocular Inflammatory Cytokines and the Effect of Intravitreal Ranibizumab Treatment. Sci. Rep. 2016, 6, 31880. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.; Li, Q. Increased Th1/Th17 Responses Contribute to Low-Grade Inflammation in Age-Related Macular Degeneration. Cell Physiol. Biochem. 2017, 44, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Qiu, A.W.; Liu, Q.H.; Wang, J.L. Blocking IL-17A Alleviates Diabetic Retinopathy in Rodents. Cell Physiol. Biochem. 2017, 41, 960–972. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wu, H.; Chen, L.; Xu, J.; Wang, M.; Li, D.; Lu, P. Effects of interleukin-17 on human retinal vascular endothelial cell capillary tube formation in vitro. Mol. Med. Rep. 2017, 16, 865–872. [Google Scholar] [CrossRef]
- Talia, D.M.; Deliyanti, D.; Agrotis, A.; Wilkinson-Berka, J.L. Inhibition of the Nuclear Receptor RORgamma and Interleukin-17A Suppresses Neovascular Retinopathy: Involvement of Immunocompetent Microglia. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1186–1196. [Google Scholar] [CrossRef]
- Hasegawa, E.; Sonoda, K.H.; Shichita, T.; Morita, R.; Sekiya, T.; Kimura, A.; Oshima, Y.; Takeda, A.; Yoshimura, T.; Yoshida, S.; et al. IL-23-independent induction of IL-17 from gammadeltaT cells and innate lymphoid cells promotes experimental intraocular neovascularization. J. Immunol. 2013, 190, 1778–1787. [Google Scholar] [CrossRef] [PubMed]
- Alivand, M.R.; Sabouni, F.; Soheili, Z.S. Probable Chemical Hypoxia Effects on Progress of CNV Through Induction of Promoter CpG Demethylation and Overexpression of IL17RC in Human RPE Cells. Curr. Eye Res. 2016, 41, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Lightman, S.; Taylor, S.R.; Bunce, C.; Longhurst, H.; Lynn, W.; Moots, R.; Stanford, M.; Tomkins-Netzer, O.; Yang, D.; Calder, V.L.; et al. Pegylated interferon-alpha-2b reduces corticosteroid requirement in patients with Behcet’s disease with upregulation of circulating regulatory T cells and reduction of Th17. Ann. Rheum. Dis. 2015, 74, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Jawad, S.; Liu, B.; Agron, E.; Nussenblatt, R.B.; Sen, H.N. Elevated serum levels of interleukin-17A in uveitis patients. Ocul. Immunol. Inflamm. 2013, 21, 434–439. [Google Scholar] [CrossRef]
- El-Asrar, A.M.; Struyf, S.; Kangave, D.; Al-Obeidan, S.S.; Opdenakker, G.; Geboes, K.; Van Damme, J. Cytokine profiles in aqueous humor of patients with different clinical entities of endogenous uveitis. Clin. Immunol. 2011, 139, 177–184. [Google Scholar] [CrossRef]
- Fraga, N.A.; Oliveira Mde, F.; Follador, I.; Rocha Bde, O.; Rego, V.R. Psoriasis and uveitis: A literature review. An. Bras. Dermatol. 2012, 87, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Pepple, K.L.; Lin, P. Targeting Interleukin-23 in the Treatment of Noninfectious Uveitis. Ophthalmology 2018, 125, 1977–1983. [Google Scholar] [CrossRef]
- Lowes, M.A.; Kikuchi, T.; Fuentes-Duculan, J.; Cardinale, I.; Zaba, L.C.; Haider, A.S.; Bowman, E.P.; Krueger, J.G. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J. Investig. Dermatol. 2008, 128, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Sepah, Y.J.; Velez, G.; Tang, P.H.; Yang, J.; Chemudupati, T.; Li, A.S.; Nguyen, Q.D.; Bassuk, A.G.; Mahajan, V.B. Proteomic analysis of intermediate uveitis suggests myeloid cell recruitment and implicates IL-23 as a therapeutic target. Am. J. Ophthalmol. Case Rep. 2020, 18, 100646. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, X.; Luo, L.; Qu, B.; Huang, X.; Xu, L.; Lin, Y.; Ye, S.; Liu, Y. Elevated serum IL-23 correlates with intraocular inflammation after cataract surgery in patients with Vogt-Koyanagi-Harada disease. Br. J. Ophthalmol. 2010, 94, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Przepiera-Bedzak, H.; Fischer, K.; Brzosko, M. Extra-Articular Symptoms in Constellation with Selected Serum Cytokines and Disease Activity in Spondyloarthritis. Mediat. Inflamm. 2016, 2016, 7617954. [Google Scholar] [CrossRef] [PubMed]
- Holan, V.; Hermankova, B.; Krulova, M.; Zajicova, A. Cytokine interplay among the diseased retina, inflammatory cells and mesenchymal stem cells—A clue to stem cell-based therapy. World J. Stem Cells 2019, 11, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Neufeld, A.H. Tumor necrosis factor-alpha: A potentially neurodestructive cytokine produced by glia in the human glaucomatous optic nerve head. Glia 2000, 32, 42–50. [Google Scholar] [CrossRef]
- Ohta, K.; Yamagami, S.; Taylor, A.W.; Streilein, J.W. IL-6 antagonizes TGF-beta and abolishes immune privilege in eyes with endotoxin-induced uveitis. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2591–2599. [Google Scholar]
- Cohen, T.; Nahari, D.; Cerem, L.W.; Neufeld, G.; Levi, B.Z. Interleukin 6 induces the expression of vascular endothelial growth factor. J. Biol. Chem. 1996, 271, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.; Zhang, W.; Lee, D.L.; Romero, M.J.; Nguyen, D.T.; Al-Shabrawey, M.; Tsai, N.T.; Liou, G.I.; Brands, M.W.; Caldwell, R.W.; et al. Role of IL-6 in angiotensin II-induced retinal vascular inflammation. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1709–1718. [Google Scholar] [CrossRef]
- Duplechain, A.; Conrady, C.D.; Patel, B.C.; Baker, S. Uveitis; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Ivashkiv, L.B. IFNgamma: Signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 545–558. [Google Scholar] [CrossRef]
- Luger, D.; Silver, P.B.; Tang, J.; Cua, D.; Chen, Z.; Iwakura, Y.; Bowman, E.P.; Sgambellone, N.M.; Chan, C.C.; Caspi, R.R. Either a Th17 or a Th1 effector response can drive autoimmunity: Conditions of disease induction affect dominant effector category. J. Exp. Med. 2008, 205, 799–810. [Google Scholar] [CrossRef]
- Wildner, G.; Diedrichs-Mohring, M. Resolution of uveitis. Semin. Immunopathol. 2019, 41, 727–736. [Google Scholar] [CrossRef]
- Charteris, D.G.; Lightman, S.L. Comparison of the expression of interferon gamma, IL2, IL4, and lymphotoxin mRNA in experimental autoimmune uveoretinitis. Br. J. Ophthalmol. 1994, 78, 786–790. [Google Scholar] [CrossRef]
- Horai, R.; Caspi, R.R. Cytokines in autoimmune uveitis. J. Interferon Cytokine Res. 2011, 31, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kwon, J.Y.; Kim, S.Y.; Jung, K.; Cho, M.L. Interferon-gamma regulates inflammatory cell death by targeting necroptosis in experimental autoimmune arthritis. Sci. Rep. 2017, 7, 10133. [Google Scholar] [CrossRef] [PubMed]
- Langer, V.; Vivi, E.; Regensburger, D.; Winkler, T.H.; Waldner, M.J.; Rath, T.; Schmid, B.; Skottke, L.; Lee, S.; Jeon, N.L.; et al. IFN-gamma drives inflammatory bowel disease pathogenesis through VE-cadherin-directed vascular barrier disruption. J. Clin. Investig. 2019, 129, 4691–4707. [Google Scholar] [CrossRef]
- Horiuchi, T.; Mitoma, H.; Harashima, S.; Tsukamoto, H.; Shimoda, T. Transmembrane TNF-alpha: Structure, function and interaction with anti-TNF agents. Rheumatology 2010, 49, 1215–1228. [Google Scholar] [CrossRef]
- Mirshahi, A.; Hoehn, R.; Lorenz, K.; Kramann, C.; Baatz, H. Anti-tumor necrosis factor alpha for retinal diseases: Current knowledge and future concepts. J. Ophthalmic Vis. Res. 2012, 7, 39–44. [Google Scholar]
- Kalliolias, G.D.; Ivashkiv, L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016, 12, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Dannappel, M.; Vlantis, K.; Kumari, S.; Polykratis, A.; Kim, C.; Wachsmuth, L.; Eftychi, C.; Lin, J.; Corona, T.; Hermance, N.; et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature 2014, 513, 90–94. [Google Scholar] [CrossRef]
- Nagineni, C.N.; Kommineni, V.K.; William, A.; Detrick, B.; Hooks, J.J. Regulation of VEGF expression in human retinal cells by cytokines: Implications for the role of inflammation in age-related macular degeneration. J. Cell Physiol. 2012, 227, 116–126. [Google Scholar] [CrossRef]
- Sanchez-Cano, D.; Callejas-Rubio, J.L.; Ruiz-Villaverde, R.; Rios-Fernandez, R.; Ortego-Centeno, N. Off-label uses of anti-TNF therapy in three frequent disorders: Behcet’s disease, sarcoidosis, and noninfectious uveitis. Mediat. Inflamm. 2013, 2013, 286857. [Google Scholar] [CrossRef]
- Levy-Clarke, G.; Jabs, D.A.; Read, R.W.; Rosenbaum, J.T.; Vitale, A.; Van Gelder, R.N. Expert panel recommendations for the use of anti-tumor necrosis factor biologic agents in patients with ocular inflammatory disorders. Ophthalmology 2014, 121, 785–796 e783. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Galisteo, R.; Gutkind, J.S. CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1) complex. J. Biol. Chem. 2009, 284, 6038–6042. [Google Scholar] [CrossRef]
- Ehrenberg, M.; Benny, O. Evolving multidimensional pharmacological approaches to CNV therapy in AMD. Curr. Eye Res. 2018, 43, 147–154. [Google Scholar] [CrossRef]
- Sasaki, F.; Koga, T.; Ohba, M.; Saeki, K.; Okuno, T.; Ishikawa, K.; Nakama, T.; Nakao, S.; Yoshida, S.; Ishibashi, T.; et al. Leukotriene B4 promotes neovascularization and macrophage recruitment in murine wet-type AMD models. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Saika, S. TGFbeta pathobiology in the eye. Lab. Investig. 2006, 86, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Senger, D.R.; Perruzzi, C.A.; Feder, J.; Dvorak, H.F. A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell lines. Cancer Res. 1986, 46, 5629–5632. [Google Scholar] [PubMed]
- Lipski, D.A.; Foucart, V.; Dewispelaere, R.; Caspers, L.E.; Defrance, M.; Bruyns, C.; Willermain, F. Retinal endothelial cell phenotypic modifications during experimental autoimmune uveitis: A transcriptomic approach. BMC Ophthalmol. 2020, 20, 106. [Google Scholar] [CrossRef] [PubMed]
- Lightman, S.; Greenwood, J. Effect of lymphocytic infiltration on the blood-retinal barrier in experimental autoimmune uveoretinitis. Clin. Exp. Immunol. 1992, 88, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Al-Latayfeh, M.; Silva, P.S.; Sun, J.K.; Aiello, L.P. Antiangiogenic therapy for ischemic retinopathies. Cold Spring Harb. Perspect. Med. 2012, 2, a006411. [Google Scholar] [CrossRef]
- Sun, Y.; Smith, L.E.H. Retinal Vasculature in Development and Diseases. Annu. Rev. Vis. Sci. 2018, 4, 101–122. [Google Scholar] [CrossRef]
- Mirando, A.C.; Lima, E.S.R.; Chu, Z.; Campochiaro, P.A.; Pandey, N.B.; Popel, A.S. Suppression of Ocular Vascular Inflammation through Peptide-Mediated Activation of Angiopoietin-Tie2 Signaling. Int. J. Mol. Sci. 2020, 21, 5142. [Google Scholar] [CrossRef]
- Silveira, R.C.; Procianoy, R.S. High plasma cytokine levels, white matter injury and neurodevelopment of high risk preterm infants: Assessment at two years. Early Hum. Dev. 2011, 87, 433–437. [Google Scholar] [CrossRef]
- Diedrichs-Mohring, M.; Kaufmann, U.; Wildner, G. The immunopathogenesis of chronic and relapsing autoimmune uveitis—Lessons from experimental rat models. Prog. Retin. Eye Res. 2018, 65, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Diedrichs-Mohring, M.; Niesik, S.; Priglinger, C.S.; Thurau, S.R.; Obermayr, F.; Sperl, S.; Wildner, G. Intraocular DHODH-inhibitor PP-001 suppresses relapsing experimental uveitis and cytokine production of human lymphocytes, but not of RPE cells. J. Neuroinflamm. 2018, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- Diedrichs-Mohring, M.; Leban, J.; Strobl, S.; Obermayr, F.; Wildner, G. A new small molecule for treating inflammation and chorioretinal neovascularization in relapsing-remitting and chronic experimental autoimmune uveitis. Investig. Ophthalmol. Vis. Sci. 2014, 56, 1147–1157. [Google Scholar] [CrossRef]
- Suzuki, H.; Onishi, H.; Wada, J.; Yamasaki, A.; Tanaka, H.; Nakano, K.; Morisaki, T.; Katano, M. VEGFR2 is selectively expressed by FOXP3high CD4+ Treg. Eur. J. Immunol. 2010, 40, 197–203. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Heier, J.S.; Do, D.V.; Mirando, A.C.; Pandey, N.B.; Sheng, H.; Heah, T. The Tie2 signaling pathway in retinal vascular diseases: A novel therapeutic target in the eye. Int. J. Retin. Vitr. 2020, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Tadros, A.; Hughes, D.P.; Dunmore, B.J.; Brindle, N.P. ABIN-2 protects endothelial cells from death and has a role in the antiapoptotic effect of angiopoietin-1. Blood 2003, 102, 4407–4409. [Google Scholar] [CrossRef]
- Jo-Watanabe, A.; Okuno, T.; Yokomizo, T. The Role of Leukotrienes as Potential Therapeutic Targets in Allergic Disorders. Int. J. Mol. Sci. 2019, 20, 3580. [Google Scholar] [CrossRef]
- Elmasry, K.; Ibrahim, A.S.; Abdulmoneim, S.; Al-Shabrawey, M. Bioactive lipids and pathological retinal angiogenesis. Br. J. Pharmacol. 2019, 176, 93–109. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Dalli, J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin. Immunol. 2015, 27, 200–215. [Google Scholar] [CrossRef]
- Spite, M.; Claria, J.; Serhan, C.N. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 2014, 19, 21–36. [Google Scholar] [CrossRef]
- Singh, R.K.; Tandon, R.; Dastidar, S.G.; Ray, A. A review on leukotrienes and their receptors with reference to asthma. J. Asthma 2013, 50, 922–931. [Google Scholar] [CrossRef]
- Satpathy, S.R.; Jala, V.R.; Bodduluri, S.R.; Krishnan, E.; Hegde, B.; Hoyle, G.W.; Fraig, M.; Luster, A.D.; Haribabu, B. Crystalline silica-induced leukotriene B4-dependent inflammation promotes lung tumour growth. Nat. Commun. 2015, 6, 7064. [Google Scholar] [CrossRef]
- Kim, N.D.; Chou, R.C.; Seung, E.; Tager, A.M.; Luster, A.D. A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis. J. Exp. Med. 2006, 203, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Poeckel, D.; Funk, C.D. The 5-lipoxygenase/leukotriene pathway in preclinical models of cardiovascular disease. Cardiovasc. Res. 2010, 86, 243–253. [Google Scholar] [CrossRef]
- Birukova, A.A.; Smurova, K.; Birukov, K.G.; Kaibuchi, K.; Garcia, J.G.; Verin, A.D. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc. Res. 2004, 67, 64–77. [Google Scholar] [CrossRef]
- Kawkitinarong, K.; Linz-McGillem, L.; Birukov, K.G.; Garcia, J.G. Differential regulation of human lung epithelial and endothelial barrier function by thrombin. Am. J. Respir. Cell Mol. Biol. 2004, 31, 517–527. [Google Scholar] [CrossRef]
- Espinosa, K.; Bosse, Y.; Stankova, J.; Rola-Pleszczynski, M. CysLT1 receptor upregulation by TGF-beta and IL-13 is associated with bronchial smooth muscle cell proliferation in response to LTD4. J. Allergy Clin. Immunol. 2003, 111, 1032–1040. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, S.R.; Park, H.S.; Jin, G.Y.; Lee, Y.C. Cysteinyl leukotriene receptor antagonist regulates vascular permeability by reducing vascular endothelial growth factor expression. J. Allergy Clin. Immunol. 2004, 114, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Pergola, C.; Koeberle, A.; Hoffmann, M.; Dehm, F.; Bramanti, P.; Cuzzocrea, S.; Werz, O.; Sautebin, L. The 5-lipoxygenase inhibitor, zileuton, suppresses prostaglandin biosynthesis by inhibition of arachidonic acid release in macrophages. Br. J. Pharmacol. 2010, 161, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, L.; Roinestad, K.; Van, T.; Springman, E.B. Recent advances in clinical development of leukotriene B4 pathway drugs. Semin. Immunol. 2017, 33, 65–73. [Google Scholar] [CrossRef]
- Gronke, L.; Beeh, K.M.; Cameron, R.; Kornmann, O.; Beier, J.; Shaw, M.; Holz, O.; Buhl, R.; Magnussen, H.; Jorres, R.A. Effect of the oral leukotriene B4 receptor antagonist LTB019 on inflammatory sputum markers in patients with chronic obstructive pulmonary disease. Pulm. Pharmacol. Ther. 2008, 21, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Konstan, M.W.; Doring, G.; Heltshe, S.L.; Lands, L.C.; Hilliard, K.A.; Koker, P.; Bhattacharya, S.; Staab, A.; Hamilton, A.; Investigators; et al. A randomized double blind, placebo controlled phase 2 trial of BIIL 284 BS (an LTB4 receptor antagonist) for the treatment of lung disease in children and adults with cystic fibrosis. J. Cyst. Fibros. 2014, 13, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.J.; Erwig, L.P.; Liversidge, J.; Forrester, J.V.; Rees, A.J.; Dick, A.D. Retinal microenvironment controls resident and infiltrating macrophage function during uveoretinitis. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2250–2257. [Google Scholar]
- Sezin, T.; Murthy, S.; Attah, C.; Seutter, M.; Holtsche, M.M.; Hammers, C.M.; Schmidt, E.; Meshrkey, F.; Mousavi, S.; Zillikens, D.; et al. Dual inhibition of complement factor 5 and leukotriene B4 synergistically suppresses murine pemphigoid disease. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Roversi, P.; Ryffel, B.; Togbe, D.; Maillet, I.; Teixeira, M.; Ahmat, N.; Paesen, G.C.; Lissina, O.; Boland, W.; Ploss, K.; et al. Bifunctional lipocalin ameliorates murine immune complex-induced acute lung injury. J. Biol. Chem. 2013, 288, 18789–18802. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eskandarpour, M.; Nunn, M.A.; Weston-Davies, W.; Calder, V.L. Immune-Mediated Retinal Vasculitis in Posterior Uveitis and Experimental Models: The Leukotriene (LT)B4-VEGF Axis. Cells 2021, 10, 396. https://doi.org/10.3390/cells10020396
Eskandarpour M, Nunn MA, Weston-Davies W, Calder VL. Immune-Mediated Retinal Vasculitis in Posterior Uveitis and Experimental Models: The Leukotriene (LT)B4-VEGF Axis. Cells. 2021; 10(2):396. https://doi.org/10.3390/cells10020396
Chicago/Turabian StyleEskandarpour, Malihe, Miles A. Nunn, Wynne Weston-Davies, and Virginia L. Calder. 2021. "Immune-Mediated Retinal Vasculitis in Posterior Uveitis and Experimental Models: The Leukotriene (LT)B4-VEGF Axis" Cells 10, no. 2: 396. https://doi.org/10.3390/cells10020396
APA StyleEskandarpour, M., Nunn, M. A., Weston-Davies, W., & Calder, V. L. (2021). Immune-Mediated Retinal Vasculitis in Posterior Uveitis and Experimental Models: The Leukotriene (LT)B4-VEGF Axis. Cells, 10(2), 396. https://doi.org/10.3390/cells10020396




