Organization of Neuropeptide Y-Immunoreactive Cells in the Mongolian gerbil (Meriones unguiculatus) Visual Cortex
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Tissue Preparation
2.2. Horseradish Peroxidase Staining
2.3. Fluorescence Immunohistochemistry
2.4. Quantitative Analysis
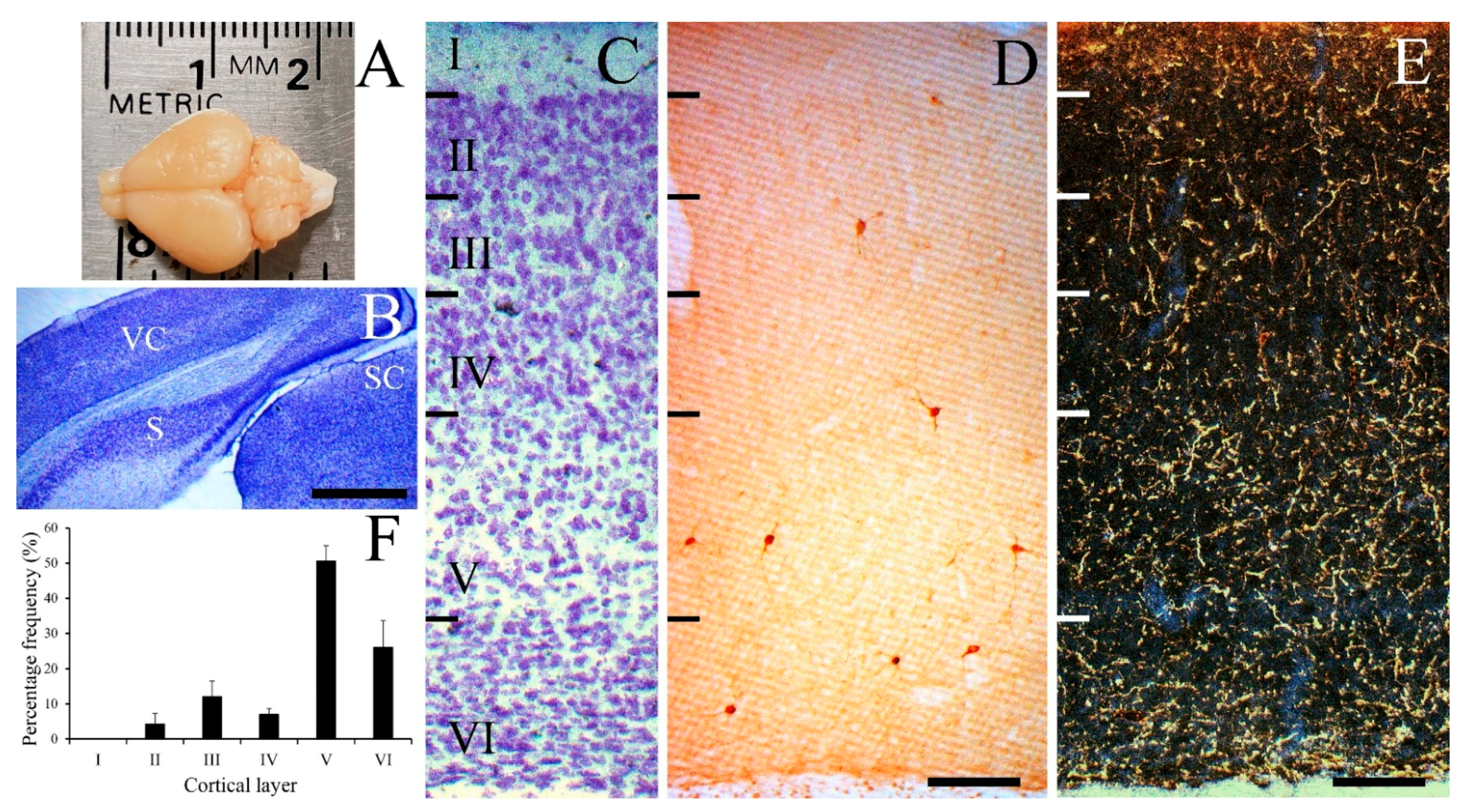
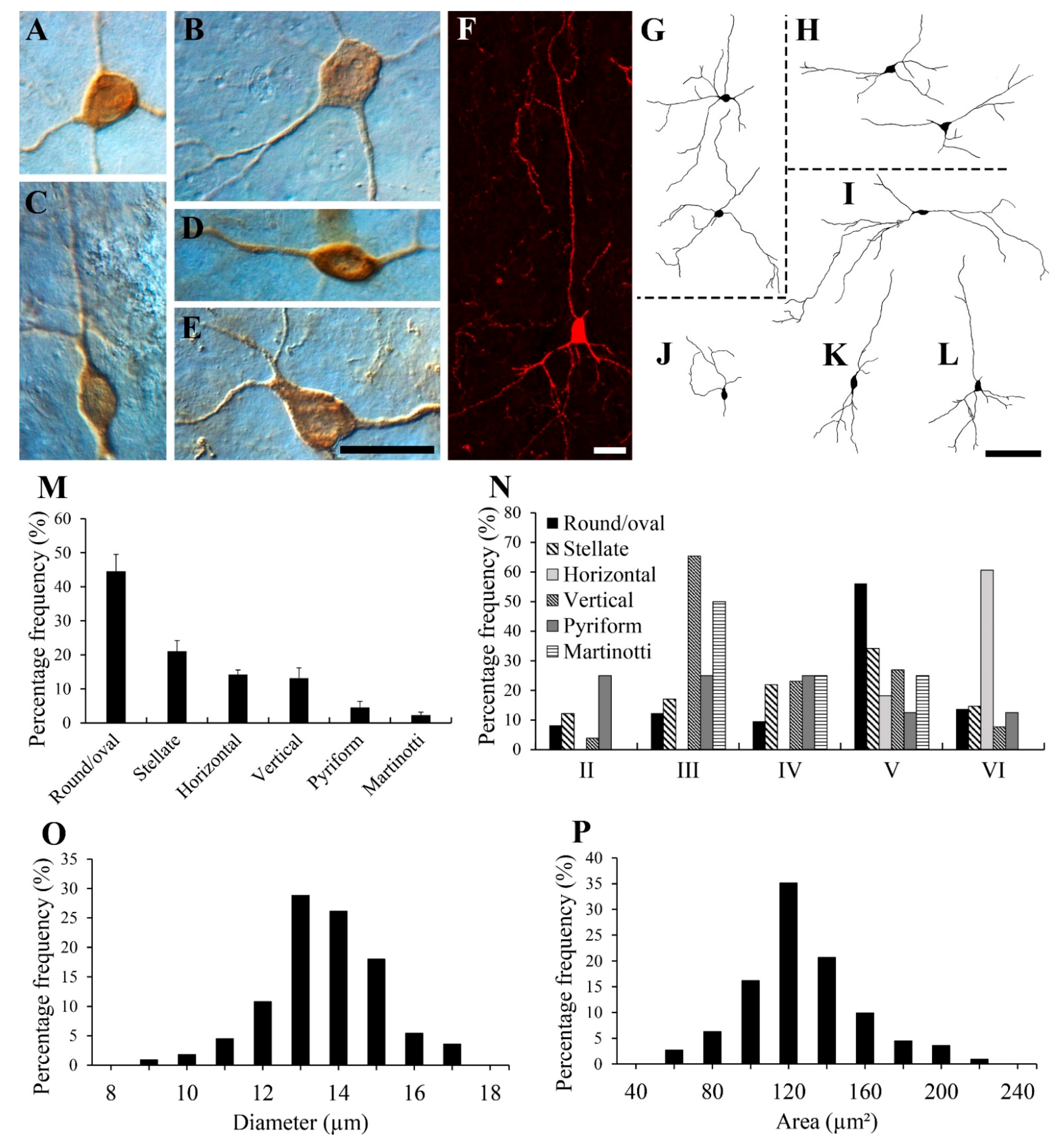
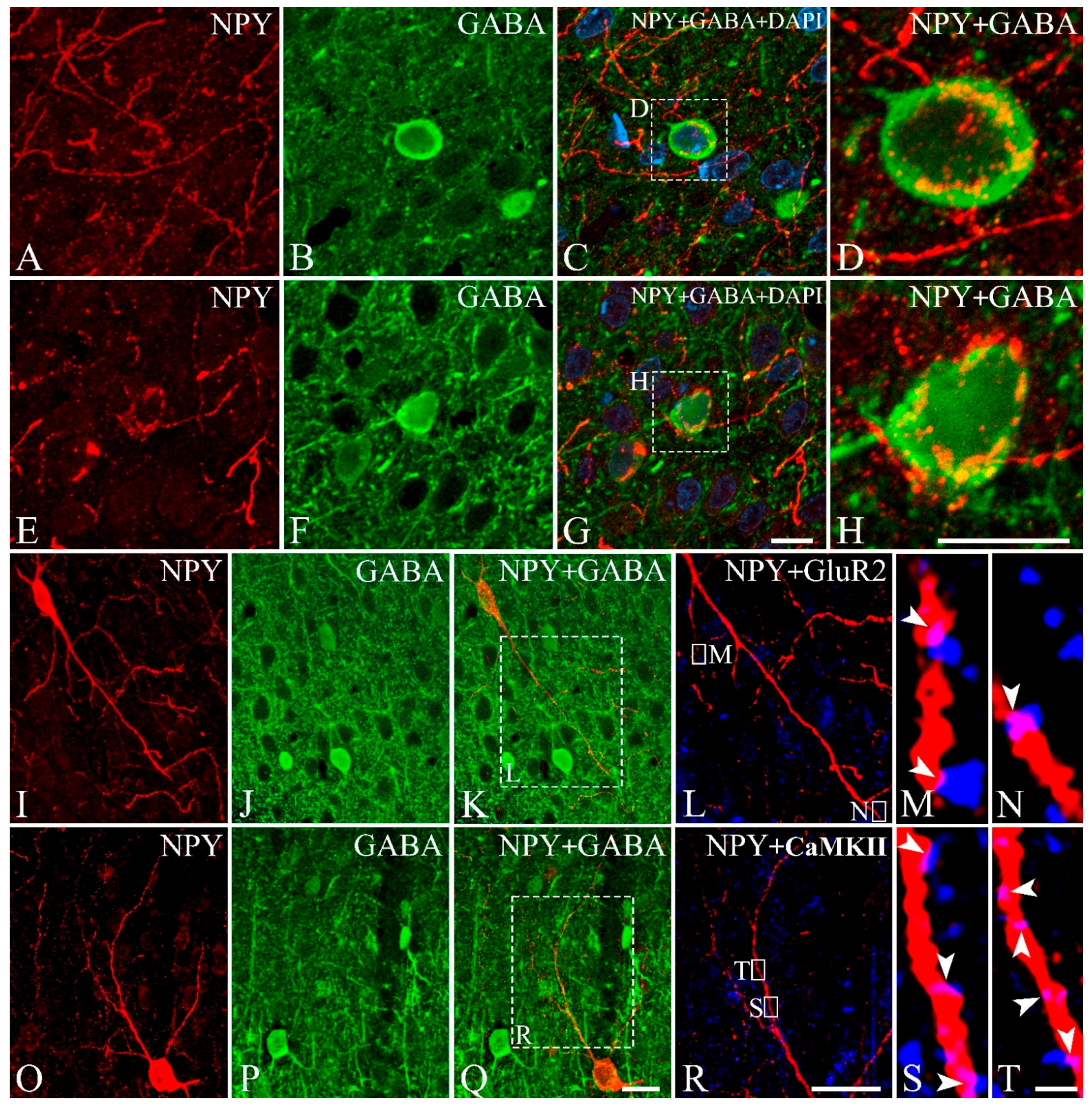
3. Results
3.1. Laminar Distribution of NPY-IR Neurons
3.2. Morphology of NPY-IR Neurons
3.3. Colocalization of NPY with GABA, CBPs, Somatostatin, GluR2, or CaMKII
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allen, Y.S.; Bloom, S.R.; Polak, J.M. The neuropeptide Y-immunoreactive neuronal system: Discovery, anatomy and involvement in neurodegenerative disease. Hum. Neurobiol. 1986, 5, 227–234. [Google Scholar] [PubMed]
- Larhammar, D.; Blomqvist, A.G.; Söderberg, C. Evolution of neuropeptide Y and its related peptides. Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1993, 106, 743–752. [Google Scholar] [CrossRef]
- Tatemoto, K. Neuropeptide Y: Complete amino acid sequence of the brain peptide. Proc. Natl. Acad. Sci. USA 1982, 79, 5485–5489. [Google Scholar] [CrossRef] [PubMed]
- Beck, B.; Pourie, G. Ghrelin, neuropeptide Y, and other feeding-regulatory peptides active in the hippocampus: Role in learning and memory. Nutr. Rev. 2013, 71, 541–561. [Google Scholar] [CrossRef]
- Hokfelt, T.; Stanic, D.; Sanford, S.D.; Gatlin, J.C.; Nilsson, I.; Paratcha, G.; Ledda, F.; Fetissov, S.; Lindfors, C.; Herzog, H.; et al. NPY and its involvement in axon guidance, neurogenesis, and feeding. Nutrition 2008, 24, 860–868. [Google Scholar] [CrossRef]
- Loh, K.; Herzog, H.; Shi, Y.C. Regulation of energy homeostasis by the NPY system. Trends Endocrinol. Metab. 2015, 26, 125–135. [Google Scholar] [CrossRef]
- Thorsell, A.; Heilig, M. Diverse functions of neuropeptide Y revealed using genetically modified animals. Neuropeptides 2002, 36, 182–193. [Google Scholar] [CrossRef]
- Decressac, M.; Barker, R.A. Neuropeptide Y and its role in CNS disease and repair. Exp. Neurol. 2012, 238, 265–272. [Google Scholar] [CrossRef]
- Flood, J.F.; Hernandez, E.N.; Morley, J.E. Modulation of memory processing by neuropeptide Y. Brain Res. 1987, 421, 280–290. [Google Scholar] [CrossRef]
- Flood, J.F.; Baker, M.L.; Hernandez, E.N.; Morley, J.E. Modulation of memory processing by neuropeptide Y varies with brain injection site. Brain Res. 1989, 503, 73–82. [Google Scholar] [CrossRef]
- Gotzsche, C.R.; Woldbye, D.P. The role of NPY in learning and memory. Neuropeptides 2016, 55, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Reichmann, F.; Holzer, P. Neuropeptide Y: A stressful review. Neuropeptides 2016, 55, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Tasan, R.O.; Verma, D.; Wood, J.; Lach, G.; Hormer, B.; de Lima, T.C.; Herzog, H.; Sperk, G. The role of neuropeptide Y in fear conditioning and extinction. Neuropeptides 2016, 55, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Wahlestedt, C.; Pich, E.M.; Koob, G.F.; Yee, F.; Heilig, M. Modulation of anxiety and neuropeptide Y-Y1 receptors by antisense oligodeoxynucleotides. Science 1993, 259, 528–531. [Google Scholar] [CrossRef]
- Albers, H.E.; Ferris, C.F. Neuropeptide Y: Role in light-dark cycle entrainment of hamster circadian rhythms. Neurosci. Lett. 1984, 50, 163–168. [Google Scholar] [CrossRef]
- Erion, R.; King, A.N.; Wu, G.; Hogenesch, J.B.; Sehgal, A. Neural clocks and neuropeptide F/Y regulate circadian gene expression in a peripheral metabolic tissue. Elife 2016, 5, e13552. [Google Scholar] [CrossRef]
- Kah, O.; Pontet, A.; Danger, J.M.; Dubourg, P.; Pelletier, G.; Vaudry, H.; Calas, A. Characterization, cerebral distribution and gonadotropin release activity of neuropeptide Y (NPY) in the goldfish. Fish Physiol. Biochem. 1989, 7, 69–76. [Google Scholar] [CrossRef]
- Kalra, S.P.; Kalra, P.S. Nutritional infertility: The role of the interconnected hypothalamic neuropeptide Y-galanin-opioid network. Front. Neuroendocrinol. 1996, 17, 371–401. [Google Scholar] [CrossRef]
- Beck, B. Neuropeptide Y in normal eating and in genetic and dietary-induced obesity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1159–1185. [Google Scholar] [CrossRef]
- Wu, Y.; He, H.; Cheng, Z.; Bai, Y.; Ma, X. The role of neuropeptide Y and peptide YY in the development of obesity via gut-brain axis. Curr. Protein. Pept. Sci. 2019, 20, 750–758. [Google Scholar] [CrossRef]
- Zhang, L.; Bijker, M.S.; Herzog, H. The neuropeptide Y system: Pathophysiological and therapeutic implications in obesity and cancer. Pharmacol. Ther. 2011, 131, 91–113. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Neves, J.; Pereira de Almeida, L.; Cavadas, C. Neuropeptide Y (NPY) as a therapeutic target for neurodegenerative diseases. Neurobiol. Dis. 2016, 95, 210–224. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.W.; Zhu, P.; Shi, Y.C.; Zhang, C.L.; Huang, X.F.; Liang, S.Y.; Song, Z.Y.; Lin, S. Current views on neuropeptide Y and diabetes-related atherosclerosis. Diab. Vasc. Dis. Res. 2017, 14, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.M.J.; Green, P.; Tapoulal, N.; Lewandowski, A.J.; Leeson, P.; Herring, N. The role of neuropeptide Y in cardiovascular health and disease. Front. Physiol. 2018, 9, 1281. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Sun, W.; Zhang, C.; Song, Z.; Lin, S. The role of neuropeptide Y in the pathophysiology of atherosclerotic cardiovascular disease. Int. J. Cardiol. 2016, 220, 235–241. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Hausken, T. The role of the neuropeptide Y (NPY) family in the pathophysiology of inflammatory bowel disease (IBD). Neuropeptides 2016, 55, 137–144. [Google Scholar] [CrossRef]
- Lay, A.C.; Barrington, A.F.; Hurcombe, J.A.; Ramnath, R.D.; Graham, M.; Lewis, P.A.; Wilson, M.C.; Heesom, K.J.; Butler, M.J.; Perrett, R.M.; et al. A role for NPY-NPY2R signaling in albuminuric kidney disease. Proc. Natl. Acad. Sci. USA 2020, 117, 15862–15873. [Google Scholar] [CrossRef]
- Zoccali, C.; D’Arrigo, G.; Leonardis, D.; Pizzini, P.; Postorino, M.; Tripepi, G.; Mallamaci, F.; van den Brand, J.; van Zuilen, A.; Wetzels, J.; et al. Neuropeptide Y and chronic kidney disease progression: A cohort study. Nephrol. Dial. Transplant. 2018, 33, 1805–1812. [Google Scholar] [CrossRef]
- Berman, N.E.; Fredrickson, E. Morphology and laminar distribution of neuropeptide Y immunoreactive neurons in the human striate cortex. Synapse 1992, 11, 20–27. [Google Scholar] [CrossRef]
- Van Reeth, O.; Goldman, S.; Schiffmann, S.; Verstappen, A.; Pelletier, G.; Vaudry, H.; Vanderhaeghen, J.J. Distribution of neuropeptide Y immunoreactivity in human visual cortex and underlying white matter. Peptides 1987, 8, 1107–1117. [Google Scholar] [CrossRef]
- Hendry, S.H.; Jones, E.G.; Emson, P.C. Morphology, distribution, and synaptic relations of somatostatin- and neuropeptide Y-immunoreactive neurons in rat and monkey neocortex. J. Neurosci. 1984, 4, 2497–2517. [Google Scholar] [CrossRef] [PubMed]
- Kuljis, R.O.; Rakic, P. Neuropeptide Y-containing neurons are situated predominantly outside cytochrome oxidase puffs in macaque visual cortex. Vis. Neurosci. 1989, 2, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Kuljis, R.O.; Rakic, P. Distribution of neuropeptide Y-containing perikarya and axons in various neocortical areas in the macaque monkey. J. Comp. Neurol. 1989, 280, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Kuljis, R.O.; Rakic, P. Multiple types of neuropeptide Y-containing neurons in primate neocortex. J. Comp. Neurol. 1989, 280, 393–409. [Google Scholar] [CrossRef]
- Hogan, D.; Berman, N.E. The development of neuropeptide Y immunoreactive neurons in cat visual cortical areas. Brain Res. Dev. Brain Res. 1992, 67, 343–369. [Google Scholar] [CrossRef]
- Wahle, P.; Meyer, G.; Albus, K. Localization of NPY-immunoreactivity in the cat’s visual cortex. Exp. Brain Res. 1986, 61, 364–374. [Google Scholar] [CrossRef]
- Wahle, P.; Meyer, G. Morphology and quantitative changes of transient NPY-ir neuronal populations during early postnatal development of the cat visual cortex. J. Comp. Neurol. 1987, 261, 165–192. [Google Scholar] [CrossRef]
- Allen, Y.S.; Adrian, T.E.; Allen, J.M.; Tatemoto, K.; Crow, T.J.; Bloom, S.R.; Polak, J.M. Neuropeptide Y distribution in the rat brain. Science 1983, 221, 877–879. [Google Scholar] [CrossRef]
- Papadopoulos, G.C.; Parnavelas, J.G.; Cavanagh, M.E. Extensive co-existence of neuropeptides in the rat visual cortex. Brain Res. 1987, 420, 95–99. [Google Scholar] [CrossRef]
- Gonchar, Y.; Wang, Q.; Burkhalter, A. Multiple distinct subtypes of GABAergic neurons in mouse visual cortex identified by triple immunostaining. Front. Neuroanat. 2008, 1, 3. [Google Scholar] [CrossRef]
- Bagnoli, P.; Fontanesi, G.; Alesci, R.; Erichsen, J.T. Distribution of neuropeptide Y, substance P, and choline acetyltransferase in the developing visual system of the pigeon and effects of unilateral retina removal. J. Comp. Neurol. 1992, 318, 392–414. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Karten, H.J. Immunohistochemical analysis of the visual wulst of the pigeon (Columba livia). J. Comp. Neurol. 1990, 300, 346–369. [Google Scholar] [CrossRef] [PubMed]
- Bennis, M.; Ba m’hamed, S.; Rio, J.P.; Le Cren, D.; Reperant, J.; Ward, R. The distribution of NPY-like immunoreactivity in the chameleon brain. Anat. Embryol. 2001, 203, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.M.; Debski, E.A. Neuropeptide Y immunoreactivity of a projection from the lateral thalamic nucleus to the optic tectum of the leopard frog. Vis. Neurosci. 1995, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Montesano, A.; Baumgart, M.; Avallone, L.; Castaldo, L.; Lucini, C.; Tozzini, E.T.; Cellerino, A.; D’Angelo, L.; de Girolamo, P. Age-related central regulation of orexin and NPY in the short-lived African killifish Nothobranchius furzeri. J. Comp. Neurol. 2019, 527, 1508–1526. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Yamamoto, T.; Nakagawa, M.; Uemura, H. Neuropeptide Y-immunoreactive neuronal system and colocalization with FMRFamide in the optic lobe and peduncle complex of the octopus (Octopus vulgaris). Cell Tissue Res. 2002, 307, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Aoki, C.; Pickel, V.M. Neuropeptide Y in the cerebral cortex and the caudate-putamen nuclei: Ultrastructural basis for interactions with GABAergic and non-GABAergic neurons. J. Neurosci. 1989, 9, 4333–4354. [Google Scholar] [CrossRef]
- Dávila, J.C.; de la Calle, A.; Gutiérrez, A.; Megías, M.; Andreu, M.J.; Guirado, S. Distribution of neuropeptide Y (NPY) in the cerebral cortex of the lizards Psammodromus algirus and podarcis hispanica: Co-localization of NPY, somatostatin, and GABA. J. Comp. Neurol. 1991, 308, 397–408. [Google Scholar] [CrossRef]
- Demeulemeester, H.; Vandesande, F.; Orban, G.A.; Brandon, C.; Vanderhaeghen, J.J. Heterogeneity of GABAergic cells in cat visual cortex. J. Neurosci. 1988, 8, 988–1000. [Google Scholar] [CrossRef]
- Baimbridge, K.G.; Celio, M.R.; Rogers, J.H. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992, 15, 303–308. [Google Scholar] [CrossRef]
- Schwaller, B. Emerging Functions of the “Ca2+ Buffers” Parvalbumin, Calbindin D-28k and Calretinin in the Brain. In Handbook of Neurochemistry and Molecular Neurobiology: Neural Protein Metabolism and Function; Lajtha, A., Banik, N., Eds.; Springer US: Boston, MA, USA, 2007; pp. 197–221. ISBN 978-0-387-30346-8. [Google Scholar] [CrossRef]
- Schwaller, B. The use of transgenic mouse models to reveal the functions of Ca2+ buffer proteins in excitable cells. Biochim. Biophys. Acta 2012, 1820, 1294–1303. [Google Scholar] [CrossRef] [PubMed]
- Fairless, R.; Williams, S.K.; Diem, R. Calcium-binding proteins as determinants of central nervous system neuronal vulnerability to disease. Int. J. Mol. Sci. 2019, 20, 2146. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, B.W.; Heizmann, C.W. The S100 family of EF-hand calcium-binding proteins: Functions and pathology. Trends Biochem. Sci. 1996, 21, 134–140. [Google Scholar] [CrossRef]
- Gonchar, Y.; Burkhalter, A. Three distinct families of GABAergic neurons in rat visual cortex. Cereb. Cortex. 1997, 7, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kong, J.H.; Kang, Y.S.; Park, W.M.; Jeong, S.A.; Park, S.M.; Lim, J.K.; Jeon, C.J. The distribution and morphology of calbindin D28K- and calretinin-immunoreactive neurons in the visual cortex of mouse. Mol. Cells 2002, 14, 143–149. [Google Scholar] [PubMed]
- Kim, T.J.; Ye, E.A.; Jeon, C.J. Distribution of AMPA glutamate receptor GluR1 subunit-immunoreactive neurons and their co-localization with calcium-binding proteins and GABA in the mouse visual cortex. Mol. Cells 2006, 21, 34–41. [Google Scholar] [PubMed]
- Batchelder, M.; Keller, L.S.; Sauer, M.B.; West, W.L. Chapter 52—Gerbils. In The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents; Suckow, M.A., Stevens, K.A., Wilson, R.P., Eds.; Academic Press: Boston, MA, USA, 2012; pp. 1131–1155. ISBN 978-0-12-380920-9. [Google Scholar] [CrossRef]
- Bleich, E.M.; Martin, M.; Bleich, A.; Klos, A. The Mongolian gerbil as a model for inflammatory bowel disease. Int. J. Exp. Pathol. 2010, 91, 281–287. [Google Scholar] [CrossRef]
- Cheal, M.L. The gerbil: A unique model for research on aging. Exp. Aging Res. 1986, 12, 3–21. [Google Scholar] [CrossRef]
- Pecková, R.; Sak, B.; Květoňová, D.; Kváč, M.; Koriťáková, E.; Foitová, I. The course of experimental giardiasis in Mongolian gerbil. Parasitol. Res. 2018, 117, 2437–2443. [Google Scholar] [CrossRef]
- Vincent, A.L.; Rodrick, G.E.; Sodeman, W.A., Jr. The pathology of the Mongolian Gerbil (Meriones unguiculatus): A review. Lab. Anim. Sci. 1979, 29, 645–651. [Google Scholar]
- Bytyqi, A.H.; Layer, P.G. Lamina formation in the Mongolian gerbil retina (Meriones unguiculatus). Anat. Embryol. 2005, 209, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Ingle, D.J. New methods for analysis of vision in the gerbil. Behavioural. Brain Research. 1981, 3, 151–173. [Google Scholar] [CrossRef]
- Macharadze, T.; Budinger, E.; Brosch, M.; Scheich, H.; Ohl, F.W.; Henschke, J.U. Early sensory loss alters the dendritic branching and spine density of supragranular pyramidal neurons in rodent primary sensory cortices. Front. Neural Circuits 2019, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Govardovskii, V.I.; Rohlich, P.; Szel, A.; Khokhlova, T.V. Cones in the retina of the Mongolian gerbil, Meriones unguiculatus: An immunocytochemical and electrophysiological study. Vision Res. 1992, 32, 19–27. [Google Scholar] [CrossRef]
- Garcia Garrido, M.; Beck, S.C.; Mühlfriedel, R.; Julien, S.; Schraermeyer, U.; Seeliger, M.W. Towards a quantitative OCT image analysis. PLoS ONE 2014, 9, e100080. [Google Scholar] [CrossRef] [PubMed]
- Huber, G.; Heynen, S.; Imsand, C.; vom Hagen, F.; Muehlfriedel, R.; Tanimoto, N.; Feng, Y.; Hammes, H.P.; Grimm, C.; Peichl, L.; et al. Novel rodent models for macular research. PLoS ONE 2010, 5, e13403. [Google Scholar] [CrossRef]
- Jeong, M.J.; Jeon, C.J. Localization of melanopsin-immunoreactive cells in the Mongolian gerbil retina. Neurosci. Res. 2015, 100, 6–16. [Google Scholar] [CrossRef]
- Luan, L.; Ren, C.; Lau, B.W.; Yang, J.; Pickard, G.E.; So, K.F.; Pu, M. Y-like retinal ganglion cells innervate the dorsal raphe nucleus in the Mongolian gerbil (Meriones unguiculatus). PLoS ONE 2011, 6, e18938. [Google Scholar] [CrossRef]
- Luan, L.; Ren, C.; Wang, W.; Nan, Y.; Gao, J.; Pu, M. Morphological properties of medial amygdala-projecting retinal ganglion cells in the Mongolian gerbil. Sci. China Life Sci. 2018, 61, 644–650. [Google Scholar] [CrossRef]
- Ren, C.; Pu, M.; Cui, Q.; So, K.F. Dendritic morphology of caudal periaqueductal gray projecting retinal ganglion cells in Mongolian gerbil (Meriones unguiculatus). PLoS ONE 2014, 9, e103306. [Google Scholar] [CrossRef][Green Version]
- Zhang, T.; Huang, L.; Zhang, L.; Tan, M.; Pu, M.; Pickard, G.E.; So, K.F.; Ren, C. ON and OFF retinal ganglion cells differentially regulate serotonergic and GABAergic activity in the dorsal raphe nucleus. Sci. Rep. 2016, 6, 26060. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.K.; Kim, J.Y.; Jeon, C.J. Immunocytochemical localization of calretinin in the superficial layers of the cat superior colliculus. Neurosci. Res. 2002, 44, 325–335. [Google Scholar] [CrossRef]
- Jeon, C.J.; Strettoi, E.; Masland, R.H. The major cell populations of the mouse retina. J. Neurosci. 1998, 18, 8936–8946. [Google Scholar] [CrossRef] [PubMed]
- Tigges, M.; Tigges, J.; McDonald, J.K.; Slattery, M.; Fernandes, A. Postnatal development of neuropeptide Y-like immunoreactivity in area 17 of normal and visually deprived rhesus monkeys. Vis. Neurosci. 1989, 2, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulos, J.; Papadopoulos, G.C.; Michaloudi, H.; Cavanagh, M.E.; Parnavelas, J.G. Postnatal development of neuropeptide Y-containing neurons in the visual cortex of normal- and dark-reared rats. Neurosci. Lett. 1992, 145, 75–78. [Google Scholar] [CrossRef]
- Cavanagh, M.E.; Parnavelas, J.G. Development of neuropeptide Y (NPY) immunoreactive neurons in the rat occipital cortex: A combined immunohistochemical-autoradiographic study. J. Comp. Neurol. 1990, 297, 553–563. [Google Scholar] [CrossRef]
- Wai, S.M.; Kindler, P.M.; Lam, E.T.; Zhang, A.; Yew, D.T. Distribution of neuropeptide Y-immunoreactive neurons in the human brainstem, cerebellum, and cortex during development. Cell Mol. Neurobiol. 2004, 24, 667–684. [Google Scholar] [CrossRef]
- Hendry, S.H.; Jones, E.G.; DeFelipe, J.; Schmechel, D.; Brandon, C.; Emson, P.C. Neuropeptide-containing neurons of the cerebral cortex are also GABAergic. Proc. Natl. Acad. Sci. USA 1984, 81, 6526–6530. [Google Scholar] [CrossRef]
- Winters, G.C.; Polese, G.; Di Cosmo, A.; Moroz, L.L. Mapping of neuropeptide Y expression in Octopus brains. J. Morphol. 2020, 281, 790–801. [Google Scholar] [CrossRef]
- Silveira, M.A.; Anair, J.D.; Beebe, N.L.; Mirjalili, P.; Schofield, B.R.; Roberts, M.T. Neuropeptide Y expression defines a novel class of GABAergic projection neuron in the inferior colliculus. J. Neurosci. 2020, 40, 4685–4699. [Google Scholar] [CrossRef]
- Jones, E.G.; Hendry, S.H. Co-localization of GABA and neuropeptides in neocortical neurons. Trends Neurosci. 1986, 9, 71–76. [Google Scholar] [CrossRef]
- Karagiannis, A.; Gallopin, T.; Dávid, C.; Battaglia, D.; Geoffroy, H.; Rossier, J.; Hillman, E.M.; Staiger, J.F.; Cauli, B. Classification of NPY-expressing neocortical interneurons. J. Neurosci. 2009, 29, 3642–3659. [Google Scholar] [CrossRef] [PubMed]
- Milner, T.A.; Wiley, R.G.; Kurucz, O.S.; Prince, S.R.; Pierce, J.P. Selective changes in hippocampal neuropeptide Y neurons following removal of the cholinergic septal inputs. J. Comp. Neurol. 1997, 386, 46–59. [Google Scholar] [CrossRef]
- Marshall, C.J.; Desroziers, E.; McLennan, T.; Campbell, R.E. Defining subpopulations of arcuate nucleus GABA neurons in male, female, and prenatally androgenized female mice. Neuroendocrinology 2017, 105, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Ke, W.; He, Q.; Wang, X.; Zheng, R.; Li, T.; Luan, G.; Long, Y.S.; Liao, W.P.; Shu, Y. Laminar distribution of neurochemically-identified interneurons and cellular co-expression of molecular markers in epileptic human cortex. Neurosci. Bull. 2018, 34, 992–1006. [Google Scholar] [CrossRef] [PubMed]
- Demeulemeester, H.; Arckens, L.; Vandesande, F.; Orban, G.A.; Heizmann, C.W.; Pochet, R. Calcium binding proteins and neuropeptides as molecular markers of GABAergic interneurons in the cat visual cortex. Exp. Brain Res. 1991, 84, 538–544. [Google Scholar] [CrossRef]
- Masland, R.H. Neuronal cell types. Curr. Biol. 2004, 14, R497–R500. [Google Scholar] [CrossRef]
- Markram, H.; Toledo-Rodriguez, M.; Wang, Y.; Gupta, A.; Silberberg, G.; Wu, C. Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci. 2004, 5, 793–807. [Google Scholar] [CrossRef]
- Huntley, M.A.; Srinivasan, K.; Friedman, B.A.; Wang, T.M.; Yee, A.X.; Wang, Y.; Kaminker, J.S.; Sheng, M.; Hansen, D.V.; Hanson, J.E. Genome-wide analysis of differential gene expression and splicing in excitatory neurons and interneuron subtypes. J. Neurosci. 2020, 40, 958–973. [Google Scholar] [CrossRef]
- Bacci, A.; Huguenard, J.R.; Prince, D.A. Differential modulation of synaptic transmission by neuropeptide Y in rat neocortical neurons. Proc. Natl. Acad. Sci. USA 2002, 99, 17125–17130. [Google Scholar] [CrossRef]
- Jackson, J.; Karnani, M.M.; Zemelman, B.V.; Burdakov, D.; Lee, A.K. Inhibitory control of prefrontal cortex by the claustrum. Neuron 2018, 99, 1029–1039.e4. [Google Scholar] [CrossRef] [PubMed]
- Grünert, U.; Lin, B.; Martin, P.R. Glutamate receptors at bipolar synapses in the inner plexiform layer of primate retina: Light microscopic analysis. J. Comp. Neurol. 2003, 466, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Jeon, C.J.; Kong, J.H.; Strettoi, E.; Rockhill, R.; Stasheff, S.F.; Masland, R.H. Pattern of synaptic excitation and inhibition upon direction-selective retinal ganglion cells. J. Comp. Neurol. 2002, 449, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Aoki, C.; Pickel, V.M. Neuropeptide Y in cortex and striatum. Ultrastructural distribution and coexistence with classical neurotransmitters and neuropeptides. Ann. N. Y. Acad. Sci. 1990, 611, 186–205. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.M.; Clark, R.M.; Hoyle, J.A.; Dickson, T.C. Pathogenic or protective? Neuropeptide Y in amyotrophic lateral sclerosis. J. Neurochem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Neves, J.; Gonçalves, N.; Cunha-Santos, J.; Simões, A.T.; den Dunnen, W.F.; Hirai, H.; Kügler, S.; Cavadas, C.; Pereira de Almeida, L. Neuropeptide Y mitigates neuropathology and motor deficits in mouse models of Machado-Joseph disease. Hum. Mol. Genet. 2015, 24, 5451–5463. [Google Scholar] [CrossRef]
- Allegra, M.; Genovesi, S.; Maggia, M.; Cenni, M.C.; Zunino, G.; Sgado, P.; Caleo, M.; Bozzi, Y. Altered GABAergic markers, increased binocularity and reduced plasticity in the visual cortex of Engrailed-2 knockout mice. Front. Cell Neurosci. 2014, 8, 163. [Google Scholar] [CrossRef]

| Antibodies | Animal | No. Sections | No. NPY Cells | No. Double | % Double (Mean ± S.D.) |
|---|---|---|---|---|---|
| GABA | #1 | 4 | 53 | 46 | 86.79 ± 5.62 |
| #2 | 4 | 84 | 77 | 91.67 ± 6.36 | |
| #3 | 4 | 64 | 57 | 89.06 ± 10.83 | |
| GABA total | 12 | 201 | 180 | 89.55 ± 8.58 | |
| Calbindin-D28K | #1 | 6 | 84 | 2 | 2.38 ± 2.73 |
| #2 | 6 | 85 | 5 | 5.88 ± 5.30 | |
| #3 | 6 | 98 | 3 | 3.06 ± 5.08 | |
| Calbindin-D28K total | 18 | 267 | 10 | 3.75 ± 1.85 | |
| Calretinin | #1 | 4 | 49 | 14 | 28.57 ± 12.88 |
| #2 | 4 | 79 | 28 | 35.44 ± 11.32 | |
| #3 | 4 | 70 | 19 | 27.14 ± 6.72 | |
| Calretinin total | 12 | 198 | 61 | 30.81 ± 4.44 | |
| Parvalbumin | #1 | 4 | 64 | 0 | 0 |
| #2 | 4 | 59 | 0 | 0 | |
| #3 | 4 | 53 | 0 | 0 | |
| Parvalbumin total | 12 | 176 | 0 | 0 | |
| #1 | 4 | 83 | 71 | 85.54 ± 0.58 | |
| Somatostatin | #2 | 4 | 78 | 69 | 88.46 ± 9.70 |
| #3 | 4 | 88 | 84 | 95.45 ± 2.38 | |
| Somatostatin total | 12 | 249 | 224 | 89.95 ± 5.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-J.; Lee, W.-T.; Jeon, C.-J. Organization of Neuropeptide Y-Immunoreactive Cells in the Mongolian gerbil (Meriones unguiculatus) Visual Cortex. Cells 2021, 10, 311. https://doi.org/10.3390/cells10020311
Lee M-J, Lee W-T, Jeon C-J. Organization of Neuropeptide Y-Immunoreactive Cells in the Mongolian gerbil (Meriones unguiculatus) Visual Cortex. Cells. 2021; 10(2):311. https://doi.org/10.3390/cells10020311
Chicago/Turabian StyleLee, Myung-Jun, Won-Tae Lee, and Chang-Jin Jeon. 2021. "Organization of Neuropeptide Y-Immunoreactive Cells in the Mongolian gerbil (Meriones unguiculatus) Visual Cortex" Cells 10, no. 2: 311. https://doi.org/10.3390/cells10020311
APA StyleLee, M.-J., Lee, W.-T., & Jeon, C.-J. (2021). Organization of Neuropeptide Y-Immunoreactive Cells in the Mongolian gerbil (Meriones unguiculatus) Visual Cortex. Cells, 10(2), 311. https://doi.org/10.3390/cells10020311





