The Functional and Mechanistic Roles of Immunoproteasome Subunits in Cancer
Abstract
1. Introduction
2. Structural and Functional Differences: Constitutive and Immunoproteasome
2.1. Composition, Assembly, and Regulation
2.2. Functions of Immunoproteasome in Immune and Non-Immune Cells
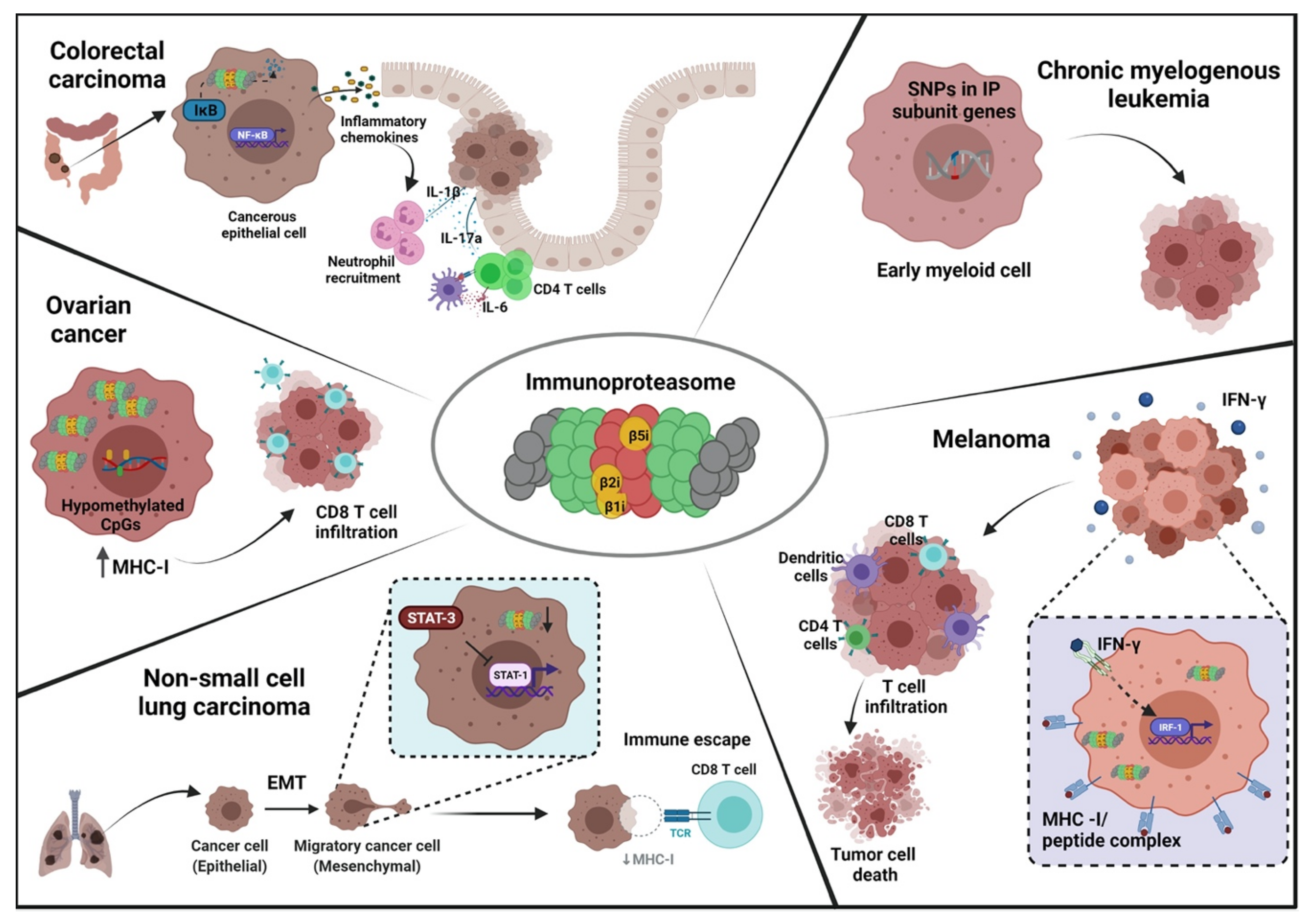
3. Functional and Mechanistic Role of Immunoproteasome Subunits in Cancer
3.1. Role of Inducible Catalytic Subunits in Cancer
3.2. Role of Regulatory Subunits in Cancer
4. Proteasome and Immunoproteasome Inhibitors in Cancer Therapy
| Immunoproteasome Subunit | Cancer | Expression in Cancer Cells | Clinical Outcome | Regulatory Mediator(s) | Use as a Functional Parameter | Therapeutic Intervention | References |
|---|---|---|---|---|---|---|---|
| PSMB8 (β5i subunit) | NSCLC | downregulated | Poor prognosis | STAT3-mTOR mediated inhibition of STAT-1 | NS | IFN-γ treatment to induce IP expression | [22,157] |
| Renal cell carcinoma | upregulated | Poor prognosis | NS | Prognostic biomarker | NS | [152] | |
| TNBC | upregulated | Survival | NS | NS | NS | [21,162] | |
| Glioma | upregulated | Poor prognosis | NS | NS | NS | [148,153] | |
| Laryngeal carcinoma | upregulated | NS | Non-receptor tyrosine kinase encoded by oncogene c-Abl | NS | Tyrosine kinase inhibitor: Nilotinib | [149] | |
| Hypopharyngeal carcinoma | upregulated | NS | Non-receptor tyrosine kinase encoded by oncogene c-Abl | NS | Tyrosine kinase inhibitor: Nilotinib | [149] | |
| Colorectal carcinoma | upregulated | Poor prognosis | Transcription factor: NFκB Proinflammatory cytokines (IL17a) and chemokines (CXCL-1/2/3) SNP encoding LMP7-K allele | NS | Inhibition of β5i with ONX-912 | [151,154,172] | |
| Gastric adenocarcinoma | upregulated | Poor prognosis | NS | Prognostic biomarker | NS | [23] | |
| Melanoma | upregulated | Survival | Cytokine: IFN-γ | Prognostic biomarker, predictive marker for ICB therapy | Overexpression of β5i via IFN-γ treatment | [156] | |
| Prostate cancer | upregulated | NS | miR-451a | NS | NS | [24] | |
| Papillary thyroid cancer | upregulated | NS | miR-451a | NS | NS | [163] | |
| Multiple myeloma | upregulated | NS | NS | NS | Selective inhibitors: PR-924, ONX-0912 | [28,204] | |
| Cervical cancer | NS | High risk | SNP | NS | NS | [172] | |
| PSMB9 (β1i subunit) | Renal cell carcinoma | downregulated | NS | NS | NS | NS | [168] |
| Metastatic breast carcinoma | downregulated | NS | NS | NS | NS | [166] | |
| NSCLC | downregulated | Poor prognosis | STAT3 -mTOR mediated inhibition of STAT-1 | Predictive marker for ICB therapy | NS | [156,169] | |
| APL | downregulated | NS | NS | NS | NS | [164] | |
| Melanoma | upregulated | Survival | NS | Predictive marker for ICB therapy | NS | [169] | |
| Ovarian carcinoma | upregulated | NS | Hypomethylated CpG islands of 6p21.3 | NS | NS | [167] | |
| Cervical cancer | NS | High risk | SNP | NS | NS | [172] | |
| Prostate cancer | upregulated | NS | NS | NS | Selective inhibitor: UK-101 | [208] | |
| Multiple myeloma | upregulated | NS | NS | NS | Selective inhibitor: IPSI-001 | [210] | |
| PSMB10 (β2i subunit) | Metastatic breast carcinoma | downregulated | NS | NS | NS | NS | [166] |
| APL | downregulated | NS | Transcription factor: PU.1 | NS | NS | [164] | |
| NSCLC | downregulated | Survival | NS | NS | NS | [157] | |
| CML | NS | High risk | SNP | NS | NS | [149] | |
| PSME1 (PA28-α subunit) | Multiple myeloma | upregulated | NS | NS | Biomarker for bortezomib treatment | NS | [26] |
| OSCC | upregulated | Poor prognosis | NS | Prognostic marker | NS | [179] | |
| Soft tissue leiomyosarcoma | upregulated | Poor prognosis | NS | Prognostic marker | NS | [27] | |
| Skin cutaneous melanoma | upregulated | Survival | NS | Prognostic marker | NS | [25] | |
| Ovarian cancer | upregulated | Poor prognosis | NS | Biomarker for tumor relapse | NS | [178] | |
| NSCLC | upregulated | Survival | NS | Predictive marker for ICB therapy | NS | [169] | |
| TNBC | upregulated | NS | CDK15 | NS | NS | [180] | |
| Melanoma | upregulated | Survival | NS | Predictive marker for ICB therapy | [169] | ||
| PSME2 (PA28-β subunit) | ESCC | downregulated | NS | NS | NS | NS | [181] |
| Ovarian cancer | upregulated | Poor prognosis | NS | Biomarker for tumor relapse | NS | [178] | |
| Melanoma | upregulated | Survival | NS | Predictive marker for ICB therapy | NS | [169] | |
| TNBC | upregulated | NS | CDK15 | NS | NS | [180] | |
| NSCLC | upregulated | Survival | NS | Predictive marker for ICB therapy | NS | [169] |
5. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Voges, D.; Zwickl, P.; Baumeister, W. The 26S Proteasome: A Molecular Machine Designed for Controlled Proteolysis. Annu. Rev. Biochem. 1999, 68, 1051–1068. [Google Scholar] [CrossRef]
- Collins, G.A.; Goldberg, A.L. The Logic of the 26S Proteasome. Cell 2017, 169, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Unno, M.; Mizushima, T.; Morimoto, Y.; Tomisugi, Y.; Tanaka, K.; Yasuoka, N.; Tsukihara, T. The Structure of the Mammalian 20S Proteasome at 2.75 Å Resolution. Structure 2002, 10, 609–618. [Google Scholar] [CrossRef]
- Rock, K.L.; Gramm, C.; Rothstein, L.; Clark, K.; Stein, R.; Dick, L.; Hwang, D.; Goldberg, A.L. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell 1994, 78, 761–771. [Google Scholar] [CrossRef]
- Aki, M.; Shimbara, N.; Takashina, M.; Akiyama, K.; Kagawa, S.; Tamura, T.; Tanahashi, N.; Yoshimura, T.; Tanaka, K.; Ichihara, A. Interferon-γ Induces Different Subunit Organizations and Functional Diversity of Proteasomes1. J. Biochem. 1994, 115, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K. The proteasome: Overview of structure and functions. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2009, 85, 12–36. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.L.; Cascio, P.; Saric, T.; Rock, K.L. The importance of the proteasome and subsequent proteolytic steps in the generation of antigenic peptides. Mol. Immunol. 2002, 39, 147–164. [Google Scholar] [CrossRef]
- Rock, K.L.; Goldberg, A.L. Degradation of cell proteins and the generation of mhc class i-presented peptides. Annu. Rev. Immunol. 1999, 17, 739–779. [Google Scholar] [CrossRef] [PubMed]
- Abele, R.; Tampé, R. The ABCs of Immunology: Structure and Function of TAP, the Transporter Associated with Antigen Processing. Physiology 2004, 19, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Pamer, E.; Cresswell, P. Mechanisms of mhc class i–restricted antigen processing. Annu. Rev. Immunol. 1998, 16, 323–358. [Google Scholar] [CrossRef]
- Boes, B.; Hengel, H.; Ruppert, T.; Multhaup, G.; Koszinowski, U.H.; Kloetzel, P.M. Interferon gamma stimulation modulates the proteolytic activity and cleavage site preference of 20S mouse proteasomes. J. Exp. Med. 1994, 179, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Gaczynska, M.; Rock, K.L.; Goldberg, A.L. γ-Interferon and expression of MHC genes regulate peptide hydrolysis by proteasomes. Nature 1993, 365, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Huber, E.M.; Basler, M.; Schwab, R.; Heinemeyer, W.; Kirk, C.J.; Groettrup, M.; Groll, M. Immuno- and Constitutive Proteasome Crystal Structures Reveal Differences in Substrate and Inhibitor Specificity. Cell 2012, 148, 727–738. [Google Scholar] [CrossRef]
- Kincaid, E.Z.; Che, J.W.; York, I.; Escobar, H.; Reyes-Vargas, E.; Delgado, J.C.; Welsh, R.M.; Karow, M.L.; Murphy, A.J.; Valenzuela, D.M.; et al. Mice completely lacking immunoproteasomes show major changes in antigen presentation. Nat. Immunol. 2012, 13, 129–135. [Google Scholar] [CrossRef]
- Winter, M.B.; La Greca, F.; Arastu-Kapur, S.; Caiazza, F.; Cimermancic, P.; Buchholz, T.J.; Anderl, J.L.; Ravalin, M.; Bohn, M.F.; Sali, A.; et al. Immunoproteasome functions explained by divergence in cleavage specificity and regulation. Elife 2017, 6, e27364. [Google Scholar] [CrossRef]
- Mishto, M.; Liepe, J.; Textoris-Taube, K.; Keller, C.; Henklein, P.; Weberruß, M.; Dahlmann, B.; Enenkel, C.; Voigt, A.; Kuckelkorn, U.; et al. Proteasome isoforms exhibit only quantitative differences in cleavage and epitope generation. Eur. J. Immunol. 2014, 44, 3508–3521. [Google Scholar] [CrossRef] [PubMed]
- Gutcher, I.; Becher, B. APC-derived cytokines and T cell polarization in autoimmune inflammation. J. Clin. Investig. 2007, 117, 1119–1127. [Google Scholar] [CrossRef]
- Kalim, K.W.; Basler, M.; Kirk, C.J.; Groettrup, M. Immunoproteasome Subunit LMP7 Deficiency and Inhibition Suppresses Th1 and Th17 but Enhances Regulatory T Cell Differentiation. J. Immunol. 2012, 189, 4182–4193. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Hwang, S.M.; Gomes, A.V. Identification of the Immunoproteasome as a Novel Regulator of Skeletal Muscle Differentiation. Mol. Cell. Biol. 2014, 34, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Rouette, A.; Trofimov, A.; Haberl, D.; Boucher, G.; Lavallée, V.P.; D’Angelo, G.; Hébert, J.; Sauvageau, G.; Lemieux, S.; Perreault, C. Expression of immunoproteasome genes is regulated by cell-intrinsic and -extrinsic factors in human cancers. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Song, I.H.; Heo, S.H.; Kim, Y.A.; Park, I.A.; Bang, W.S.; Park, H.S.; Gong, G.; Lee, H.J. Expression of immunoproteasome subunit LMP7 in breast cancer and its association with immune-related markers. Cancer Res. Treat. 2019, 51, 80–89. [Google Scholar] [CrossRef]
- Kiuchi, T.; Tomaru, U.; Ishizu, A.; Imagawa, M.; Iwasaki, S.; Suzuki, A.; Otsuka, N.; Ohhara, Y.; Kinoshita, I.; Matsuno, Y.; et al. Expression of the immunoproteasome subunit β5i in non-small cell lung carcinomas. J. Clin. Pathol. 2020, 74, 300–306. [Google Scholar] [CrossRef]
- Kwon, C.H.; Park, H.J.; Choi, Y.R.; Kim, A.; Kim, H.W.; Choi, J.H.; Hwang, C.S.; Lee, S.J.; Choi, C.I.; Jeon, T.Y.; et al. PSMB8 and PBK as potential gastric cancer subtype-specific biomarkers associated with prognosis. Oncotarget 2016, 7, 21454. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, H.Z.; Jiang, Y.J.; Xu, L.Q. miR-451a is downregulated and targets PSMB8 in prostate cancer. Kaohsiung J. Med Sci. 2020, 36, 494–500. [Google Scholar] [CrossRef]
- Wang, Q.; Pan, F.; Li, S.; Huang, R.; Wang, X.; Wang, S.; Liao, X.; Li, D.; Zhang, L. The prognostic value of the proteasome activator subunit gene family in skin cutaneous melanoma. J. Cancer 2019, 10, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- Dytfeld, D.; Luczak, M.; Wrobel, T.; Usnarska-Zubkiewicz, L.; Brzezniakiewicz, K.; Jamroziak, K.; Giannopoulos, K.; Przybylowicz-Chalecka, A.; Ratajczak, B.; Czerwinska-Rybak, J.; et al. Comparative proteomic profiling of refractory/relapsed multiple myeloma reveals biomarkers involved in resistance to bortezomib-based therapy. Oncotarget 2016, 7, 56726–56736. [Google Scholar] [CrossRef]
- Lou, S.; Cleven, A.H.G.; Balluff, B.; de Graaff, M.; Kostine, M.; Bruijn, I.B.; McDonnell, L.A.; Bovée, J.V.M.G. High nuclear expression of proteasome activator complex subunit 1 predicts poor survival in soft tissue leiomyosarcomas. Clin. Sarcoma Res. 2016, 6, 17. [Google Scholar] [CrossRef]
- Singh, A.V.; Bandi, M.; Aujay, M.A.; Kirk, C.J.; Hark, D.E.; Raje, N.; Chauhan, D.; Anderson, K.C. PR-924, a selective inhibitor of the immunoproteasome subunit LMP-7, blocks multiple myeloma cell growth both in vitro and in vivo. Br. J. Haematol. 2011, 152, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ju, D.; Jarois, T.; Xie, Y. Diminished feedback regulation of proteasome expression and resistance to proteasome inhibitors in breast cancer cells. Breast Cancer Res. Treat. 2008, 107, 267–274. [Google Scholar] [CrossRef]
- Blum, J.S.; Wearsch, P.A.; Cresswell, P. Pathways of Antigen Processing. Annu. Rev. Immunol. 2013, 31, 443–473. [Google Scholar] [CrossRef]
- Eggensperger, S.; Tampé, R. The transporter associated with antigen processing: A key player in adaptive immunity. Biol. Chem. 2015, 396, 1059–1072. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.K.; Monaco, J.J. Homology of proteasome subunits to a major histocompatibility complex-linked LMP gene. Nature 1991, 353, 664–667. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, J.; Brown, M.G.; Finley, D.; Monaco, J.J. MHC-linked LMP gene products specifically alter peptidase activities of the proteasome. Nature 1993, 365, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Yokota, K.; Kagawa, S.; Shimbara, N.; Tamura, T.; Akioka, H.; Nothwang, H.; Noda, C.; Tanaka, K.; Ichihara, A. cDNA cloning and interferon gamma down-regulation of proteasomal subunits X and Y. Science 1994, 265, 1231–1234. [Google Scholar] [CrossRef]
- Groll, M.; Ditzel, L.; Löwe, J.; Stock, D.; Bochtler, M.; Bartunik, H.D.; Huber, R. Structure of 20S proteasome from yeast at 2.4Å resolution. Nature 1997, 386, 463–471. [Google Scholar] [CrossRef]
- DeMartino, G.N.; Slaughter, C.A. The Proteasome, a Novel Protease Regulated by Multiple Mechanisms. J. Biol. Chem. 1999, 274, 22123–22126. [Google Scholar] [CrossRef]
- Ortiz-Navarrete, V.; Seelig, A.; Gernold, M.; Frentzel, S.; Kloetzel, P.M.; Hämmerling, G.J. Subunit of the “20S” proteasome (multicatalytic proteinase) encoded by the major histocompatibility complex. Nature 1991, 353, 662–664. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.; Powis, S.H.; Glynne, R.; Radley, E.; Beck, S.; Trowsdale, J. Second proteasome-related gene in the human MHC class II region. Nature 1991, 353, 667–668. [Google Scholar] [CrossRef] [PubMed]
- Rechsteiner, M.; Hill, C.P. Mobilizing the proteolytic machine: Cell biological roles of proteasome activators and inhibitors. Trends Cell Biol. 2005, 15, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.Y.; Tanahashi, N.; Akiyama, K.; Hisamatsu, H.; Noda, C.; Tanaka, K.; Chung, C.H.; Shibmara, N.; Willy, P.J.; Mott, J.D.; et al. Primary structures of two homologous subunits of PA28, a γ-interferon-inducible protein activator of the 20S proteasome. FEBS Lett. 1995, 366, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Tanahashi, N.; Yokota, K.; Ahn, J.Y.; Chung, C.H.; Fujiwara, T.; Takahashi, E.; DeMartino, G.N.; Slaughter, C.A.; Toyonaga, T.; Yamamura, K.; et al. Molecular properties of the proteasome activator PA28 family proteins and γ-interferon regulation. Genes Cells 1997, 2, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.J.; Palanimurugan, R.; Matias, A.C.; Ramos, P.C.; Dohmen, R.J. Catalytic Mechanism and Assembly of the Proteasome. Chem. Rev. 2009, 109, 1509–1536. [Google Scholar] [CrossRef] [PubMed]
- Hirano, Y.; Hendil, K.B.; Yashiroda, H.; Iemura, S.; Nagane, R.; Hioki, Y.; Natsume, T.; Tanaka, K.; Murata, S. A heterodimeric complex that promotes the assembly of mammalian 20S proteasomes. Nature 2005, 437, 1381–1385. [Google Scholar] [CrossRef] [PubMed]
- Yashiroda, H.; Mizushima, T.; Okamoto, K.; Kameyama, T.; Hayashi, H.; Kishimoto, T.; Niwa, S.; Kasahara, M.; Kurimoto, E.; Sakata, E.; et al. Crystal structure of a chaperone complex that contributes to the assembly of yeast 20S proteasomes. Nat. Struct. Mol. Biol. 2008, 15, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Griffin, T.A.; Nandi, D.; Cruz, M.; Fehling, H.J.; Kaer, L.V.; Monaco, J.J.; Colbert, R.A. Immunoproteasome Assembly: Cooperative Incorporation of Interferon γ (IFN-γ)–inducible Subunits. J. Exp. Med. 1998, 187, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Groettrup, M.; Standera, S.; Stohwasser, R.; Kloetzel, P.M. The subunits MECL-1 and LMP2 are mutually required for incorporation into the 20S proteasome. Proc. Natl. Acad. Sci. USA 1997, 94, 8970–8975. [Google Scholar] [CrossRef]
- Kingsbury, D.J.; Griffin, T.A.; Colbert, R.A. Novel Propeptide Function in 20 S Proteasome Assembly Influences β Subunit Composition. J. Biol. Chem. 2000, 275, 24156–24162. [Google Scholar] [CrossRef]
- Heink, S.; Ludwig, D.; Kloetzel, P.-M.; Kruger, E. From The Cover: IFN- -induced immune adaptation of the proteasome system is an accelerated and transient response. Proc. Natl. Acad. Sci. USA 2005, 102, 9241–9246. [Google Scholar] [CrossRef]
- Ramos, P.C.; Höckendorff, J.; Johnson, E.S.; Varshavsky, A.; Dohmen, R.J. Ump1p Is Required for Proper Maturation of the 20S Proteasome and Becomes Its Substrate upon Completion of the Assembly. Cell 1998, 92, 489–499. [Google Scholar] [CrossRef]
- Früh, K.; Gossen, M.; Wang, K.; Bujard, H.; Peterson, P.A.; Yang, Y. Displacement of housekeeping proteasome subunits by MHC-encoded LMPs: A newly discovered mechanism for modulating the multicatalytic proteinase complex. EMBO J. 1994, 13, 3236–3244. [Google Scholar] [CrossRef]
- Masters, E.I.; Pratt, G.; Förster, A.; Hill, C.P. Purification and analysis of recombinant 11S activators of the 20S proteasome: Trypanosoma brucei PA26 and human PA28 alpha, PA28 beta, and PA28 gamma. Methods Enzym. 2005, 398, 306–321. [Google Scholar] [CrossRef]
- Cascio, P. Properties of the hybrid form of the 26S proteasome containing both 19S and PA28 complexes. EMBO J. 2002, 21, 2636–2645. [Google Scholar] [CrossRef] [PubMed]
- Kopp, F.; Dahlmann, B.; Kuehn, L. Reconstitution of hybrid proteasomes from purified PA700–20 S complexes and PA28αβ activator: Ultrastructure and peptidase activities. J. Mol. Biol. 2001, 313, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Hendil, K.B.; Khan, S.; Tanaka, K. Simultaneous binding of PA28 and PA700 activators to 20 S proteasomes. Biochem. J. 1998, 332, 749–754. [Google Scholar] [CrossRef]
- Guillaume, B.; Chapiro, J.; Stroobant, V.; Colau, D.; Holle, B.V.; Parvizi, G.; Bousquet-Dubouch, M.-P.; Theate, I.; Parmentier, N.; Van den Eynde, B.J. Two abundant proteasome subtypes that uniquely process some antigens presented by HLA class I molecules. Proc. Natl. Acad. Sci. USA 2010, 107, 18599–18604. [Google Scholar] [CrossRef]
- Gaczynska, M.; Goldberg, A.L.; Tanaka, K.; Hendil, K.B.; Rock, K.L. Proteasome Subunits X and Y Alter Peptidase Activities in Opposite Ways to the Interferon-γ-induced Subunits LMP2 and LMP7. J. Biol. Chem. 1996, 271, 17275–17280. [Google Scholar] [CrossRef]
- Orlowski, M.; Wilk, S. Catalytic Activities of the 20 S Proteasome, a Multicatalytic Proteinase Complex. Arch. Biochem. Biophys. 2000, 383, 1–16. [Google Scholar] [CrossRef]
- Namiki, S.; Nakamura, T.; Oshima, S.; Yamazaki, M.; Sekine, Y.; Tsuchiya, K.; Okamoto, R.; Kanai, T.; Watanabe, M. IRF-1 mediates upregulation of LMP7 by IFN-γ and concerted expression of immunosubunits of the proteasome. FEBS Lett. 2005, 579, 2781–2787. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F. Molecular Mechanisms of IFN-γ to Up-Regulate MHC Class I Antigen Processing and Presentation. Int. Rev. Immunol. 2009, 28, 239–260. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee-Kishore, M.; Kishore, R.; Hicklin, D.J.; Marincola, F.M.; Ferrone, S. Different Requirements for Signal Transducer and Activator of Transcription 1α and Interferon Regulatory Factor 1 in the Regulation of Low Molecular Mass Polypeptide 2 and Transporter Associated with Antigen Processing 1 Gene Expression. J. Biol. Chem. 1998, 273, 16177–16183. [Google Scholar] [CrossRef]
- Foss, G.S.; Larsen, F.; Solheim, J.; Prydz, H. Constitutive and interferon-γ-induced expression of the human proteasome subunit multicatalytic endopeptidase complex-like 11EMBL accession number for MECL1 cDNA: Y13640.1. Biochim. Biophys. Acta 1998, 1402, 17–28. [Google Scholar] [CrossRef][Green Version]
- Bose, S.; Brooks, P.; Mason, G.G.F.; Rivett, A.J. γ-Interferon decreases the level of 26 S proteasomes and changes the pattern of phosphorylation. Biochem. J. 2001, 353, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Jäkel, S.; Kuckelkorn, U.; Szalay, G.; Plötz, M.; Textoris-Taube, K.; Opitz, E.; Klingel, K.; Stevanovic, S.; Kandolf, R.; Kotsch, K.; et al. Differential Interferon Responses Enhance Viral Epitope Generation by Myocardial Immunoproteasomes in Murine Enterovirus Myocarditis. Am. J. Pathol. 2009, 175, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.-C.; Seifert, U.; Kato, T.; Rice, C.M.; Feinstone, S.M.; Kloetzel, P.-M.; Rehermann, B. Virus-induced type I IFN stimulates generation of immunoproteasomes at the site of infection. J. Clin. Investig. 2006, 116, 3006–3014. [Google Scholar] [CrossRef]
- Szalay, G.; Meiners, S.; Voigt, A.; Lauber, J.; Spieth, C.; Speer, N.; Sauter, M.; Kuckelkorn, U.; Zell, A.; Klingel, K.; et al. Ongoing Coxsackievirus Myocarditis Is Associated with Increased Formation and Activity of Myocardial Immunoproteasomes. Am. J. Pathol. 2006, 168, 1542–1552. [Google Scholar] [CrossRef] [PubMed]
- Gavilán, M.P.; Castaño, A.; Torres, M.; Portavella, M.; Caballero, C.; Jiménez, S.; García-Martínez, A.; Parrado, J.; Vitorica, J.; Ruano, D. Age-related increase in the immunoproteasome content in rat hippocampus: Molecular and functional aspects. J. Neurochem. 2009, 108, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Kotamraju, S.; Matalon, S.; Matsunaga, T.; Shang, T.; Hickman-Davis, J.M.; Kalyanaraman, B. Upregulation of immunoproteasomes by nitric oxide: Potential antioxidative mechanism in endothelial cells. Free Radic. Biol. Med. 2006, 40, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Callahan, M.K.; Wohlfert, E.A.; Ménoret, A.; Srivastava, P.K. Heat Shock Up-Regulates lmp2 and lmp7 and Enhances Presentation of Immunoproteasome-Dependent Epitopes. J. Immunol. 2006, 177, 8393–8399. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Martin, S.; Dimayuga, E.; Bruce-Keller, A.J.; Keller, J.N. LMP2 Knock-Out Mice Have Reduced Proteasome Activities and Increased Levels of Oxidatively Damaged Proteins. Antioxid. Redox Signal. 2006, 8, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.S.; Oubrahim, H.; Stadtman, E.R. Inhibition of apoptosis in acute promyelocytic leukemia cells leads to increases in levels of oxidized protein and LMP2 immunoproteasome. Proc. Natl. Acad. Sci. USA 2004, 101, 11560–11565. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zanelli, E.; Smart, M.; David, C. Genomic Organization and Tissue Expression of Mouse Proteasome Gene Lmp-2. Genomics 1993, 16, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.L.; White, L.C.; Kelly, A.; Beck, S.; Trowsdale, J.; Ting, J.P. Coordinate regulation of the human TAP1 and LMP2 genes from a shared bidirectional promoter. J. Exp. Med. 1995, 181, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- James, A.B. Regulation of the Neuronal Proteasome by Zif268 (Egr1). J. Neurosci. 2006, 26, 1624–1634. [Google Scholar] [CrossRef] [PubMed]
- De Verteuil, D.; Muratore-Schroeder, T.L.; Granados, D.P.; Fortier, M.-H.; Hardy, M.-P.; Bramoullé, A.; Caron, É.; Vincent, K.; Mader, S.; Lemieux, S.; et al. Deletion of Immunoproteasome Subunits Imprints on the Transcriptome and Has a Broad Impact on Peptides Presented by Major Histocompatibility Complex I molecules. Mol. Cell. Proteom. 2010, 9, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, B.; Stroobant, V.; Bousquet-Dubouch, M.-P.; Colau, D.; Chapiro, J.; Parmentier, N.; Dalet, A.; den Eynde, B.J.V. Analysis of the Processing of Seven Human Tumor Antigens by Intermediate Proteasomes. J. Immunol. 2012, 189, 3538–3547. [Google Scholar] [CrossRef] [PubMed]
- Fehling, H.; Swat, W.; Laplace, C.; Kuhn, R.; Rajewsky, K.; Muller, U.; Boehmer, H. von MHC class I expression in mice lacking the proteasome subunit LMP-7. Science 1994, 265, 1234–1237. [Google Scholar] [CrossRef]
- Sijts, A.J.A.M.; Standera, S.; Toes, R.E.M.; Ruppert, T.; Beekman, N.J.C.M.; van Veelen, P.A.; Ossendorp, F.A.; Melief, C.J.M.; Kloetzel, P.M. MHC Class I Antigen Processing of an Adenovirus CTL Epitope Is Linked to the Levels of Immunoproteasomes in Infected Cells. J. Immunol. 2000, 164, 4500–4506. [Google Scholar] [CrossRef] [PubMed]
- Kaert, L.V.; Ashton-Rickardt, P.G.; Eichelberger, M.; Gaczynska, M.; Nagashima, K.; Rock, K.L.; Goldberg, A.L.; Doherty, P.C.; Tonegawa, S. Altered peptidase and viral-specific T cell response in LMP2 mutant mice. Immunity 1994, 1, 533–541. [Google Scholar] [CrossRef]
- Cerundolo, V.; Kelly, A.; Elliott, T.; Trowsdale, J.; Townsend, A. Genes encoded in the major histocompatibility complex affecting the generation of peptides for TAP transport. Eur. J. Immunol. 1995, 25, 554–562. [Google Scholar] [CrossRef]
- Valmori, D.; Gileadi, U.; Servis, C.; Dunbar, P.R.; Cerottini, J.-C.; Romero, P.; Cerundolo, V.; Lévy, F. Modulation of Proteasomal Activity Required for the Generation of a Cytotoxic T Lymphocyte–defined Peptide Derived from the Tumor Antigen MAGE-3. J. Exp. Med. 1999, 189, 895–906. [Google Scholar] [CrossRef]
- Sewell, A.K.; Price, D.A.; Teisserenc, H.; Booth, B.L.; Gileadi, U.; Flavin, F.M.; Trowsdale, J.; Phillips, R.E.; Cerundolo, V. IFN-γ Exposes a Cryptic Cytotoxic T Lymphocyte Epitope in HIV-1 Reverse Transcriptase. J. Immunol. 1999, 162, 7075. [Google Scholar]
- Chapiro, J.; Claverol, S.; Piette, F.; Ma, W.; Stroobant, V.; Guillaume, B.; Gairin, J.-E.; Morel, S.; Burlet-Schiltz, O.; Monsarrat, B.; et al. Destructive cleavage of antigenic peptides either by the immunoproteasome or by the standard proteasome results in differential antigen presentation. J. Immunol. 2006, 176, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Morel, S.; Lévy, F.; Burlet-Schiltz, O.; Brasseur, F.; Probst-Kepper, M.; Peitrequin, A.-L.; Monsarrat, B.; Velthoven, R.V.; Cerottini, J.-C.; Boon, T.; et al. Processing of Some Antigens by the Standard Proteasome but Not by the Immunoproteasome Results in Poor Presentation by Dendritic Cells. Immunity 2000, 12, 107–117. [Google Scholar] [CrossRef]
- Chapatte, L.; Ayyoub, M.; Morel, S.; Peitrequin, A.-L.; Lévy, N.; Servis, C.; Van den Eynde, B.J.; Valmori, D.; Lévy, F. Processing of tumor-associated antigen by the proteasomes of dendritic cells controls in vivo T-cell responses. Cancer Res. 2006, 66, 5461–5468. [Google Scholar] [CrossRef] [PubMed]
- Gileadi, U.; Moins-Teisserenc, H.T.; Correa, I.; Booth, B.L.; Dunbar, P.R.; Sewell, A.K.; Trowsdale, J.; Phillips, R.E.; Cerundolo, V. Generation of an Immunodominant CTL Epitope Is Affected by Proteasome Subunit Composition and Stability of the Antigenic Protein. J. Immunol. 1999, 163, 6045. [Google Scholar] [PubMed]
- Sijts, A.J.A.M.; Ruppert, T.; Rehermann, B.; Schmidt, M.; Koszinowski, U.; Kloetzel, P.-M. Efficient Generation of a Hepatitis B Virus Cytotoxic T Lymphocyte Epitope Requires the Structural Features of Immunoproteasomes. J. Exp. Med. 2000, 191, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Basler, M.; Lauer, C.; Moebius, J.; Weber, R.; Przybylski, M.; Kisselev, A.F.; Tsu, C.; Groettrup, M. Why the Structure but Not the Activity of the Immunoproteasome Subunit Low Molecular Mass Polypeptide 2 Rescues Antigen Presentation. J. Immunol. 2012, 189, 1868–1877. [Google Scholar] [CrossRef]
- Murata, S.; Sasaki, K.; Kishimoto, T.; Niwa, S.-I.; Hayashi, H.; Takahama, Y.; Tanaka, K. Regulation of CD8+ T Cell Development by Thymus-Specific Proteasomes. Science 2007, 316, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Van den Eynde, B.J.; Morel, S. Differential processing of class-I-restricted epitopes by the standard proteasome and the immunoproteasome. Curr. Opin. Immunol. 2001, 13, 147–153. [Google Scholar] [CrossRef]
- Basler, M.; Kirk, C.J.; Groettrup, M. The immunoproteasome in antigen processing and other immunological functions. Curr. Opin. Immunol. 2013, 25, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Murata, S.; Takahama, Y.; Kasahara, M.; Tanaka, K. The immunoproteasome and thymoproteasome: Functions, evolution and human disease. Nat. Immunol. 2018, 19, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Ebstein, F.; Textoris-Taube, K.; Keller, C.; Golnik, R.; Vigneron, N.; Van den Eynde, B.J.; Schuler-Thurner, B.; Schadendorf, D.; Lorenz, F.K.M.; Uckert, W.; et al. Proteasomes generate spliced epitopes by two different mechanisms and as efficiently as non-spliced epitopes. Sci. Rep. 2016, 6, 24032. [Google Scholar] [CrossRef]
- Vigneron, N.; Stroobant, V.; Chapiro, J.; Ooms, A.; Degiovanni, G.; Morel, S.; van der Bruggen, P.; Boon, T.; Van den Eynde, B.J. An antigenic peptide produced by peptide splicing in the proteasome. Science 2004, 304, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Hanada, K.; Yewdell, J.W.; Yang, J.C. Immune recognition of a human renal cancer antigen through post-translational protein splicing. Nature 2004, 427, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Warren, E.H.; Vigneron, N.J.; Gavin, M.A.; Coulie, P.G.; Stroobant, V.; Dalet, A.; Tykodi, S.S.; Xuereb, S.M.; Mito, J.K.; Riddell, S.R.; et al. An antigen produced by splicing of noncontiguous peptides in the reverse order. Science 2006, 313, 1444–1447. [Google Scholar] [CrossRef]
- Michaux, A.; Larrieu, P.; Stroobant, V.; Fonteneau, J.-F.; Jotereau, F.; Van den Eynde, B.J.; Moreau-Aubry, A.; Vigneron, N. A spliced antigenic peptide comprising a single spliced amino acid is produced in the proteasome by reverse splicing of a longer peptide fragment followed by trimming. J. Immunol. 2014, 192, 1962–1971. [Google Scholar] [CrossRef]
- Dalet, A.; Robbins, P.F.; Stroobant, V.; Vigneron, N.; Li, Y.F.; El-Gamil, M.; Hanada, K.; Yang, J.C.; Rosenberg, S.A.; Van den Eynde, B.J. An antigenic peptide produced by reverse splicing and double asparagine deamidation. Proc. Natl. Acad. Sci. USA 2011, 108, E323–E331. [Google Scholar] [CrossRef]
- Berkers, C.R.; de Jong, A.; Schuurman, K.G.; Linnemann, C.; Geenevasen, J.A.J.; Schumacher, T.N.M.; Rodenko, B.; Ovaa, H. Peptide Splicing in the Proteasome Creates a Novel Type of Antigen with an Isopeptide Linkage. J. Immunol. 2015, 195, 4075–4084. [Google Scholar] [CrossRef] [PubMed]
- Liepe, J.; Marino, F.; Sidney, J.; Jeko, A.; Bunting, D.E.; Sette, A.; Kloetzel, P.M.; Stumpf, M.P.H.; Heck, A.J.R.; Mishto, M. A large fraction of HLA class I ligands are proteasome-generated spliced peptides. Science 2016, 354, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Vigneron, N.; Stroobant, V.; Ferrari, V.; Abi Habib, J.; Van den Eynde, B.J. Production of spliced peptides by the proteasome. Mol. Immunol. 2019, 113, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Admon, A. Are There Indeed Spliced Peptides in the Immunopeptidome? Mol. Cell Proteom. 2021, 20, 100099. [Google Scholar] [CrossRef] [PubMed]
- Dubiel, W.; Pratt, G.; Ferrell, K.; Rechsteiner, M. Purification of an 11 S regulator of the multicatalytic protease. J. Biol. Chem. 1992, 267, 22369–22377. [Google Scholar] [CrossRef]
- Ma, C.P.; Slaughter, C.A.; DeMartino, G.N. Identification, purification, and characterization of a protein activator (PA28) of the 20 S proteasome (macropain). J. Biol. Chem. 1992, 267, 10515–10523. [Google Scholar] [CrossRef]
- Zhang, Z.; Clawson, A.; Realini, C.; Jensen, C.C.; Knowlton, J.R.; Hill, C.P.; Rechsteiner, M. Identification of an activation region in the proteasome activator REG. Proc. Natl. Acad. Sci. USA 1998, 95, 2807–2811. [Google Scholar] [CrossRef]
- Realini, C.; Jensen, C.C.; Zhang, Z.; Johnston, S.C.; Knowlton, J.R.; Hill, C.P.; Rechsteiner, M. Characterization of Recombinant REGα, REGβ, and REGγ Proteasome Activators. J. Biol. Chem. 1997, 272, 25483–25492. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, L.; Dahlmann, B. Proteasome Activator PA28 and Its Interaction with 20 S Proteasomes. Arch. Biochem. Biophys. 1996, 329, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; von Kampen, J.; Slaughter, C.A.; DeMartino, G.N. Relative Functions of the α and β Subunits of the Proteasome Activator, PA28. J. Biol. Chem. 1997, 272, 27994–28000. [Google Scholar] [CrossRef] [PubMed]
- Dick, T.P.; Ruppert, T.; Groettrup, M.; Kloetzel, P.M.; Kuehn, L.; Koszinowski, U.H.; Stevanovic, S.; Schild, H.; Rammensee, H.-G. Coordinated Dual Cleavages Induced by the Proteasome Regulator PA28 Lead to Dominant MHC Ligands. Cell 1996, 86, 253–262. [Google Scholar] [CrossRef]
- Groettrup, M.; Soza, A.; Eggers, M.; Kuehn, L.; Dick, T.P.; Schild, H.; Rammensee, H.-G.; Koszinowski, U.H.; Kloetzel, P.-M. A role for the proteasome regulator PA28α in antigen presentation. Nature 1996, 381, 166–168. [Google Scholar] [CrossRef]
- Raule, M.; Cerruti, F.; Benaroudj, N.; Migotti, R.; Kikuchi, J.; Bachi, A.; Navon, A.; Dittmar, G.; Cascio, P. PA28αβ Reduces Size and Increases Hydrophilicity of 20S Immunoproteasome Peptide Products. Chem. Biol. 2014, 21, 470–480. [Google Scholar] [CrossRef]
- Respondek, D.; Voss, M.; Kühlewindt, I.; Klingel, K.; Krüger, E.; Beling, A. PA28 modulates antigen processing and viral replication during coxsackievirus B3 infection. PLoS ONE 2017, 12, e0173259. [Google Scholar] [CrossRef]
- Chen, W.; Norbury, C.C.; Cho, Y.; Yewdell, J.W.; Bennink, J.R. Immunoproteasomes Shape Immunodominance Hierarchies of Antiviral Cd8+ T Cells at the Levels of T Cell Repertoire and Presentation of Viral Antigens. J. Exp. Med. 2001, 193, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Moebius, J.; van den Broek, M.; Groettrup, M.; Basler, M. Immunoproteasomes are essential for survival and expansion of T cells in virus-infected mice. Eur. J. Immunol. 2010, 40, 3439–3449. [Google Scholar] [CrossRef] [PubMed]
- Davignon, J.-L.; Hayder, M.; Baron, M.; Boyer, J.-F.; Constantin, A.; Apparailly, F.; Poupot, R.; Cantagrel, A. Targeting monocytes/macrophages in the treatment of rheumatoid arthritis. Rheumatology 2013, 52, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Muchamuel, T.; Basler, M.; Aujay, M.A.; Suzuki, E.; Kalim, K.W.; Lauer, C.; Sylvain, C.; Ring, E.R.; Shields, J.; Jiang, J.; et al. A selective inhibitor of the immunoproteasome subunit LMP7 blocks cytokine production and attenuates progression of experimental arthritis. Nat. Med. 2009, 15, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Rockwell, C.E.; Monaco, J.J.; Qureshi, N. A Critical Role for the Inducible Proteasomal Subunits LMP7 and MECL1 in Cytokine Production by Activated Murine Splenocytes. Pharmacology 2012, 89, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Hensley, S.E.; Zanker, D.; Dolan, B.P.; David, A.; Hickman, H.D.; Embry, A.C.; Skon, C.N.; Grebe, K.M.; Griffin, T.A.; Chen, W.; et al. Unexpected Role for the Immunoproteasome Subunit LMP2 in Antiviral Humoral and Innate Immune Responses. J. Immunol. 2010, 184, 4115–4122. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Faustman, D. Essential Role of Human Leukocyte Antigen-encoded Proteasome Subunits in NF-κB Activation and Prevention of Tumor Necrosis Factor-α-induced Apoptosis. J. Biol. Chem. 2000, 275, 5238–5247. [Google Scholar] [CrossRef] [PubMed]
- Visekruna, A.; Slavova, N.; Dullat, S.; Gröne, J.; Kroesen, A.-J.; Ritz, J.-P.; Buhr, H.-J.; Steinhoff, U. Expression of catalytic proteasome subunits in the gut of patients with Crohn’s disease. Int. J. Colorectal Dis. 2009, 24, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.R.; Lee, N.-R.; Han, S.; Wu, Y.; Sharma, L.K.; Carmony, K.C.; Marks, J.; Lee, D.-M.; Ban, J.-O.; Wehenkel, M.; et al. Revisiting the role of the immunoproteasome in the activation of the canonical NF-κB pathway. Mol. BioSystems 2012, 8, 2295–2302. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Faustman, D. NOD Mice Are Defective in Proteasome Production and Activation of NF-κB. Mol. Cell. Biol. 1999, 19, 8646–8659. [Google Scholar] [CrossRef] [PubMed]
- Volkov, A.; Hagner, S.; Löser, S.; Alnahas, S.; Raifer, H.; Hellhund, A.; Garn, H.; Steinhoff, U. β5i Subunit Deficiency of the Immunoproteasome Leads to Reduced Th2 Response in OVA Induced Acute Asthma. PLoS ONE 2013, 8, e60565. [Google Scholar] [CrossRef]
- Frausto, R.F.; Crocker, S.J.; Eam, B.; Whitmire, J.K.; Whitton, J.L. Myelin oligodendrocyte glycoprotein peptide-induced experimental allergic encephalomyelitis and T cell responses are unaffected by immunoproteasome deficiency. J. Neuroimmunol. 2007, 192, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.; Gonzalez, E.; Visekruna, A.; Kuhl, A.A.; Loddenkemper, C.; Mollenkopf, H.; Kaufmann, S.H.E.; Steinhoff, U.; Joeris, T. Targeting the proteasome: Partial inhibition of the proteasome by bortezomib or deletion of the immunosubunit LMP7 attenuates experimental colitis. Gut 2010, 59, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Basler, M.; Dajee, M.; Moll, C.; Groettrup, M.; Kirk, C.J. Prevention of Experimental Colitis by a Selective Inhibitor of the Immunoproteasome. J. Immunol. 2010, 185, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, Y.; Nakahara, M.; Shimamura, M.; Horie, I.; Arima, K.; Abiru, N. Prophylactic and therapeutic efficacies of a selective inhibitor of the immunoproteasome for Hashimoto’s thyroiditis, but not for Graves’ hyperthyroidism, in mice. Clin. Exp. Immunol. 2012, 168, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Arima, K.; Kinoshita, A.; Mishima, H.; Kanazawa, N.; Kaneko, T.; Mizushima, T.; Ichinose, K.; Nakamura, H.; Tsujino, A.; Kawakami, A.; et al. Proteasome assembly defect due to a proteasome subunit beta type 8 (PSMB8) mutation causes the autoinflammatory disorder, Nakajo-Nishimura syndrome. Proc. Natl. Acad. Sci. USA 2011, 108, 14914–14919. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, A.; Maekawa, Y.; Uehara, H.; Izumi, K.; Kawachi, I.; Nishizawa, M.; Toyoshima, Y.; Takahashi, H.; Standley, D.M.; Tanaka, K.; et al. A mutation in the immunoproteasome subunit PSMB8 causes autoinflammation and lipodystrophy in humans. J. Clin. Investig. 2011, 121, 4150–4160. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Xing, C.; DeMartino, G.N.; Mizrachi, D.; Hernandez, M.D.; Sousa, A.B.; de Villarreal, L.M.; dos Santos, H.G.; Garg, A. PSMB8 Encoding the β5i Proteasome Subunit Is Mutated in Joint Contractures, Muscle Atrophy, Microcytic Anemia, and Panniculitis-Induced Lipodystrophy Syndrome. Am. J. Hum. Genet. 2010, 87, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Sarrabay, G.; Méchin, D.; Salhi, A.; Boursier, G.; Rittore, C.; Crow, Y.; Rice, G.; Tran, T.-A.; Cezar, R.; Duffy, D.; et al. PSMB10, the last immunoproteasome gene missing for PRAAS. J. Allergy Clin. Immunol. 2020, 145, 1015–1017.e6. [Google Scholar] [CrossRef]
- Brehm, A.; Liu, Y.; Sheikh, A.; Marrero, B.; Omoyinmi, E.; Zhou, Q.; Montealegre, G.; Biancotto, A.; Reinhardt, A.; de Jesus, A.A.; et al. Additive loss-of-function proteasome subunit mutations in CANDLE/PRAAS patients promote type I IFN production. J. Clin. Investig. 2016, 126, 795. [Google Scholar] [CrossRef]
- Poli, M.C.; Ebstein, F.; Nicholas, S.K.; de Guzman, M.M.; Forbes, L.R.; Chinn, I.K.; Mace, E.M.; Vogel, T.P.; Carisey, A.F.; Benavides, F.; et al. Heterozygous Truncating Variants in POMP Escape Nonsense-Mediated Decay and Cause a Unique Immune Dysregulatory Syndrome. Am. J. Hum. Genet. 2018, 102, 1126–1142. [Google Scholar] [CrossRef]
- De Jesus, A.A.; Brehm, A.; VanTries, R.; Pillet, P.; Parentelli, A.-S.; Montealegre Sanchez, G.A.; Deng, Z.; Paut, I.K.; Goldbach-Mansky, R.; Krüger, E. Novel proteasome assembly chaperone mutations in PSMG2/PAC2 cause the autoinflammatory interferonopathy CANDLE/PRAAS4. J. Allergy Clin. Immunol. 2019, 143, 1939–1943.e8. [Google Scholar] [CrossRef] [PubMed]
- Keller, I.E.; Vosyka, O.; Takenaka, S.; Kloß, A.; Dahlmann, B.; Willems, L.I.; Verdoes, M.; Overkleeft, H.S.; Marcos, E.; Adnot, S.; et al. Regulation of Immunoproteasome Function in the Lung. Sci. Rep. 2015, 5, 10230. [Google Scholar] [CrossRef] [PubMed]
- Baldovino, S.; Piccinini, M.; Anselmino, A.; Ramondetti, C.; Rinaudo, M.T.; Costanzo, P.; Sena, L.M.; Roccatello, D. Structural and functional properties of proteasomes purified from the human kidney. J. Nephrol. 2006, 19, 710–716. [Google Scholar] [PubMed]
- Vasuri, F.; Capizzi, E.; Bellavista, E.; Mishto, M.; Santoro, A.; Fiorentino, M.; Capri, M.; Cescon, M.; Grazi, G.L.; Grigioni, W.F.; et al. Studies on immunoproteasome in human liver. Part I: Absence in fetuses, presence in normal subjects, and increased levels in chronic active hepatitis and cirrhosis. Biochem. Biophys. Res. Commun. 2010, 397, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Tabaczewski, P.; Truscott, S.M.; Kaer, L.V.; Stroynowski, I. Hepatocytes Express Abundant Surface Class I MHC and Efficiently Use Transporter Associated with Antigen Processing, Tapasin, and Low Molecular Weight Polypeptide Proteasome Subunit Components of Antigen Processing and Presentation Pathway. J. Immunol. 2005, 175, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Noda, C.; Tanahashi, N.; Shimbara, N.; Hendil, K.B.; Tanaka, K. Tissue Distribution of Constitutive Proteasomes, Immunoproteasomes, and PA28 in Rats. Biochem. Biophys. Res. Commun. 2000, 277, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wang, H.; Lee, I.H.; Modi, S.; Wang, X.; Du, J.; Mitch, W.E. PTEN Inhibition Improves Muscle Regeneration in Mice Fed a High-Fat Diet. Diabetes 2010, 59, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Zu, L.; Bedja, D.; Fox-Talbot, K.; Gabrielson, K.L.; Kaer, L.V.; Becker, L.C.; Cai, Z.P. Evidence for a role of immunoproteasomes in regulating cardiac muscle mass in diabetic mice. J. Mol. Cell. Cardiol. 2010, 49, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Seifert, U.; Bialy, L.P.; Ebstein, F.; Bech-Otschir, D.; Voigt, A.; Schröter, F.; Prozorovski, T.; Lange, N.; Steffen, J.; Rieger, M.; et al. Immunoproteasomes Preserve Protein Homeostasis upon Interferon-Induced Oxidative Stress. Cell 2010, 142, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Van Deventer, S.; Neefjes, J. The Immunoproteasome Cleans up after Inflammation. Cell 2010, 142, 517–518. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pickering, A.M.; Koop, A.L.; Teoh, C.Y.; Ermak, G.; Grune, T.; Davies, K.J.A. The immunoproteasome, the 20S proteasome and the PA28αβ proteasome regulator are oxidative-stress-adaptive proteolytic complexes. Biochem. J. 2010, 432, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Deshaies, R.J. Proteotoxic crisis, the ubiquitin-proteasome system, and cancer therapy. BMC Biol. 2014, 12, 94. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Brewer, M.D.; Guo, L.; Wang, R.; Jiang, P.; Yang, X. Enhanced Degradation of Misfolded Proteins Promotes Tumorigenesis. Cell Rep. 2017, 18, 3143–3154. [Google Scholar] [CrossRef] [PubMed]
- Kumatori, A.; Tanaka, K.; Inamura, N.; Sone, S.; Ogura, T.; Matsumoto, T.; Tachikawa, T.; Shin, S.; Ichihara, A. Abnormally high expression of proteasomes in human leukemic cells. Proc. Natl. Acad. Sci. USA 1990, 87, 7071–7075. [Google Scholar] [CrossRef]
- Petrocca, F.; Altschuler, G.; Tan, S.M.; Mendillo, M.L.; Yan, H.; Jerry, D.J.; Kung, A.L.; Hide, W.; Ince, T.A.; Lieberman, J. A Genome-wide siRNA Screen Identifies Proteasome Addiction as a Vulnerability of Basal-like Triple-Negative Breast Cancer Cells. Cancer Cell 2013, 24, 182–196. [Google Scholar] [CrossRef]
- Yang, B.-Y.; Song, J.-W.; Sun, H.; Xing, J.-C.; Yang, Z.-H.; Wei, C.-Y.; Xu, T.-Y.; Yu, Z.-N.; Zhang, Y.-N.; Wang, Y.-F.; et al. PSMB8 regulates glioma cell migration, proliferation, and apoptosis through modulating ERK1/2 and PI3K/AKT signaling pathways. Biomed. Pharmacother. 2018, 100, 205–212. [Google Scholar] [CrossRef]
- Chen, N.X.; Liu, K.; Liu, X.; Zhang, X.X.; Han, D.Y. Induction and Regulation of the Immunoproteasome Subunit b5i (PSMB8) in Laryngeal and Hypopharyngeal Carcinoma Cells. Med. Sci. Monit. 2020, 26, e926110-1. [Google Scholar] [CrossRef]
- Seliger, B.; Höhne, A.; Knuth, A.; Bernhard, H.; Ehring, B.; Tampé, R.; Huber, C. Reduced membrane major histocompatibility complex class I density and stability in a subset of human renal cell carcinomas with low TAP and LMP expression. Clin. Cancer Res. 1996, 2, 1427–1433. [Google Scholar] [PubMed]
- Vachharajani, N.; Joeris, T.; Luu, M.; Hartmann, S.; Pautz, S.; Jenike, E.; Pantazis, G.; Prinz, I.; Hofer, M.J.; Steinhoff, U.; et al. Prevention of colitis-associated cancer by selective targeting of immunoproteasome subunit LMP7. Oncotarget 2017, 8, 50447–50459. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, Ż.; Niezgoda, M.; Młynarczyk, G.; Acewicz, M.; Kasacka, I. Comparative Assessment of the WNT/β-Catenin Pathway, CacyBP/SIP, and the Immunoproteasome Subunit LMP7 in Various Histological Types of Renal Cell Carcinoma. Front. Oncol. 2020, 10, 2530. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Cheng, Y.C.; Tsai, W.C.; Chen, Y. PSMB8 inhibition decreases tumor angiogenesis in glioblastoma through vascular endothelial growth factor A reduction. Cancer Sci. 2020, 111, 4142–4153. [Google Scholar] [CrossRef]
- Koerner, J.; Brunner, T.; Groettrup, M. Inhibition and deficiency of the immunoproteasome subunit LMP7 suppress the development and progression of colorectal carcinoma in mice. Oncotarget 2017, 8, 50873–50888. [Google Scholar] [CrossRef] [PubMed]
- Leister, H.; Luu, M.; Staudenraus, D.; Krol, A.L.; Mollenkopf, H.-J.; Sharma, A.; Schmerer, N.; Schulte, L.N.; Bertrams, W.; Schmeck, B.; et al. Pro- and Antitumorigenic Capacity of Immunoproteasomes in Shaping the Tumor Microenvironment. Cancer Immunol. Res. 2021, 9, 682–692. [Google Scholar] [CrossRef]
- Kalaora, S.; Lee, J.S.; Barnea, E.; Levy, R.; Greenberg, P.; Alon, M.; Yagel, G.; Eli, G.B.; Oren, R.; Peri, A.; et al. Immunoproteasome expression is associated with better prognosis and response to checkpoint therapies in melanoma. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Tripathi, S.C.; Peters, H.L.; Taguchi, A.; Katayama, H.; Wang, H.; Momin, A.; Jolly, M.K.; Celiktas, M.; Rodriguez-Canales, J.; Liu, H.; et al. Immunoproteasome deficiency is a feature of non-small cell lung cancer with a mesenchymal phenotype and is associated with a poor outcome. Proc. Natl. Acad. Sci. USA 2016, 113, E1555–E1564. [Google Scholar] [CrossRef]
- Shoji, T.; Kikuchi, E.; Kikuchi, J.; Takashima, Y.; Furuta, M.; Takahashi, H.; Tsuji, K.; Maeda, M.; Kinoshita, I.; Dosaka-Akita, H.; et al. Evaluating the immunoproteasome as a potential therapeutic target in cisplatin-resistant small cell and non-small cell lung cancer. Cancer Chemother. Pharmacol. 2020, 85, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Busse, A.; Kraus, M.; Na, I.K.; Rietz, A.; Scheibenbogen, C.; Driessen, C.; Blau, I.W.; Thiel, E.; Keilholz, U. Sensitivity of tumor cells to proteasome inhibitors is associated with expression levels and composition of proteasome subunits. Cancer 2008, 112, 659–670. [Google Scholar] [CrossRef]
- Niewerth, D.; Kaspers, G.J.L.; Assaraf, Y.G.; van Meerloo, J.; Kirk, C.J.; Anderl, J.; Blank, J.L.; van de Ven, P.M.; Zweegman, S.; Jansen, G.; et al. Interferon-γ-induced upregulation of immunoproteasome subunit assembly overcomes bortezomib resistance in human hematological cell lines. J. Hematol. Oncol. 2014, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chng, W.-J. Novel mechanism of drug resistance to proteasome inhibitors in multiple myeloma. World J. Clin. Oncol. 2019, 10, 303–306. [Google Scholar] [CrossRef]
- Adwal, A.; Croft, P.K.-D.; Shakya, R.; Lim, M.; Kalaw, E.; Taege, L.D.; Reed, A.E.M.; Lakhani, S.R.; Callen, D.F.; Saunus, J.M. Tradeoff between metabolic i-proteasome addiction and immune evasion in triple-negative breast cancer. Life Sci. Alliance 2020, 3, e201900562. [Google Scholar] [CrossRef]
- Fan, X.; Zhao, Y. miR-451a inhibits cancer growth, epithelial-mesenchymal transition and induces apoptosis in papillary thyroid cancer by targeting PSMB8. J. Cell. Mol. Med. 2019, 23, 8067–8075. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-W.; Wang, P.; Liu, J.-Q.; Zhang, H.; Xi, W.-D.; Jia, X.-H.; Wang, K.-K. Coordinated regulation of the immunoproteasome subunits by PML/RARα and PU.1 in acute promyelocytic leukemia. Oncogene 2014, 33, 2700–2708. [Google Scholar] [CrossRef] [PubMed]
- Bruzzoni-Giovanelli, H.; González, J.R.; Sigaux, F.; Villoutreix, B.O.; Cayuela, J.M.; Guilhot, J.; Preudhomme, C.; Guilhot, F.; Poyet, J.-L.; Rousselot, P. Genetic polymorphisms associated with increased risk of developing chronic myelogenous leukemia. Oncotarget 2015, 6, 36269–36277. [Google Scholar] [CrossRef] [PubMed]
- Mirandola, P.; Micheloni, C.; Solenghi, E.; Artico, M.; Soda, G.; Zanelli, G.; Pelusi, G.; Fiorini, T.; Cocco, L.; Vitale, M.; et al. Expression of HLA class I antigen and proteasome subunits LMP-2 and LMP-10 in primary vs. metastatic breast carcinoma lesions. Int. J. Oncol. 2004, 25, 1625–1629. [Google Scholar]
- Wang, C.; Cicek, M.S.; Charbonneau, B.; Kalli, K.R.; Armasu, S.M.; Larson, M.C.; Konecny, G.E.; Winterhoff, B.; Fan, J.B.; Bibikova, M.; et al. Tumor hypomethylation at 6p21.3 associates with longer time to recurrence of high-grade serous epithelial ovarian cancer. Cancer Res. 2014, 74, 3084–3091. [Google Scholar] [CrossRef] [PubMed]
- Seliger, B.; Atkins, D.; Bock, M.; Ritz, U.; Ferrone, S.; Huber, C.; Störkel, S. Characterization of human lymphocyte antigen class I antigen-processing machinery defects in renal cell carcinoma lesions with special emphasis on transporter-associated with antigen-processing down-regulation. Clin. Cancer Res. 2003, 9, 1721–1727. [Google Scholar] [PubMed]
- Thompson, J.C.; Davis, C.; Deshpande, C.; Hwang, W.T.; Jeffries, S.; Huang, A.; Mitchell, T.C.; Langer, C.J.; Albelda, S.M. Gene signature of antigen processing and presentation machinery predicts response to checkpoint blockade in non-small cell lung cancer (NSCLC) and melanoma. J. ImmunoTherapy Cancer 2020, 8, e000974. [Google Scholar] [CrossRef] [PubMed]
- Liew, P.L.; Huang, R.L.; Weng, Y.C.; Fang, C.L.; Huang, T.H.-M.; Lai, H.C. Distinct methylation profile of mucinous ovarian carcinoma reveals susceptibility to proteasome inhibitors. Int. J. Cancer 2018, 143, 355–367. [Google Scholar] [CrossRef]
- Fellerhoff, B.; Gu, S.; Laumbacher, B.; Nerlich, A.G.; Weiss, E.H.; Glas, J.; Kopp, R.; Johnson, J.P.; Wank, R. The LMP7-K allele of the immunoproteasome exhibits reduced transcript stability and predicts high risk of colon cancer. Cancer Res. 2011, 71, 7145–7154. [Google Scholar] [CrossRef]
- Li, C.; Dai, S.; Yan, Z.; Zhang, X.; Liu, S.; Wang, X.; Wang, J.; Shi, L.; Yao, Y. Genetic polymorphisms of proteasome subunit genes of the MHC-I antigen-presenting system are associated with cervical cancer in a Chinese Han population. Hum. Immunol. 2020, 81, 445–451. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, N.; van Helden, M.J.G.; Textoris-Taube, K.; Chiba, T.; Topham, D.J.; Kloetzel, P.M.; Zaiss, D.M.W.; Sijts, A.J.A.M. PA28 and the proteasome immunosubunits play a central and independent role in the production of MHC class I-binding peptides in vivo. Eur. J. Immunol. 2011, 41, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sijts, A.J.A.M.; Song, M.; Janek, K.; Nussbaum, A.K.; Kral, S.; Schirle, M.; Stevanovic, S.; Paschen, A.; Schild, H.; et al. Expression of the proteasome activator PA28 rescues the presentation of a cytotoxic T lymphocyte epitope on melanoma cells. Cancer Res. 2002, 62, 2875–2882. [Google Scholar]
- Sijts, E.J.A.M.; Kloetzel, P.M. The role of the proteasome in the generation of MHC class I ligands and immune responses. Cell Mol. Life Sci. 2011, 68, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Cascio, P. PA28αβ: The enigmatic magic ring of the proteasome? Biomolecules 2014, 4, 566–584. [Google Scholar] [CrossRef] [PubMed]
- Ossendorp, F.; Fu, N.; Camps, M.; Granucci, F.; Gobin, S.J.P.; van den Elsen, P.J.; Schuurhuis, D.; Adema, G.J.; Lipford, G.B.; Chiba, T.; et al. Differential Expression Regulation of the α and β Subunits of the PA28 Proteasome Activator in Mature Dendritic Cells. J. Immunol. 2005, 174, 7815–7822. [Google Scholar] [CrossRef] [PubMed]
- Longuespée, R.; Boyon, C.; Castellier, C.; Jacquet, A.; Desmons, A.; Kerdraon, O.; Vinatier, D.; Fournier, I.; Day, R.; Salzet, M. The C-terminal fragment of the immunoproteasome PA28S (Reg alpha) as an early diagnosis and tumor-relapse biomarker: Evidence from mass spectrometry profiling. Histochem. Cell Biol. 2012, 138, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Jiang, Y.; Xie, L.; Jiang, L.; Li, J.; Sun, C.; Xu, H.; Wang, R.; Zhou, M.; Zhou, Y.; et al. Overexpression of proteasomal activator PA28α serves as a prognostic factor in oral squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2016, 35, 35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, S.; Dai, X.; Gong, K.; Song, K.; Tai, F.; Shi, J. PA28α/β Promote Breast Cancer Cell Invasion and Metastasis via Down-Regulation of CDK15. Front. Oncol. 2019, 9, 1283. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Xu, L.; Fang, W.M.; Han, J.Y.; Wang, K.; Zhu, K.S. Identification of PA28β as a potential novel biomarker in human esophageal squamous cell carcinoma. Tumor Biol. 2017, 39, 1010428317719780. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Ebstein, F.; Bürger, E.; Textoris-Taube, K.; Gorny, X.; Urban, S.; Zhao, F.; Dannenberg, T.; Sucker, A.; Keller, C.; et al. The proteasome immunosubunits, PA28 and ER-aminopeptidase 1 protect melanoma cells from efficient MART-126-35-specific T-cell recognition. Eur. J. Immunol. 2015, 45, 3257–3268. [Google Scholar] [CrossRef]
- Sánchez-Martín, D.; Martínez-Torrecuadrada, J.; Teesalu, T.; Sugahara, K.N.; Alvarez-Cienfuegos, A.; Ximénez-Embún, P.; Fernández-Periáñez, R.; Martín, M.T.; Molina-Privado, I.; Ruppen-Cañás, I.; et al. Proteasome activator complex PA28 identified as an accessible target in prostate cancer by in vivo selection of human antibodies. Proc. Natl. Acad. Sci. USA 2013, 110, 13791–13796. [Google Scholar] [CrossRef] [PubMed]
- Almond, J.B.; Cohen, G.M. The proteasome: A novel target for cancer chemotherapy. Leukemia 2002, 16, 433–443. [Google Scholar] [CrossRef]
- Kisselev, A.F.; van der Linden, W.A.; Overkleeft, H.S. Proteasome Inhibitors: An Expanding Army Attacking a Unique Target. Chem. Biol. 2012, 19, 99–115. [Google Scholar] [CrossRef]
- Kisselev, A.F.; Groettrup, M. Subunit specific inhibitors of proteasomes and their potential for immunomodulation. Curr. Opin. Chem. Biol. 2014, 23, 16–22. [Google Scholar] [CrossRef]
- Ettari, R.; Zappalà, M.; Grasso, S.; Musolino, C.; Innao, V.; Allegra, A. Immunoproteasome-selective and non-selective inhibitors: A promising approach for the treatment of multiple myeloma. Pharmacol. Ther. 2018, 182, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Sherman, D.J.; Li, J. Proteasome Inhibitors: Harnessing Proteostasis to Combat Disease. Molecules 2020, 25, 671. [Google Scholar] [CrossRef] [PubMed]
- Kane, R.C.; Bross, P.F.; Farrell, A.T.; Pazdur, R. Velcade ®: U.S. FDA Approval for the Treatment of Multiple Myeloma Progressing on Prior Therapy. Oncologist 2003, 8, 508–513. [Google Scholar] [CrossRef]
- Adams, J.; Palombella, V.J.; Sausville, E.A.; Johnson, J.; Destree, A.; Lazarus, D.D.; Maas, J.; Pien, C.S.; Prakash, S.; Elliott, P.J. Proteasome Inhibitors: A Novel Class of Potent and Effective Antitumor Agents. Cancer Res. 1999, 59, 2615. [Google Scholar] [PubMed]
- Argyriou, A.A.; Bruna, J.; Marmiroli, P.; Cavaletti, G. Chemotherapy-induced peripheral neurotoxicity (CIPN): An update. Crit. Rev. Oncol. Hematol. 2012, 82, 51–77. [Google Scholar] [CrossRef]
- Muchtar, E.; Gertz, M.A.; Magen, H. A practical review on carfilzomib in multiple myeloma. Eur. J. Haematol. 2016, 96, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.; Tian, Z.; Zhou, B.; Kuhn, D.; Orlowski, R.; Raje, N.; Richardson, P.; Anderson, K.C. In Vitro and In Vivo Selective Antitumor Activity of a Novel Orally Bioavailable Proteasome Inhibitor MLN9708 against Multiple Myeloma Cells. Clin. Cancer Res. 2011, 17, 5311–5321. [Google Scholar] [CrossRef]
- Kraus, M.; Bader, J.; Geurink, P.P.; Weyburne, E.S.; Mirabella, A.C.; Silzle, T.; Shabaneh, T.B.; van der Linden, W.A.; de Bruin, G.; Haile, S.R.; et al. The novel 2-selective proteasome inhibitor LU-102 synergizes with bortezomib and carfilzomib to overcome proteasome inhibitor resistance of myeloma cells. Haematologica 2015, 100, 1350–1360. [Google Scholar] [CrossRef] [PubMed]
- Altun, M.; Galardy, P.J.; Shringarpure, R.; Hideshima, T.; LeBlanc, R.; Anderson, K.C.; Ploegh, H.L.; Kessler, B.M. Effects of PS-341 on the Activity and Composition of Proteasomes in Multiple Myeloma Cells. Cancer Res. 2005, 65, 7896–7901. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wu, Y.; Zhou, X.; Xu, J.; Zhu, W.; Shu, Y.; Liu, P. Efficacy of therapy with bortezomib in solid tumors: A review based on 32 clinical trials. Future Oncol. 2014, 10, 1795–1807. [Google Scholar] [CrossRef] [PubMed]
- Dirix, L. A phase II study of the combination of endocrine treatment and bortezomib in patients with endocrine-resistant metastatic breast cancerA phase II study of the combination of endocrine treatment and bortezomib in patients with endocrine-resistant metastatic breast cancer. Oncol. Rep. 2011, 27, 657–663. [Google Scholar] [CrossRef]
- Chen, D.; Frezza, M.; Schmitt, S.; Kanwar, J.; Dou, Q.P. Bortezomib as the First Proteasome Inhibitor Anticancer Drug: Current Status and Future Perspectives. Curr. Cancer Drug Targets 2011, 11, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Merin, N.; Kelly, K. Clinical Use of Proteasome Inhibitors in the Treatment of Multiple Myeloma. Pharmaceuticals 2014, 8, 1–20. [Google Scholar] [CrossRef]
- Parlati, F.; Lee, S.J.; Aujay, M.; Suzuki, E.; Levitsky, K.; Lorens, J.B.; Micklem, D.R.; Ruurs, P.; Sylvain, C.; Lu, Y.; et al. Carfilzomib can induce tumor cell death through selective inhibition of the chymotrypsin-like activity of the proteasome. Blood 2009, 114, 3439–3447. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.F.; Hanke, N.T.; Sands, B.J.; Carbajal, L.; Anderl, J.L.; Garland, L.L. Carfilzomib demonstrates broad anti-tumor activity in pre-clinical non-small cell and small cell lung cancer models. J. Exp. Clin. Cancer Res. 2014, 33, 111. [Google Scholar] [CrossRef] [PubMed]
- Niewerth, D.; van Meerloo, J.; Jansen, G.; Assaraf, Y.G.; Hendrickx, T.C.; Kirk, C.J.; Anderl, J.L.; Zweegman, S.; Kaspers, G.J.L.; Cloos, J. Anti-leukemic activity and mechanisms underlying resistance to the novel immunoproteasome inhibitor PR-924. Biochem. Pharmacol. 2014, 89, 43–51. [Google Scholar] [CrossRef]
- Kim, K.B.; Myung, J.; Sin, N.; Crews, C.M. Proteasome inhibition by the natural products epoxomicin and dihydroeponemycin: Insights into specificity and potency. Bioorganic Med. Chem. Lett. 1999, 9, 3335–3340. [Google Scholar] [CrossRef]
- Chauhan, D.; Singh, A.V.; Aujay, M.; Kirk, C.J.; Bandi, M.; Ciccarelli, B.; Raje, N.; Richardson, P.; Anderson, K.C. A novel orally active proteasome inhibitor ONX 0912 triggers in vitro and in vivo cytotoxicity in multiple myeloma. Blood 2010, 116, 4906–4915. [Google Scholar] [CrossRef] [PubMed]
- Ruschak, A.M.; Slassi, M.; Kay, L.E.; Schimmer, A.D. Novel Proteasome Inhibitors to Overcome Bortezomib Resistance. JNCI J. Natl. Cancer Inst. 2011, 103, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Busch, M.; Friese-Hamim, M.; Crosignani, S.; Fuchss, T.; Musil, D.; Rohdich, F.; Sanderson, M.P.; Seenisamy, J.; Walter-Bausch, G.; et al. Structure-Based Optimization and Discovery of M3258, a Specific Inhibitor of the Immunoproteasome Subunit LMP7 (β5i). J. Med. Chem. 2021, 64, 10230–10245. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, M.P.; Friese-Hamim, M.; Walter-Bausch, G.; Busch, M.; Gaus, S.; Musil, D.; Rohdich, F.; Zanelli, U.; Downey-Kopyscinski, S.L.; Mitsiades, C.S.; et al. M3258 Is a Selective Inhibitor of the Immunoproteasome Subunit LMP7 (β5i) Delivering Efficacy in Multiple Myeloma Models. Mol. Cancer 2021, 20, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Wehenkel, M.; Ban, J.-O.; Ho, Y.-K.; Carmony, K.C.; Hong, J.T.; Kim, K.B. A selective inhibitor of the immunoproteasome subunit LMP2 induces apoptosis in PC-3 cells and suppresses tumour growth in nude mice. Br. J. Cancer 2012, 107, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.K.; Bargagna-Mohan, P.; Wehenkel, M.; Mohan, R.; Kim, K.-B. LMP2-Specific Inhibitors: Chemical Genetic Tools for Proteasome Biology. Chem. Biol. 2007, 14, 419–430. [Google Scholar] [CrossRef]
- Kuhn, D.J.; Hunsucker, S.A.; Chen, Q.; Voorhees, P.M.; Orlowski, M.; Orlowski, R.Z. Targeted inhibition of the immunoproteasome is a potent strategy against models of multiple myeloma that overcomes resistance to conventional drugs and nonspecific proteasome inhibitors. Blood 2009, 113, 4667–4676. [Google Scholar] [CrossRef] [PubMed]
- Downey-Kopyscinski, S.; Daily, E.W.; Gautier, M.; Bhatt, A.; Florea, B.I.; Mitsiades, C.S.; Richardson, P.G.; Driessen, C.; Overkleeft, H.S.; Kisselev, A.F. An inhibitor of proteasome β2 sites sensitizes myeloma cells to immunoproteasome inhibitors. Blood Adv. 2018, 2, 2443–2451. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripathi, S.C.; Vedpathak, D.; Ostrin, E.J. The Functional and Mechanistic Roles of Immunoproteasome Subunits in Cancer. Cells 2021, 10, 3587. https://doi.org/10.3390/cells10123587
Tripathi SC, Vedpathak D, Ostrin EJ. The Functional and Mechanistic Roles of Immunoproteasome Subunits in Cancer. Cells. 2021; 10(12):3587. https://doi.org/10.3390/cells10123587
Chicago/Turabian StyleTripathi, Satyendra Chandra, Disha Vedpathak, and Edwin Justin Ostrin. 2021. "The Functional and Mechanistic Roles of Immunoproteasome Subunits in Cancer" Cells 10, no. 12: 3587. https://doi.org/10.3390/cells10123587
APA StyleTripathi, S. C., Vedpathak, D., & Ostrin, E. J. (2021). The Functional and Mechanistic Roles of Immunoproteasome Subunits in Cancer. Cells, 10(12), 3587. https://doi.org/10.3390/cells10123587






