Comparative Mapping of the Macrochromosomes of Eight Avian Species Provides Further Insight into Their Phylogenetic Relationships and Avian Karyotype Evolution
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Chromosome Preparation
2.2. Preparation of BAC Probes for FISH
2.3. Fluorescence In Situ Hybridisation (FISH)
2.4. Karyotype Analysis and Ideogram Generation
2.5. MLGO (Maximum Likelihood for Gene Order Analysis) Analysis for Ancestral Genome Reconstruction
2.6. GRIMM (Genome Rearrangements In Man and Mouse) Analysis for Chromosome Rearrangement
2.7. Mathematical Analyses
3. Results
3.1. Karyotypes and Ideograms for Eight Avian Species
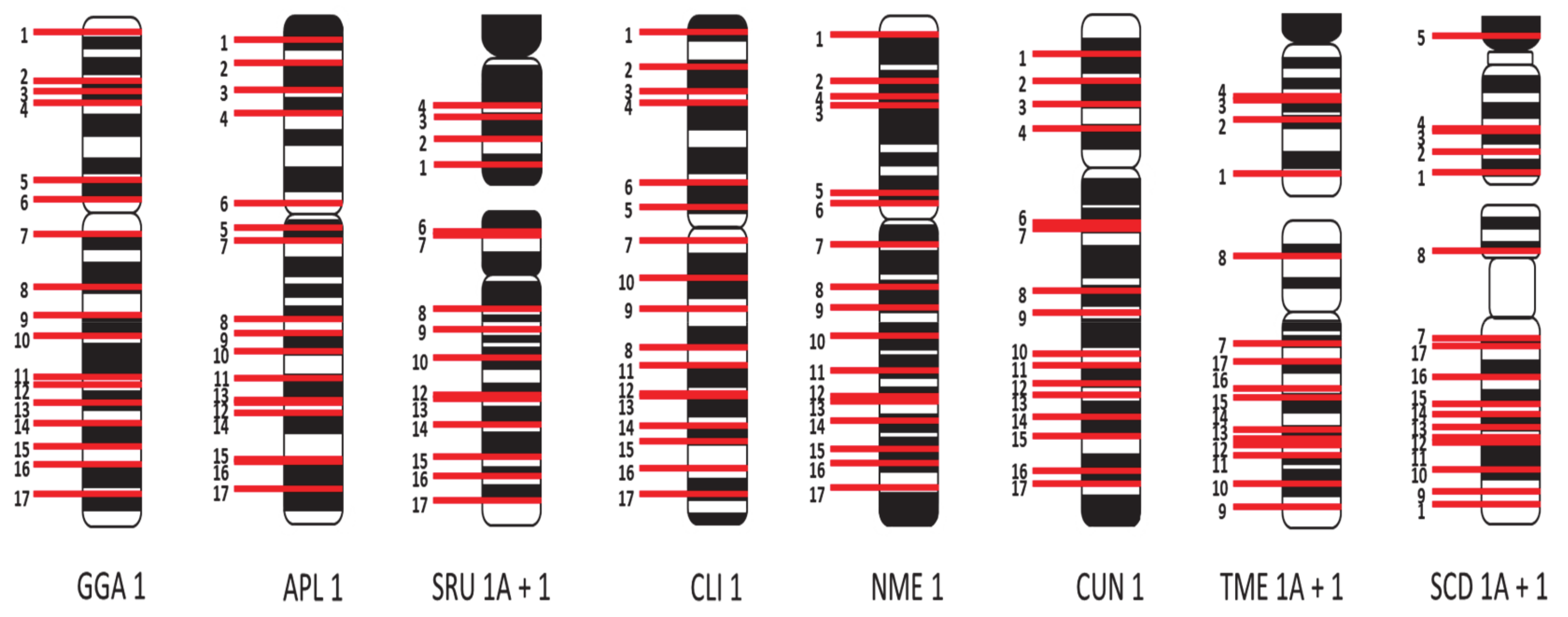
3.2. Application of a Panel of 74 Selected Chicken BACs for the Fine Mapping of Macrochromosome Homologs 1–9 and Z
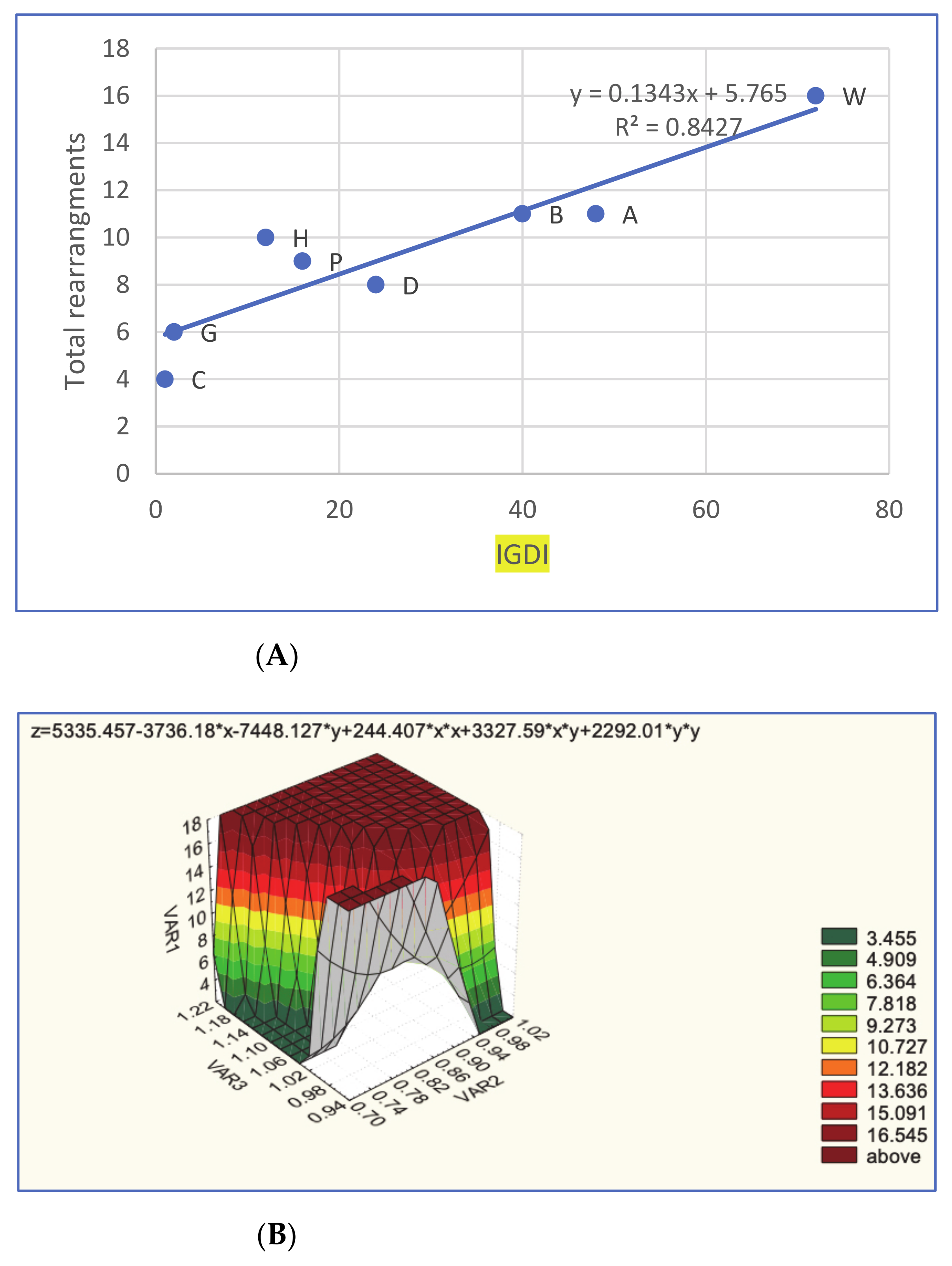
3.3. Reconstructing the Neognathae Ancestor and Rearrangements
4. Discussion
4.1. Comparative Macrochromosome Maps
4.2. Chromosome Paints vs. BAC Mapping
4.3. Centromere Position
4.4. A Potential Tool for Genome Assembly
4.5. Phylogenetics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christidis, L.B. Aves. In Animal Cytogenetics; John, B., Ed.; Gebrüder Bornträger: Berlin, Germany; Stuttgart, Germany, 1990; Volume 4, 116p. [Google Scholar]
- Masabanda, J.S.; Burt, D.W.; O’Brien, P.C.; Vignal, A.; Fillon, V.; Walsh, P.S.; Cox, H.; Tempest, H.G.; Smith, J.; Habermann, F.; et al. Molecular cytogenetic definition of the chicken genome: The first complete avian karyotype. Genetics 2004, 166, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.K.; Robertson, L.B.; Tempest, H.G.; Skinner, B.M. The evolution of the avian genome as revealed by comparative molecular cytogenetics. Cytogenet. Genome Res. 2007, 117, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, R.; Ferguson-Smith, M.A.; de Oliveira, E.H.C. Karyotype evolution in birds: From conventional staining to chromosome painting. Genes 2018, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Stiller, J.; Zhang, G. Comparative phylogenomics, a stepping stone for bird biodiversity studies. Diversity 2019, 11, 115. [Google Scholar] [CrossRef]
- Shetty, S.; Griffin, D.K.; Graves, J.A. Comparative painting reveals strong chromosome homology over 80 million years of bird evolution. Chromosome Res. 1999, 7, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Nanda, I.; Shan, Z.; Schartl, M.; Burt, D.W.; Koehler, M.; Nothwang, H.; Grützner, F.; Paton, I.R.; Windsor, D.; Dunn, I.; et al. 300 million years of conserved synteny between chicken Z and human chromosome 9. Nat. Genet. 1999, 21, 258–259. [Google Scholar] [CrossRef]
- Nanda, I.; Benisch, P.; Fetting, D.; Haaf, T.; Schmid, M. Synteny conservation of chicken macrochromosomes 1–10 in different avian lineages revealed by cross-species chromosome painting. Cytogenet. Genome Res. 2011, 132, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Raudsepp, T.; Houck, M.L.; O’Brien, P.C.; Ferguson-Smith, M.A.; Ryder, O.A.; Chowdhary, B.P. Cytogenetic analysis of California condor (Gymnogyps californianus) chromosomes: Comparison with chicken (Gallus gallus) macrochromosomes. Cytogenet. Genome Res. 2002, 98, 54–60. [Google Scholar] [CrossRef]
- Shibusawa, M.; Nishida-Umehara, C.; Masabanda, J.; Griffin, D.K.; Isobe, T.; Matsuda, Y. Chromosome rearrangements between chicken and guinea fowl defined by comparative chromosome painting and FISH mapping of DNA clones. Cytogenet. Genome Res. 2002, 98, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Arnold, A.P. Chromosomal polymorphism and comparative painting analysis in the zebra finch. Chromosome Res. 2005, 13, 47–56. [Google Scholar] [CrossRef]
- Nishida, C.; Ishijima, J.; Kosaka, A.; Tanabe, H.; Habermann, F.A.; Griffin, D.K.; Matsuda, Y. Characterization of chromosome structures of Falconinae (Falconidae, Falconiformes, Aves) by chromosome painting and delineation of chromosome rearrangements during their differentiation. Chromosome Res. 2008, 16, 171–181. [Google Scholar] [CrossRef]
- Damas, J.; O’Connor, R.; Farré, M.; Lenis, V.P.E.; Martell, H.J.; Mandawala, A.; Fowler, K.; Joseph, S.; Swain, M.T.; Griffin, D.K.; et al. Upgrading short-read animal genome assemblies to chromosome level using comparative genomics and a universal probe set. Genome Res. 2017, 27, 875–884. [Google Scholar] [CrossRef]
- Lichter, P.; Tang, C.J.; Call, K.; Hermanson, G.; Evans, G.A.; Housman, D.; Ward, D.C. High-resolution mapping of human chromosome 11 by in situ hybridization with cosmid clones. Science 1990, 247, 64–69. [Google Scholar] [CrossRef]
- O’Connor, R.E.; Farré, M.; Joseph, S.; Damas, J.; Kiazim, L.; Jennings, R.; Bennett, S.; Slack, E.A.; Allanson, E.; Larkin, D.M.; et al. Chromosome-level assembly reveals extensive rearrangement in saker falcon and budgerigar, but not ostrich, genomes. Genome Biol. 2018, 19, 171. [Google Scholar] [CrossRef] [PubMed]
- Romanov, M.N.; Farré, M.; Lithgow, P.E.; Fowler, K.E.; Skinner, B.M.; O’Connor, R.; Fonseka, G.; Backström, N.; Matsuda, Y.; Nishida, C.; et al. Reconstruction of gross avian genome structure, organization and evolution suggests that the chicken lineage most closely resembles the dinosaur avian ancestor. BMC Genom. 2014, 15, 1060. [Google Scholar] [CrossRef] [PubMed]
- Romanov, M.N.; O’Connor, R.; Skinner, B.M.; Martell, H.; Farré, M.; Larkin, D.M.; Griffin, D.K. Comparative Cytogenomics Enhanced with Bioinformatic Tools Provides Further Insights into Genome Evolution of Birds and Other Amniotes. In Proceedings of the 2nd Annual Food, Nutrition and Agriculture Genomics Congress, London, UK, 29–30 April 2015. [Google Scholar]
- Schmid, M.; Smith, J.; Burt, D.W.; Aken, B.L.; Antin, P.B.; Archibald, A.L.; Ashwell, C.; Blackshear, P.J.; Boschiero, C.; Brown, C.T.; et al. Third Report on Chicken Genes and Chromosomes 2015. Cytogenet. Genome Res. 2015, 145, 78–179. [Google Scholar] [CrossRef] [PubMed]
- Ricklefs, R.E. Species richness and morphological diversity of passerine birds. Proc. Natl. Acad. Sci. USA 2012, 109, 14482–14487. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, S.; Searcy, W.A.; Peters, S. Brain development, song learning and mate choice in birds: A review and experimental test of the “nutritional stress hypothesis”. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2002, 188, 1003–1014. [Google Scholar] [CrossRef]
- Olson, C.R.; Mello, C.V. Significance of vitamin A to brain function, behavior and learning. Mol. Nutr. Food Res. 2010, 54, 489–495. [Google Scholar] [CrossRef]
- Frankl-Vilches, C.; Kuhl, H.; Werber, M.; Klages, S.; Kerick, M.; Bakker, A.; de Oliveira, E.H.; Reusch, C.; Capuano, F.; Vowinckel, J.; et al. Using the canary genome to decipher the evolution of hormone-sensitive gene regulation in seasonal singing birds. Genome Biol. 2015, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Hoodless, A. Studies of West Palearctic birds. 195. Eurasian Woodcock Scolopax rusticola. BR Birds 1995, 88, 578–592. [Google Scholar]
- Braña, F.; Prieto, L.; González-Quirós, P. Habitat change and timing of dusk flight in the Eurasian woodcock: A trade-off between feeding and predator avoidance? Ann. Zool. Fenn. 2010, 47, 206–214. [Google Scholar] [CrossRef]
- Duriez, O.; Eraud, C.; Barbraud, C.; Ferrand, Y. Factors affecting population dynamics of Eurasian woodcocks wintering in France: Assessing the efficiency of a hunting-free reserve. Biol. Conserv. 2005, 122, 89–97. [Google Scholar] [CrossRef]
- BirdLife International. Species Factsheet: Chlamydotis Undulata. 2020. Available online: http://datazone.birdlife.org/species/factsheet/african-houbara-chlamydotis-undulata (accessed on 24 December 2020).
- Vickrey, A.I.; Bruders, R.; Kronenberg, Z.; Mackey, E.; Bohlender, R.J.; Maclary, E.T.; Maynez, R.; Osborne, E.J.; Johnson, K.P.; Huff, C.D.; et al. Introgression of regulatory alleles and a missense coding mutation drive plumage pattern diversity in the rock pigeon. eLife 2018, 7, e34803. [Google Scholar] [CrossRef] [PubMed]
- Jourdain, E.; Gunnarsson, G.; Wahlgren, J.; Latorre-Margalef, N.; Bröjer, C.; Sahlin, S.; Svensson, L.; Waldenström, J.; Lundkvist, A.; Olsen, B. Influenza virus in a natural host, the mallard: Experimental infection data. PLoS ONE 2010, 5, e8935. [Google Scholar] [CrossRef]
- Rao, M.; Morisson, M.; Faraut, T.; Bardes, S.; Fève, K.; Labarthe, E.; Fillon, V.; Huang, Y.; Li, N.; Vignal, A. A duck RH panel and its potential for assisting NGS genome assembly. BMC Genom. 2012, 13, 513. [Google Scholar] [CrossRef] [PubMed]
- Hedges, S.B.; Marin, J.; Suleski, M.; Paymer, M.; Kumar, S. Tree of life reveals clock-like speciation and diversification. Mol. Biol. Evol. 2015, 32, 835–845. [Google Scholar] [CrossRef]
- O’Connor, R.E.; Romanov, M.N.; Kiazim, L.G.; Barrett, P.M.; Farré, M.; Damas, J.; Ferguson-Smith, M.; Valenzuela, N.; Larkin, D.M.; Griffin, D.K. Reconstruction of the diapsid ancestral genome permits chromosome evolution tracing in avian and non-avian dinosaurs. Nat. Commun. 2018, 9, 1883. [Google Scholar] [CrossRef]
- O’Connor, R.E.; Kiazim, L.; Skinner, B.; Fonseka, G.; Joseph, S.; Jennings, R.; Larkin, D.M.; Griffin, D.K. Patterns of microchromosome organization remain highly conserved throughout avian evolution. Chromosoma 2019, 128, 21–29. [Google Scholar] [CrossRef]
- Ladjali-Mohammedi, K.; Bitgood, J.J.; Tixier-Boichard, M.; Ponce De Leon, F.A. International system for standardized avian karyotypes (ISSAK): Standardized banded karyotypes of the domestic fowl (Gallus domesticus). Cytogenet. Cell Genet. 1999, 86, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Rasband, W.S. ImageJ: Image processing and analysis in Java. ASCL 2012. [Google Scholar]
- Hu, F.; Lin, Y.; Tang, J. MLGO: Phylogeny reconstruction and ancestral inference from gene-order data. BMC Bioinform. 2014, 15, 354. [Google Scholar] [CrossRef]
- Prum, R.O.; Berv, J.S.; Dornburg, A.; Field, D.J.; Townsend, J.P.; Lemmon, E.M.; Lemmon, A.R. A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature 2015, 526, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Cepas, J.; Serra, F.; Bork, P. ETE 3: Reconstruction, analysis, and visualization of phylogenomic data. Mol. Biol. Evol. 2016, 33, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Tesler, G. GRIMM: Genome rearrangements web server. Bioinformatics 2002, 18, 492–493. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Suleski, M.; Hedges, S.B. TimeTree: A resource for timelines, timetrees, and divergence times. Mol. Biol. Evol. 2017, 34, 1812–1819. [Google Scholar] [CrossRef] [PubMed]
- Fillon, V.; Vignoles, M.; Crooijmans, R.P.; Groenen, M.A.; Zoorob, R.; Vignal, A. FISH mapping of 57 BAC clones reveals strong conservation of synteny between Galliformes and Anseriformes. Anim. Genet. 2007, 38, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Hammar, B.O. The karyotypes of thirty-one birds. Hereditas 1970, 65, 29–58. [Google Scholar] [CrossRef]
- Dos Santos, M.D.; Kretschmer, R.; Frankl-Vilches, C.; Bakker, A.; Gahr, M.; O’Brien, P.C.; Ferguson-Smith, M.A.; de Oliveira, E.H. Comparative cytogenetics between two important songbird models: The zebra finch and the canary. PLoS ONE 2017, 12, e0170997. [Google Scholar] [CrossRef]
- Mahiddine-Aoudjit, L.; Boucekkine, O.; Ladjali-Mohammedi, K. Banding cytogenetics of the vulnerable species Houbara bustard (Otidiformes) and comparative analysis with the Domestic fowl. Comp. Cytogenet. 2019, 13, 1–17. [Google Scholar] [CrossRef]
- Bourque, G.; Zdobnov, E.M.; Bork, P.; Pevzner, P.A.; Tesler, G. Comparative architectures of mammalian and chicken genomes reveal highly variable rates of genomic rearrangements across different lineages. Genome Res. 2005, 15, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, R.; Gunski, R.J.; Garnero Adel, V.; Furo Ide, O.; O’Brien, P.C.; Ferguson-Smith, M.A.; de Oliveira, E.H. Molecular cytogenetic characterization of multiple intrachromosomal rearrangements in two representatives of the genus Turdus (Turdidae, Passeriformes). PLoS ONE 2014, 9, e103338. [Google Scholar] [CrossRef]
- Burt, D.W.; Bruley, C.; Dunn, I.C.; Jones, C.T.; Ramage, A.; Law, A.S.; Morrice, D.R.; Paton, I.R.; Smith, J.; Windsor, D.; et al. The dynamics of chromosome evolution in birds and mammals. Nature 1999, 402, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Barton, N.H. Chromosomal speciation and molecular divergence--accelerated evolution in rearranged chromosomes. Science 2003, 300, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.V.; Kingan, S.B.; Calkins, J.D.; Balakrishnan, C.N.; Jennings, W.B.; Swanson, W.J.; Sorenson, M.D. Speciation in birds: Genes, geography, and sexual selection. Proc. Natl. Acad. Sci. USA 2005, 102, 6550–6557. [Google Scholar] [CrossRef]
- Noor, M.A.; Grams, K.L.; Bertucci, L.A.; Reiland, J. Chromosomal inversions and the reproductive isolation of species. Proc. Natl. Acad. Sci. USA 2001, 98, 12084–12088. [Google Scholar] [CrossRef] [PubMed]
- Rieseberg, L.H. Chromosomal rearrangements and speciation. Trends Ecol. Evol. 2001, 16, 351–358. [Google Scholar] [CrossRef]
- Pevzner, P.; Tesler, G. Human and mouse genomic sequences reveal extensive breakpoint reuse in mammalian evolution. Proc. Natl. Acad. Sci. USA 2003, 100, 7672–7677. [Google Scholar] [CrossRef] [PubMed]
- Larkin, D.M.; Pape, G.; Donthu, R.; Auvil, L.; Welge, M.; Lewin, H.A. Breakpoint regions and homologous synteny blocks in chromosomes have different evolutionary histories. Genome Res. 2009, 19, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Bovine Genome Sequencing and Analysis Consortium. The genome sequence of taurine cattle: A window to ruminant biology and evolution. Science 2009, 324, 522–528. [Google Scholar] [CrossRef]
- Groenen, M.A.; Archibald, A.L.; Uenishi, H.; Tuggle, C.K.; Takeuchi, Y.; Rothschild, M.F.; Rogel-Gaillard, C.; Park, C.; Milan, D.; Megens, H.J.; et al. Analyses of pig genomes provide insight into porcine demography and evolution. Nature 2012, 491, 393–398. [Google Scholar] [CrossRef]
- Farré, M.; Narayan, J.; Slavov, G.T.; Damas, J.; Auvil, L.; Li, C.; Jarvis, E.D.; Burt, D.W.; Griffin, D.K.; Larkin, D.M. Novel insights into chromosome evolution in birds, archosaurs, and reptiles. Genome Biol. Evol. 2016, 8, 2442–2451. [Google Scholar] [CrossRef] [PubMed]
- Farré, M.; Kim, J.; Proskuryakova, A.A.; Zhang, Y.; Kulemzina, A.I.; Li, Q.; Zhou, Y.; Xiong, Y.; Johnson, J.L.; Perelman, P.L.; et al. Evolution of gene regulation in ruminants differs between evolutionary breakpoint regions and homologous synteny blocks. Genome Res. 2019, 29, 576–589. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.K.; Haberman, F.; Masabanda, J.; O’Brien, P.; Bagga, M.; Sazanov, A.; Smith, J.; Burt, D.W.; Ferguson-Smith, M.; Wienberg, J. Micro- and macrochromosome paints generated by flow cytometry and microdissection: Tools for mapping the chicken genome. Cytogenet. Cell Genet. 1999, 87, 278–281. [Google Scholar] [CrossRef]
- Hansmann, T.; Nanda, I.; Volobouev, V.; Yang, F.; Schartl, M.; Haaf, T.; Schmid, M. Cross-species chromosome painting corroborates microchromosome fusion during karyotype evolution of birds. Cytogenet. Genome Res. 2009, 126, 281–304. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; O’Brien, P.C.; Ng, B.L.; Fu, B.; Volobouev, V.; Carter, N.P.; Ferguson-Smith, M.A.; Yang, F. Avian comparative genomics: Reciprocal chromosome painting between domestic chicken (Gallus gallus) and the stone curlew (Burhinus oedicnemus, Charadriiformes)—An atypical species with low diploid number. Chromosome Res. 2009, 17, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Guttenbach, M.; Nanda, I.; Feichtinger, W.; Masabanda, J.S.; Griffin, D.K.; Schmid, M. Comparative chromosome painting of chicken autosomal paints 1–9 in nine different bird species. Cytogenet. Genome Res. 2003, 103, 173–184. [Google Scholar] [CrossRef]
- Derjusheva, S.; Kurganova, A.; Habermann, F.; Gaginskaya, E. High chromosome conservation detected by comparative chromosome painting in chicken, pigeon and passerine birds. Chromosome Res. 2004, 12, 715–723. [Google Scholar] [CrossRef]
- Shibusawa, M.; Nishida-Umehara, C.; Tsudzuki, M.; Masabanda, J.; Griffin, D.K.; Matsuda, Y. A comparative karyological study of the blue-breasted quail (Coturnix chinensis, Phasianidae) and California quail (Callipepla californica, Odontophoridae). Cytogenet. Genome Res. 2004, 106, 82–90. [Google Scholar] [CrossRef]
- Jackson, R.C. Interspecific hybridization in Haplopappus and its bearing on chromosome evolution in the Blepharodon section. Am. J. Bot. 1962, 49, 119–132. [Google Scholar] [CrossRef]
- Jackson, R.C. The karyotype in systematics. Annu. Rev. Ecol. Evol. Syst. 1971, 2, 327–368. [Google Scholar] [CrossRef]
- Rocchi, M.; Archidiacono, N.; Schempp, W.; Capozzi, O.; Stanyon, R. Centromere repositioning in mammals. Heredity 2012, 108, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Houde, P. Special Issue: Genomic Analyses of Avian Evolution. Diversity 2019, 11, 178. [Google Scholar] [CrossRef]
- Romanov, M.N.; Kiazim, L.; O’Connor, R.; Griffin, D.K. Current Molecular Genetic and Genomic Technologies in the Field of Studying the Avian Biology. In Proceedings of the Molecular Genetic Technologies for Analysis of Gene Expression of Productivity and Resistance to Diseases of Animals, Materials of the 2nd International Conference, Moscow, Russia, 25 December 2020; Sel’skokhozyaistvennye Tekhnologii: Moscow, Russia, 2020; pp. 34–44. [Google Scholar] [CrossRef]
- Romanov, M.N.; Farré-Belmonte, M.; Lithgow, P.E.; O’Connor, R.; Fowler, K.E.; Larkin, D.M.; Griffin, D.K. In silico Reconstruction of Chromosomal Rearrangements and an Avian Ancestral Karyotype. In Proceedings of the International Plant and Animal Genome XXII Conference, San Diego, CA, USA, 10–14 January 2014; Scherago International: San Diego, CA, USA, 2014. [Google Scholar]
- RDocumentation. The R Base Package by R-core R-core@R-project.org; Base v3.6.2. Scale Function. 2019. Available online: https://www.rdocumentation.org/packages/base/versions/3.6.2/topics/scale (accessed on 22 December 2020).
- Shitikov, V.K.; Mastitsky, S.E. 10.4.2 Fuzzy k-Means Method (Fuzzy Analysis Clustering). In Classification, Regression, Data Mining Algorithms Using R; Electronic Book; 2017; Available online: https://ranalytics.github.io/data-mining/104-Other-Clustering-Methods.html#sec_10_4_2 (accessed on 22 December 2020).
- Fanny: Fuzzy Analysis Clustering. Documentation for Package ‘Cluster’ Version 2.1.0. “Finding Groups in Data”: Cluster Analysis Extended Rousseeuw et al. 2019. Available online: https://rdrr.io/cran/cluster/man/fanny.html (accessed on 22 December 2020).
- Suzuki, R.; Shimodaira, H. Pvclust: An R package for assessing the uncertainty in hierarchical clustering. Bioinformatics 2006, 22, 1540–1542. [Google Scholar] [CrossRef]
- Zhao, Q.; Hautamaki, V.; Fränti, P. Knee Point Detection in BIC for Detecting the Number of Clusters. In International Conference on Advanced Concepts for Intelligent Vision Systems—ACIVS 2008; Lecture Notes in Computer Science; Blanc-Talon, J., Bourennane, S., Philips, W., Popescu, D., Scheunders, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 5259, pp. 664–673. ISBN 978-3-540-88457-6. [Google Scholar]
- Smith, M.R. TreeDist: Distances between Phylogenetic Trees (Version v0.0.0.9000). Zenodo 2019. Available online: https://zenodo.org/record/3528124 (accessed on 24 December 2020). [CrossRef]
- Smith, M.R. Information theoretic Generalized Robinson-Foulds metrics for comparing phylogenetic trees. Bioinformatics 2020. [Google Scholar] [CrossRef]
- Smith, M.R. Visualise a Matching–VisualizeMatching. TreeDist 1.2.1. 2020. Available online: https://ms609.github.io/TreeDist/reference/VisualizeMatching.html (accessed on 22 December 2020).
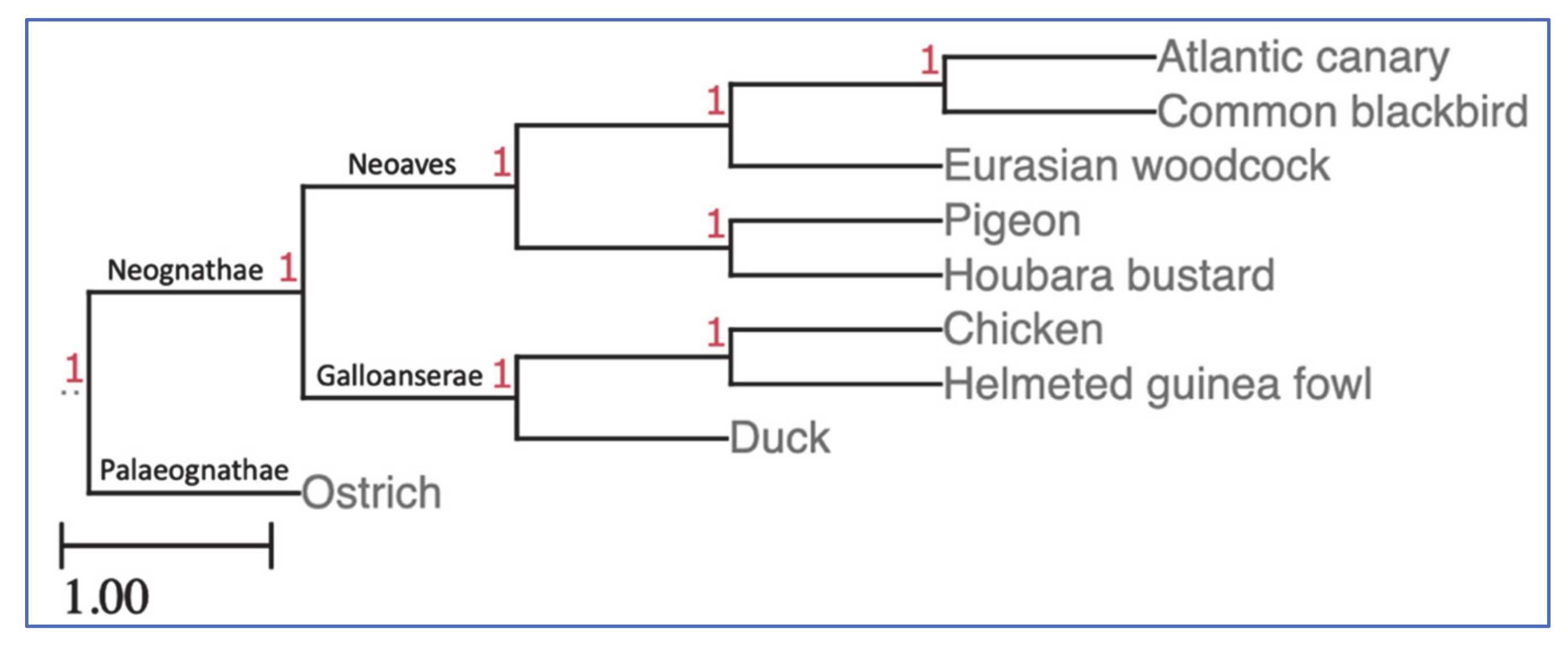
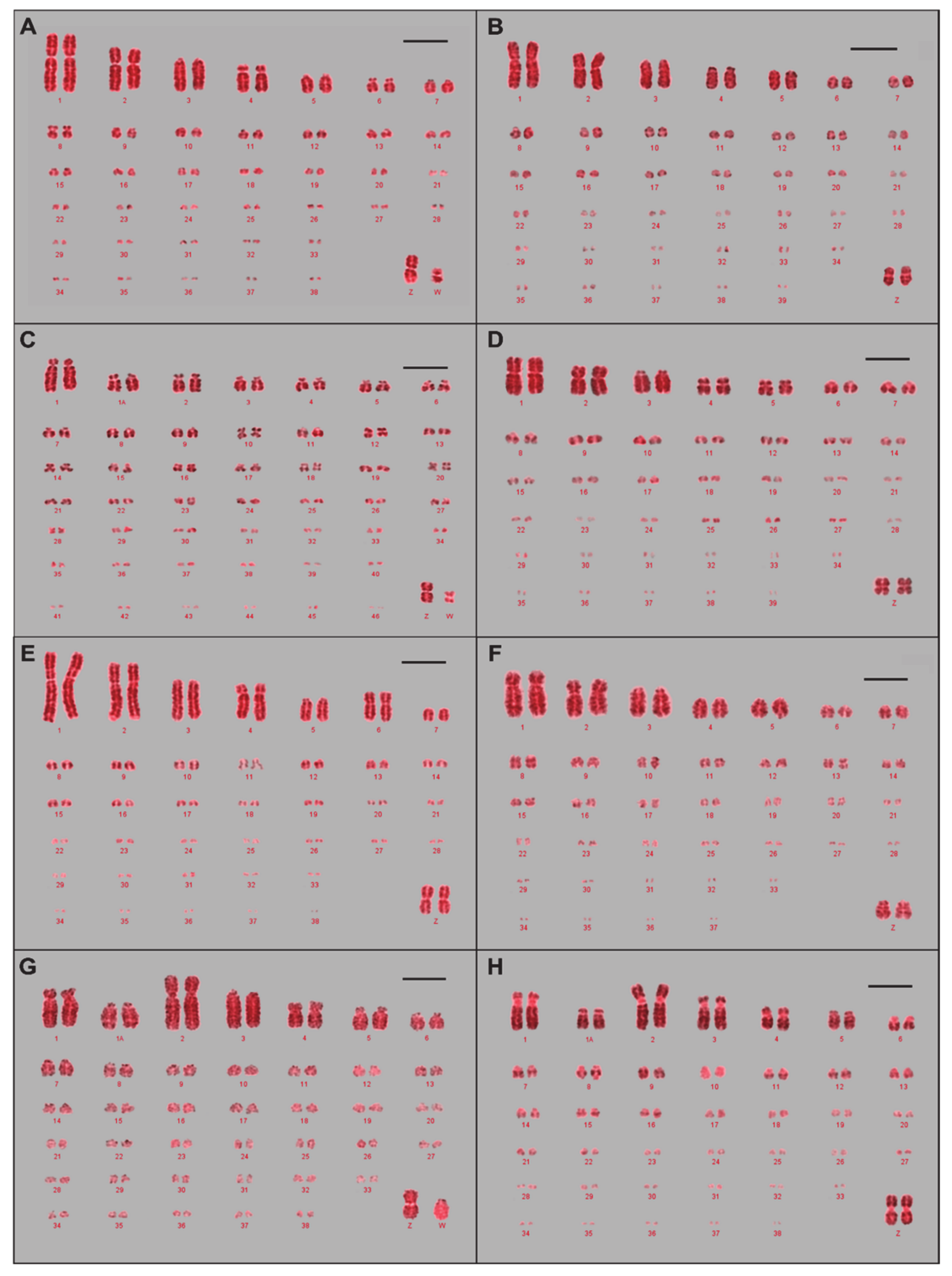
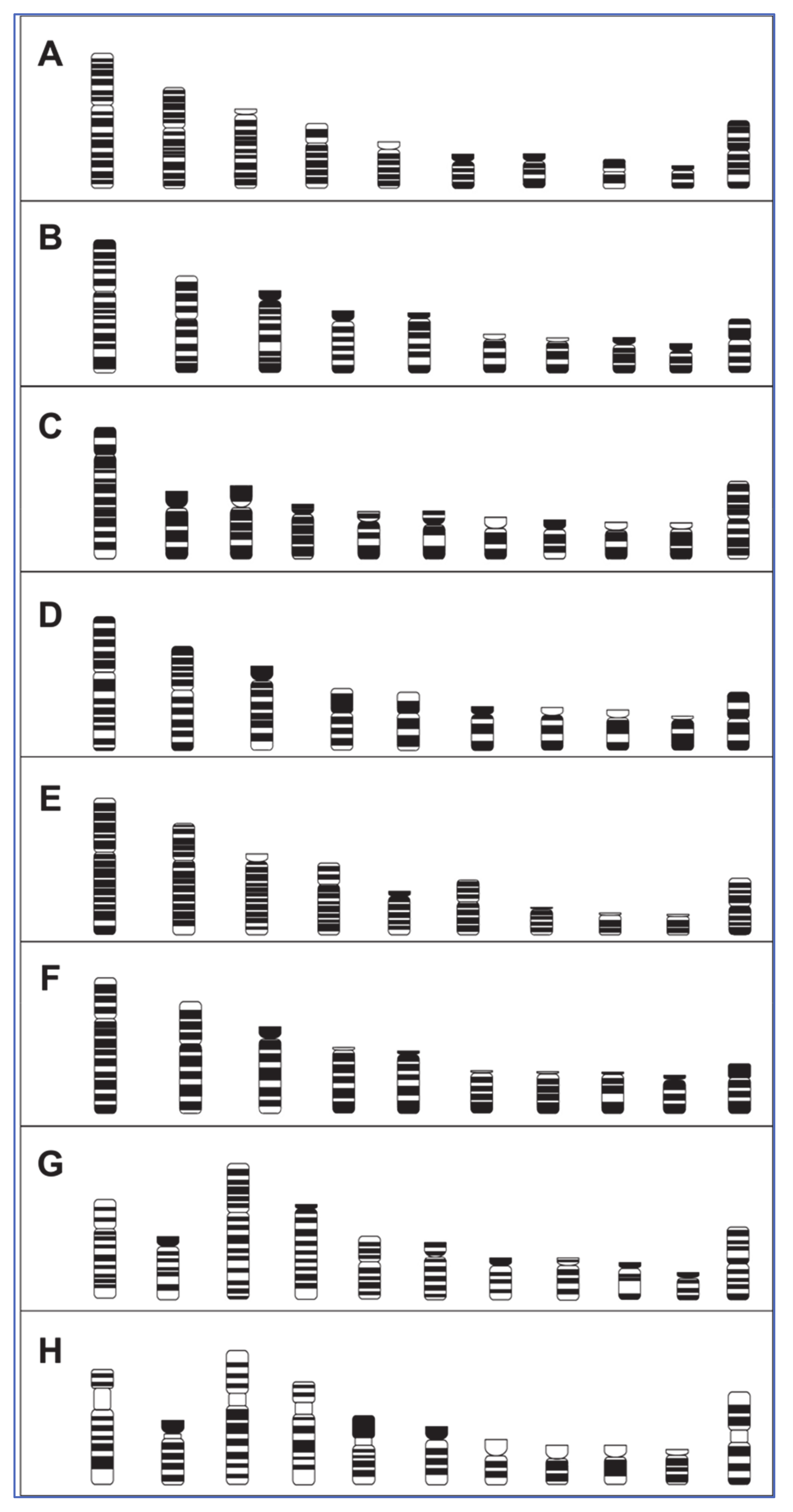
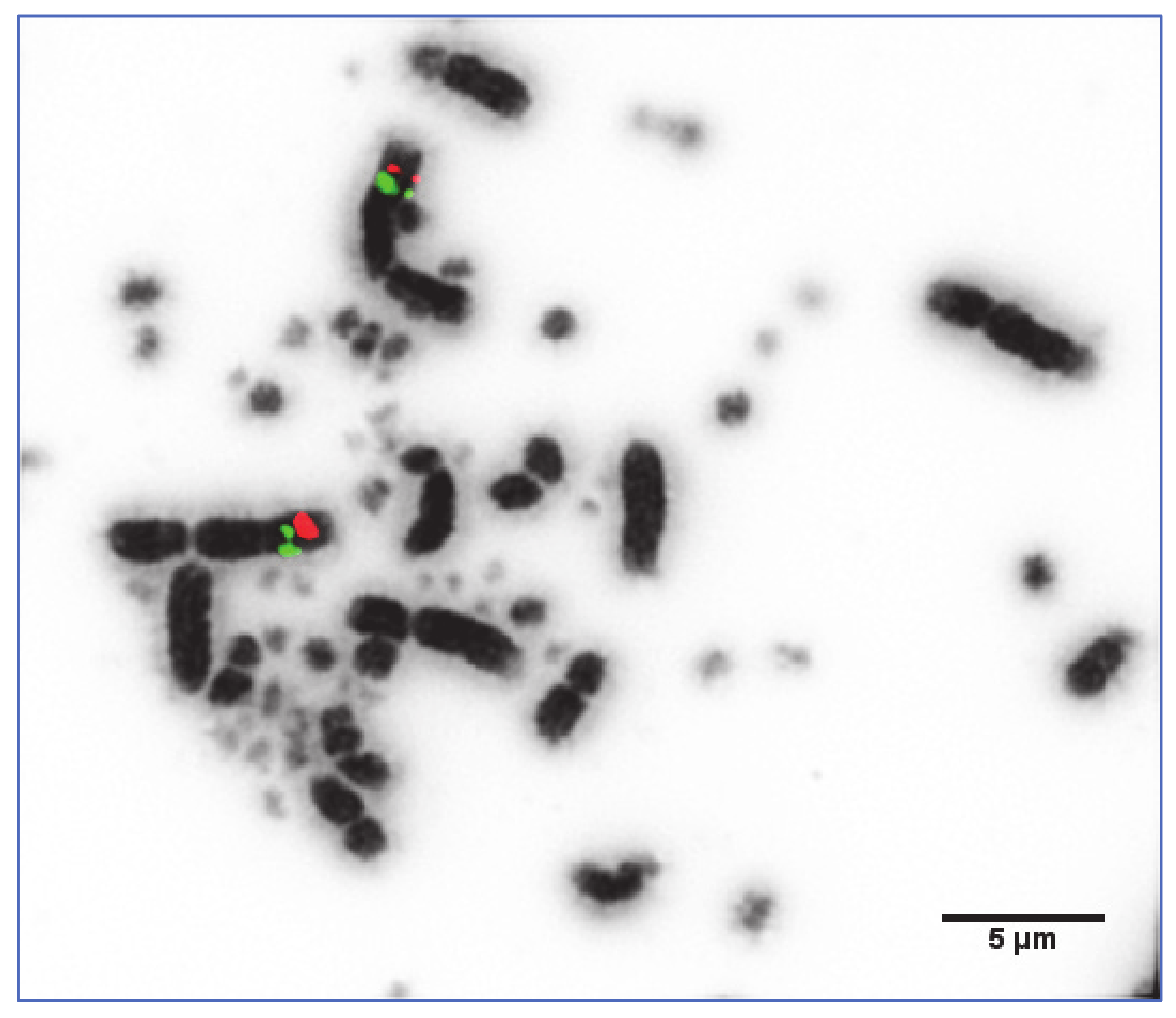

| Infraclass | Order | Common Name | 2n | Divergence 1 (Mya 2) | Hybridisation Success Rate (%) |
|---|---|---|---|---|---|
| Neognathae | Galliformes | chicken | 78 3 | – | 100 |
| Galliformes | helmeted guinea fowl | 78 3 | 47 | 100 | |
| Anseriformes | duck (mallard) | 80 4 | 80 | 85.1 | |
| Columbiformes | rock dove (pigeon) | 80 5 | 98 | 93.2 | |
| Otidiformes | houbara bustard | 76 6 | 98 | 87.8 | |
| Passeriformes | common blackbird | 80 7 | 98 | 78.4 | |
| Passeriformes | Atlantic canary | 80 8 | 98 | 73.0 | |
| Charadriiformes | Eurasian woodcock | 96 6 | 98 | 73.0 | |
| Palaeognathae | Struthioniformes | ostrich 9 | 80 6 | 140 | 83.8 |
| Species | Inversions | Duplications | Intra-Chromosomal Translocations | Inter-Chromosomal Translocations | Fusions | Fissions | Total |
|---|---|---|---|---|---|---|---|
| Chicken | 3 | 1 | 4 | ||||
| Guinea fowl | 4 | 2 | 6 | ||||
| Duck | 8 | 8 | |||||
| Houbara | 9 | 9 | |||||
| Pigeon | 11 | 11 | |||||
| Blackbird | 9 | 1 | 10 | ||||
| Canary | 4 | 2 | 2 | 2 | 1 | 11 | |
| Woodcock | 8 | 3 | 5 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiazim, L.G.; O’Connor, R.E.; Larkin, D.M.; Romanov, M.N.; Narushin, V.G.; Brazhnik, E.A.; Griffin, D.K. Comparative Mapping of the Macrochromosomes of Eight Avian Species Provides Further Insight into Their Phylogenetic Relationships and Avian Karyotype Evolution. Cells 2021, 10, 362. https://doi.org/10.3390/cells10020362
Kiazim LG, O’Connor RE, Larkin DM, Romanov MN, Narushin VG, Brazhnik EA, Griffin DK. Comparative Mapping of the Macrochromosomes of Eight Avian Species Provides Further Insight into Their Phylogenetic Relationships and Avian Karyotype Evolution. Cells. 2021; 10(2):362. https://doi.org/10.3390/cells10020362
Chicago/Turabian StyleKiazim, Lucas G., Rebecca E. O’Connor, Denis M. Larkin, Michael N. Romanov, Valery G. Narushin, Evgeni A. Brazhnik, and Darren K. Griffin. 2021. "Comparative Mapping of the Macrochromosomes of Eight Avian Species Provides Further Insight into Their Phylogenetic Relationships and Avian Karyotype Evolution" Cells 10, no. 2: 362. https://doi.org/10.3390/cells10020362
APA StyleKiazim, L. G., O’Connor, R. E., Larkin, D. M., Romanov, M. N., Narushin, V. G., Brazhnik, E. A., & Griffin, D. K. (2021). Comparative Mapping of the Macrochromosomes of Eight Avian Species Provides Further Insight into Their Phylogenetic Relationships and Avian Karyotype Evolution. Cells, 10(2), 362. https://doi.org/10.3390/cells10020362










