Shear Stress Alterations Activate BMP4/pSMAD5 Signaling and Induce Endothelial Mesenchymal Transition in Varicose Veins
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Endothelial Cell Culture and Flow Exposure
2.3. RNA Extraction, cDNA Synthesis, and Quantitative Real-Time PCR
2.4. Histology and Immunohistochemistry
2.5. Western Blotting
2.6. Immunofluorescence Analysis
2.7. Inhibitor Assays
2.8. Statistical Analysis
3. Results
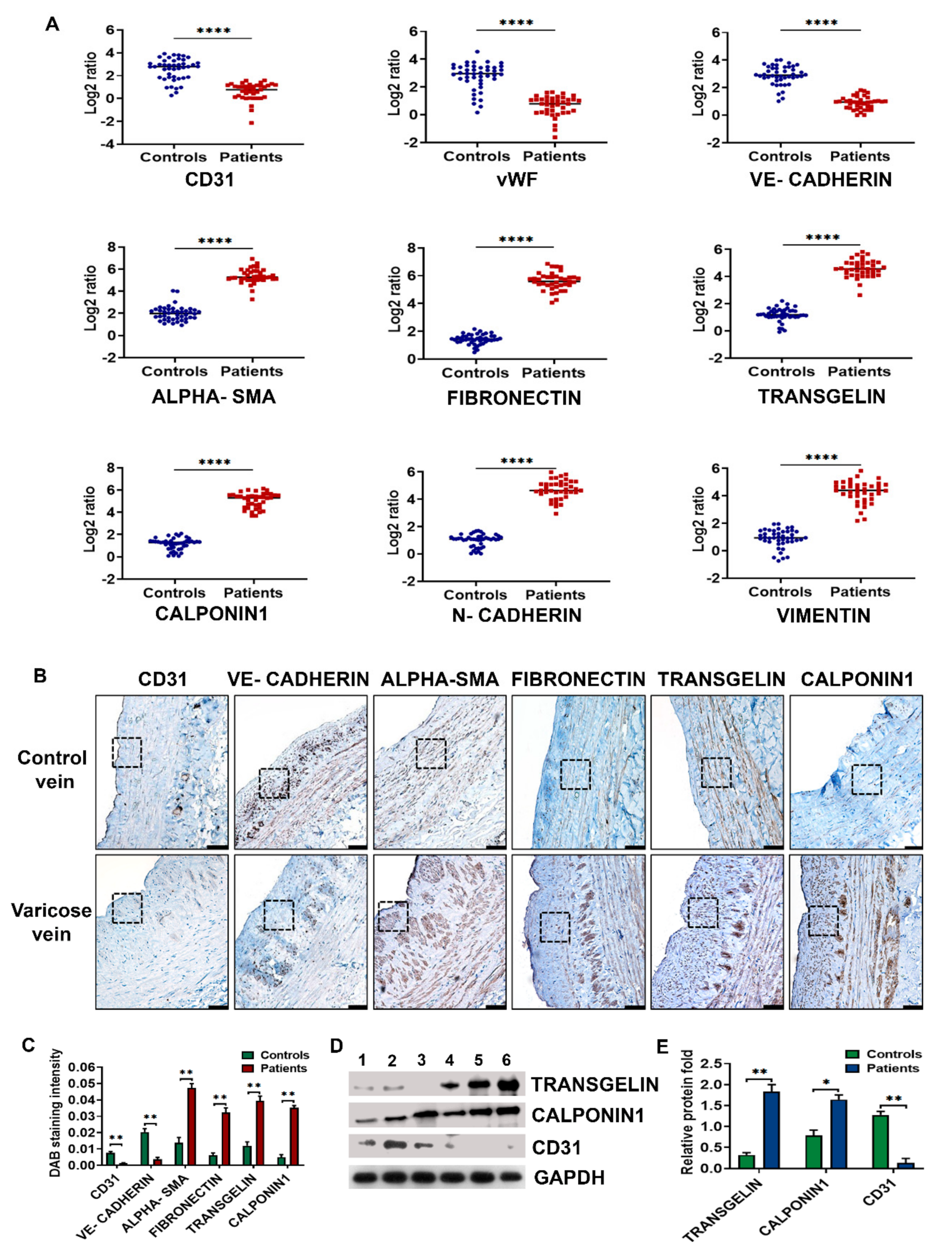
3.1. Neointima of Varicose Veins Express High Levels of Mesenchymal Markers
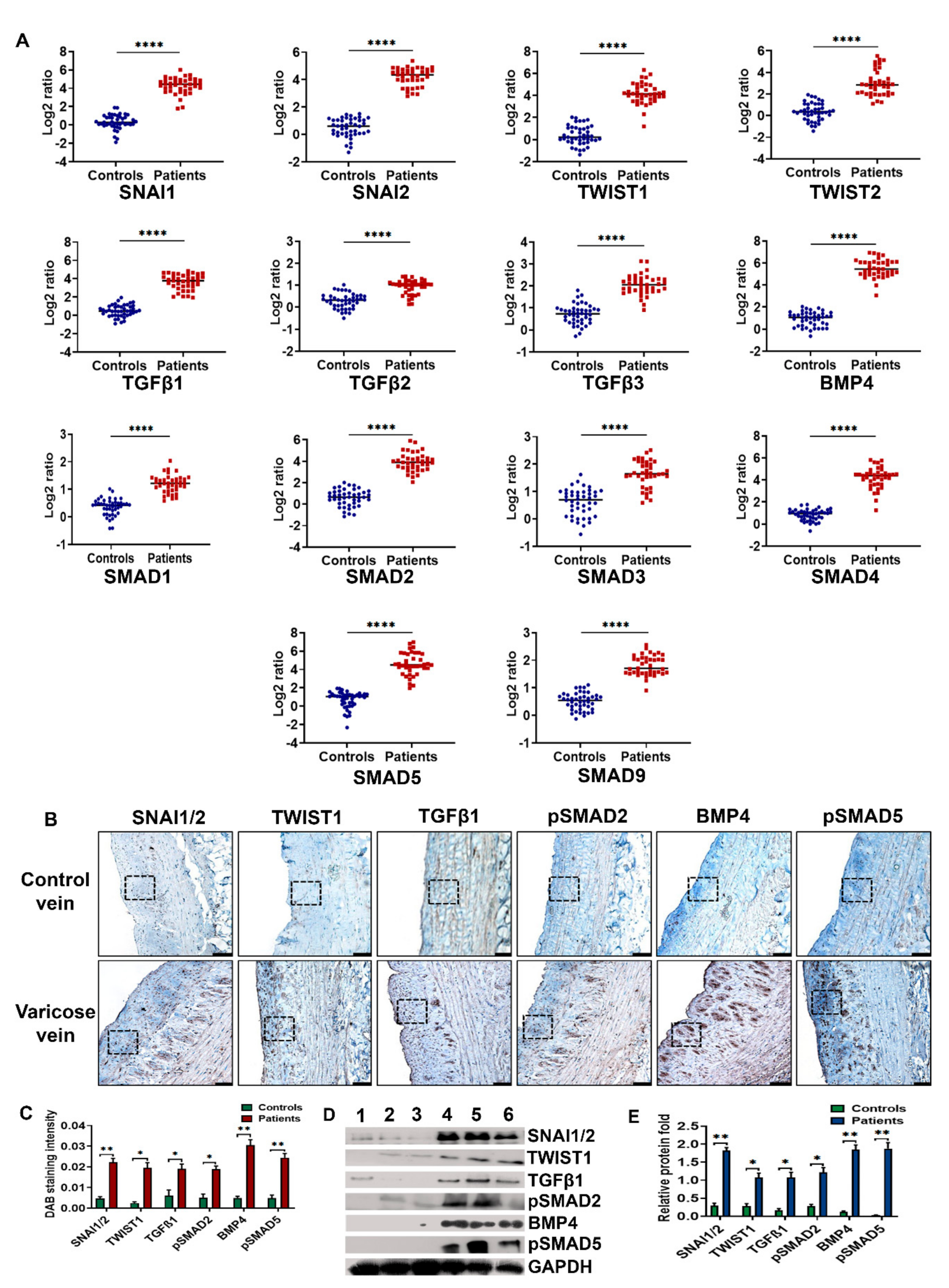
3.2. BMP4-pSMAD5-SNAI1/2 Signaling Is Upregulated in Varicose Veins
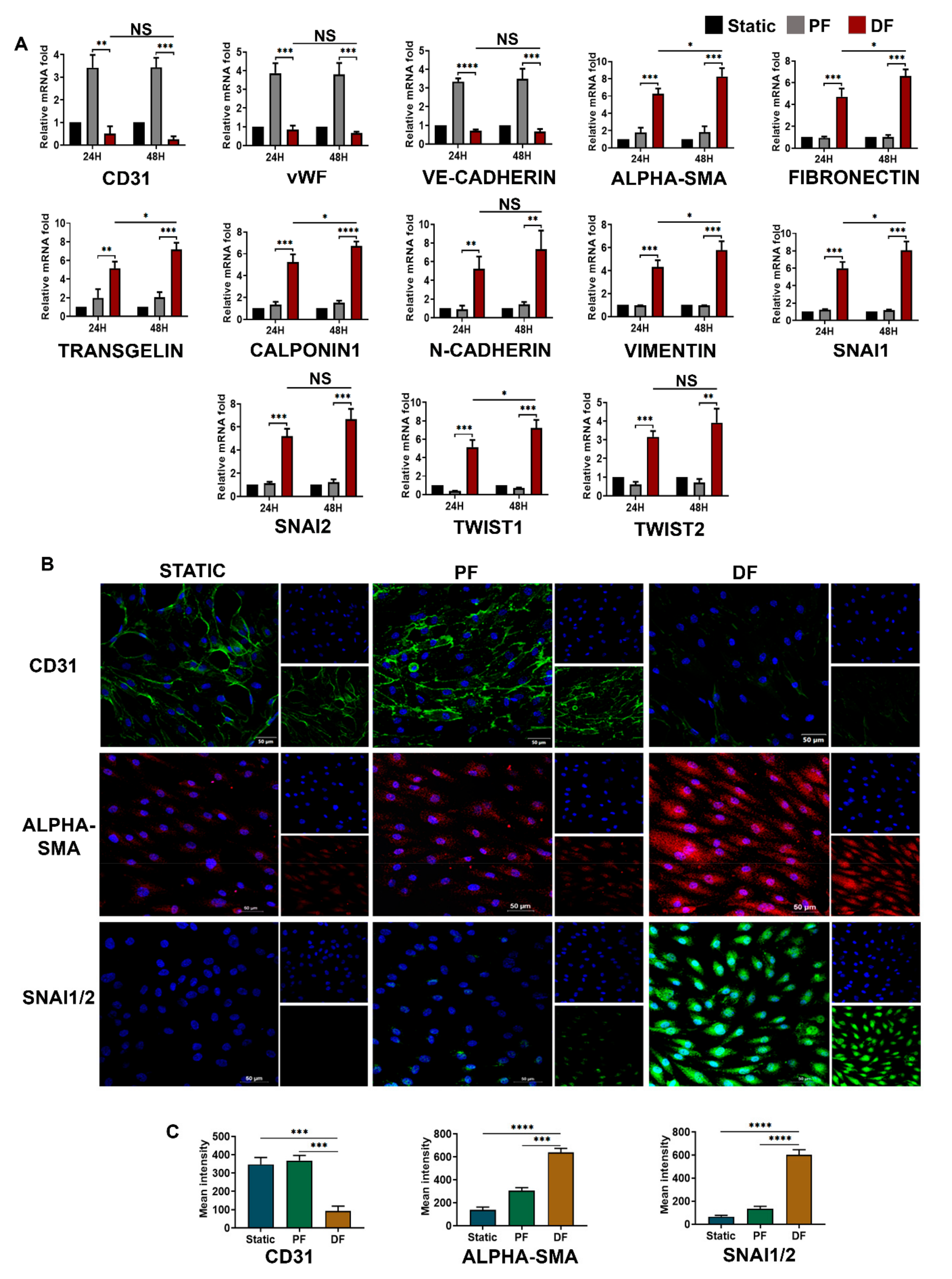
3.3. Disturbed Venous Flow Induces EndMT in Venous Endothelial Cells
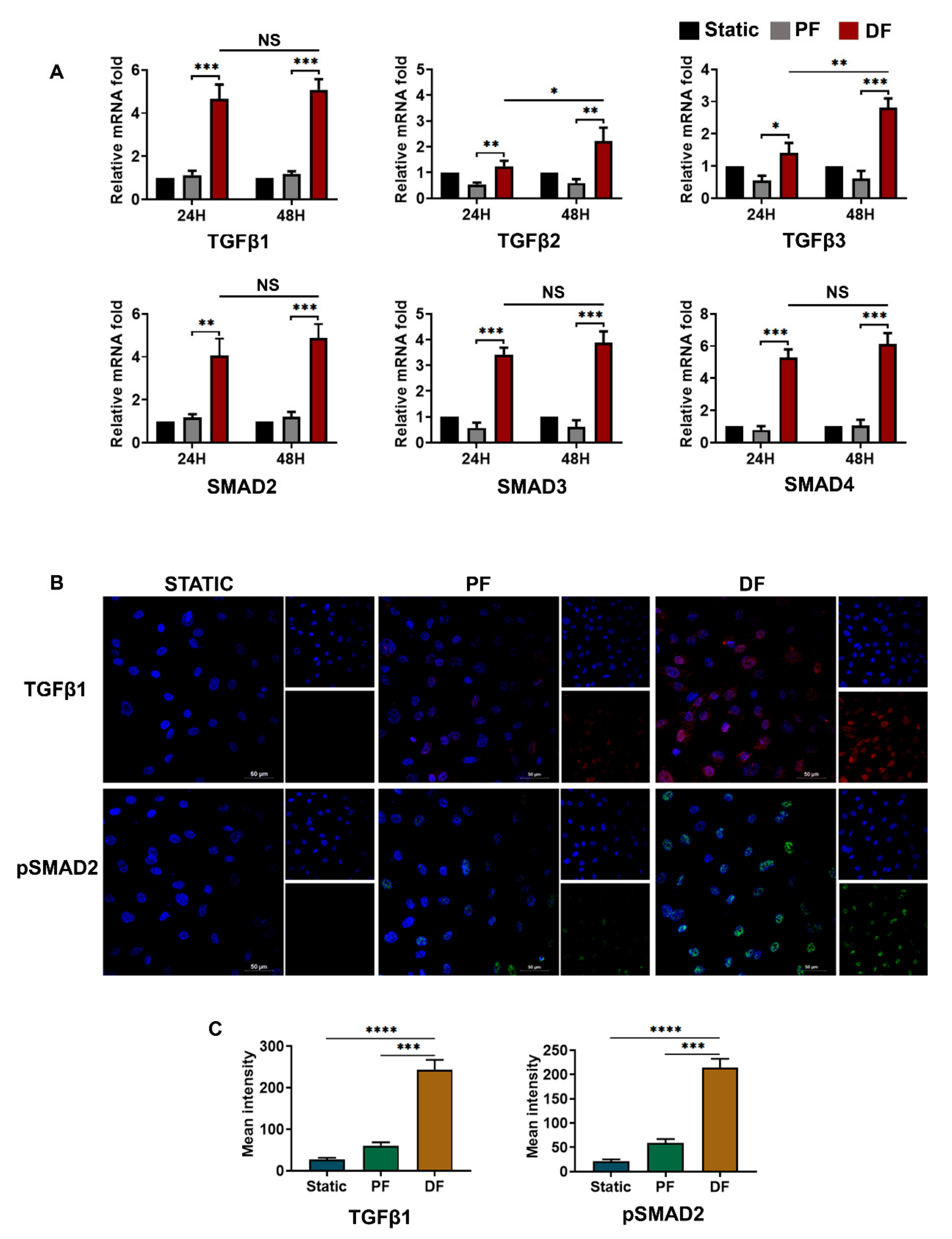
3.4. TGFβ1-pSMAD2 Signaling in Venous Endothelial Cells Exposed to Disturbed Flow
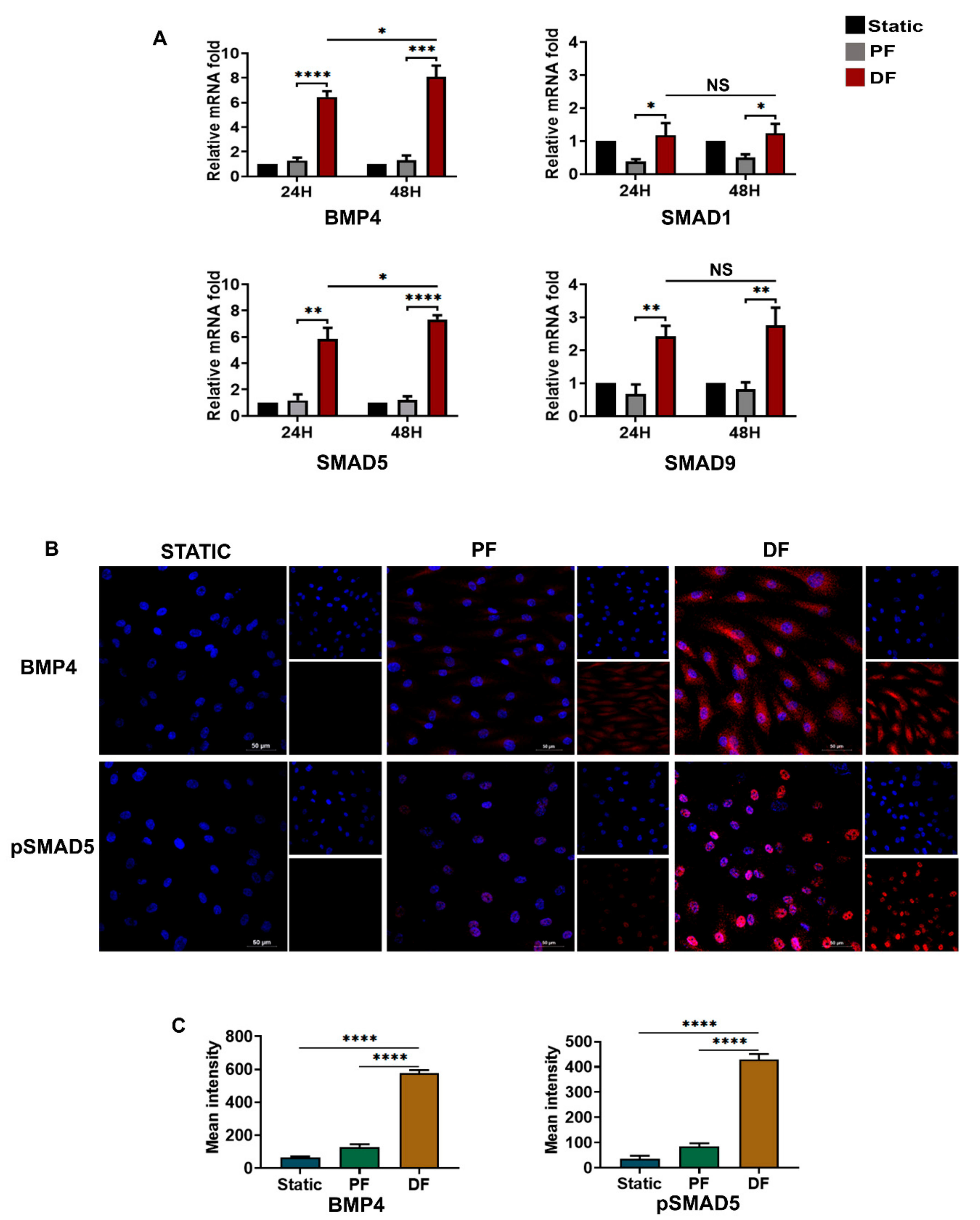
3.5. BMP4-pSMAD5 Is Significantly Expressed in Venous Endothelial Cells Exposed to Disturbed Flow
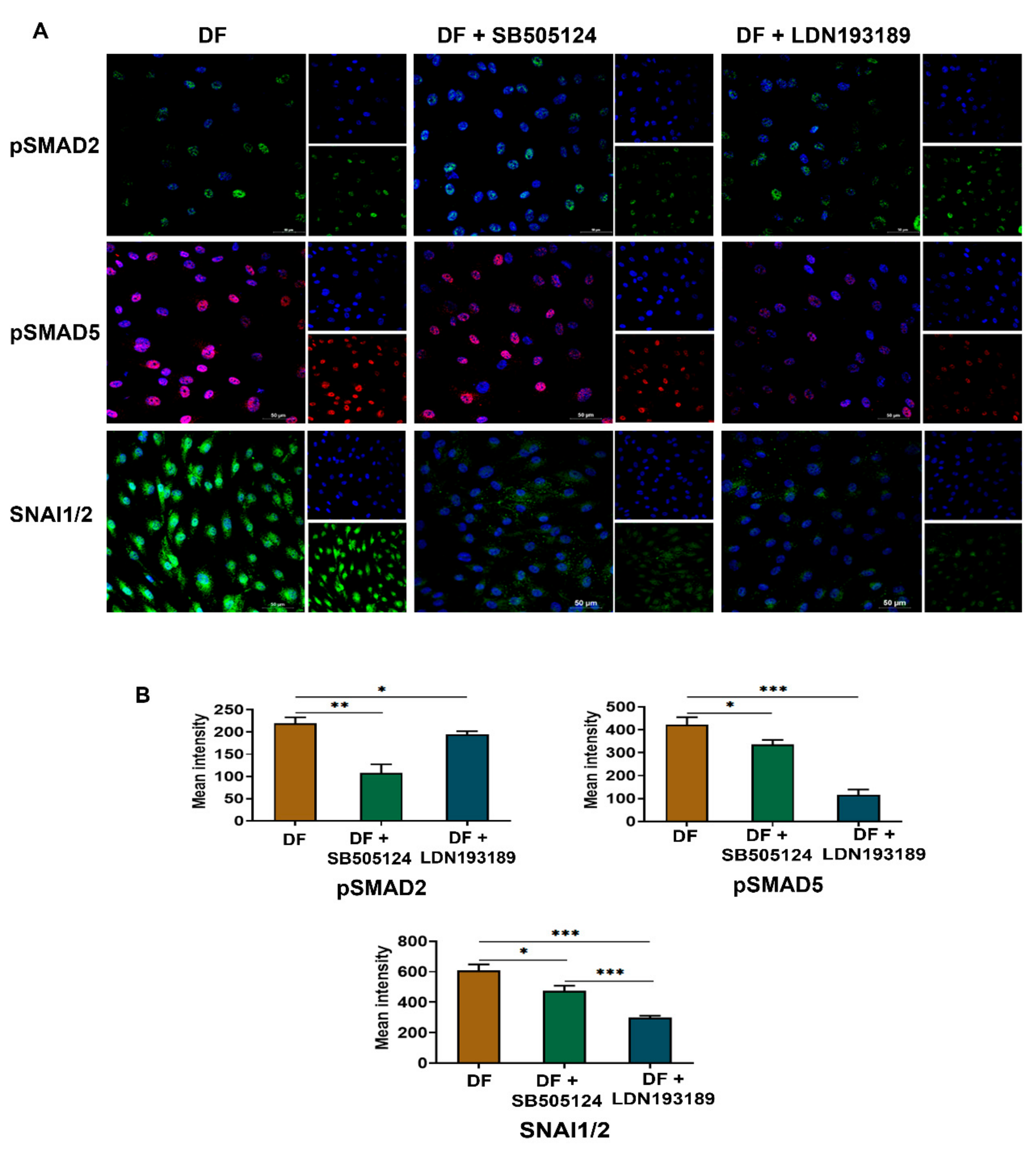
3.6. LDN193189 Abrogates SNAI1/2 Expression Due to Disturbed Flow
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALK | Activin receptor-like kinase |
| BMP | Bone morphogenic protein |
| Dil-Ac-LDL | Dil-labeled acetylated low-density lipoprotein |
| EndMT | Endothelial mesenchymal transition |
| HUVEC | Human umbilical vein endothelial cells |
| IBIDI | Integrated bio diagnostics |
| pSMAD | Phospho SMAD |
| SMAD | Mothers against decapentaplegic homolog 3 |
| SMC | Smooth muscle cells |
| TGFβ | Transforming growth factor β |
| vWF | von Willebrand factor |
References
- Vuylsteke, M.E.; Thomis, S.; Guillaume, G.; Modliszewski, M.-L.; Weides, N.; Staelens, I. Epidemiological study on chronic venous disease in Belgium and Luxembourg: Prevalence, risk factors, and symptomatology. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 432–439. [Google Scholar] [CrossRef]
- Raffetto, J.; Khalil, R. Mechanisms of varicose vein formation: Valve dysfunction and wall dilation. Phlebology 2008, 23, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, R.T.; Raffetto, J.D. Chronic venous insufficiency. Circulation 2005, 111, 2398–2409. [Google Scholar] [CrossRef] [PubMed]
- Chiu, J.J.; Chien, S. Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiol. Rev. 2011, 91, 327–387. [Google Scholar] [CrossRef] [PubMed]
- Lurie, F. Physiology and pathophysiology of the venous system. PanVascular Med. 2014, 1–19. [Google Scholar] [CrossRef]
- Surendran, S.; Girijamma, A.; Nair, R.; Ramegowda, K.S.; Nair, D.H.; Thulaseedharan, J.V.; Lakkappa, R.B.; Kamalapurkar, G.; Kartha, C.C. Forkhead box C2 promoter variant c.-512C> T is associated with increased susceptibility to chronic venous diseases. PLoS ONE 2014, 9, e90682. [Google Scholar] [CrossRef]
- Li, M.; Stenmark, K.R.; Shandas, R.; Tan, W. Effects of pathological flow on pulmonary artery endothelial production of vasoactive mediators and growth factors. J. Vasc. Res. 2009, 46, 561–571. [Google Scholar] [CrossRef]
- Wu, C.C.; Chao, Y.C.; Chen, C.N.; Chien, S.; Chen, Y.C.; Chien, C.C.; Chiu, J.J.; Yen, B.L. Synergism of biochemical and mechanical stimuli in the differentiation of human placenta-derived multipotent cells into endothelial cells. J. Biomech. 2008, 41, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Fukuyama, M.; Satoh, K.; Fujimura, Y.; Yoshioka, A.; Takahashi, H.; Handa, M.; Kawai, Y.; Watanabe, K.; Ikeda, Y. Low shear stress can initiate von Willebrand factor-dependent platelet aggregation in patients with type IIB and platelet-type von Willebrand disease. J. Clin. Investig. 1993, 92, 1555–1558. [Google Scholar] [CrossRef][Green Version]
- Hathcock, J.J. Flow effects on coagulation and thrombosis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1729–1737. [Google Scholar] [CrossRef]
- Papaioannou, T.G.; Stefanadis, C. Vascular wall shear stress: Basic principles and methods. Hell. J. Cardiol. 2005, 46, 9–15. [Google Scholar]
- Moonen, J.R.A.; Lee, E.S.; Schmidt, M.; Maleszewska, M.; Koerts, J.A.; Brouwer, L.A.; Van Kooten, T.G.; Van Luyn, M.J.; Zeebregts, C.J.; Krenning, G. Endothelial-to-mesenchymal transition contributes to fibro-proliferative vascular disease and is modulated by fluid shear stress. Cardiovasc. Res. 2015, 108, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Souilhol, C.; Harmsen, M.C.; Evans, P.C.; Krenning, G. Endothelial–mesenchymal transition in atherosclerosis. Cardiovasc. Res. 2018, 114, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Ranchoux, B.; Antigny, F.; Rucker-Martin, C.; Hautefort, A.; Péchoux, C.; Bogaard, H.J.; Dorfmüller, P.; Remy, S.; Lecerf, F.; Planté, S. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation 2015, 131, 1006–1018. [Google Scholar] [CrossRef]
- Zhang, C.P.; Ding, X.X.; Tian, T.; Li, B.J.; Wang, C.Y.; Jiang, S.S.; Shao, J.Q.; Yuan, Y.L.; Tian, Y.; Zhang, M. Impaired lipophagy in endothelial cells with prolonged exposure to oxidized low-density lipoprotein. Mol. Med. Rep. 2020, 22, 2665–2672. [Google Scholar] [CrossRef]
- Matveeva, V.; Khanova, M.; Sardin, E.; Antonova, L.; Barbarash, O. Endovascular interventions permit isolation of endothelial colony-forming cells from peripheral blood. Int. J. Mol. Sci. 2018, 19, 3453. [Google Scholar] [CrossRef]
- Crum, R.J.; Krane, C. Pravastatin& [trade] Prevents Venous Shear Stress Dependent Induction of Aquaporin 1 Protein Expression in Human Umbilical Vein Endothelial Cells in vitro. Fed. Am. Soc. Exp. Biol. J. 2017, 31, lb639. [Google Scholar]
- Surendran, S.; Ramegowda, K.S.; Suresh, A.; Raj, S.B.; Lakkappa, R.K.B.; Kamalapurkar, G.; Radhakrishnan, N.; Kartha, C.C. Arterialization and anomalous vein wall remodeling in varicose veins is associated with upregulated FoxC2-Dll4 pathway. Lab. Investig. 2016, 96, 399–408. [Google Scholar] [CrossRef]
- Tekari, A.; Luginbuehl, R.; Hofstetter, W.; Egli, R.J. Transforming growth factor beta signaling is essential for the autonomous formation of cartilage-like tissue by expanded chondrocytes. PLoS ONE 2015, 10, e0120857. [Google Scholar]
- Sanvitale, C.E.; Kerr, G.; Chaikuad, A.; Ramel, M.-C.; Mohedas, A.H.; Reichert, S.; Wang, Y.; Triffitt, J.T.; Cuny, G.D.; Yu, P.B. A new class of small molecule inhibitor of BMP signaling. PLoS ONE 2013, 8, e62721. [Google Scholar] [CrossRef] [PubMed]
- Anto, R.J.; Mukhopadhyay, A.; Denning, K.; Aggarwal, B.B. Curcumin (diferuloylmethane) induces apoptosis through activation of caspase-8, BID cleavage and cytochrome c release: Its suppression by ectopic expression of Bcl-2 and Bcl-xl. Carcinogenesis 2002, 23, 143–150. [Google Scholar] [CrossRef]
- Morikawa, M.; Derynck, R.; Miyazono, K. TGF-β and the TGF-β family: Context-dependent roles in cell and tissue physiology. Cold Spring Harb. Perspect. Biol. 2016, 8, a021873. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Troncone, L.; Augur, Z.M.; Kim, S.S.; McNeil, M.E.; Paul, B.Y. The role of bone morphogenetic protein signaling in vascular calcification. Bone 2020, 141, 115542. [Google Scholar] [CrossRef]
- Mihajlović, J.; Diehl, L.A.; Hochhaus, A.; Clement, J.H. Inhibition of bone morphogenetic protein signaling reduces viability, growth and migratory potential of non-small cell lung carcinoma cells. J. Cancer Res. Clin. Oncol. 2019, 145, 2675–2687. [Google Scholar] [CrossRef]
- Cooley, B.C.; Nevado, J.; Mellad, J.; Yang, D.; Hilaire, C.S.; Negro, A.; Fang, F.; Chen, G.; San, H.; Walts, A.D. TGF-β signaling mediates endothelial-to-mesenchymal transition (EndMT) during vein graft remodeling. Sci. Transl. Med. 2014, 6, 227ra34. [Google Scholar] [CrossRef] [PubMed]
- Hoch, J.R.; Stark, V.K.; Turnipseed, W.D. The temporal relationship between the development of vein graft intimal hyperplasia and growth factor gene expression. J. Vasc. Surg. 1995, 22, 51–58. [Google Scholar] [CrossRef]
- Wang, L.; Luo, J.Y.; Li, B.; Tian, X.Y.; Chen, L.J.; Huang, Y.; Liu, J.; Deng, D.; Lau, C.W.; Wan, S. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature 2016, 540, 579–582. [Google Scholar] [CrossRef]
- Lin, Q.; Zhao, J.; Zheng, C.; Chun, J. Roles of notch signaling pathway and endothelial-mesenchymal transition in vascular endothelial dysfunction and atherosclerosis. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6485–6491. [Google Scholar]
- Li, H.; Zhao, Q.; Chang, L.; Wei, C.; Bei, H.; Yin, Y.; Chen, M.; Wang, H.; Liang, J.; Wu, Y. LncRNA MALAT1 modulates ox-LDL induced EndMT through the Wnt/β-catenin signaling pathway. Lipids Health Dis. 2019, 18, 62. [Google Scholar] [CrossRef]
- Pascual, G.; Mendieta, C.; García-Honduvilla, N.; Corrales, C.; Bellón, J.M.; Buján, J. TGF-β1 upregulation in the aging varicose vein. J. Vasc. Res. 2007, 44, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Kowalewski, R.; Malkowski, A.; Sobolewski, K.; Gacko, M. Evaluation of transforming growth factor-β signaling pathway in the wall of normal and varicose veins. Pathobiology 2010, 77, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Gu, J.; Li, Y.; Ma, Q.; Feng, J.; Lu, S. Transforming growth factor-β1 and inducible nitric oxide synthase signaling were involved in effects of prostaglandin E2 on progression of lower limb varicose veins. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 9, 1535–1544. [Google Scholar] [CrossRef]
- Riser, B.L.; Cortes, P.; Heilig, C.; Grondin, J.; Ladson-Wofford, S.; Patterson, D.; Narins, R.G. Cyclic stretching force selectively up-regulates transforming growth factor-beta isoforms in cultured rat mesangial cells. Am. J. Pathol. 1996, 148, 1915. [Google Scholar] [PubMed]
- Ligi, D.; Croce, L.; Mosti, G.; Raffetto, J.D.; Mannello, F. Chronic venous insufficiency: Transforming growth factor-β isoforms and soluble endoglin concentration in different states of wound healing. Int. J. Mol. Sci. 2017, 18, 2206. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.L.; Cooke, M.E.; Alliston, T. ECM stiffness primes the TGFβ pathway to promote chondrocyte differentiation. Mol. Biol. Cell 2012, 23, 3731–3742. [Google Scholar] [CrossRef]
- Uemura, M.; Nagasawa, A.; Terai, K. Yap/Taz transcriptional activity in endothelial cells promotes intramembranous ossification via the BMP pathway. Sci. Rep. 2016, 6, 27473. [Google Scholar] [CrossRef]
- Sorescu, G.P.; Sykes, M.; Weiss, D.; Platt, M.O.; Saha, A.; Hwang, J.; Boyd, N.; Boo, Y.C.; Vega, J.D.; Taylor, W.R. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. J. Biol. Chem. 2003, 278, 31128–31135. [Google Scholar] [CrossRef]
- Jo, H.; Song, H. Disturbed Shear Stress Induces Inflammation and Atherosclerosis-Role of BMP4 as a Mechanosensitive and Inflammatory Cytokine. J. Korean Diabetes 2005, 29, 271–281. [Google Scholar]
- Zhou, J.; Lee, P.L.; Tsai, C.-S.; Lee, C.I.; Yang, T.L.; Chuang, H.-S.; Lin, W.W.; Lin, T.E.; Lim, S.H.; Wei, S.Y. Force-specific activation of Smad1/5 regulates vascular endothelial cell cycle progression in response to disturbed flow. Proc. Natl. Acad. Sci. USA 2012, 109, 7770–7775. [Google Scholar] [CrossRef]
- Neal, A.; Nornes, S.; Payne, S.; Wallace, M.D.; Fritzsche, M.; Louphrasitthiphol, P.; Wilkinson, R.N.; Chouliaras, K.M.; Liu, K.; Plant, K. Venous identity requires BMP signalling through ALK3. Nat. Commun. 2019, 10, 453. [Google Scholar] [CrossRef]
- Walshe, T.E.; dela Paz, N.G.; D’Amore, P.A. The role of shear-induced transforming growth factor-β signaling in the endothelium. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2608–2617. [Google Scholar] [CrossRef] [PubMed]
- Moon, B.S.; Yoon, J.Y.; Kim, M.Y.; Lee, S.H.; Choi, T.; Choi, K.Y. Bone morphogenetic protein 4 stimulates neuronal differentiation of neuronal stem cells through the ERK pathway. Exp. Mol. Med. 2009, 41, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, Y.; Zhang, J.; Yu, D.; Li, A.; Song, H.; Zhang, W.; Davis, D.; Gilbert, M.R.; Liu, F. Autocrine BMP4 Signaling Enhances Tumor Aggressiveness via Promoting Wnt/β-Catenin Signaling in IDH1-mutant Gliomas. Transl. Oncol. 2020, 13, 125–134. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandran Latha, K.; Sreekumar, A.; Beena, V.; S.S., B.R.; Lakkappa, R.B.; Kalyani, R.; Nair, R.; Kalpana, S.R.; Kartha, C.C.; Surendran, S. Shear Stress Alterations Activate BMP4/pSMAD5 Signaling and Induce Endothelial Mesenchymal Transition in Varicose Veins. Cells 2021, 10, 3563. https://doi.org/10.3390/cells10123563
Chandran Latha K, Sreekumar A, Beena V, S.S. BR, Lakkappa RB, Kalyani R, Nair R, Kalpana SR, Kartha CC, Surendran S. Shear Stress Alterations Activate BMP4/pSMAD5 Signaling and Induce Endothelial Mesenchymal Transition in Varicose Veins. Cells. 2021; 10(12):3563. https://doi.org/10.3390/cells10123563
Chicago/Turabian StyleChandran Latha, Karthika, Ahalya Sreekumar, Vyshna Beena, Binil Raj S.S., RaviKumar B. Lakkappa, Ravi Kalyani, Radhakrishnan Nair, Saligrama Ramegowda Kalpana, Chandrasekharan C. Kartha, and Sumi Surendran. 2021. "Shear Stress Alterations Activate BMP4/pSMAD5 Signaling and Induce Endothelial Mesenchymal Transition in Varicose Veins" Cells 10, no. 12: 3563. https://doi.org/10.3390/cells10123563
APA StyleChandran Latha, K., Sreekumar, A., Beena, V., S.S., B. R., Lakkappa, R. B., Kalyani, R., Nair, R., Kalpana, S. R., Kartha, C. C., & Surendran, S. (2021). Shear Stress Alterations Activate BMP4/pSMAD5 Signaling and Induce Endothelial Mesenchymal Transition in Varicose Veins. Cells, 10(12), 3563. https://doi.org/10.3390/cells10123563







