Cytoprotective Effects of Taurine on Heat-Induced Bovine Mammary Epithelial Cells In Vitro
Abstract
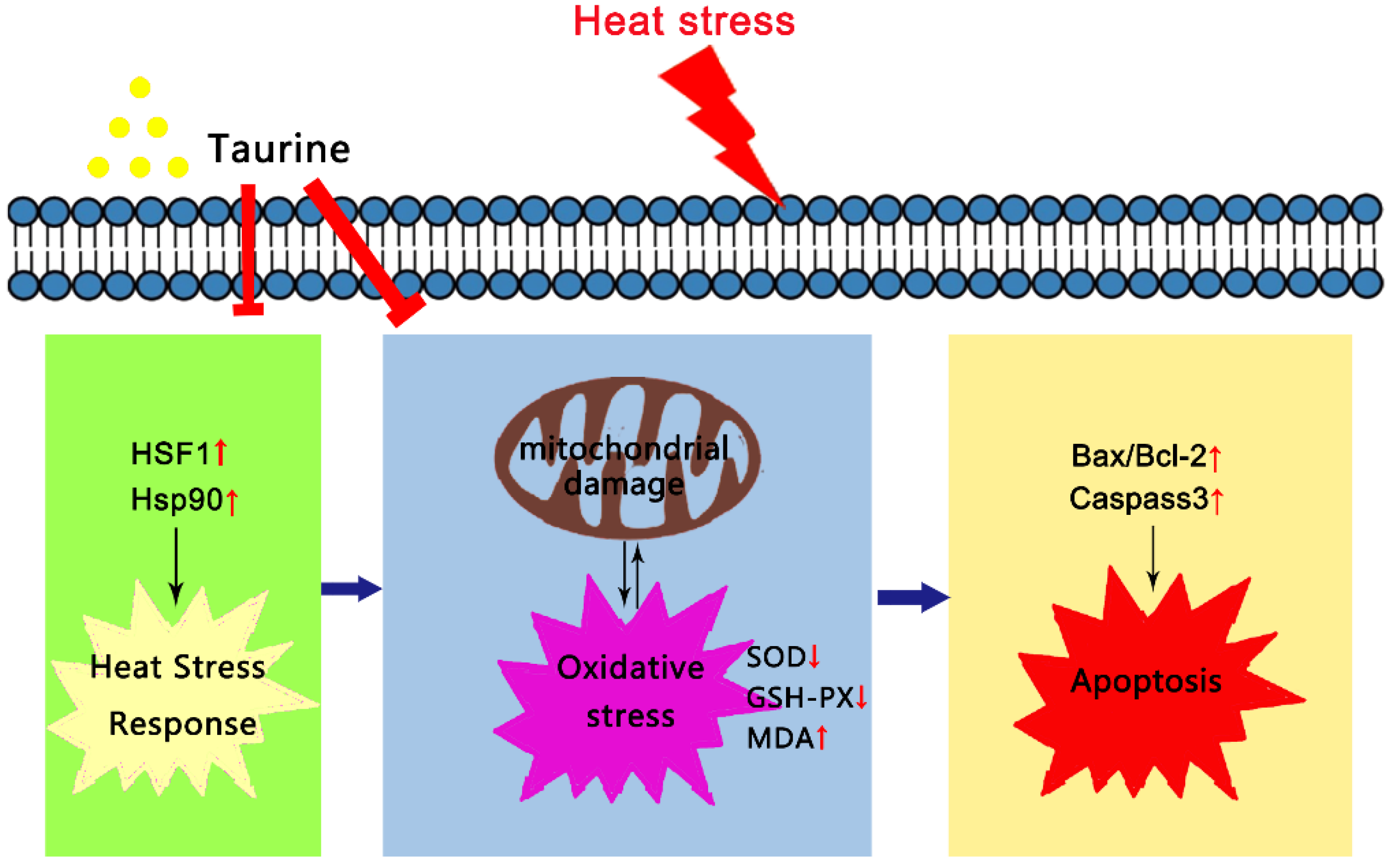
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture and Treatment
2.3. Cell Viability
2.4. Ultrastructural Observation
2.5. Detection of Mitochondrial Complex I Activity
2.6. Antioxidant Capacity Measurement
2.7. Detection of Apoptosis
2.8. RNA Isolation and Quantitative Real-Time PCR (qPCR)
2.9. Immunofluorescent Staining
2.10. Western Blotting Assay
2.11. Statistical Analysis
3. Results
3.1. Cell Viability and Change in Cell Morphology of Mammary Epithelial Cells after Heat-Induced Treatment
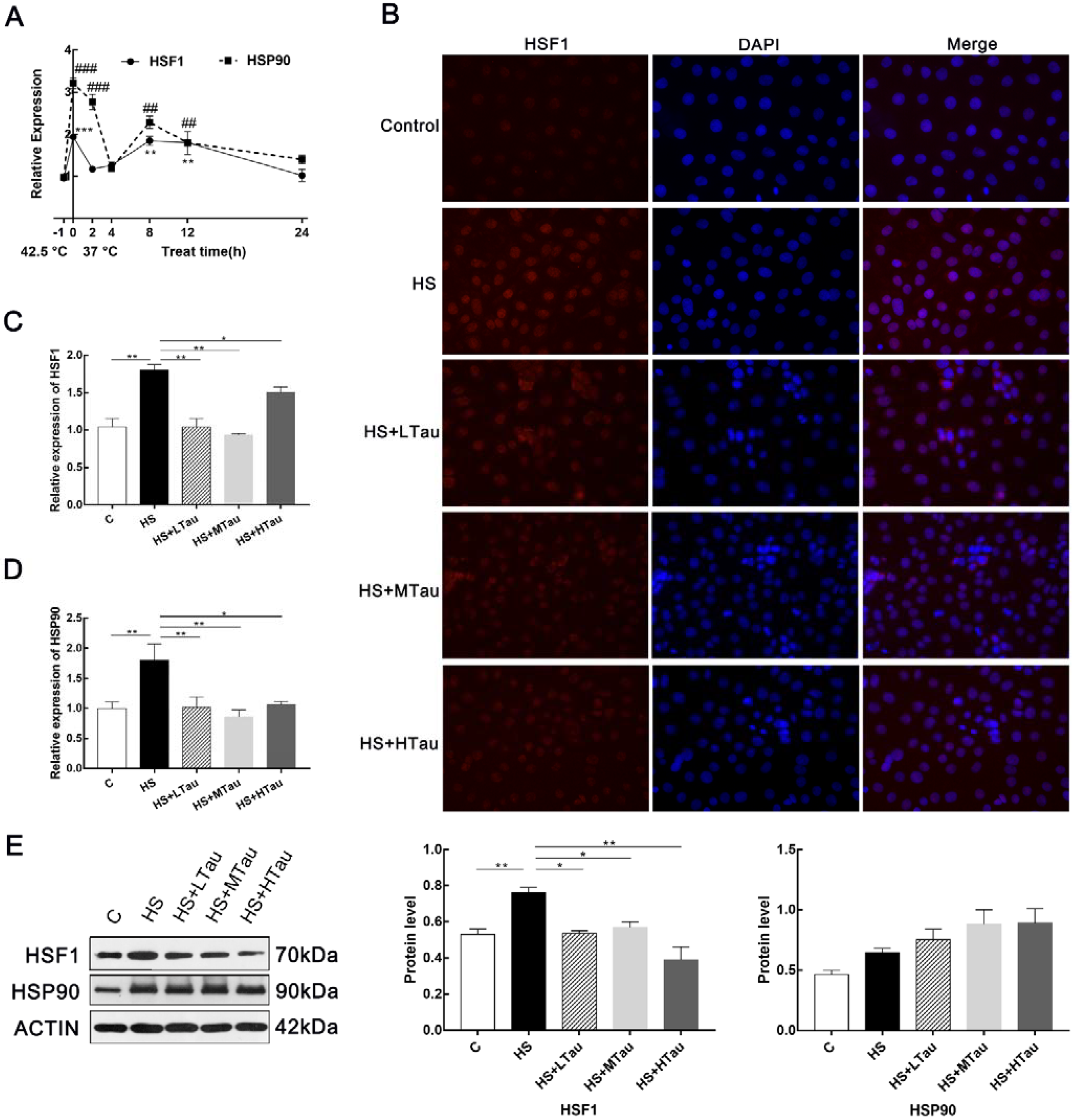
3.2. Taurine Alleviates Heat Shock Response in MAC-T Cells
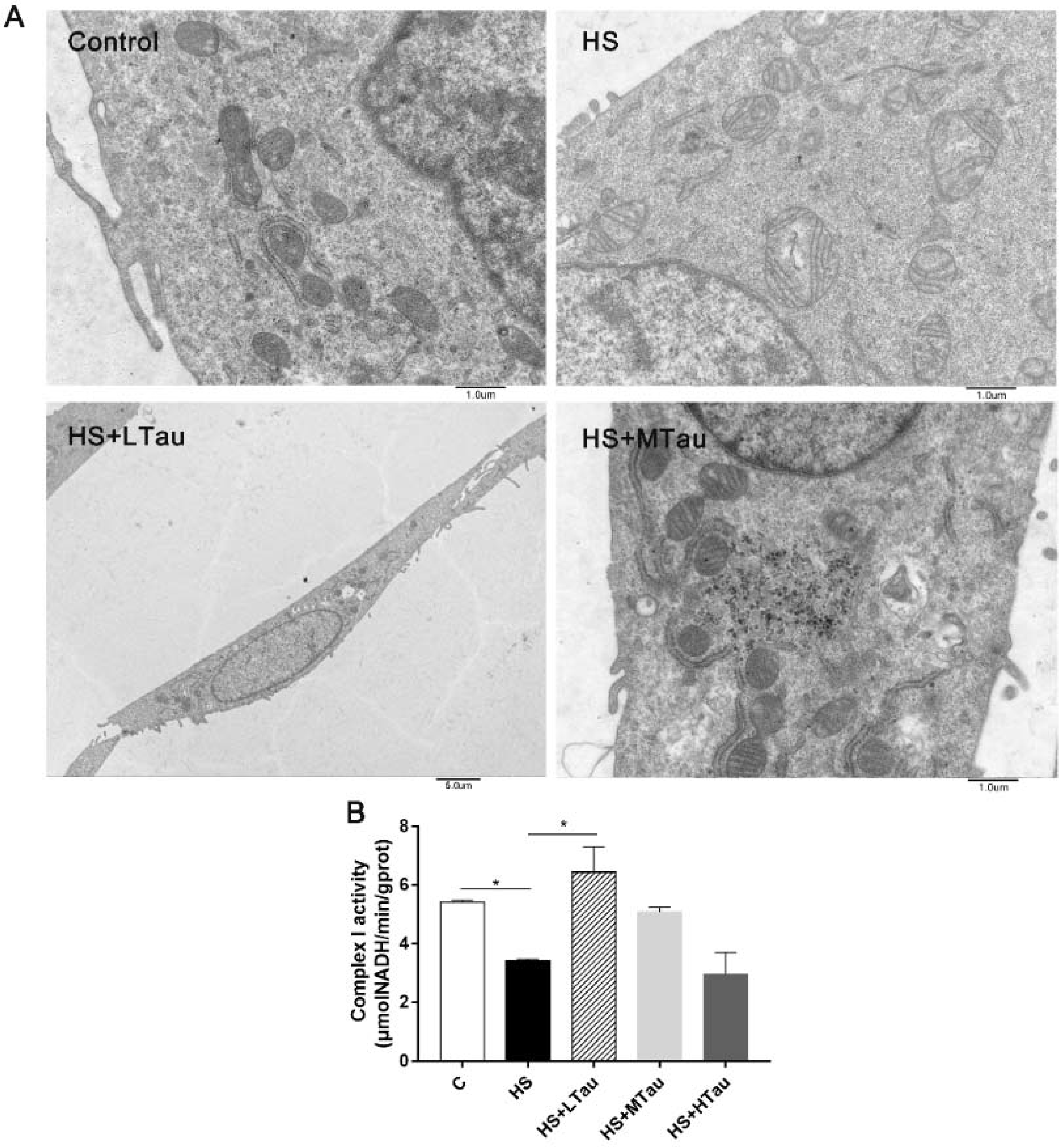
3.3. Taurine Relieves Structural Damage and the Decline in the Complex I Activity of Mitochondria under Heat Shock
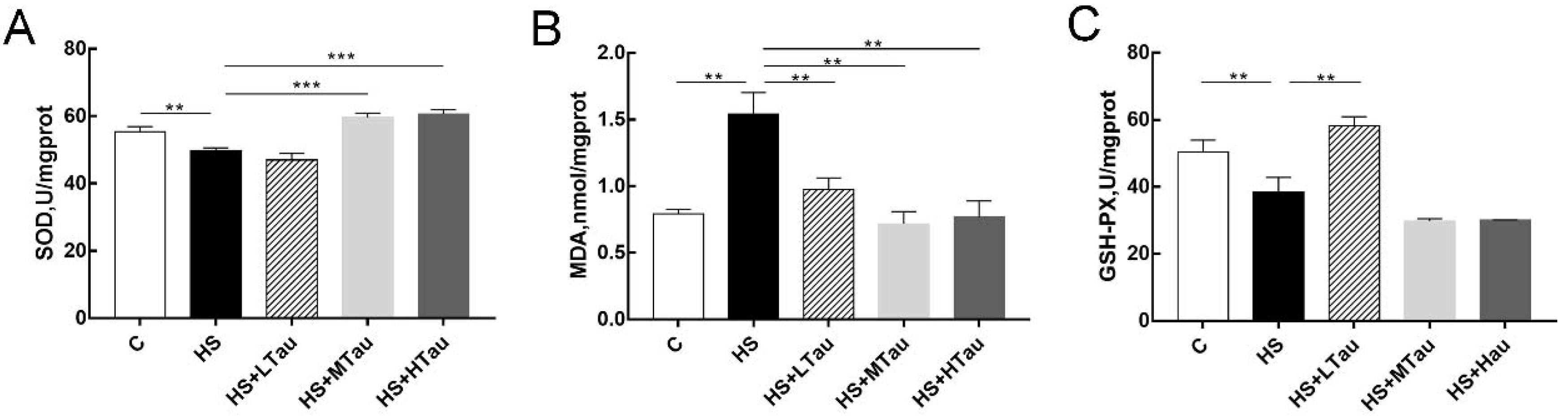
3.4. Taurine Enhances Antioxidant Capacity of Heat-Induced MAC-T Cells
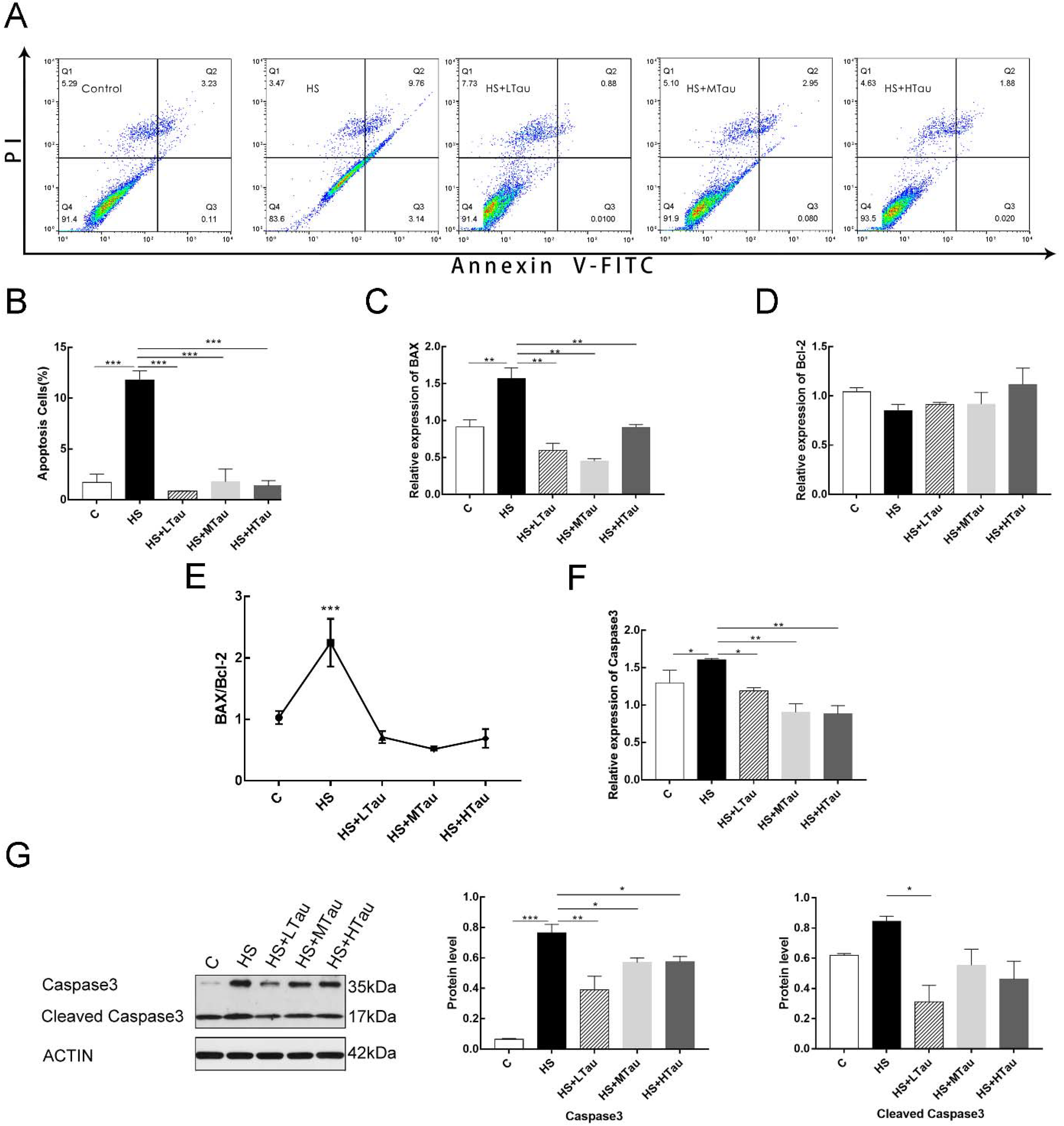
3.5. Taurine Reduces Cell Apoptosis Induced by Heat Shock
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMECs | bovine mammary epithelial cells |
| MAC-T | mammary alveolar cells-large T antigen, a bovine mammary epithelial cell (BMEC) line |
| MTT | measuring 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| PI | propidium iodide |
| DAPI | 4′,6-Diamidino-2-phenylindole dihydrochloride |
| RPS9 | ribosomal protein S9 |
| UXT | ubiquitously expressed prefoldin like chaperone |
| RPS15 | ribosomal protein S15 |
| HSF1 | heat shock transcription factor 1 |
| HSP90 | heat shock protein 90 |
| BAX | BCL2 associated X, apoptosis regulator |
| BCL2 | BCL2 apoptosis regulator |
| SOD | superoxide dismutase |
| GSH-PX | glutathione peroxidase |
| MDA | malondialdehyde |
References
- Sejian, V.; Bhatta, R.; Gaughan, J.B.; Dunshea, F.R.; Lacetera, N. Review: Adaptation of animals to heat stress. Animal 2018, 12, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Bernabucci, U.; Biffani, S.; Buggiotti, L.; Vitali, A.; Lacetera, N.; Nardone, A. The effects of heat stress in Italian Holstein dairy cattle. J. Dairy Sci. 2014, 97, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Nardone, A.; Ronchi, B.; Lacetera, N.; Ranieri, M.S.; Bernabucci, U. Effects of climate changes on animal production and sustainability of livestock systems. Livest. Sci. 2010, 130, 57–69. [Google Scholar] [CrossRef]
- Hammami, H.; Bormann, J.; M’hamdi, N.; Montaldo, H.H.; Gengler, N. Evaluation of heat stress effects on production traits and somatic cell score of Holsteins in a temperate environment. J. Dairy Sci. 2013, 96, 1844–1855. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.L.; Smith, T.; Rude, B.J.; Ward, S.H. Short communication: Comparison of the effects of heat stress on milk and component yields and somatic cell score in Holstein and Jersey cows. J. Dairy Sci. 2013, 96, 3028–3033. [Google Scholar] [CrossRef] [PubMed]
- Key, N.D.; Sneeringer, S.; Marquardt, D. Climate change, heat stress, and US. dairy production. Econ. Res. Rep. 2014. [Google Scholar] [CrossRef]
- Tao, S.; Orellana, R.M.; Weng, X.; Marins, T.N.; Dahl, G.E.; Bernard, J.K. Symposium review: The influences of heat stress on bovine mammary gland function. J. Dairy Sci. 2018, 101, 5642–5654. [Google Scholar] [CrossRef]
- Li, L.; Sun, Y.; Wu, J.; Li, X.; Luo, M.; Wang, G. The global effect of heat on gene expression in cultured bovine mammary epithelial cells. Cell Stress Chaperones 2015, 20, 381–389. [Google Scholar] [CrossRef]
- Weng, X.; Monteiro, A.P.A.; Guo, J. Effects of heat stress and dietary zinc source on performance and mammary epithelial integrity of lactating dairy cows. J. Dairy Sci. 2018, 101, 2617–2630. [Google Scholar] [CrossRef]
- Islam, M.A.; Noguchi, Y.; Taniguchi, S.; Yonekura, S. Protective effects of 5-Aminolevulinic acid on heat stress in bovine mammary epithelial cells. Asian Australas. J. Anim. Sci. 2020. [Google Scholar] [CrossRef]
- Xiao, Y.; Rungruang, S.; Hall, L.W.; Collier, J.L.; Dunshea, F.R.; Collier, R.J. Effects of niacin and betaine on bovine mammary and uterine cells exposed to thermal shock in vitro. J. Dairy Sci. 2017, 100, 4025–4037. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.A.K.; Duque, M.; Wang, L.; Shahzad, K.; Olivera, M.; Loor, J.J. Enhanced supply of methionine or arginine alters mechanistic target of rapamycin signaling proteins, messenger RNA, and microRNA abundance in heat-stressed bovine mammary epithelial cells in vitro. J. Dairy Sci. 2019, 102, 2469–2480. [Google Scholar] [CrossRef]
- Huxtable, R.J. Physiological actions of taurine. Physiol. Rev. 1992, 72, 101. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.; Azuma, J.; Takahashi, K.; Mozaffari, M. Why is taurine cytoprotective? Adv. Exp. Med. Biol. 2003, 526, 307. [Google Scholar] [PubMed]
- Haider, S.; Sajid, I.; Batool, Z.; Madiha, S.; Khaliq, S. Supplementation of taurine insulates against oxidative stress, confers neuroprotection and attenuates memory impairment in noise stress exposed male Wistar rats. Neurochem. Res. 2020, 45, 2762–2774. [Google Scholar] [CrossRef]
- Cui, Y.; Wu, G.; Wang, Z.; Huang, F.; Ning, Z.; Chu, L.; Yang, S.; Lv, Q.; Hu, J. Effects of taurine on broiler aortic endothelial apoptosis induced by heat stress. Taurine 2019, 1155, 391–406. [Google Scholar]
- Lak, S.; Ostadrahimi, A.; Nagili, B.; Asghari-Jafarabadi, M.; Djafarzadeh, R. Anti-inflammatory effect of taurine in burned patients. Adv. Pharm. Bull. 2015, 5, 531–536. [Google Scholar] [CrossRef]
- Schuller-Levis, G.B.; Park, E. Taurine and its chloramine: Modulators of immunity. Neurochem. Res. 2004, 29, 117–126. [Google Scholar] [CrossRef]
- Menzie, J.; Pan, C.; Prentice, H.; Wu, J.Y. Taurine and central nervous system disorders. Amino Acids 2014, 46, 31–46. [Google Scholar] [CrossRef]
- Bernabucci, U.; Lacetera, N.; Baumgard, L.H.; Rhoads, R.P.; Ronchi, B.; Nardone, A. Metabolic and hormonal acclimation to heat stress in domesticated ruminants. Animal 2010, 4, 1167–1183. [Google Scholar] [CrossRef]
- Rhoads, M.L.; Kim, J.W.; Collier, R.J.; Crooker, B.A.; Boisclair, Y.R.; Baumgard, L.H.; Rhoads, R.P. Effects of heat stress and nutrition on lactating holstein cows: II. Aspects of hepatic growth hormone responsiveness. J. Dairy Sci. 2010, 93, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.L.; Wall, E. Dairy cattle in a temperate climate: The effects of weather on milk yield and composition depend on management. Animal 2015, 9, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Y.; Li, L.; Han, Z.; Mao, S.; Wang, G. Betaine protects against heat exposure-induced oxidative stress and apoptosis in bovine mammary epithelial cells via regulation of ROS production. Cell Stress Chaperones 2019, 24, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Shao, J.; Li, Y.; Li, Y.; Zhao, F.; Liu, J.; Liu, H. Protective effects of inorganic and organic selenium on heat stress in bovine mammary epithelial cells. Oxidative Med. Cell. Longev. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Roy, A.; Sil, P.C. Tertiary butyl hydroperoxide induced oxidative damage in mice erythrocytes: Protection by taurine. Pathophysiology 2012, 19, 137–148. [Google Scholar] [CrossRef]
- Rosa, F.T.; Freitas, E.C.; Deminice, R.; Jordo, A.A.; Marchini, J.S. Oxidative stress and inflammation in obesity after taurine supplementation: A double-blind, placebo-controlled study. Eur. J. Nutr. 2013, 53, 823–830. [Google Scholar] [CrossRef]
- Li, M.; Reynolds, C.M.; Gray, C.; Patel, R.; Vickers, M.H. Long-term effects of a maternal high-fat: High-fructose diet on offspring growth and metabolism and impact of maternal taurine supplementation. J. Dev. Orig. Health Dis. 2019, 11, 419–426. [Google Scholar] [CrossRef]
- Miao, J.; Fa, Y.; Gu, B.; Zhu, W.; Zou, S. Taurine attenuates lipopolysaccharide-induced disfunction in mouse mammary epithelial cells. Cytokine 2012, 59, 35–40. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Z.; Chen, B.; Bu, Q.; Lu, W.; Zhu, Y.; Deng, Y.; Shao, X.; Hou, J.; Zhao, J.; et al. Taurine attenuates methamphetamine-induced autophagy and apoptosis in PC12 cells through mTOR signaling pathway. Toxicol. Lett. 2012, 215, 1–7. [Google Scholar] [CrossRef]
- Richter, K.; Haslbeck, M.; Buchner, J. The heat shock response: Life on the verge of death. Mol. Cell 2010, 40, 253–266. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, Y.; Zheng, N. The effect of heat stress on gene expression and synthesis of heat-shock and milk proteins in bovine mammary epithelial cells. Anim. Sci. J. 2016, 87, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Belal, S.A.; Kang, D.R.; Cho, E.S.R.; Park, G.; Shim, K. Taurine reduces heat stress by regulating the expression of heat shock proteins in broilers exposed to chronic heat. Braz. J. Poult. Sci. 2018, 20, 479–486. [Google Scholar] [CrossRef]
- Cadenas, E.; Davies, K.J. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef]
- Lu, Z.; He, X.; Ma, B.; Lin, Z.; Li, J.; Yun, J.; Zhou, G.; Feng, G. Dietary taurine supplementation improves breast meat quality in chronic heat-stressed broilers via activating the Nrf2 pathway and protecting mitochondria from oxidative attack. J. Sci. Food Agric. 2019, 99, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Jong, C.J.; Azuma, J.; Schaffer, S. Mechanism underlying the antioxidant activity of taurine: Prevention of mitochondrial oxidant production. Amino Acids 2012, 42, 2223–2232. [Google Scholar] [CrossRef]
- Belhadj Slimen, I.; Najar, T.; Ghram, A.; Abdrrabba, M. Heat stress effects on livestock: Molecular, cellular and metabolic aspects, a review. J. Anim. Physiol. Anim. Nutr. 2016, 100, 401–412. [Google Scholar] [CrossRef]
- Gb, D. Biochemica and physiological changes during thermal stress in bovines. J. Vet. Sci. Technol. 2013, 3, 423–430. [Google Scholar] [CrossRef]
- Aengwanich, W.; Kongbuntad, W.; Boonsorn, T. Effects of shade on physiological changes, oxidative stress, and total antioxidant power in Thai Brahman cattle. Int. J. Biometeorol. 2011, 55, 741–748. [Google Scholar] [CrossRef]
- Balkan, J. Taurine has a protective effect against thioacetamide-induced liver cirrhosis by decreasing oxidative stress. Hum. Exp. Toxicol. 2001, 20, 251–254. [Google Scholar] [CrossRef]
- Kirino, Y.; Yasukawa, T.; Ohta, S.; Akira, S.; Ishihara, K.; Watanabe, K. Codon-specific translational defect caused by a wobble modification deficiency in mutant tRNA from a human mitochondrial disease. Proc. Natl. Acad. Sci. USA 2004, 101, 15070–15075. [Google Scholar] [CrossRef]
- Davies, K.J.A. The broad spectrum of responses to oxidants in proliferating cells: A new paradigm for oxidative stress. Iubmb Life 1999, 48, 41–47. [Google Scholar]
- Qian, L.; Song, X.; Ren, H.; Jingbo, G.; Cheng, S. Mitochondrial mechanism of heat stress-induced injury in rat cardiomyocyte. Cell Stress Chaperones 2004, 9, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Mao, X.; Li, H.; Liu, D.; Chen, X. Regulation of taurine in OTA-induced apoptosis and autophagy. Toxicon 2020, 181, 82–90. [Google Scholar] [CrossRef] [PubMed]

| Genes | GenBank Number | Primer Sequence (5′-3′) |
|---|---|---|
| RPS9 | NM_001101152.2 | Forward: TCTTGGTTTCCAGAGCGTTG |
| Reverse: ATACTCGCCGATCAGCTTCA | ||
| UXT | NM_001037471.2 | Forward: CGCTACGAGGCTTTCATCTCT |
| Reverse: CGAGTGGTTAGCTTCCTGGAG | ||
| RPS15 | NM_001024541.2 | Forward: CAAGATGGCGGAAGTGGAAC |
| Reverse: GTAGCTGGTCGAGGTCTACG | ||
| BAX | NM_173894.1 | Forward: CTGAGCGAGTGTCTGAAGCG |
| Reverse: ACAGCTGCGATCATCCTCTG | ||
| BCL2 | NM_001166486.1 | Forward: AGGCTGGGACGCCTTTG |
| Reverse: GGGCTTCACTTATGGCCCAG | ||
| Caspase3 | XM_015473877.2 | Forward: TGGCGAAATGCAAAGAACGG |
| Reverse: TGTGAGCGTGCTTTTTCAGC | ||
| HSF1 | NM_001076809.1 | Forward: AGCACGCCCAGCAACAGAAAG |
| Reverse: CCGCCGTCGTTCAGCATCAG | ||
| HSP90 | NM_008302.3 | Forward: GATGGAAGAGGAGGAGGTGGAGAC |
| Reverse: AGGGCGTCAGACGAGTTTGAAATC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, H.; Li, T.; Yu, Y.; Zhou, N.; Kou, H.; Guo, Y.; Yang, L.; Yan, P. Cytoprotective Effects of Taurine on Heat-Induced Bovine Mammary Epithelial Cells In Vitro. Cells 2021, 10, 258. https://doi.org/10.3390/cells10020258
Bai H, Li T, Yu Y, Zhou N, Kou H, Guo Y, Yang L, Yan P. Cytoprotective Effects of Taurine on Heat-Induced Bovine Mammary Epithelial Cells In Vitro. Cells. 2021; 10(2):258. https://doi.org/10.3390/cells10020258
Chicago/Turabian StyleBai, Hui, Tingting Li, Yan Yu, Ningcong Zhou, Huijuan Kou, Yingying Guo, Liang Yang, and Peishi Yan. 2021. "Cytoprotective Effects of Taurine on Heat-Induced Bovine Mammary Epithelial Cells In Vitro" Cells 10, no. 2: 258. https://doi.org/10.3390/cells10020258
APA StyleBai, H., Li, T., Yu, Y., Zhou, N., Kou, H., Guo, Y., Yang, L., & Yan, P. (2021). Cytoprotective Effects of Taurine on Heat-Induced Bovine Mammary Epithelial Cells In Vitro. Cells, 10(2), 258. https://doi.org/10.3390/cells10020258





