Clever Experimental Designs: Shortcuts for Better iPSC Differentiation
Abstract
1. Introduction
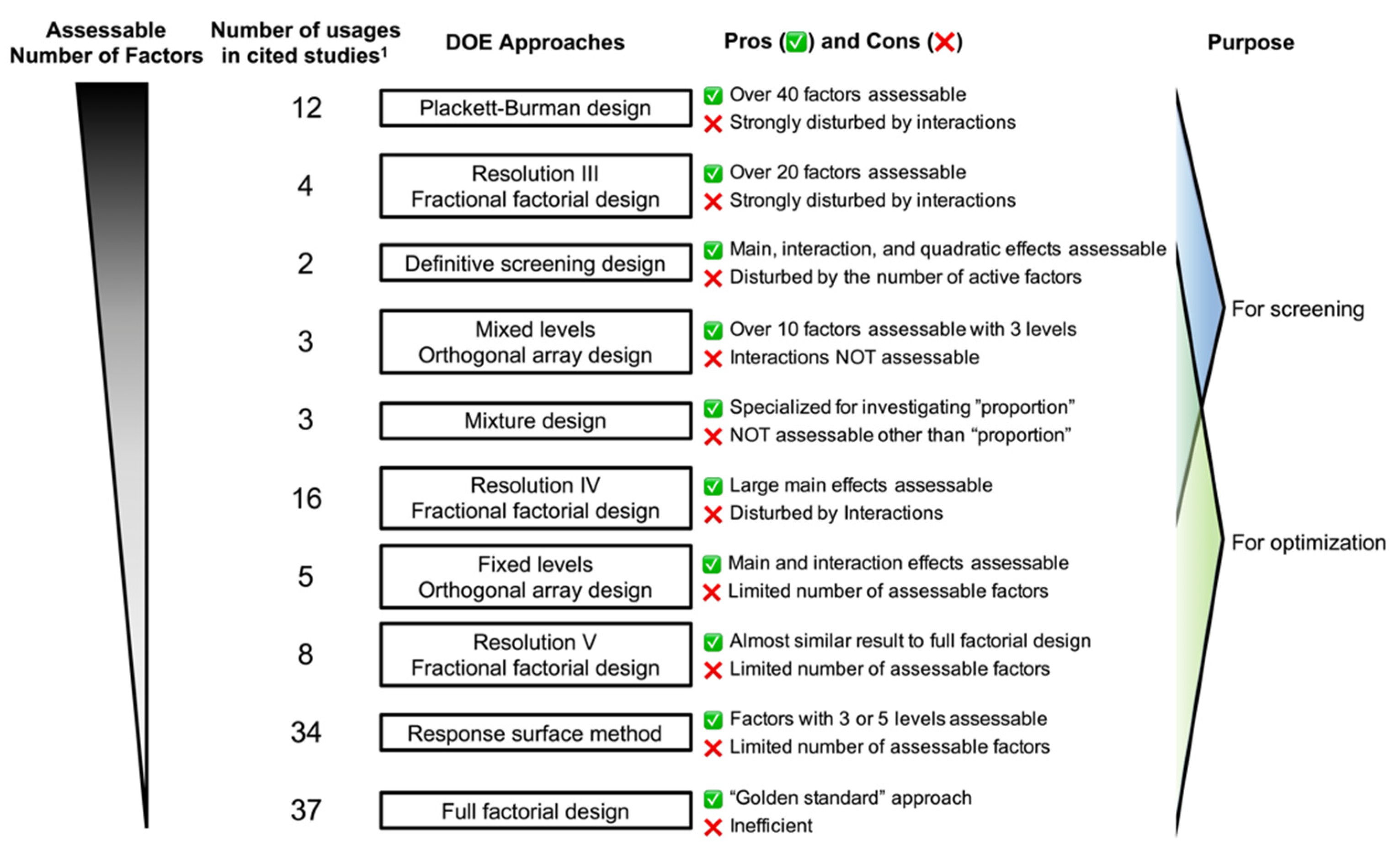
2. DOE Approaches
2.1. One Factor at a Time Approach
2.2. Full Factorial Design
2.3. Fractional Factorial Design
2.4. Orthogonal Array Designs
2.5. Response Surface Method (RSM)
2.6. Definitive Screening Design (DSD)
2.7. Mixture Design
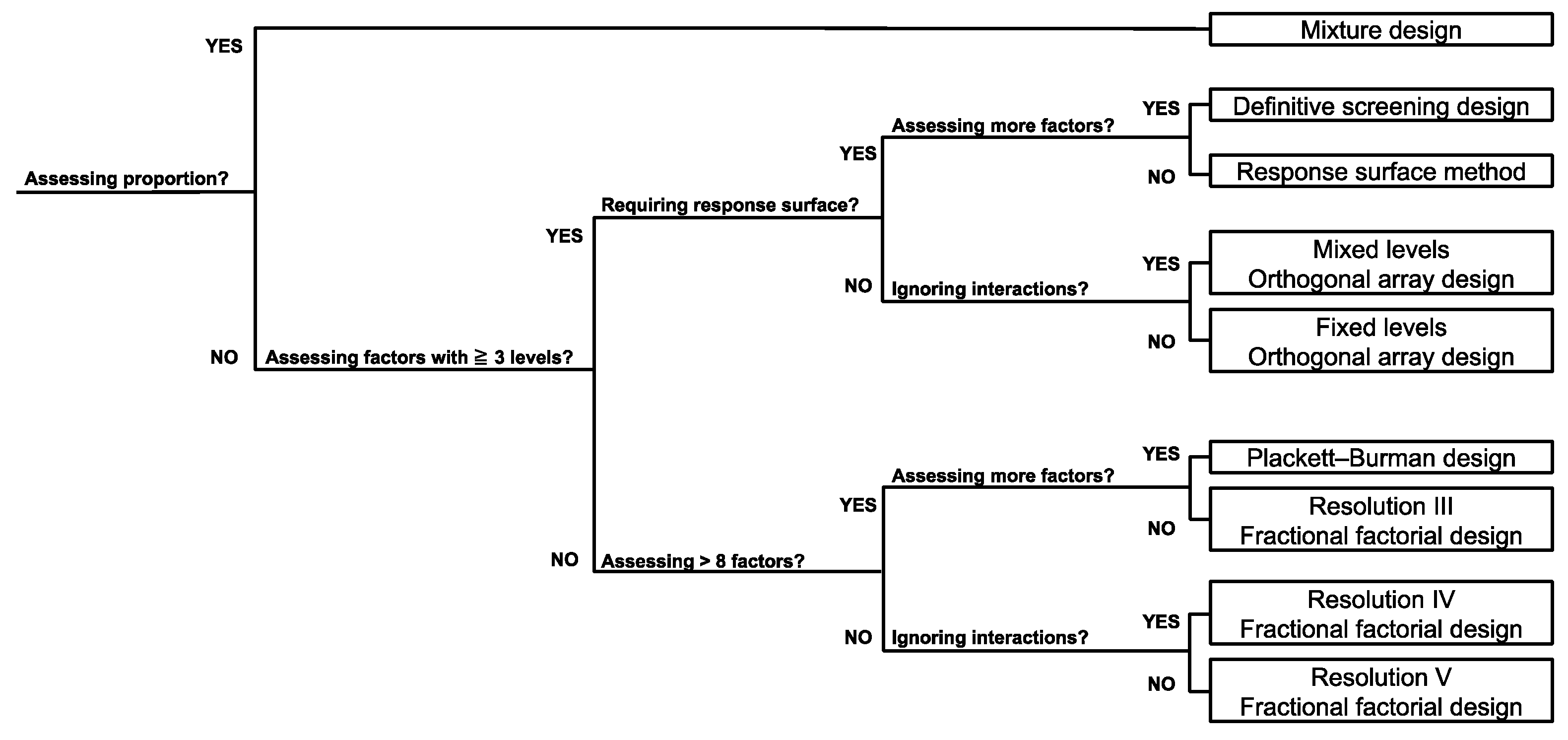
2.8. Selection of DOE Approach
3. Stem Cell Expansion and Differentiation
3.1. PSC Expansion and Differentiation
3.2. MSC and HSC Expansion, and Differentiation
4. CHO Cell Expansion
5. Other Cell Expansion, Cell Differentiation, and Cell-Material Development Processes
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Yamanaka, S. Pluripotent Stem Cell-Based Cell Therapy—Promise and Challenges. Cell Stem Cell 2020, 27, 523–531. [Google Scholar] [CrossRef]
- Umezawa, A.; Sato, Y.; Kusakawa, S.; Amagase, R.; Akutsu, H.; Nakamura, K. Research and Development Strategy for Future Embryonic Stem Cell-Based Therapy in Japan. JMA J. 2020, 3, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Vickaryous, M.K.; Hall, B.K. Human cell type diversity, evolution, development, and classification with special reference to cells derived from the neural crest. Biol. Rev. Camb. Philos. Soc. 2006, 81, 425–455. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kino-Oka, M. Designing a blueprint for next-generation stem cell bioprocessing development. Biotechnol. Bioeng. 2020, 117, 832–843. [Google Scholar] [CrossRef]
- Rohani, L.; Johnson, A.A.; Naghsh, P.; Rancourt, D.E.; Ulrich, H.; Holland, H. Concise Review: Molecular Cytogenetics and Quality Control: Clinical Guardians for Pluripotent Stem Cells. Stem Cells Transl. Med. 2018, 7, 867–875. [Google Scholar] [CrossRef]
- Sullivan, S.; Stacey, G.N.; Akazawa, C.; Aoyama, N.; Baptista, R.; Bedford, P.; Bennaceur Griscelli, A.; Chandra, A.; Elwood, N.; Girard, M.; et al. Quality control guidelines for clinical-grade human induced pluripotent stem cell lines. Regen. Med. 2018, 13, 859–866. [Google Scholar] [CrossRef]
- Ortuño-Costela, M.D.C.; Cerrada, V.; García-López, M.; Gallardo, M.E. The Challenge of Bringing iPSCs to the Patient. Int. J. Mol. Sci. 2019, 20, 6305. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Fan, Z.; Lin, Y.; Wang, T.Y. Serum-Free Medium for Recombinant Protein Expression in Chinese Hamster Ovary Cells. Front. Bioeng. Biotechnol. 2021, 9, 172. [Google Scholar] [CrossRef]
- Brown, K.; Loh, K.M.; Nusse, R. Live Imaging Reveals that the First Division of Differentiating Human Embryonic Stem Cells Often Yields Asymmetric Fates. Cell Rep. 2017, 21, 301–307. [Google Scholar] [CrossRef]
- Fowler, J.L.; Ang, L.T.; Loh, K.M. A critical look: Challenges in differentiating human pluripotent stem cells into desired cell types and organoids. Wiley Interdiscip. Rev. Dev. Biol. 2019, 9, e368. [Google Scholar] [CrossRef]
- Yao, T.; Asayama, Y. Animal-cell culture media: History, characteristics, and current issues. Reprod. Med. Biol. 2017, 16, 99–117. [Google Scholar] [CrossRef]
- Lin, Z.; Xiao, Z.; Lan, L.; Ya, T.; Liu, M.; Qu, K.; Wang, Z.; Zeng, Z.L.; Lin, X.L.; Tan, L.L.; et al. MicroRNAs: Important Regulators of Induced Pluripotent Stem Cell Generation and Differentiation. Stem Cell Rev. Rep. 2018, 14, 71–81. [Google Scholar] [CrossRef]
- Matsumoto, R.; Yamamoto, T.; Takahashi, Y. Complex organ construction from human pluripotent stem cells for biological research and disease modeling with new emerging techniques. Int. J. Mol. Sci. 2021, 22, 10184. [Google Scholar] [CrossRef]
- Kirouac, D.C.; Zandstra, P.W. The Systematic Production of Cells for Cell Therapies. Cell Stem Cell 2008, 3, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Toms, D.; Deardon, R.; Ungrin, M. Climbing the mountain: Experimental design for the efficient optimization of stem cell bioprocessing. J. Biol. Eng. 2017, 11, 35. [Google Scholar] [CrossRef]
- Hanrahan, G.; Lu, K. Application of factorial and response surface methodology in modern experimental design and optimization. Crit. Rev. Anal. Chem. 2006, 36, 141–151. [Google Scholar] [CrossRef]
- Shaw, R.; Festing, M.F.W.; Peers, I.; Furlong, L. Use of factorial designs to optimize animal experiments and reduce animal use. ILAR J. 2002, 43, 223–232. [Google Scholar] [CrossRef]
- Mandenius, C.-F.; Brundin, A. Bioprocess optimization using design-of-experiments methodology. Biotechnol. Prog. 2008, 24, 1191–1203. [Google Scholar] [CrossRef]
- Gündoʇdu, T.K.; Deniz, I.; Çalişkan, G.; Şahin, E.S.; Azbar, N. Experimental design methods for bioengineering applications. Crit. Rev. Biotechnol. 2016, 36, 368–388. [Google Scholar] [CrossRef]
- Singh, V.; Haque, S.; Niwas, R.; Srivastava, A.; Pasupuleti, M.; Tripathi, C.K.M. Strategies for fermentation medium optimization: An in-depth review. Front. Microbiol. 2017, 7, 2087. [Google Scholar] [CrossRef] [PubMed]
- Uhoraningoga, A.; Kinsella, G.K.; Henehan, G.T.; Ryan, B.J. The goldilocks approach: A review of employing design of experiments in prokaryotic recombinant protein production. Bioengineering 2018, 5, 89. [Google Scholar] [CrossRef] [PubMed]
- Politis, S.N.; Colombo, P.; Colombo, G.; Rekkas, D.M. Design of experiments (DoE) in pharmaceutical development. Drug Dev. Ind. Pharm. 2017, 43, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Sommeregger, W.; Sissolak, B.; Kandra, K.; von Stosch, M.; Mayer, M.; Striedner, G. Quality by control: Towards model predictive control of mammalian cell culture bioprocesses. Biotechnol. J. 2017, 12, 1600546. [Google Scholar] [CrossRef]
- Puskeiler, R.; Kreuzmann, J.; Schuster, C.; Didzus, K.; Bartsch, N.; Hakemeyer, C.; Schmidt, H.; Jacobs, M.; Wolf, S. The way to a design space for an animal cell culture process according to Quality by Design (QbD). BMC Proc. 2011, 5, P12. [Google Scholar] [CrossRef] [PubMed]
- Marasco, D.M.; Gao, J.; Griffiths, K.; Froggatt, C.; Wang, T.; Wei, G. Development and characterization of a cell culture manufacturing process using quality by design (QbD) principles. Adv. Biochem. Eng. Biotechnol. 2014, 139, 93–121. [Google Scholar] [CrossRef] [PubMed]
- Czitrom, V. One-factor-at-a-time versus designed experiments. Am. Stat. 1999, 53, 126–131. [Google Scholar] [CrossRef]
- Li, X.; Sudarsanam, N.; Frey, D.D. Regularities in data from factorial experiments. Complexity 2006, 11, 32–45. [Google Scholar] [CrossRef]
- Collins, L.M.; Dziak, J.J.; Li, R. Design of Experiments with Multiple Independent Variables: A Resource Management Perspective on Complete and Reduced Factorial Designs. Psychol. Methods 2009, 14, 202–224. [Google Scholar] [CrossRef]
- Rao, R.S.; Kumar, C.G.; Prakasham, R.S.; Hobbs, P.J. The Taguchi methodology as a statistical tool for biotechnological applications: A critical appraisal. Biotechnol. J. 2008, 3, 510–523. [Google Scholar] [CrossRef]
- Taguchi, G. System of Experimental Design: Engineering Methods to Optimize Quality and Minimize Costs; UNIPUB/Kraus International Publications: Millwood, NY, USA, 1987; ISBN 0527916218. [Google Scholar]
- Taguchi, G.; Chowdhury, S.; Wu, Y. Taguchi’s Quality Engineering Handbook; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; ISBN 9780470258354. [Google Scholar]
- Jones, B. 21st century screening experiments: What, why, and how. Qual. Eng. 2016, 28, 98–106. [Google Scholar] [CrossRef]
- Galvan, D.; Effting, L.; Cremasco, H.; Conte-Junior, C.A. Recent Applications of Mixture Designs in Beverages, Foods, and Pharmaceutical Health: A Systematic Review and Meta-Analysis. Foods 2021, 10, 1941. [Google Scholar] [CrossRef] [PubMed]
- Prudhomme, W.; Daley, G.Q.; Zandstra, P.; Lauffenburger, D.A. Multivariate proteomic analysis of murine embryonic stem cell self-renewal versus differentation signaling. Proc. Natl. Acad. Sci. USA 2004, 101, 2900–2905. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M.M.; Meng, G.; Rancourt, D.E.; Gates, I.D.; Kallos, M.S. Factorial experimental design for the culture of human embryonic stem cells as aggregates in stirred suspension bioreactors reveals the potential for interaction effects between bioprocess parameters. Tissue Eng. Part C Methods 2014, 20, 76–89. [Google Scholar] [CrossRef]
- Barbosa, H.S.C.; Fernandes, T.G.; Dias, T.P.; Diogo, M.M.; Cabral, J.M.S. New insights into the mechanisms of embryonic stem cell self-renewal under Hypoxia: A multifactorial analysis approach. PLoS ONE 2012, 7, e38963. [Google Scholar] [CrossRef]
- Marinho, P.A.; Chailangkarn, T.; Muotri, A.R. Systematic optimization of human pluripotent stem cells media using Design of Experiments. Sci. Rep. 2015, 5, 9834. [Google Scholar] [CrossRef]
- Ratcliffe, E.; Hourd, P.; Guijarro-Leach, J.; Rayment, E.; Williams, D.J.; Thomas, R.J. Application of response surface methodology to maximize the productivity of scalable automated human embryonic stem cell manufacture. Regen. Med. 2013, 8, 39–48. [Google Scholar] [CrossRef]
- Badenes, S.M.; Fernandes, T.G.; Cordeiro, C.S.M.; Boucher, S.; Kuninger, D.; Vemuri, M.C.; Diogo, M.M.; Cabral, J.M.S. Defined essential 8′ medium and vitronectin efficiently support scalable xeno-free expansion of human induced pluripotent stem cells in stirred microcarrier culture systems. PLoS ONE 2016, 11, e0151264. [Google Scholar] [CrossRef]
- Chang, K.H.; Zandstra, P.W. Quantitative screening of embryonic stem cell differentiation: Endoderm formation as a model. Biotechnol. Bioeng. 2004, 88, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Flaim, C.J.; Chien, S.; Bhatia, S.N. An extracellular matrix microarray for probing cellular differentiation. Nat. Methods 2005, 2, 119–125. [Google Scholar] [CrossRef]
- Flaim, C.J.; Teng, D.; Chien, S.; Bhatia, S.N. Combinatorial signaling microenvironments for studying stem cell fate. Stem Cells Dev. 2008, 17, 29–39. [Google Scholar] [CrossRef]
- Kumar, N.; Richter, J.; Cutts, J.; Bush, K.T.; Trujillo, C.; Nigam, S.K.; Gaasterland, T.; Brafman, D.; Willert, K. Generation of an expandable intermediate mesoderm restricted progenitor cell line from human pluripotent stem cells. eLife 2015, 4, e08413. [Google Scholar] [CrossRef] [PubMed]
- Hallam, D.; Hilgen, G.; Dorgau, B.; Zhu, L.; Yu, M.; Bojic, S.; Hewitt, P.; Schmitt, M.; Uteng, M.; Kustermann, S.; et al. Human-Induced Pluripotent Stem Cells Generate Light Responsive Retinal Organoids with Variable and Nutrient-Dependent Efficiency. Stem Cells 2018, 36, 1535–1551. [Google Scholar] [CrossRef]
- Li, L.; Tan, D.; Liu, S.; Jiao, R.; Yang, X.; Li, F.; Wu, H.; Huang, W. Optimization of Factor Combinations for Stem Cell Differentiations on a Design-of-Experiment Microfluidic Chip. Anal. Chem. 2020, 92, 14228–14235. [Google Scholar] [CrossRef] [PubMed]
- Songstad, A.E.; Worthington, K.S.; Chirco, K.R.; Giacalone, J.C.; Whitmore, S.S.; Anfinson, K.R.; Ochoa, D.; Cranston, C.M.; Riker, M.J.; Neiman, M.; et al. Connective Tissue Growth Factor Promotes Efficient Generation of Human Induced Pluripotent Stem Cell-Derived Choroidal Endothelium. Stem Cells Transl. Med. 2017, 6, 1533–1546. [Google Scholar] [CrossRef]
- Yasui, R.; Sekine, K.; Yamaguchi, K.; Furukawa, Y.; Taniguchi, H. Robust parameter design of human induced pluripotent stem cell differentiation protocols defines lineage-specific induction of anterior-posterior gut tube endodermal cells. Stem Cells 2021, 39, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Carmichael, S.T.; Lowry, W.E.; Segura, T. Hydrogel design of experiments methodology to optimize hydrogel for iPSC-NPC culture. Adv. Healthc. Mater. 2015, 4, 534–539. [Google Scholar] [CrossRef]
- Jung, J.P.; Hu, D.; Domian, I.J.; Ogle, B.M. An integrated statistical model for enhanced murine cardiomyocyte differentiation via optimized engagement of 3D extracellular matrices. Sci. Rep. 2015, 5, 18705. [Google Scholar] [CrossRef]
- Esteban, P.P.; Patel, H.; Veraitch, F.; Khalife, R. Optimization of the nutritional environment for differentiation of human-induced pluripotent stem cells using design of experiments—A proof of concept. Biotechnol. Prog. 2021, 37, e3143. [Google Scholar] [CrossRef]
- Hu, K.; Yu, Y. Metabolite availability as a window to view the early embryo microenvironment in vivo. Mol. Reprod. Dev. 2017, 84, 1027–1038. [Google Scholar] [CrossRef]
- Thomas, R.J.; Hourd, P.C.; Williams, D.J. Application of process quality engineering techniques to improve the understanding of the in vitro processing of stem cells for therapeutic use. J. Biotechnol. 2008, 136, 148–155. [Google Scholar] [CrossRef]
- Jakobsen, R.B.; Østrup, E.; Zhang, X.; Mikkelsen, T.S.; Brinchmann, J.E. Analysis of the effects of five factors relevant to in vitro chondrogenesis of human mesenchymal stem cells using factorial design and high throughput mRNA-profiling. PLoS ONE 2014, 9, e96615. [Google Scholar] [CrossRef]
- Liu, C.H.; Wu, M.L.; Hwang, S.M. Optimization of serum free medium for cord blood mesenchymal stem cells. Biochem. Eng. J. 2007, 33, 1–9. [Google Scholar] [CrossRef]
- Fan, X.; Liu, T.; Liu, Y.; Ma, X.; Cui, Z. Optimization of primary culture condition for mesenchymal stem cells derived from umbilical cord blood with factorial design. Biotechnol. Prog. 2009, 25, 499–507. [Google Scholar] [CrossRef]
- Decaris, M.L.; Leach, J.K. Design of experiments approach to engineer cell-secreted matrices for directing osteogenic differentiation. Ann. Biomed. Eng. 2011, 39, 1174–1185. [Google Scholar] [CrossRef]
- Kwon, S.S.; Kim, H.; Shin, S.J.; Lee, S.Y. Optimization of tenocyte lineage-related factors from tonsil-derived mesenchymal stem cells using response surface methodology. J. Orthop. Surg. Res. 2020, 15, 109. [Google Scholar] [CrossRef]
- Petzer, A.L.; Zandstra, P.W.; Piret, J.M.; Eaves, C.J. Differential cytokine effects on primitive (CD34+CD38-) human hematopoietic cells: Novel responses to Flt3-ligand and thrombopoietin. J. Exp. Med. 1996, 183, 2551–2558. [Google Scholar] [CrossRef]
- Zandstra, P.W.; Conneally, E.; Petzer, A.L.; Piret, J.M.; Eaves, C.J. Cytokine manipulation of primitive human hematopoietic cell self-renewal. Proc. Natl. Acad. Sci. USA 1997, 94, 4698–4703. [Google Scholar] [CrossRef]
- Zandstra, P.W.; Conneally, E.; Piret, J.M.; Eaves, C.J. Ontogeny-associated changes in the cytokine responses of primitive human haemopoietic cells. Br. J. Haematol. 1998, 101, 770–778. [Google Scholar] [CrossRef]
- Panuganti, S.; Schlinker, A.C.; Lindholm, P.F.; Papoutsakis, E.T.; Miller, W.M. Three-stage ex vivo expansion of high-ploidy megakaryocytic cells: Toward large-scale platelet production. Tissue Eng. Part A 2013, 19, 998–1014. [Google Scholar] [CrossRef]
- Yao, C.L.; Liu, C.H.; Chu, I.M.; Hsieh, T.B.; Hwang, S.M. Factorial designs combined with the steepest ascent method to optimize serum-free media for ex vivo expansion of human hematopoietic progenitor cells. Enzyme Microb. Technol. 2003, 33, 343–352. [Google Scholar] [CrossRef]
- Yao, C.L.; Chu, I.M.; Hsieh, T.B.; Hwang, S.M. A systematic strategy to optimize ex vivo expansion medium for human hematopoietic stem cells derived from umbilical cord blood mononuclear cells. Exp. Hematol. 2004, 32, 720–727. [Google Scholar] [CrossRef]
- Case, J.; Rice, A.; Wood, J.; Gaudry, L.; Vowels, M.; Nordon, R.E. Characterization of cytokine interactions by flow cytometry and factorial analysis. Cytometry 2001, 43, 69–81. [Google Scholar] [CrossRef]
- Chen, T.W.; Yao, C.L.; Chu, I.M.; Chuang, T.L.; Hsieh, T.B.; Hwang, S.M. Large generation of megakaryocytes from serum-free expanded human CD34+ cells. Biochem. Biophys. Res. Commun. 2009, 378, 112–117. [Google Scholar] [CrossRef]
- Hsu, S.C.; Lu, L.C.; Chan, K.Y.; Huang, C.H.; Cheng, S.L.; Chan, Y.S.; Yang, Y.S.; Lai, Y.T.; Yao, C.L. Large-scale production and directed induction of functional dendritic cells ex vivo from serum-free expanded human hematopoietic stem cells. Cytotherapy 2019, 21, 755–768. [Google Scholar] [CrossRef]
- Irhimeh, M.R.; Fitton, J.H.; Ko, K.H.; Lowenthal, R.M.; Nordon, R.E. Formation of an adherent hematopoietic expansion culture using fucoidan. Ann. Hematol. 2011, 90, 1005–1015. [Google Scholar] [CrossRef]
- Andrade, P.Z.; Dos Santos, F.; Almeida-Porada, G.; Lobato Da Silva, C.; Joaquim, J.M. Systematic delineation of optimal cytokine concentrations to expand hematopoietic stem/progenitor cells in co-culture with mesenchymal stem cells. Mol. Biosyst. 2010, 6, 1207–1215. [Google Scholar] [CrossRef]
- Audet, J.; Miller, C.L.; Eaves, C.J.; Piret, J.M. Common and distinct features of cytokine effects on hematopoietic stem and progenitor cells revealed by dose-response surface analysis. Biotechnol. Bioeng. 2002, 80, 393–404. [Google Scholar] [CrossRef]
- Cortin, V.; Garnier, A.; Pineault, N.; Lemieux, R.; Boyer, L.; Proulx, C. Efficient in vitro megakaryocyte maturation using cytokine cocktails optimized by statistical experimental design. Exp. Hematol. 2005, 33, 1182–1191. [Google Scholar] [CrossRef]
- Lim, M.; Ye, H.; Panoskaltsis, N.; Drakakis, E.M.; Yue, X.; Cass, A.E.G.; Radomska, A.; Mantalaris, A. Intelligent bioprocessing for haemotopoietic cell cultures using monitoring and design of experiments. Biotechnol. Adv. 2007, 25, 353–368. [Google Scholar] [CrossRef]
- Deshpande, R.R.; Wittmann, C.; Heinzle, E. Microplates with integrated oxygen sensing for medium optimization in animal cell culture. Cytotechnology 2004, 46, 1–8. [Google Scholar] [CrossRef]
- Mora, A.; Nabiswa, B.; Duan, Y.; Zhang, S.; Carson, G.; Yoon, S. Early integration of Design of Experiment (DOE) and multivariate statistics identifies feeding regimens suitable for CHO cell line development and screening. Cytotechnology 2019, 71, 1137–1153. [Google Scholar] [CrossRef] [PubMed]
- Sandadi, S.; Ensari, S.; Kearns, B. Application of fractional factorial designs to screen active factors for antibody production by Chinese hamster ovary cells. Biotechnol. Prog. 2006, 22, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Castro, P.M.L.; Hayter, P.M.; Ison, A.P.; Bull, A.T. Application of a statistical design to the optimization of culture medium for recombinant interferon-gamma production by Chinese hamster ovary cells. Appl. Microbiol. Biotechnol. 1992, 38, 84–90. [Google Scholar] [CrossRef]
- Castro, P.M.; Ison, A.P.; Hayter, P.M.; Bull, A.T. CHO cell growth and recombinant interferon-γ production: Effects of BSA, Pluronic and lipids. Cytotechnology 1995, 19, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Kim, N.S.; Lee, G.M. Development of a serum-free medium for the production of humanized antibody from Chinese hamster ovary cells using a statistical design. Vitr. Cell. Dev. Biol. Anim. 1998, 34, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Kim, N.S.; Lee, G.M. Development of a serum-free medium for dihydrofolate reductase-deficient Chinese hamster ovary cells (DG44) using a statistical design: Beneficial effect of weaning of cells. In Vitro Cell. Dev. Biol. Anim. 1999, 35, 178–182. [Google Scholar] [CrossRef]
- Lee, G.M.; Kim, E.J.; Kim, N.S.; Yoon, S.K.; Ahn, Y.H.; Song, J.Y. Development of a serum-free medium for the production of erythropoietin by suspension culture of recombinant Chinese hamster ovary cells using a statistical design. J. Biotechnol. 1999, 69, 85–93. [Google Scholar] [CrossRef]
- Sandadi, S.; Ensari, S.; Kearns, B. Heuristic optimization of antibody production by Chinese hamster ovary cells. Biotechnol. Prog. 2005, 21, 1537–1542. [Google Scholar] [CrossRef]
- Liu, C.H.; Chang, T.Y. Rational development of serum-free medium for Chinese hamster ovary cells. Process Biochem. 2006, 41, 2314–2319. [Google Scholar] [CrossRef]
- Parampalli, A.; Eskridge, K.; Smith, L.; Meagher, M.M.; Mowry, M.C.; Subramanian, A. Developement of serum-free media in CHO-DG44 cells using a central composite statistical design. Cytotechnology 2007, 54, 57–68. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, G.M. Development of serum-free medium supplemented with hydrolysates for the production of therapeutic antibodies in CHO cell cultures using design of experiments. Appl. Microbiol. Biotechnol. 2009, 83, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, H.; Liu, M.; Zhang, T.; Zhang, J.; Wang, X.; Xiang, W. Rational development of a serum-free medium and fed-batch process for a GS-CHO cell line expressing recombinant antibody. Cytotechnology 2013, 65, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, H.; Watari, A.; Shinoda, Y.; Okamoto, H.; Takuma, S. Application of a Quality by Design approach to the cell culture process of monoclonal antibody production, resulting in the establishment of a Design space. J. Pharm. Sci. 2013, 102, 4274–4283. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Deng, X.; Poon, H.F.; Liu, X.; Tan, W.S.; Zhou, Y.; Fan, L. Chinese hamster ovary cell performance enhanced by a rational divide-and-conquer strategy for chemically defined medium development. J. Biosci. Bioeng. 2015, 120, 690–696. [Google Scholar] [CrossRef]
- Torkashvand, F.; Vaziri, B.; Maleknia, S.; Heydari, A.; Vossoughi, M.; Davami, F.; Mahboudi, F. Designed amino acid feed in improvement of production and quality targets of a therapeutic monoclonal antibody. PLoS ONE 2015, 10, e0140597. [Google Scholar] [CrossRef]
- Brunner, M.; Fricke, J.; Kroll, P.; Herwig, C. Investigation of the interactions of critical scale-up parameters (pH, pO2 and pCO2) on CHO batch performance and critical quality attributes. Bioprocess Biosyst. Eng. 2017, 40, 251–263. [Google Scholar] [CrossRef]
- Mayrhofer, P.; Reinhart, D.; Castan, A.; Kunert, R. Rapid development of clone-specific, high-performing perfusion media from established feed supplements. Biotechnol. Prog. 2020, 36, e2933. [Google Scholar] [CrossRef]
- Puente-Massaguer, E.; Badiella, L.; Gutiérrez-Granados, S.; Cervera, L.; Gòdia, F. A statistical approach to improve compound screening in cell culture media. Eng. Life Sci. 2019, 19, 315–327. [Google Scholar] [CrossRef]
- Fouladiha, H.; Marashi, S.A.; Torkashvand, F.; Mahboudi, F.; Lewis, N.E.; Vaziri, B. A metabolic network-based approach for developing feeding strategies for CHO cells to increase monoclonal antibody production. Bioprocess Biosyst. Eng. 2020, 43, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Didier, C.; Etcheverrigaray, M.; Kratje, R.; Goicoechea, H.C. Crossed mixture design and multiple response analysis for developing complex culture media used in recombinant protein production. Chemom. Intell. Lab. Syst. 2007, 86, 1–9. [Google Scholar] [CrossRef]
- Rouiller, Y.; Périlleux, A.; Collet, N.; Jordan, M.; Stettler, M.; Broly, H. A high-throughput media design approach for high performance mammalian fed-batch cultures. MAbs 2013, 5, 501–511. [Google Scholar] [CrossRef]
- Nie, L.; Gao, D.; Jiang, H.; Gou, J.; Li, L.; Hu, F.; Guo, T.; Wang, H.; Qu, H. Development and Qualification of a Scale-Down Mammalian Cell Culture Model and Application in Design Space Development by Definitive Screening Design. AAPS PharmSciTech 2019, 20, 246. [Google Scholar] [CrossRef]
- Wu, S.; Rish, A.J.; Skomo, A.; Zhao, Y.; Drennen, J.K.; Anderson, C.A. Rapid serum-free/suspension adaptation: Medium development using a definitive screening design for Chinese hamster ovary cells. Biotechnol. Prog. 2021, 37, e3154. [Google Scholar] [CrossRef]
- Legmann, R.; Benoit, B.; Fedechko, R.W.; Deppeler, C.L.; Srinivasan, S.; Robins, R.H.; Mccormick, E.L.; Ferrick, D.A.; Rodgers, S.T.; Russo, A.P. A Strategy for clone selection under different production conditions. Biotechnol. Prog. 2011, 27, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.; Voisard, D.; Berthoud, A.; Tercier, L.; Kleuser, B.; Baer, G.; Broly, H. Cell culture medium improvement by rigorous shuffling of components using media blending. Cytotechnology 2013, 65, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, T.; Yasuda, S.; Tachi, S.; Matsuyama, S.; Kusakawa, S.; Tano, K.; Miura, T.; Matsuyama, A.; Sato, Y. SALL3 expression balance underlies lineage biases in human induced pluripotent stem cell differentiation. Nat. Commun. 2019, 10, 2175. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.M.; Lim, B. A precarious balance: Pluripotency factors as lineage specifiers. Cell Stem Cell 2011, 8, 363–369. [Google Scholar] [CrossRef]
- Hoesli, C.A.; Johnson, J.D.; Piret, J.M. Purified human pancreatic duct cell culture conditions defined by serum-free high-content growth factor screening. PLoS ONE 2012, 7, e33999. [Google Scholar] [CrossRef]
- Chen, X.M.; Elisia, I.; Kitts, D.D. Defining conditions for the co-culture of Caco-2 and HT29-MTX cells using Taguchi design. J. Pharmacol. Toxicol. Methods 2010, 61, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.P.; Moyano, J.V.; Collier, J.H. Multifactorial optimization of endothelial cell growth using modular synthetic extracellular matrices. Integr. Biol. 2011, 3, 185–196. [Google Scholar] [CrossRef]
- Jeon, M.K.; Lim, J.B.; Lee, G.M. Development of a serum-free medium for in vitro expansion of human cytotoxic T lymphocytes using a statistical design. BMC Biotechnol. 2010, 10, 70. [Google Scholar] [CrossRef]
- Zhao, A.; Chen, F.; Ning, C.; Wu, H.; Song, H.; Wu, Y.; Chen, R.; Zhou, K.; Xu, X.; Lu, Y.; et al. Use of real-time cellular analysis and Plackett-Burman design to develop the serum-free media for PC-3 prostate cancer cells. PLoS ONE 2017, 12, e0185470. [Google Scholar] [CrossRef]
- Lee, E.; Lim, Z.R.; Chen, H.Y.; Yang, B.X.; Lam, A.T.L.; Chen, A.K.L.; Sivalingam, J.; Reuveny, S.; Loh, Y.H.; Oh, S.K.W. Defined Serum-Free Medium for Bioreactor Culture of an Immortalized Human Erythroblast Cell Line. Biotechnol. J. 2018, 13, e1700567. [Google Scholar] [CrossRef] [PubMed]
- Petiot, E.; Fournier, F.; Gény, C.; Pinton, H.; Marc, A. Rapid screening of serum-free media for the growth of adherent vero cells by using a small-scale and non-invasive tool. Appl. Biochem. Biotechnol. 2010, 160, 1600–1615. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, K.; Rourou, S.; Loukil, H.; Majoul, S.; Kallel, H. Optimization of virus yield as a strategy to improve rabies vaccine production by Vero cells in a bioreactor. J. Biotechnol. 2006, 121, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, J.G.; Dhurjati, P. Fractional Factorial Study of Hybridoma Behavior. 2. Kinetics of Nutrient Uptake and Waste Production. Biotechnol. Prog. 1993, 9, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Moran, E.B.; McGowan, S.T.; McGuire, J.M.; Frankland, J.E.; Oyebade, I.A.; Waller, W.; Archer, L.C.; Morris, L.O.; Pandya, J.; Nathan, S.R.; et al. A systematic approach to the validation of process control parameters for monoclonal antibody production in fed-batch culture of a murine myeloma. Biotechnol. Bioeng. 2000, 69, 242–255. [Google Scholar] [CrossRef]
- Kallel, H.; Zaïri, H.; Rourou, S.; Essafi, M.; Barbouche, R.; Dellagi, K.; Fathallah, D.M. Use of Taguchi’s methods as a basis to optimize hybridoma cell line growth and antibody production in a spinner flask. Cytotechnology 2002, 39, 9–14. [Google Scholar] [CrossRef]
- Liu, G.; Kawaguchi, H.; Ogasawara, T.; Asawa, Y.; Kishimoto, J.; Takahashi, T.; Chung, U.I.; Yamaoka, H.; Asato, H.; Nakamura, K.; et al. Optimal combination of soluble factors for tissue engineering of permanent cartilage from cultured human chondrocytes. J. Biol. Chem. 2007, 282, 20407–20415. [Google Scholar] [CrossRef]
- Enochson, L.; Brittberg, M.; Lindahl, A. Optimization of a chondrogenic medium through the use of factorial design of experiments. Biores. Open Access 2012, 1, 306–313. [Google Scholar] [CrossRef]
- Chen, Y.; Bloemen, V.; Impens, S.; Moesen, M.; Luyten, F.P.; Schrooten, J. Characterization and optimization of cell seeding in scaffolds by factorial design: Quality by design approach for Skeletal tissue engineering. Tissue Eng. Part C Methods 2011, 17, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Kuterbekov, M.; Machillot, P.; Baillet, F.; Jonas, A.M.; Glinel, K.; Picart, C. Design of experiments to assess the effect of culture parameters on the osteogenic differentiation of human adipose stromal cells. Stem Cell Res. Ther. 2019, 10, 256. [Google Scholar] [CrossRef]
- Chen, W.L.K.; Likhitpanichkul, M.; Ho, A.; Simmons, C.A. Integration of statistical modeling and high-content microscopy to systematically investigate cell-substrate interactions. Biomaterials 2010, 31, 2489–2497. [Google Scholar] [CrossRef]
- Dong, J.; Mandenius, C.F.; Lübberstedt, M.; Urbaniak, T.; Nüssler, A.K.N.; Knobeloch, D.; Gerlach, J.C.; Zeilinger, K. Evaluation and optimization of hepatocyte culture media factors by design of experiments (DoE) methodology. Cytotechnology 2008, 57, 251–261. [Google Scholar] [CrossRef][Green Version]
- Li, Q.; Cheung, W.H.; Chow, K.L.; Ellis-Behnke, R.G.; Chau, Y. Factorial analysis of adaptable properties of self-assembling peptide matrix on cellular proliferation and neuronal differentiation of pluripotent embryonic carcinoma. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 748–756. [Google Scholar] [CrossRef]
- Pasovic, L.; Utheim, T.P.; Reppe, S.; Khan, A.Z.; Jackson, C.J.; Thiede, B.; Berg, J.P.; Messelt, E.B.; Eidet, J.R. Improvement of Storage Medium for Cultured Human Retinal Pigment Epithelial Cells Using Factorial Design. Sci. Rep. 2018, 8, 5688. [Google Scholar] [CrossRef]
- Reppe, S.; Jackson, C.J.; Ringstad, H.; Tønseth, K.A.; Bakke, H.; Eidet, J.R.; Utheim, T.P. High throughput screening of additives using factorial design to promote survival of stored cultured epithelial sheets. Stem Cells Int. 2018, 2018, 6545876. [Google Scholar] [CrossRef]
- Khosravi, R.; Hosseini, S.N.; Javidanbardan, A.; Khatami, M.; Kaghazian, H.; Mousavi Nasab, S.D. Optimization of non-detergent treatment for enveloped virus inactivation using the Taguchi design of experimental methodology (DOE). Prep. Biochem. Biotechnol. 2019, 49, 686–694. [Google Scholar] [CrossRef]
- Kleman, M.I.; Oellers, K.; Lullau, E. Optimal conditions for freezing cho-s and HEK293-EBNA cell lines: Influence of ME2SO, freeze density, and pei-mediated transfection on devitalization and growth of cells, and expression of recombinant protein. Biotechnol. Bioeng. 2008, 100, 911–922. [Google Scholar] [CrossRef]
- Kennedy, M.; Krouse, D. Strategies for improving fermentation medium performance: A review. J. Ind. Microbiol. Biotechnol. 1999, 23, 456–475. [Google Scholar] [CrossRef]
- Hook, A.L.; Anderson, D.G.; Langer, R.; Williams, P.; Davies, M.C.; Alexander, M.R. High throughput methods applied in biomaterial development and discovery. Biomaterials 2010, 31, 187–198. [Google Scholar] [CrossRef]
- Rankin, S.A.; Mccracken, K.W.; Luedeke, D.M.; Han, L.; Wells, J.M.; Shannon, J.M.; Zorn, A.M. Timing is everything: Reiterative Wnt, BMP and RA signaling regulate developmental competence during endoderm organogenesis. Dev. Biol. 2017, 434, 121–132. [Google Scholar] [CrossRef]
- Debevec, V.; Srčič, S.; Horvat, M. Scientific, statistical, practical, and regulatory considerations in design space development. Drug Dev. Ind. Pharm. 2018, 44, 349–364. [Google Scholar] [CrossRef]
- Papaneophytou, C. Design of Experiments as a Tool for Optimization in Recombinant Protein Biotechnology: From Constructs to Crystals. Mol. Biotechnol. 2019, 61, 873–891. [Google Scholar] [CrossRef]
- Sasamata, M.; Shimojo, D.; Fuse, H.; Nishi, Y.; Sakurai, H.; Nakahata, T.; Yamagishi, Y.; Sasaki-Iwaoka, H. Establishment of a Robust Platform for Induced Pluripotent Stem Cell Research Using Maholo LabDroid. SLAS Technol. 2021, 26, 441–453. [Google Scholar] [CrossRef]
- Rodriguez-Granrose, D.; Jones, A.; Loftus, H.; Tandeski, T.; Heaton, W.; Foley, K.T.; Silverman, L. Design of experiment (DOE) applied to artificial neural network architecture enables rapid bioprocess improvement. Bioprocess Biosyst. Eng. 2021, 44, 1301–1308. [Google Scholar] [CrossRef]
- Zou, M.; Zhou, Z.W.; Fan, L.; Zhang, W.J.; Zhao, L.; Liu, X.P.; Wang, H.B.; Tan, W.S. A novel method based on nonparametric regression with a Gaussian kernel algorithm identifies the critical components in CHO media and feed optimization. J. Ind. Microbiol. Biotechnol. 2020, 47, 63–72. [Google Scholar] [CrossRef]
- Grzesik, P.; Warth, S.C. One-Time Optimization of Advanced T Cell Culture Media Using a Machine Learning Pipeline. Front. Bioeng. Biotechnol. 2021, 9, 614324. [Google Scholar] [CrossRef]
| Cells | Experimental Design | Number of Factors | Factors | Year | Ref. |
|---|---|---|---|---|---|
| Murine ESC | Full factorial 24 | 4 | LIF, FGF4, Fibronectin, Laminin | 2004 | [34] |
| Full factorial 23 | 3 | FGF4, Fibronectin, Laminin | |||
| Human ESC | Full factorial 32 | 2 | Seeding density, Agitation speed | 2014 | [35] |
| Murine ESC | RSM | 3 | CHIR99021, LIF, PD0325901 | 2012 | [36] |
| Human ESC | RSM | 4 | Seeding density, Media volume, Media exchange time, Duration between passages | 2013 | [38] |
| Human iPSC | RSM | 2 | bFGF, NRG1β1 | 2015 | [37] |
| Human iPSC | RSM | 2 | Seeding density, Agitation speed | 2016 | [39] |
| Cells | Purpose | Experimental Design | Number of Factors | Factors | Year | Ref. |
|---|---|---|---|---|---|---|
| Murine ESC | Endodermal differentiation | Full factorial 25 | 5 | Glucose, Insulin, bFGF, Retinoic acid, EGF | 2004 | [40] |
| Full factorial 32 | 2 | Retinoic acid, EGF | ||||
| Murine ESC | Hepatocyte differentiation | Full factorial 25 | 5 | Collagen I, Collagen III, Collagen IV, Laminin, Fibronectin | 2005 | [41] |
| Murine ESC | Cardiac cell differentiation | Full factorial 25 | 5 | Collagen I, Collagen III, Collagen IV, Laminin, Fibronectin | 2008 | [42] |
| Full factorial 24 | 4 | Wnt3a, Activin A, BMP4, FGF4 | ||||
| Human iPSC | Mesodermal progenitor differentiation | Full factorial 27 | 7 | Collagen I, Collagen III, Collagen IV, Collagen V, Laminin, Fibronectin, Vitronectin | 2015 | [43] |
| Human iPSC | Retinal organoid differentiation | Full factorial 25 | 5 | Initial cell density, 1-Thioglycerol, BMP4, KSR, Lipids | 2018 | [44] |
| Full factorial 24 | 4 | Initial cell density, CHIR99201, BMP4, SU5402 | ||||
| Human iPSC | Definitive endoderm differentiation | 24−1 Resolution IV | 4 | Activin A, GDF8, Wortmannin, CHIR99201 | 2020 | [45] |
| Human iPSC | Choroidal endothelium cell differentiation | L12 | 5 | CTGF, CTNNB1, SHC1, TWEAKR, VEGFB | 2017 | [46] |
| Human iPSC | Four endodermal cell differentiation | L18 | 8 | Retinoic acid, CHIR99201(early phase), bFGF(later phase), Sodium butyrate, bFGF(early phase), CHIR99201(later phase), (LDN193189, BMP4), A-83-01 | 2021 | [47] |
| Human iPSC-derived | Mature neuron differentiation | RSM | 3 | RGD, YIGSR, IKVAV 1 | 2015 | [48] |
| neural progenitor cell | RSM | 2 | RGD, IKVAV 1 | |||
| RSM | 2 | RGD, IKVAV 1 | ||||
| Murine iPSC | Cardiomyocyte differentiation | Full factorial 23 | 3 | Collagen I, Laminin, Fibronectin | 2015 | [49] |
| RSM | 3 | Collagen I, Laminin, Fibronectin | ||||
| Full factorial 23 | 3 | Collagen I, Fibronectin, TSP1 | ||||
| Human iPSC | Trilineage bifurcation | RSM | 3 | O2 tension, Glucose, Pyruvate | 2021 | [50] |
| Purpose | Experimental Design | Number of Factors | Factors | Year | Ref. |
|---|---|---|---|---|---|
| MSC expansion | Full factorial 24 | 4 | Seeding density, Fetal calf serum, Media volume, Culture time | 2008 | [52] |
| Chondrocyte differentiation | Full factorial 25 | 5 | TGFβ1, BMP2, DEX, FGF2, IGF1 | 2014 | [53] |
| MSC expansion | 24−1 Resolution IV | 4 | Hydrocortisone, bFGF, Human albumin, SITE supplement 1 | 2007 | [54] |
| MSC expansion | L8 | 4 | Seeding density, Cytokines 2, Serum, Stromal cells | 2009 | [55] |
| L8 | 6 | SCF, TPO, FL, IL-3, GM-CSF, G-CSF | |||
| Osteoblast differentiation | RSM | 4 | Culture duration, O2 tension, Seeding density, Two media 3 | 2011 | [56] |
| Tenocyte differentiation | RSM | 2 | TGFβ3, Culture days | 2020 | [57] |
| Purpose | Experimental Design | Number of Factors | Factors | Year | Ref. |
|---|---|---|---|---|---|
| LTC-IC and CFC bifurcation | Full factorial 25 | 5 | FL, SF, IL-3, IL-6, (G-CSF, NGFβ) | 1996 | [58] |
| LTC-IC and CFC bifurcation | Full factorial 23 | 3 | FL, SF, IL-3 | 1997 | [59] |
| LTC-IC and CFC bifurcation | Full factorial 26 | 6 | FL, SF, IL-3, (IL-6, sIL-6R), TPO, IL-1 | 1998 | [60] |
| Megakaryocyte and platelet differentiation | Full factorial 24 | 4 | SCF, IL-3, IL-6, IL-9 | 2013 | [61] |
| HSC expansion | 29−5 Resolution III | 9 | TPO, IL-3, SCF, FL, G-CSF, GM-CSF, IL-6, sIL-6R, EPO | 2003 | [62] |
| 24−1 Resolution IV | 4 | TPO, IL-3, SCF, FL | |||
| 28−4 Resolution IV | 8 | Albumax, BSA, TF, Glutamine, Hydrocortisone, Peptone, 2-ME, Insulin | |||
| 24 | 4 | BSA, Insulin, TF, 2-ME | |||
| 27−3 Resolution IV | 7 | TPO, IL-3, SCF, FL, G-CSF, GM-CSF, IL-6 | |||
| HSC expansion | Full factorial 24 | 4 | BSA, Insulin, TF, 2-ME | 2004 | [63] |
| 210−6 Resolution III | 10 | TPO, IL-3, SCF, FL, IL-11, IL-6, GM-CSF, G-CSF, SCGF, HGF | |||
| Erythroid cell, granulocyte, and megakaryocyte differentiation | 27−3 Resolution IV | 7 | FL, SCF, IL-3, (MGDF, G-CSF), IL-11, IL-6, EPO | 2001 | [64] |
| Full factorial 24 | 4 | IL-3, IL-11, IL-6, EPO | |||
| Megakaryocyte differentiation | 28−3 Resolution IV | 8 | TPO, IL-3, SCF, FL, IL-11, IL-6, GM-CSF, IL-9 | 2009 | [65] |
| Dendritic cell differentiation | 28−4 Resolution IV | 8 | SCF, FL, IL-1β, GM-CSF, TNFα, IL-4, IL-6, TGFβ1 | 2019 | [66] |
| 25−1 Resolution V | 5 | SCF, FL, IL-1β, GM-CSF, TNFα | |||
| HSC differentiation ability | 25−1 Resolution V | 5 | SCF, FL, TPO, SDF-1, Fucoidan | 2011 | [67] |
| Full factorial 23 | 3 | SCF, FL, TPO | |||
| HSC expansion | RSM | 4 | SCF, FL, TPO, LIF | 2010 | [68] |
| LTC-IC and CFC bifurcation | Full factorial 25 | 5 | IL-11, SF, FL, TPO, Temperature | 2002 | [69] |
| RSM | 3 | IL-11, SF, FL | |||
| Megakaryocyte and platelet differentiation | Plackett–Burman | 11 | SCF, FL, IL-11, MIP-1α, IL-1α, IL-1β, IL-8, IFN-γ, VEGF, MCP-1, β-thromboglobuline | 2005 | [70] |
| Plackett–Burman | 9 | IL-9, IL-8, IL-6, IL-1α, IL-1β, SCF, FL, MIP-1α, IFN-γ | |||
| 25−1 Resolution V | 5 | SCF, FL, IL-6, IL-9, EPO | |||
| Full factorial 24 | 4 | SCF, FL, IL-6, IL-9 | |||
| RSM | 4 | TPO, SCF, IL-6, IL-9 |
| Experimental Design | Number of Factors | Factors | Year | Ref. |
|---|---|---|---|---|
| Full factorial 23 | 3 | Glucose, Glutamine, Inorganic salts | 2004 | [72] |
| Full factorial 25 | 5 | Feed volume at days 3, 5, 7, 10, and 12 | 2019 | [73] |
| 25−1 Resolution V | 5 | Sodium hypoxanthine-thymidine, Antioxidant, ITS 1, Fatty acids supplement, Polyamines supplement | 2006 | [74] |
| 24−1 Resolution IV | 5 | Amino acid feed, Glucose Feed, Temperature, pH | 2011 | [96] |
| Full factorial 31 × 22 | 3 | Glucose feed, Temperature shift, pH control frequency | ||
| Plackett–Burman | 20 | BSA, Transferrin, Insulin, Sodium pyruvate, Putrescine, Glucose, Ala, Arg, Asn, Asp, Cys, Gln, Glu, Gly, Ser, Met, (Pro, His, Hydroxyproline), (Thr, Val, Ile), (Leu, Trp, Lys), (Phe, Tyr) | 1992 | [75] |
| Plackett–Burman | 4 | Oleic acid, Linoleic acid, Cholesterol, (Choline, Ethanolamine) | 1995 | [76] |
| Plackett–Burman | 21 | Ala, Arg, (Asn, Asp), Cys, Gln, Glu, Gly, Ser, Met, (Phe, Tyr), (Thr, Val, Ile), (Leu, Trp, Lys), (Pro, His), Insulin, Transferrin, Ethanolamine, Pluronic F68, Phosphatidylcholine, Putrescine, Linoleic acid, Hydrocortisone | 1998 | [77] |
| Plackett–Burman | 21 | Ala, Arg, (Asn, Asp), Cys, Gln, Glu, Gly, Ser, Met, (Phe, Tyr), (Thr, Val, Ile), (Leu, Trp, Lys), (Pro, His), Insulin, Transferrin, Ethanolamine, Pluronic F68, Phosphatidylcholine, Hydrocortisone, Sodium selenite, Glutathione | 1999 | [78] |
| Plackett–Burman | 21 | Ala, Arg, (Asn, Asp), Cys, Gln, Glu, Gly, Ser, Met, (Phe, Tyr), (Thr, Val, Ile), (Leu, Trp, Lys), (Pro, His), Sodium selenite, Insulin, Transferrin, Hydrocortisone, Ethanolamine, Phosphatidylcholine, Glutathione, Pluronic F68 | 1999 | [79] |
| RSM | 2 | Glucose, Gln | 2005 | [80] |
| RSM | 2 | Glucose, NaCl | ||
| 27−3 Resolution IV | 7 | Insulin, Meat peptone, Yeast extract, SerEx, BSA, Linoleic acid–BSA, Dextran sulfate | 2006 | [81] |
| RSM | 2 | Insulin, SerEx | ||
| RSM | 5 | Gln, Essential amino acids supplement, Non-essential amino acids supplement, ITS 1, Lipids | 2007 | [82] |
| RSM | 3 | Yeastolate, Soy, Wheat | 2009 | [83] |
| Plackett–Burman | 17 | Ethanolamine, Sodium selenite, Putrescine, Hydrocortisone, Lipids, Sodium pyruvate, Ascorbic acid, Glutathione, Choline chloride, D-calcium pantothenate, Folic acid, Niacinamide, Pyridoxine-hydrochloride, Riboflavin, Thiamine hydrochloride, Cyanocobalamin, I-inositol | 2013 | [84] |
| RSM | 3 | Lipids, Putrescine, Ammonium ferric citrate | ||
| RSM | 3 | Temperature, pH, Seeding density, Culture duration | 2013 | [85] |
| RSM | 3 | Glucose, Asn, Gln | 2015 | [86] |
| Plackett–Burman | 19 | 19 amino acids (Gln excluded) | 2015 | [87] |
| RSM | 4 | Asp, Glu, Arg, Gly | ||
| RSM | 3 | pH, O2 tension, CO2 tension | 2017 | [88] |
| 28−4 Resolution IV | 8 | 8 kinds of commercial supplements | 2020 | [89] |
| RSM | 4 | 4 kinds of commercial supplements | ||
| Full factorial 23 | 3 | 3 kinds of commercial supplements | ||
| Plackett–Burman | 8 | Sodium selenite, Transferrin, Albumin, Insulin, Tocopherol, Tween 80, Fatty acids, Synthetic cholesterol | 2019 | [90] |
| Box–Behnken RSM | 3 | Transferrin, Insulin, Tween 80 | ||
| Plackett–Burman | 15 | Gln, Asp, Lys, Trp, Thr, Val, His, Vitamin B1, Thymidine, Deoxy-cytidine, 3-methyl-oxobutyrate, Deoxy-guanosine, Vitamin B6, Vitamin A, Arachidonate | 2020 | [91] |
| RSM | 2 | Thr, Arachidonate | ||
| Mixture Design | 6 | Hexoses, Energy provider compounds | 2007 | [92] |
| Mixture Design | 20 | 20 amino acids | 2013 | [97] |
| Mixture Design | 43 | 19 amino acids (Gln excluded), Disodium phosphate, Magnesium sulfate, Calcium chloride, Myo-inositol, Sodium pyruvate, D-biotin, Choline Chloride, Folic acid, Niacinamide, D-pantothenic acid, Potassium chloride, Pyridoxine, Riboflavin, Thiamine, Ferric ammonium citrate, Vitamin B12, Hypoxanthine, Thymidine, Putrescine, Ethanolamine, Zinc sulfate, Cupric sulfate, Pluronic, Sodium selenite | 2013 | [93] |
| DSD | 5 | pH, Shifted temperature, Seeding density, Viable cell density at first feeding, Viable cell density at temperature shift | 2019 | [94] |
| DSD | 6 | DMEM fraction, Cellgro trace element A, Cellgro trace element B, Insulin, Ca2+, Mg2+ | 2021 | [95] |
| Cells | Experimental Design | Number of Factors | Factors | Year | Ref. |
|---|---|---|---|---|---|
| Human pancreatic duct cell | Full factorial 25 | 5 | bFGF, EGF, HGF, KGF, VEGF | 2012 | [100] |
| Vero | 210−6 Resolution III | 10 | (20 amino acids, Vitamin B1, Magnesium sulfate, Sodium phosphate), (Vitamins H, B2, and B9, Thymidine, Uracil, Xanthine, Hypoxanthine), (Vitamins B12, B3, and B7, Choline chloride, Pyridoxal), (Vitamins B3, B6, and BX, Putrescin), (Vitamins A, D2, and K3, Linoleic acids, Lipoic acids), (Deoxyribose, Adenine, Adenosine, Ethanolamine), (Plant and yeast extracts, EGF, Insulin), (Sodium citrate, Ferric chloride), (Glucose, Pyruvate), (Other) | 2010 | [106] |
| Murine hybridoma | 29−4 Resolution IV | 9 | Serum, Dissolved oxygen, Temperature, pH, Glucose, Glutamine, Lactate, Ammonium, Base medium concentration | 1993 | [108] |
| Murine myeloma | 25−1 Resolution V | 5 | pH, Temperature, Dissolved oxygen, Early/late feed regime, Seeding density | 2000 | [109] |
| Murine hybridoma | L8 | 4 | Stirring speed, Fetal bovine or calf serum, Serum concentration, Glucose and glutamine supplement | 2002 | [110] |
| Vero | L8 | 4 | Cytodex 1, Regulation of glucose, Initial glucose, Gln | 2006 | [107] |
| Caco-2 and HT29-MTX cells | L18 | 4 | MEM or DMEM medium, Seeding time, Seeding density, and Caco-2/HT29-MTX ratio | 2010 | [101] |
| Human umbilical vein endothelial cell (HUVEC) | Full factorial 24 | 4 | RGDS, IKVAV, YIGSR, Q11 1 | 2011 | [102] |
| RSM | 3 | RGDS, IKVAV, YIGSR 1 | |||
| Human peripheral blood mononuclear cell | 24−1 Resolution IV | 4 | Phosphatidyl choline, Polyamine supplement, Antioxidant supplement, Cholesterol | 2010 | [103] |
| RSM | 2 | Polyamine supplement, Cholesterol | |||
| Human prostate cancer cells | Plackett–Burman | 16 | Transferrin, Sodium selenite, Sodium L-ascorbate, Ferric citrate, L-glutathione, BSA, EGF, bFGF, Ethanolamine, Linoleic acid, Arachidonate, Thioglycerol, Hydrocortisone, Yeast hydrolysate, Penicillin-Streptomycin Solution, Succinic Acid | 2017 | [104] |
| RSM | 3 | EGF, FGF, Linoleic acid | |||
| Immortalized human erythroblast | 29−4 Resolution IV | 9 | BSA, EPO, Holo-transferrin, Hydrocortisone, Insulin, Fatty acid supplement, Lipid mixture solution, Non-essential amino acids supplement, SCF | 2018 | [105] |
| RSM | 3 | BSA, EPO, Fatty acid supplement |
| Cells | Purpose | Experimental Design | Number of Factors | Factors | Year | Ref. |
|---|---|---|---|---|---|---|
| Mouse pluripotent embryonic carcinoma | Neuronal cell differentiation | Full factorial 23 | 3 | 2D- or 3D-culture, IKVAV 1, ECM stiffness | 2012 | [117] |
| Human chondrocytes | Cartilage differentiation | 212−4 Resolution VI | 12 | BMP2, Insulin, IGF1, Testosterone, Parathyroid hormone, IL-1RA, Growth hormone, 17β-estradiol, Triiodothyronine, 1α-25-dihydroxy vitamin D3, FGF2, DEX | 2007 | [111] |
| Human chondrocytes | Articular chondrocyte differentiation | 25−1 Resolution V | 5 | TGFβ1, ASC, ITS, DEX, Linoleic acid | 2012 | [112] |
| Full factorial 23 | 3 | TGFβ1, DEX, Glucose | ||||
| Full factorial 22 | 2 | DEX, Glucose | ||||
| Human bone progenitor cells | Skeletal tissue development | 25−1 Resolution V | 5 | Medium volume, Seeding density, Human periosteum-derived cell or osteosarcoma cell, Seeding timing, Foamed titanium or 3D fiber-deposited titanium | 2011 | [113] |
| Human adipose-derived stromal cells | Osteoblast differentiation | 12 × 12 Hadamard matrix 2 | 8 | Two human adipose-derived stromal cells suppliers, Seeding density, DMEM/F12 or DMEM, Human platelet lysate or Fetal bovine serum, L-ascorbate-2-phosphate, β-glycerophosphate, DEX, BMP9 | 2019 | [114] |
| Human hepatoma cell | Hepatocyte differentiation | 27−4 Resolution III | 7 | Human serum albumin, HGF, Oncostatin M, DEX, FGF4, EGF, Nicotinamide | 2008 | [116] |
| RSM | 3 | Oncostatin M, HGF, FGF4 | ||||
| Murine embryonic fibroblast cell | Osteoblast differentiation | RSM | 2 | Matrix stiffness, Collagen I | 2010 | [115] |
| Cells | Purpose | Experimental Design | Number of Factors | Factors | Year | Ref. |
|---|---|---|---|---|---|---|
| Human retinal pigment epithelial cells | Cell storage condition | Full factorial 25 | 5 | Adenosine, Allopurinol, β-Glycerophosphate, L-Ascorbic acid, Taurine | 2018 | [118] |
| Human epithelial cell sheets | Cell storage condition | 210−4 Resolution IV | 10 | 1% Glycerol, L-Ascorbic acid, Allopurinol, Sodium pyruvate, Adenosine, Taurine, L-Glutathione, Hydrocortizone, LiCl, Antimycin-A | 2018 | [119] |
| 210−4 Resolution IV | 10 | 0.75% Glycerol, 3% Glycerol, Icilin, Menthol, Dimethyl (S)-(−)-malate, Methyl pyruvate, N-Acetyl-L-Cys, Insulin, Acetovanillone, N-(2-Mercaptopropionyl)glycine | ||||
| Full factorial 55 | 5 | L-Carnosine, Dimethyl sulfoxide, Fenoldopam mesylate, Glycerol, LIF | ||||
| Full factorial 55 | 5 | Glycerol, Aspirin, Melatonin, Lactic acid, ATP | ||||
| Vero | Virus inactivation | L9 | 4 | Temperature, Treatment time, pH, Ethanol | 2019 | [120] |
| CHO cell and HEK293 | Cell freezing and refreezing condition | RSM | 3 | Freezing density, Dimethyloxide, Seeding density | 2008 | [121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasui, R.; Sekine, K.; Taniguchi, H. Clever Experimental Designs: Shortcuts for Better iPSC Differentiation. Cells 2021, 10, 3540. https://doi.org/10.3390/cells10123540
Yasui R, Sekine K, Taniguchi H. Clever Experimental Designs: Shortcuts for Better iPSC Differentiation. Cells. 2021; 10(12):3540. https://doi.org/10.3390/cells10123540
Chicago/Turabian StyleYasui, Ryota, Keisuke Sekine, and Hideki Taniguchi. 2021. "Clever Experimental Designs: Shortcuts for Better iPSC Differentiation" Cells 10, no. 12: 3540. https://doi.org/10.3390/cells10123540
APA StyleYasui, R., Sekine, K., & Taniguchi, H. (2021). Clever Experimental Designs: Shortcuts for Better iPSC Differentiation. Cells, 10(12), 3540. https://doi.org/10.3390/cells10123540






