Co-Expression Network Analysis of Micro-RNAs and Proteins in the Alzheimer’s Brain: A Systematic Review of Studies in the Last 10 Years
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Search Strategy
2.1.2. Search Terms and Databases
2.1.3. Data Extraction
2.1.4. Quality Appraisal of Papers
2.1.5. Meta-Analyses
2.2. Pathway Analysis
2.3. Predicting miRNA-Protein Interactions through Inverse Relationships
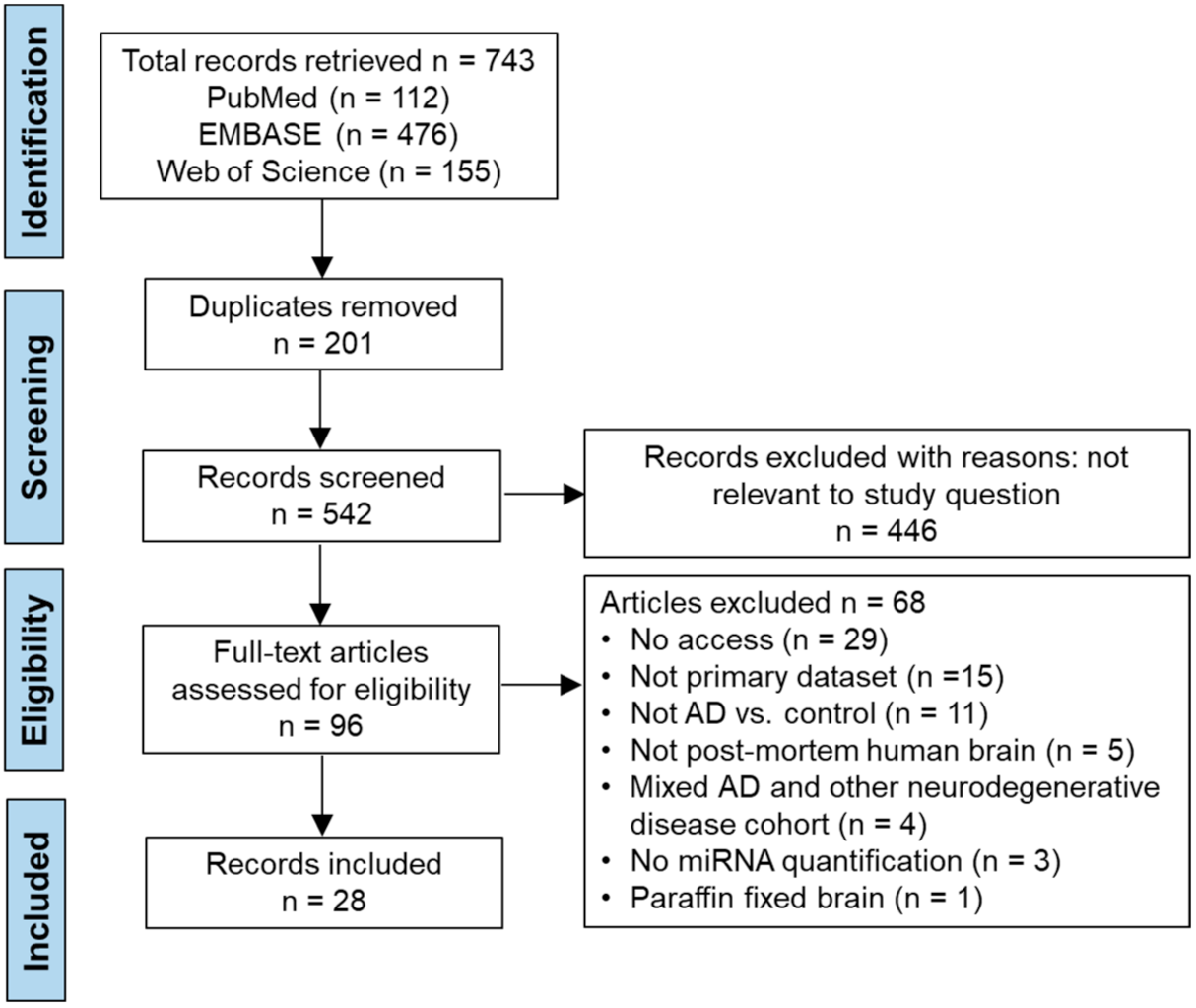
3. Results
3.1. Study Characteristics
3.2. Data Extraction
3.3. AXIS Quality Appraisal
3.4. Risk of Bias
3.5. Meta-Analyses
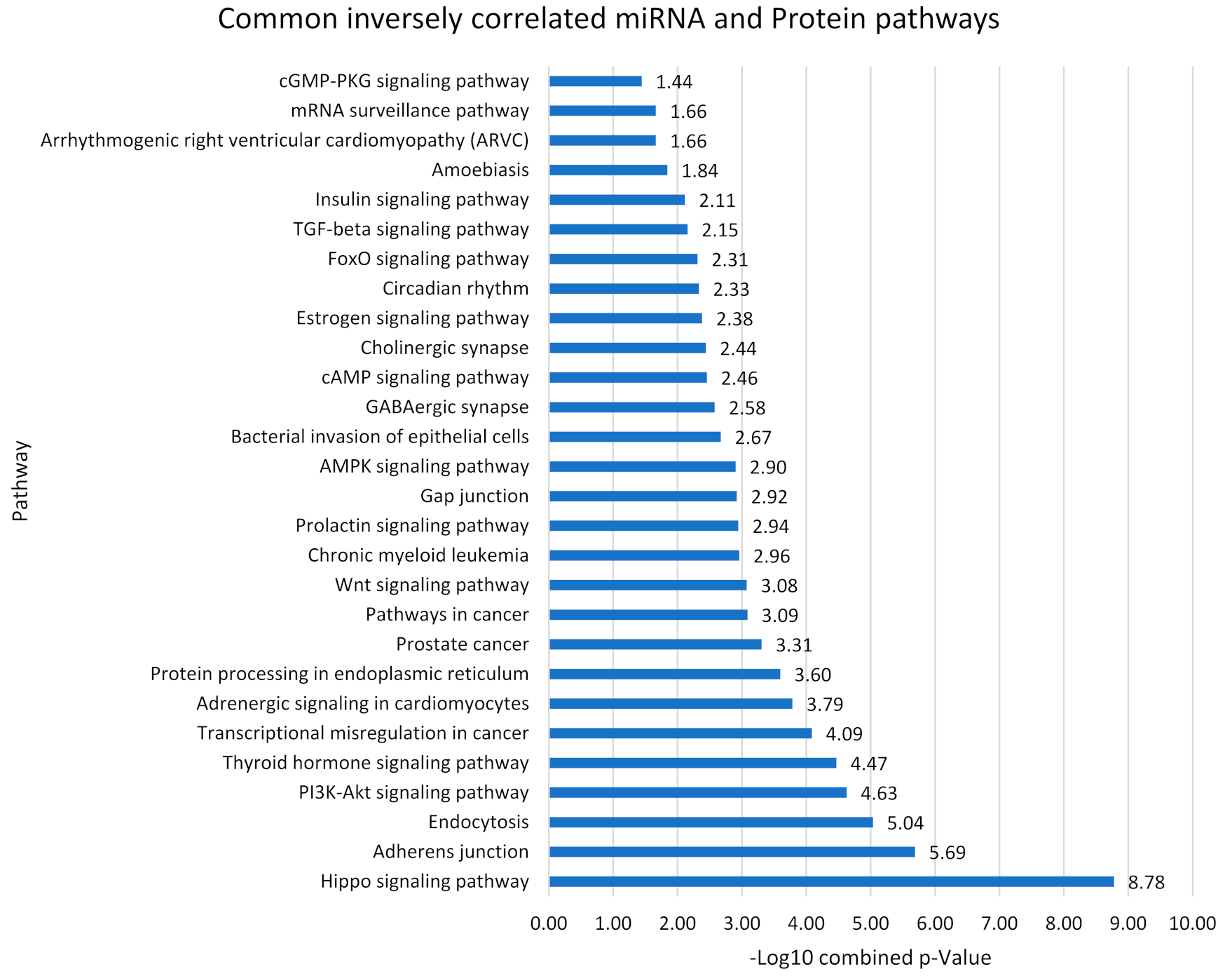
3.6. Pathways Analysis
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimers Dement. 2020, 16, 391–460. [Google Scholar] [CrossRef]
- Hebert, L.E.; Weuve, J.; Scherr, P.A.; Evans, D.A. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013, 80, 1778–1783. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart disease and stroke statistics—2020 update: A report from the american heart association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Jennings, L.A.; Hollands, S.; Keeler, E.; Wenger, N.S.; Reuben, D.B. The effects of dementia care co-management on acute care, hospice, and long-term care utilization. J. Am. Geriatr. Soc. 2020, 68, 2500–2507. [Google Scholar] [CrossRef]
- Yu, H.; Wang, X.; He, R.; Liang, R.; Zhou, L. Measuring the caregiver burden of caring for community-residing people with Alzheimer’s disease. PLoS ONE 2015, 10, e0132168. [Google Scholar] [CrossRef]
- GBD 2016 Dementia Collaborators. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019, 18, 88–106. [Google Scholar] [CrossRef]
- Viña, J.; Lloret, A. Why women have more Alzheimer’s disease than men: Gender and mitochondrial toxicity of amyloid-beta peptide. J. Alzheimers Dis. 2010, 20 (Suppl. 2), S527–S533. [Google Scholar] [CrossRef] [PubMed]
- Farrer, L.A.; Cupples, L.A.; Haines, J.L.; Hyman, B.; Kukull, W.A.; Mayeux, R.; Myers, R.H.; Pericak-Vance, M.A.; Risch, N.; van Duijn, C.M. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer disease meta analysis consortium. JAMA 1997, 278, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.A.; Bennett, D.A.; Wilson, R.S.; Bienias, J.L.; Morris, M.C.; Scherr, P.A.; Hebert, L.E.; Aggarwal, N.; Beckett, L.A.; Joglekar, R.; et al. Incidence of Alzheimer disease in a biracial urban community: Relation to apolipoprotein E allele status. Arch. Neurol. 2003, 60, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.A.; Xu, W.; Gaglioti, A.H.; Holt, J.B.; Croft, J.B.; Mack, D.; McGuire, L.C. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimers Dement. 2019, 15, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Breteler, M.M.; Claus, J.J.; Grobbee, D.E.; Hofman, A. Cardiovascular disease and distribution of cognitive function in elderly people: The rotterdam study. BMJ 1994, 308, 1604–1608. [Google Scholar] [CrossRef]
- Wilson, R.S.; Krueger, K.R.; Arnold, S.E.; Schneider, J.A.; Kelly, J.F.; Barnes, L.L.; Tang, Y.; Bennett, D.A. Loneliness and risk of Alzheimer disease. Arch. Gen. Psychiatry 2007, 64, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, R.F.; Albert, M.S.; Alonso, A.; Coker, L.H.; Coresh, J.; Davis, S.M.; Deal, J.A.; McKhann, G.M.; Mosley, T.H.; Sharrett, A.R.; et al. Associations between midlife vascular risk factors and 25-year incident dementia in the atherosclerosis risk in communities (ARIC) cohort. JAMA Neurol. 2017, 74, 1246–1254. [Google Scholar] [CrossRef]
- Zhao, M.; Veeranki, S.P.; Magnussen, C.G.; Xi, B. Recommended physical activity and all cause and cause specific mortality in US adults: Prospective cohort study. BMJ 2020, 370, m2031. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.M.; Yates, P.O.; Marcyniuk, B. Correlation between senile plaque and neurofibrillary tangle counts in cerebral cortex and neuronal counts in cortex and subcortical structures in Alzheimer’s disease. Neurosci. Lett. 1985, 56, 51–55. [Google Scholar] [CrossRef]
- Hardy, J.; Allsop, D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol. Sci. 1991, 12, 383–388. [Google Scholar] [CrossRef]
- Rubin, R. Recently approved alzheimer drug raises questions that might never be answered. JAMA 2021, 326, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Makin, S. The amyloid hypothesis on trial. Nature 2018, 559, S4–S7. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Das, S.; Hyman, B.T. APOE and Alzheimer’s disease: Advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 2021, 20, 68–80. [Google Scholar] [CrossRef]
- Mirzaei, S.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Saleki, H.; Ranjbar, A.; Saleh, S.H.S.; Bagherian, M.; Sharifzadeh, S.O.; Hushmandi, K.; et al. Regulation of nuclear Factor-KappaB (NF-κB) signaling pathway by non-coding RNAs in cancer: Inhibiting or promoting carcinogenesis? Cancer Lett. 2021, 509, 63–80. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Hushmandi, K.; Hashemi, F.; Moghadam, E.R.; Owrang, M.; Hashemi, F.; Makvandi, P.; Goharrizi, M.A.S.B.; Najafi, M.; et al. Lung cancer cells and their sensitivity/resistance to cisplatin chemotherapy: Role of microRNAs and upstream mediators. Cell. Signal. 2021, 78, 109871. [Google Scholar] [CrossRef]
- Mirzaei, S.; Zarrabi, A.; Asnaf, S.E.; Hashemi, F.; Zabolian, A.; Hushmandi, K.; Raei, M.; Goharrizi, M.A.S.B.; Makvandi, P.; Samarghandian, S.; et al. The role of microRNA-338-3p in cancer: Growth, invasion, chemoresistance, and mediators. Life Sci. 2021, 268, 119005. [Google Scholar] [CrossRef]
- Catalanotto, C.; Cogoni, C.; Zardo, G. MicroRNA in control of gene expression: An overview of nuclear functions. Int. J. Mol. Sci. 2016, 17, 1712. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.D.; Li, L.; Chan, W.Y. MicroRNAs: Key regulators in the central nervous system and their implication in neurological diseases. Int. J. Mol. Sci. 2016, 17, 842. [Google Scholar] [CrossRef] [PubMed]
- Im, H.I.; Kenny, P.J. MicroRNAs in neuronal function and dysfunction. Trends Neurosci. 2012, 35, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Hébert, S.S.; Sergeant, N.; Buée, L. MicroRNAs and the regulation of tau metabolism. Int. J. Alzheimers Dis. 2012, 2012, 406561. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Jun, S.; Rellick, S.; Quintana, D.D.; Cavendish, J.Z.; Simpkins, J.W. Expression of microRNA-34a in Alzheimer’s disease brain targets genes linked to synaptic plasticity, energy metabolism, and resting state network activity. Brain Res. 2016, 1646, 139–151. [Google Scholar] [CrossRef]
- Long, J.M.; Maloney, B.; Rogers, J.T.; Lahiri, D.K. Novel upregulation of amyloid-β precursor protein (APP) by microRNA-346 via targeting of APP mRNA 5′-untranslated region: Implications in Alzheimer’s disease. Mol. Psychiatry 2019, 24, 345–363. [Google Scholar] [CrossRef]
- Han, C.; Guo, L.; Yang, Y.; Guan, Q.; Shen, H.; Sheng, Y.; Jiao, Q. Mechanism of microRNA-22 in regulating neuroinflammation in Alzheimer’s disease. Brain Behav. 2020, 10, e01627. [Google Scholar] [CrossRef]
- Nelson, P.T.; Wang, W.X. MiR-107 is reduced in Alzheimer’s disease brain neocortex: Validation study. J. Alzheimers Dis. 2010, 21, 75–79. [Google Scholar] [CrossRef]
- Fang, M.; Wang, J.; Zhang, X.; Geng, Y.; Hu, Z.; Rudd, J.A.; Ling, S.; Chen, W.; Han, S. The miR-124 regulates the expression of BACE1/β-secretase correlated with cell death in Alzheimer’s disease. Toxicol. Lett. 2012, 209, 94–105. [Google Scholar] [CrossRef] [PubMed]
- El Fatimy, R.; Li, S.; Chen, Z.; Mushannen, T.; Gongala, S.; Wei, Z.; Balu, D.T.; Rabinovsky, R.; Cantlon, A.; Elkhal, A.; et al. MicroRNA-132 provides neuroprotection for tauopathies via multiple signaling pathways. Acta Neuropathol. 2018, 136, 537–555. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Yuan, K.; Tong, X.; Hu, J.; Song, Z.; Zhang, G.; Fang, X.; Zhang, W. MiR-16 attenuates β-amyloid-induced neurotoxicity through targeting β-site amyloid precursor protein-cleaving enzyme 1 in an Alzheimer’s disease cell model. Neuroreport 2018, 29, 1365–1372. [Google Scholar] [CrossRef]
- Hanna, J.; Hossain, G.S.; Kocerha, J. The Potential for microRNA Therapeutics and Clinical Research. Front Genet. 2019, 10, 478. [Google Scholar] [CrossRef]
- Ghaffari, M.; Sanadgol, N.; Abdollahi, M.A. Systematic review of current progresses in the nucleic acid-based therapies for neurodegeneration with implications for Alzheimer’s disease. Mini. Rev. Med. Chem. 2020, 20, 1499–1517. [Google Scholar] [CrossRef]
- Nazem, A.; Mansoori, G.A. Nanotechnology for Alzheimer’s disease detection and treatment. Insci. J. 2011, 1, 169–193. [Google Scholar] [CrossRef]
- Lanford, R.E.; Hildebrandt-Eriksen, E.S.; Petri, A.; Persson, R.; Lindow, M.; Munk, M.E.; Kauppinen, S.; Ørum, H. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science 2010, 327, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Downes, M.J.; Brennan, M.L.; Williams, H.C.; Dean, R.S. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 2016, 6, e011458. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Vlachos, I.S.; Kostoulas, N.; Vergoulis, T.; Georgakilas, G.; Reczko, M.; Maragkakis, M.; Paraskevopoulou, M.D.; Prionidis, K.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA miRPath v.2.0: Investigating the combinatorial effect of microRNAs in pathways. Nucleic Acids Res. 2012, 40, W498–W504. [Google Scholar] [CrossRef] [PubMed]
- Paraskevopoulou, M.D.; Georgakilas, G.; Kostoulas, N.; Vlachos, I.S.; Vergoulis, T.; Reczko, M.; Filippidis, C.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-microT web server v5.0: Service integration into miRNA functional analysis workflows. Nucleic Acids Res. 2013, 41, W169–W173. [Google Scholar] [CrossRef]
- Annese, A.; Manzari, C.; Lionetti, C.; Picardi, E.; Horner, D.S.; Chiara, M.; Caratozzolo, M.F.; Tullo, A.; Fosso, B.; Pesole, G.; et al. Whole transcriptome profiling of late-onset Alzheimer’s disease patients provides insights into the molecular changes involved in the disease. Sci. Rep. 2018, 8, 4282. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Vella, L.J.; Barnham, K.J.; McLean, C.; Masters, C.L.; Hill, A.F. Small RNA fingerprinting of Alzheimer’s disease frontal cortex extracellular vesicles and their comparison with peripheral extracellular vesicles. J. Extracell. Vesicles 2020, 9, 1766822. [Google Scholar] [CrossRef] [PubMed]
- Chopra, N.; Wang, R.; Maloney, B.; Nho, K.; Beck, J.S.; Pourshafie, N.; Niculescu, A.; Saykin, A.J.; Rinaldi, C.; Counts, S.E.; et al. MicroRNA-298 reduces levels of human amyloid-β precursor protein (APP), β-site APP-converting enzyme 1 (BACE1) and specific tau protein moieties. Mol. Psychiatry 2020. [Google Scholar] [CrossRef]
- Culpan, D.; Kehoem, P.G.; Love, S. Tumour necrosis factor-α (TNF-α) and miRNA expression in frontal and temporal neocortex in Alzheimer’s disease and the effect of TNF-α on miRNA expression in vitro. Int. J. Mol. Epidemiol. Genet. 2011, 2, 156–162. [Google Scholar] [PubMed]
- Gong, G.; An, F.; Wang, Y.; Bian, M.; Yu, L.J.; Wei, C. miR-15b represses BACE1 expression in sporadic Alzheimer’s disease. Oncotarget 2017, 8, 91551–91557. [Google Scholar] [CrossRef]
- Hébert, S.S.; Wang, W.X.; Zhu, Q.; Nelson, P.T. A study of small RNAs from cerebral neocortex of pathology-verified Alzheimer’s disease, dementia with lewy bodies, hippocampal sclerosis, frontotemporal lobar dementia, and non-demented human controls. J. Alzheimers Dis. 2013, 35, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Henriques, A.D.; Machado-Silva, W.; Leite, R.; Suemoto, C.K.; Leite, K.; Srougi, M.; Pereira, A.C.; Jacob-Filho, W.; Nóbrega, O.T.; Brazilian Aging Brain Study Group. Genome-wide profiling and predicted significance of post-mortem brain microRNA in Alzheimer’s disease. Mech. Ageing Dev. 2020, 191, 111352. [Google Scholar] [CrossRef]
- Kumar, S.; Reddy, P.H. MicroRNA-455-3p as a Potential Biomarker for Alzheimer’s disease: An update. Front. Aging Neurosci. 2018, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Vijayan, M.; Reddy, P.H. MicroRNA-455-3p as a potential peripheral biomarker for Alzheimer’s disease. Hum. Mol. Genet. 2017, 26, 3808–3822. [Google Scholar] [CrossRef] [PubMed]
- Lau, P.; Bossers, K.; Janky, R.; Salta, E.; Frigerio, C.S.; Barbash, S.; Rothman, R.; Sierksma, A.S.; Thathiah, A.; Greenberg, D.; et al. Alteration of the microRNA network during the progression of Alzheimer’s disease. EMBO Mol. Med. 2013, 5, 1613–1634. [Google Scholar] [CrossRef]
- Lei, X.; Lei, L.; Zhang, Z.; Zhang, Z.; Cheng, Y. Downregulated miR-29c correlates with increased BACE1 expression in sporadic Alzheimer’s disease. Int. J. Clin. Exp. Pathol. 2015, 8, 1565–1574. [Google Scholar] [PubMed]
- Li, J.; Chen, W.; Yi, Y.; Tong, Q. miR-219-5p inhibits tau phosphorylation by targeting TTBK1 and GSK-3β in Alzheimer’s disease. J. Cell Biochem. 2019, 120, 9936–9946. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Y.; Zhang, L. MicroRNA-132 promotes neurons cell apoptosis and activates Tau phosphorylation by targeting GTDC-1 in Alzheimer’s disease. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8523–8532. [Google Scholar] [CrossRef]
- Llorens, F.; Thüne, K.; Andrés-Benito, P.; Tahir, W.; Ansoleaga, B.; Hernández-Ortega, K.; Martí, E.; Zerr, I.; Ferrer, I. MicroRNA expression in the locus coeruleus, entorhinal cortex, and hippocampus at early and middle stages of braak neurofibrillary tangle pathology. J. Mol. Neurosci. 2017, 63, 206–215. [Google Scholar] [CrossRef]
- Moncini, S.; Lunghi, M.; Valmadre, A.; Grasso, M.; Del Vescovo, V.; Riva, P.; Denti, M.A.; Venturin, M. The miR-15/107 Family of microRNA genes regulates CDK5R1/p35 with Implications for Alzheimer’s disease pathogenesis. Mol. Neurobiol. 2017, 54, 4329–4342. [Google Scholar] [CrossRef]
- Müller, M.; Kuiperij, H.B.; Claassen, J.A.; Küsters, B.; Verbeek, M.M. MicroRNAs in Alzheimer’s disease: Differential expression in hippocampus and cell-free cerebrospinal fluid. Neurobiol. Aging 2014, 35, 152–158. [Google Scholar] [CrossRef]
- Pichler, S.; Gu, W.; Hartl, D.; Gasparoni, G.; Leidinger, P.; Keller, A.; Meese, E.; Mayhaus, M.; Hampel, H.; Riemenschneider, M. The miRNome of Alzheimer’s disease: Consistent downregulation of the miR-132/212 cluster. Neurobiol. Aging 2017, 50, e1–e167. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Zhang, J.; He, F.P.; Bao, W.X.; Zheng, T.T.; Zhou, D.M.; Pan, H.Y.; Zhang, H.; Zhang, X.Q.; He, X.; et al. Down-regulated expression of microRNA-338-5p contributes to neuropathology in Alzheimer’s disease. FASEB J. 2019, 33, 4404–4417. [Google Scholar] [CrossRef]
- Santa-Maria, I.; Alaniz, M.E.; Renwick, N.; Cela, C.; Fulga, T.A.; Van Vactor, D.; Tuschl, T.; Clark, L.N.; Shelanski, M.L.; McCabe, B.D.; et al. Dysregulation of microRNA-219 promotes neurodegeneration through post-transcriptional regulation of tau. J. Clin. Investig. 2015, 125, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, D.; Huang, H.Z.; Wang, Z.H.; Hou, T.Y.; Yang, X.; Pang, P.; Wei, N.; Zhou, Y.F.; Dupras, M.J.; et al. A novel MicroRNA-124/PTPN1 signal pathway mediates synaptic and memory deficits in Alzheimer’s disease. Biol. Psychiatry 2018, 83, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.K.; Veremeyko, T.; Patel, N.; Lemere, C.A.; Walsh, D.M.; Esau, C.; Vanderburg, C.; Krichevsky, A.M. De-repression of FOXO3a death axis by microRNA-132 and -212 causes neuronal apoptosis in Alzheimer’s disease. Hum. Mol. Genet. 2013, 22, 3077–3092. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Wu, Y.; Li, L.; Liu, C. MicroRNA-425-5p promotes tau phosphorylation and cell apoptosis in Alzheimer’s disease by targeting heat shock protein B8. J. Neural. Transm. 2020, 127, 339–346. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, J.; Liu, B.; Cui, Q.; Wang, Y. Primate-specific miR-603 is implicated in the risk and pathogenesis of Alzheimer’s disease. Aging 2016, 8, 272–290. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, Y.; Alexandrov, P.N.; Jaber, V.; Lukiw, W.J. Deficiency in the ubiquitin conjugating enzyme UBE2A in Alzheimer’s Disease (AD) is linked to deficits in a natural circular miRNA-7 sponge (circRNA; ciRS-7). Genes 2016, 7, 116. [Google Scholar] [CrossRef]
- Zhao, Y.; Bhattacharjee, S.; Jones, B.M.; Dua, P.; Alexandrov, P.N.; Hill, J.M.; Lukiw, W.J. Regulation of TREM2 expression by an NF-κB-sensitive miRNA-34a. Neuroreport 2013, 24, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Beckelman, B.C.; Zhou, X.; Keene, C.D.; Ma, T. Impaired eukaryotic elongation factor 1A expression in Alzheimer’s disease. Neurodegener. Dis. 2016, 16, 39–43. [Google Scholar] [CrossRef]
- Chiu, C.; Miller, M.C.; Monahan, R.; Osgood, D.P.; Stopa, E.G.; Silverberg, G.D. P-glycoprotein expression and amyloid accumulation in human aging and Alzheimer’s disease: Preliminary observations. Neurobiol. Aging 2015, 36, 2475–2482. [Google Scholar] [CrossRef]
- Shepherd, C.E.; Affleck, A.J.; Bahar, A.Y.; Carew-Jones, F.; Gregory, G.; Small, D.H.; Halliday, G.M. Alzheimer’s amyloid-β and tau protein accumulation is associated with decreased expression of the LDL receptor-associated protein in human brain tissue. Brain Behav. 2020, 10, e01672. [Google Scholar] [CrossRef]
- Chen, C.H.; Zhou, W.; Liu, S.; Deng, Y.; Cai, F.; Tone, M.; Tone, Y.; Tong, Y.; Song, W. Increased NF-κB signalling up-regulates BACE1 expression and its therapeutic potential in Alzheimer’s disease. Int. J. Neuropsychopharmacol. 2012, 15, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Holler, C.J.; Davis, P.R.; Beckett, T.L.; Platt, T.L.; Webb, R.L.; Head, E.; Murphy, M.P. Bridging integrator 1 (BIN1) protein expression increases in the Alzheimer’s disease brain and correlates with neurofibrillary tangle pathology. J. Alzheimers Dis. 2014, 42, 1221–1227. [Google Scholar] [CrossRef]
- Walker, D.G.; Whetzel, A.M.; Lue, L.F. Expression of suppressor of cytokine signaling genes in human elderly and Alzheimer’s disease brains and human microglia. Neuroscience 2015, 302, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Glennon, E.B.; Whitehouse, I.J.; Miners, J.S.; Kehoe, P.G.; Love, S.; Kellett, K.A.; Hooper, N.M. BIN1 is decreased in sporadic but not familial Alzheimer’s disease or in aging. PLoS ONE 2013, 8, e78806. [Google Scholar] [CrossRef] [PubMed]
- Byman, E.; Schultz, N.; Netherlands Brain Bank; Fex, M.; Wennström, M. Brain alpha-amylase: A novel energy regulator important in Alzheimer disease? Brain Pathol. 2018, 28, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Camats-Perna, J.; Medeiros, R.; Anggono, V.; Widagdo, J. Altered Expression of the m6A Methyltransferase METTL3 in Alzheimer’s disease. eNeuro 2020, 7, ENEURO.0125-20.2020. [Google Scholar] [CrossRef] [PubMed]
- Yoo, I.D.; Park, M.W.; Cha, H.W.; Yoon, S.; Boonpraman, N.; Yi, S.S.; Moon, J.S. Elevated CLOCK and BMAL1 contribute to the impairment of aerobic glycolysis from astrocytes in Alzheimer’s disease. Int. J. Mol. Sci. 2020, 21, 7862. [Google Scholar] [CrossRef]
- Chen, J.; Wang, M.; Turko, I.V. Mass spectrometry quantification of clusterin in the human brain. Mol. Neurodegener. 2012, 7, 41. [Google Scholar] [CrossRef]
- Gu, J.; Hu, W.; Tan, X.; Qu, S.; Chu, D.; Gong, C.X.; Iqbal, K.; Liu, F. Elevation of casein kinase 1ε associated with TDP-43 and tau pathologies in Alzheimer’s disease. Brain Pathol. 2020, 30, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Patassini, S.; Rustogi, N.; Riba-Garcia, I.; Hale, B.D.; Phillips, A.M.; Waldvogel, H.; Haines, R.; Bradbury, P.; Stevens, A.; et al. Regional protein expression in human Alzheimer’s brain correlates with disease severity. Commun. Biol. 2019, 2, 43. [Google Scholar] [CrossRef]
- Batkulwar, K.; Godbole, R.; Banarjee, R.; Kassaar, O.; Williams, R.J.; Kulkarni, M.J. Advanced glycation end products modulate amyloidogenic APP processing and tau phosphorylation: A mechanistic link between glycation and the development of Alzheimer’s disease. ACS Chem. Neurosci. 2018, 9, 988–1000. [Google Scholar] [CrossRef] [PubMed]
- Ilic, K.; Mlinac-Jerkovic, K.; Jovanov-Milosevic, N.; Simic, G.; Habek, N.; Bogdanovic, N.; Kalanj-Bognar, S. Hippocampal expression of cell-adhesion glycoprotein neuroplastin is altered in Alzheimer’s disease. J. Cell Mol. Med. 2019, 23, 1602–1607. [Google Scholar] [CrossRef] [PubMed]
- Lue, L.F.; Schmitz, C.T.; Serrano, G.; Sue, L.I.; Beach, T.G.; Walker, D.G. TREM2 protein expression changes correlate with Alzheimer’s disease neurodegenerative pathologies in post-mortem temporal cortices. Brain Pathol. 2015, 25, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Bekris, L.M.; Galloway, N.M.; Montine, T.J.; Schellenberg, G.D.; Yu, C.E. APOE mRNA and protein expression in postmortem brain are modulated by an extended haplotype structure. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2010, 153B, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Caušević, M.; Farooq, U.; Lovestone, S.; Killick, R. β-Amyloid precursor protein and tau protein levels are differently regulated in human cerebellum compared to brain regions vulnerable to Alzheimer’s type neurodegeneration. NeuroSci. Lett. 2010, 485, 162–166. [Google Scholar] [CrossRef]
- Campanari, M.L.; Navarrete, F.; Ginsberg, S.D.; Manzanares, J.; Sáez-Valero, J.; García-Ayllón, M.S. Increased expression of readthrough acetylcholinesterase variants in the brains of Alzheimer’s disease patients. J. Alzheimers Dis. 2016, 53, 831–841. [Google Scholar] [CrossRef]
- Bartolotti, N.; Bennett, D.A.; Lazarov, O. Reduced pCREB in Alzheimer’s disease prefrontal cortex is reflected in peripheral blood mononuclear cells. Mol. Psychiatry 2016, 21, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Jin, N.; Qian, W.; Yin, X.; Zhang, L.; Iqbal, K.; Grundke-Iqbal, I.; Gong, C.X.; Liu, F. CREB regulates the expression of neuronal glucose transporter 3: A possible mechanism related to impaired brain glucose uptake in Alzheimer’s disease. Nucleic Acids Res. 2013, 41, 3240–3256. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Cuevas, E.; Raymick, J.; Kanungo, J.; Sarkar, S. Downregulation of 14-3-3 Proteins in Alzheimer’s disease. Mol. Neurobiol. 2020, 57, 32–40. [Google Scholar] [CrossRef]
- Ginsberg, S.D.; Mufson, E.J.; Counts, S.E.; Wuu, J.; Alldred, M.J.; Nixon, R.A.; Che, S. Regional selectivity of rab5 and rab7 protein upregulation in mild cognitive impairment and Alzheimer’s disease. J. Alzheimers Dis. 2010, 22, 631–639. [Google Scholar] [CrossRef]
- Wang, S.; Wang, R.; Chen, L.; Bennett, D.A.; Dickson, D.W.; Wang, D.S. Expression and functional profiling of neprilysin, insulin-degrading enzyme, and endothelin-converting enzyme in prospectively studied elderly and Alzheimer’s brain. J. Neurochem. 2010, 115, 47–57. [Google Scholar] [CrossRef]
- Sengupta, U.; Montalbano, M.; McAllen, S.; Minuesa, G.; Kharas, M.; Kayed, R. Formation of toxic oligomeric assemblies of RNA-binding protein: Musashi in Alzheimer’s disease. Acta Neuropathol. Commun. 2018, 6, 113. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Qi, X.L.; Cao, Y.; Yu, W.F.; Ravid, R.; Winblad, B.; Pei, J.J.; Guan, Z.Z. Elevations in the levels of NF-κB and inflammatory chemotactic factors in the brains with Alzheimer’s disease—One mechanism may involve α3 nicotinic acetylcholine receptor. Curr. Alzheimer Res. 2016, 13, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Kondratova, A.A.; Kondratov, R.V. The circadian clock and pathology of the ageing brain. Nat. Rev. Neurosci. 2012, 13, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Numano, R.; Abe, M.; Hida, A.; Takahashi, R.; Ueda, M.; Block, G.D.; Sakaki, Y.; Menaker, M.; Tei, H. Resetting central and peripheral circadian oscillators in transgenic rats. Science 2000, 288, 682–685. [Google Scholar] [CrossRef]
- Buhr, E.D.; Takahashi, J.S. Molecular components of the Mammalian circadian clock. In Circadian Clocks; Springer: Berlin/Heidelberg, Germany, 2013; Volume 217, pp. 3–27. [Google Scholar] [CrossRef]
- Cronin, P.; McCarthy, M.J.; Lim, A.S.P.; Salmon, D.P.; Galasko, D.; Masliah, E.; De Jager, F.L.; Bennett, D.A.; Desplats, P. Circadian alterations during early stages of Alzheimer’s disease are associated with aberrant cycles of DNA methylation in BMAL1. Alzheimers Dement. 2017, 13, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Moon, M. Aβ-induced degradation of BMAL1 and CBP leads to circadian rhythm disruption in Alzheimer’s disease. Mol. Neurodegener. 2015, 10, 13. [Google Scholar] [CrossRef]
- Belanger, V.; Picard, N.; Cermakian, N. The circadian regulation of Presenilin-2 gene expression. Chronobiol. Int. 2006, 23, 747–766. [Google Scholar] [CrossRef]
- Chiou, Y.Y.; Yang, Y.; Rashid, N.; Ye, R.; Selby, C.P.; Sancar, A. Mammalian period represses and de-represses transcription by displacing CLOCK-BMAL1 from promoters in a Cryptochrome-dependent manner. Proc. Natl. Acad. Sci. USA 2016, 113, E6072–E6079. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, T.; Zhang, Y.; Qin, X. Molecular basis for the regulation of the circadian clock kinases CK1δ and CK1ε. Cell Signal. 2017, 31, 58–65. [Google Scholar] [CrossRef]
- Zhou, L.; Bryant, C.D.; Loudon, A.; Palmer, A.A.; Vitaterna, M.H.; Turek, F.W. The circadian clock gene Csnk1e regulates rapid eye movement sleep amount, and nonrapid eye movement sleep architecture in mice. Sleep 2014, 37, 785–793C. [Google Scholar] [CrossRef]
- Walton, K.M.; Fisher, K.; Rubitski, D.; Marconi, M.; Meng, Q.J.; Sládek, M.; Adams, J.; Bass, M.; Chandrasekaran, R.; Butler, T.; et al. Selective inhibition of casein kinase 1 epsilon minimally alters circadian clock period. J. Pharmacol. Exp. Ther. 2009, 330, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Harvey, K.F.; Pfleger, C.M.; Hariharan, I.K. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 2003, 114, 457–467. [Google Scholar] [CrossRef]
- Meng, Z.; Moroishi, T.; Guan, K.L. Mechanisms of Hippo pathway regulation. Genes Dev. 2016, 30, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Ye, X.; Yu, J.; Li, L.; Li, W.; Li, S.; Yu, J.; Lin, J.D.; Wang, C.Y.; Chinnaiyan, A.M.; et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008, 22, 1962–1971. [Google Scholar] [CrossRef] [PubMed]
- Praskova, M.; Khoklatchev, A.; Ortiz-Vega, S.; Avruch, J. Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. Biochem. J. 2004, 381 Pt 2, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Deng, J. Ubiquitination-deubiquitination in the Hippo signaling pathway. Oncol. Rep. 2019, 41, 1455–1475. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, L.; Tumaneng, K.; Wang, C.Y.; Guan, K.L. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev. 2010, 24, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Schittek, B.; Sinnberg, T. Biological functions of casein kinase 1 isoforms and putative roles in tumorigenesis. Mol. Cancer 2014, 13, 231. [Google Scholar] [CrossRef]
- Dolek, N.; Saylisoy, S.; Ozbabalik, D.; Adapinar, B. Comparison of hippocampal volume measured using magnetic resonance imaging in Alzheimer’s disease, vascular dementia, mild cognitive impairment and pseudodementia. J. Int. Med. Res. 2012, 40, 717–725. [Google Scholar] [CrossRef]
- Boopathy, G.T.K.; Hong, W. Role of hippo pathway-YAP/TAZ signaling in angiogenesis. Front. Cell Dev. Biol. 2019, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Qing, J.; Liu, X.; Wu, Q.; Zhou, M.; Zhang, Y.; Mazhar, M.; Huang, X.; Wang, L.; He, F. Hippo/YAP pathway plays a critical role in effect of GDNF against Aβ-induced inflammation in microglial cells. DNA Cell Biol. 2020, 39, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Gogia, N.; Chimata, A.V.; Deshpande, P.; Singh, A.; Singh, A. Hippo signaling: Bridging the gap between cancer and neurodegenerative disorders. Neural Regen. Res. 2021, 16, 643–652. [Google Scholar] [CrossRef] [PubMed]



| Inclusion | Exclusion |
|---|---|
| Alzheimer’s disease | Braak score < iv CERAD score ≥ 3 Other neurodegenerative diseases—Parkinson’s Disease, Lewy body pathology, Huntington’s Disease, Mild Cognitive Impairment, normal aging, inflammatory diseases (Multiple Sclerosis), PART |
| Post-mortem brain analysis | Plasma, serum, CSF, Saliva, cell-lines, transfected tissues, tissue biopsy |
| qRT-PCR or protein analyses | RNAseq, microarray analysis, in-situ hybridization |
| Qualitative and quantitative analysis | Study focusing on post-translational modifications, mutations, allelic variants, study including treatment or intervention |
| Human | Animals, cell lines |
| Male and female participants | None |
| Age-matched controls compared to AD | Single cohort studies, case studies, non-age-matched controls |
| Age ≥ 60 | Age < 60 |
| All patient ethnicities | No ethnicities were excluded |
| Primary research | Reviews, meta-analyses, bioinformatics studies using previously collected data, conference abstracts, clinical trials |
| Sample size n ≥ 3 | Sample size n < 3 |
| Published in peer-reviewed journals | Non-peer-reviewed |
| English language | Not written in English |
| (A): Up and Downregulation of miRNAs | ||||||
|---|---|---|---|---|---|---|
| Author | AD group (N; Mean Age; M:F) | Control group (N; Mean Age; M:F) | Brain Regions | Methods | miRNA Upregulated | miRNA Downregulated |
| Annese et al. 2018 [43] | 14; 74; 8:5 | 14; 77; 8:5 | HC; MTG; MFG | qRT-PCR | miR-10a-5p, miR-28-3p | miR-132-3p, miR-132-5p, miR-184, miR-212-3p, miR-212-5p, miR-34c-3p, miR-375, miR-539-5p |
| Cheng et al. 2020 [44] | 8; 76; 3:8 | 8; 67; 4:5 | FC; BDE | qRT-PCR | miR-17-5p, miR-18a-5p, miR-190a-5p, miR-219a-2-3p, miR-3157-5p, miR-374b-5p, miR-374c-3p, miR-548, miR-550a-3p, miR-550b-2-5p | miR-4284, miR-5001-3p, miR-132-5p |
| Chopra et al. 2020 [45] | 29; 84; 11:18 | 25; 86; 9:16 | TC; CB | qRT-PCR | miR-298 | |
| Culpan et al. 2011 [46] | 12; 82; 5:7 | 6; 88; 5:1 | FNC; TNC | qRT-PCR | miR-128a, miR-128b | |
| Gong et al. 2017 [47] | 40; -; - | 35; -; - | FC | qRT-PCR | miR-15b | |
| Herbert et al. 2013 [48] | 8; 78; 5:3 | 8; 71; 5:3 | STG; MTG | qRT-PCR | miR-132-3p, miR-100 | |
| Henriques et al. 2020 [49] | 16; 81; 4:12 | 18; 78: 6:12 | STG; MTG | qRT-PCR | miR-3651 | miR-1202, miR-30e-3p, miR-365b-5p, miR-4286, miR-4443, miR-4449, miR-664-3p, miR-767-5p, |
| Kumar et al. 2018 [50] | 27; 80; 14:13 | 15; 79; 8:7 | FC | qRT-PCR | miR-455-3p | |
| Kumar et al. 2017 [51] | 12; 80; 4:8 | 5; 73; 3:2 | FC | qRT-PCR | miR-3613-3p, miR-455-3p, miR-4674, miR-6722 | miR-122-5p |
| Lau et al. 2013 [52] | 41; -; - | 23; -; - | FC; HC | qRT-PCR | miR-142-3p, miR-200a-3p, miR-27a-3p, miR-92b-3p | miR-124-3p, miR-128, miR-129-2-3p, miR-129-5p, miR-132-3p miR-136-5p, miR-138-5p |
| Lei et al. 2015 [53] | 31; 78; 18:13 | 29; 80; 16:13 | FC | qRT-PCR | miR-29c | |
| Li et al. 2019 [54] | 30; 88; 18:12 | 30; 87; 20:10 | FC | qRT-PCR | miR-219-5p | |
| Liu et al. 2019 [55] | 10; -; - | 10; -; - | - | qRT-PCR | miR-132 | |
| Llorens et al. 2017 [56] | 25; -; - | 25; -; - | LC; EC; HC | qRT-PCR | miR-124-3p, miR-132-3p, miR-143-3p, miR-27a-3p | miR-124-3p |
| Long et al. 2019 [28] | 15; 84; - | 5; 84; - | FC | qRT-PCR | miR-346 | |
| Moncini et al. 2016 [57] | 12; 78; 7:3 | 11; 82; 4:7 | HC; TC | qRT-PCR | miR-103, miR-107, miR-15b, miR-16, miR-195 | |
| Muller et al. 2014 [58] | 10; 78; 7:3 | 11; 83; 4:7 | HC | qRT-PCR | miR-16 miR-146 | miR-16 miR-146 miR-107 miR-128a |
| Pichker et al. 2017 [59] | 39; 80; 15:24 | 25; 65; 15:10 | TC; PFC | qRT-PCR | miR-132 miR-212-3p | |
| Qian et al. 2019 [60] | 12; 81; - | 11; 82; - | HC | qRT-PCR | miR-338-5p | |
| Santa-Maria et al. 2015 [61] | 7; 93; 3:4 | 20; 89; 9:11 | FC | qRT-PCR | miR-219-5p | |
| Sarkar et al. 2016 [27] | 13; 76; 6:7 | 10; 77; 5:5 | TC; FC; CB | qRT-PCR | miR-146a | miR-132 |
| Wang et al. 2018 [62] | 12; 86; 3:9 | 12; 86; 1:11 | TC; HC | qRT-PCR | miR-124 | |
| Wong et al. 2013 [63] | 16; 81; 6:10 | 16; 77; 10:6 | TC | qRT-PCR | miR-132 miR-212 | |
| Yuan et al. 2020 [64] | 10; 75; 6:4 | 10; 80; 6:4 | - | qRT-PCR | miR-425-5p | |
| Zhang et al. 2016 [65] | 7; 87; 3:4 | 7; 87; 1:16 | HC | qRT-PCR | miR-603 | |
| Zhao et al. 2016 [66] | 12; 74; - | 6; 72; - | TC; HC | qRT-PCR | miR-7 miR146a miR-155 | |
| Zhao et al. 2013 [67] | 3; 72; - | 3; 72; - | HC | qRT-PCR | miR-34a miR-146a miR-125b miR-155 | |
| Zhong et al. 2018 [33] | 30; 87; - | 20; 87; - | FC | qRT-PCR | miR-16 | |
| (B): Up and downregulation of proteins | ||||||
| Author | AD group (N; Mean Age; M:F) | Control group (N; Mean Age; M:F) | Brain Regions | Methods | Protein Upregulated | Protein Downregulated |
| Beckelman et al. 2016 [68] | 5; 82-98; 2:3 | 5; 78-97; 3:2 | TC | WB, IHC | EEF1A1 | |
| Chiu et al. 2015 [69] | 7; 82.9; 3:4 | 8; 61-91; 10:4 | HP | IHC | ABCB1 (P-Glycoprotein) | |
| Shepherd et al. 2020 [70] | 17; 78; - | 16; 74; - | TC | WB, ELISA | APP, MAPT | RAP |
| Chen et al. 2012 [71] | 18; 74-89; - | 13; 68-69; - | HP, FL, TL, CB | ELISA | NF-κb, BACE1 | |
| Holler et al. 2014 [72] | 52; 85.9; 19:33 | 19; 85.2; 5:14 | HP | Immunoblot/IHC | BIN1 | |
| Walker et al. 2015 [73] | 12; 78,9; 6:6 | 12; 84; 9:3 | TC | WB | SOCS4, SOCS7 | |
| Glennon et al. 2013 [74] | 24; 69-96; 6:18 | 24; 76.4; 14:10 | HP | Immunoblot | BIN1 | |
| Byman et al. 2018 [75] | 12; 63-96; 3:9 | 8; 60-102; 5:3 | HP, IP, IT, FC, SMTG | ELISA, IHC | AMY1A | |
| Huang et al. 2020 [76] | 26; 88.6; 12:14 | 19; 90.3; 9:10 | FC | WB, IP, IHC, IF | RBM15B | METTL3 |
| Yoo et al. 2020 [77] | 3; 72; 0:3 | 3; 65; 2:1 | FC | IF | CLOCK, BMAL1 | |
| Chen et al. 2012 [78] | 12; 68-92; 8:4 | 12; 81-92; 9:3 | FC, TC, PC, OC | Mass spectrometry | CLU | |
| Gu et al. 2020 [79] | 10; 76.6; 6:4 | 9; 79.22; 4:6 | FC | WB, IHC | CK1ε | TDP43 |
| Xu et al. 2019 [80] | 9; 60-80; 6:3 | 9; 61-78; 5:4 | HP, EC, CG, SCx, MCx, CB | MS | AGT, AHNAK, ALAD, ANXA5, AQP4, ASAH1, BAG3, C3, CHGA, CLU, CP, DBI, DKK3, ESD, FGA, FGB, FGG, GJA1, H3F3A, HDGF, HIST1H1C, HIST1H1E, HP, HPX, HRSP12, HSPA1A, HSPB1, IGHA1, IGHG1, IGKC, ISYNA1, ITIH4, MAOB, MAP4, MARCKS, MECP2, NAMPT, NUCKS1, ORM1, PADI2, PAICS, PBXIP1, PCBD1, PLIN3, PNPO, PRDX1, PRDX6, S100A1, S100A11, S100A6, S100A9, SAA1, SELENBP1, SERPINA1, SERPINA3, SERPING1, SPR, STOM, TPD52L1 | ACTN2, ADAP1, AP1G1, CADPS, CAP2, CIRBP, CORO1A, CORO2B, CRAT, DLAT, DLG4, DNAJC6, DNM3, DUSP3, EEF1B2, FARSB, GAS7, GLS, GRPEL1, HGS, HOMER1, HSPA4L, IARS2, IDH3G, IPO7, KIAA0513, KIF5C, LONP1, LRPPRC, LZTFL1, MAPRE3, NDUFA10, NECAB1, OAT, OGDH, OGDHL, OTUB1, OXCT1, PAFAH1B1, PDHX, PDIA3, PHYHIPL, PPME1, PPP2R1A, PTPA, PREP, PRKRA, RAP1GDS1, RGS7, RPH3A, SARS2, SCAI, SDR39U1, SGTB, SH3GL1, SLIRP, SMS, STXBP1, STXBP3, SUCLA2, SUCLG1, TIMM44, TLN2, TRAP1, VPS35, YARS, YWHAG, YWHAH, YWHAQ |
| Batkulwar et al. 2018 [81] | 3; 84.3; - | 3; 89.3; - | FC | MS | CML, Cathepsin B, AEP, RAGE, TAU | |
| Ilic et al. 2019 [82] | 6; 77.8; 2:4 | 6; 75.5; 2:4 | - | IHC | NPTN | |
| Lue et al. 2015 [83] | 11; 82.46; 9:13 | 11; 85.4; 7:4 | FC | Immunoblot | TREM2, DAP12, IBA1, CASP3 | SNAP25, PSD95 |
| Bekris et al. 2010 [84] | 8; 60-93; 5:3 | 8; 79-94; 4:4 | HP | WB | APOE | |
| Causevic et al. 2010 [85] | 4; 82-97; - | 4; 81-86; - | HP | WB | IDE | |
| Campanari et al. 2016 [86] | 19; 75-85; 8:11 | 22; 65-73; 12:10 | FC | WB | ACHE | |
| Bartolotti et al. 2016 [87] | 21; 93.1; 0:21 | 20; 93.49; 0:20 | CB, FC | WB | CREB, CBP, EP300 | |
| Jin et al. 2013 [88] | 7; 86.29; 1:6 | 7; 86.6; 2:5 | FC | WB | GLUT3 | |
| Gu et al. 2020 [89] | 12; 75-98; 3:9 | 12; 61-100; 3:9 | TC | WB, & IHC | YWHAG, YWHAH (14-3-3 Proteins) | |
| Ginsberg et al. 2010 [90] | 38; 84.6; 14:24 | 27; 80.8; 5:12 | PFC | Quantitative immunoblot | RAB5A, RAB7A | |
| Wang et al. 2010 [91] | 10; 87.3; 3:7 | 10; 80.5; 7:3 | HP, EC, CG, SCx, MCx, CB | WB | NEP, IDE | |
| Sengupta et al. 2018 [92] | 4; 75-83; 3:1 | 4; 70-79; 2:2 | HP, BF, FC, CB, STR | WB, IF | MSI1, MSI2 | |
| Liao, et al. 2016 [93] | 10; 81.8; 4:6 | 7; 83.6; 3:4 | MTG | WB, IHC, ELISA | NF-κB, MCP-1, MIP1α | |
| Common Pathways | miRNA p Value | Protein p Value | miRNA (−log (p Value) | miRNA-Protein Inverse Relation |
|---|---|---|---|---|
| Hippo signaling pathway | 7.91 × 10−8 | 0.021 | 7.1 | ↓ miR-320a [43,44], miR-329-3p [52], miR-495-3p [52] ↑ CSNK1E [79] |
| ↑ miR-3613-3p [51], miR-200a-3p [52], miR-199a-3p [44,52], miR-199b-3p [52], miR-23a-3p [44,52], miR-425-5p [52,64], miR-34c-3p [43,44,56] ↓ YWHAG [80,89] | ||||
| ↑ miR-3613-3p [51] ↓ YWHAH [80,89] | ||||
| ↑ miR-27a-3p [52,56], miR-455-3p [50,51] ↓ YWHAQ [80] | ||||
| ↑ miR-150-5p [52], ↓ PPP2R1A [80] | ||||
| Pathways in cancer | 9.57 × 10−6 | - | 5 | ↑ miR-3613-3p [51], miR-23a-3p [44,52], miR-550a-3p [34] ↓ CREBBP [87] |
| ↑ miR-603 [65], miR-3613-3p [51] ↓ EP300 [92] | ||||
| Adherends junction | 2.33 × 10−5 | - | 4.6 | ↑ miR-23a-3p [44,52] ↓ ACTN2 [80] |
| ↑ miR-23a-3p [44,52], miR-3613-3p [51], miR-550a-3p [34] ↓ CREBBP [87] | ||||
| ↑ miR-603 [65], miR-3613-3p [51] ↓ EP300 [87] | ||||
| Wnt signaling pathway | 0.001 | - | 3.1 | ↓ miR-495-3p [52], miR-329-3p [53], miR-320a [43,44], ↑ CSNK1E [79] |
| ↑ miR-603 [65], miR-3613-3p [51] ↓ EP300 [87] | ||||
| ↑ miR-3613-3p [51], miR-23a-3p [44,52], miR-550a-3p [34] ↓ CREBBP [87] | ||||
| PI3K-Akt signaling pathway | 0.001 | - | 3 | ↑ miR-27a-3p [52,56], miR-10a-5p [43], miR-374b-5p [34], miR-155-5p [66,67], miR-200a-3p [52], miR-3613-3p [51], miR-362-3p [52], miR-425-5p [52,64] ↓ CREBBP [87] |
| ↑ miR-150-5p [52] ↓ PPP2R1A [80] | ||||
| ↑ miR-199a-3p [44,52], miR-199b-3p [52], miR-200a-3p [52], miR-3613-3p [51], miR-23a-3p [44,52], miR-425-5p [52,64], miR-34c-3p [43,44,56] ↓ YWHAG [80,89] | ||||
| ↑ miR-3613-3p [51] ↓ YWHAH [80,89] | ||||
| ↑ miR-27a-3p [52,56], miR-455-3p [50,51] ↓ YWHAQ [70] | ||||
| GABAergic | 0.001 | - | 3 | ↑ miR-200a-3p [52], miR-9-5p [27], miR-125b-5p [67] ↓ GLS [80] |
| Estrogen signaling pathway | 0.002 | - | 2.7 | ↑ miR-155-5p [66,67], miR-27a-3p [52,56], miR-3613-3p [51], miR-374b-5p [34], miR-10a-5p [43], miR-200a-3p [52], miR-425-5p [52,64], miR-362-3p [52] ↓ CREB1 [87] |
| Thyroid hormone signaling pathway | 0.002 | - | 2.7 | ↑ miR-3613-3p [51], miR-23a-3p [44,52], miR-550a-3p [34] ↓ CREBBP [87] |
| ↑ miR-155-5p [62,66,67], miR-27a-3p [52,56], miR-3613-3p [51], miR-374b-5p [34], miR-10a-5p [43], miR-200a-3p [52], miR-425-5p [52,64], miR-362-3p [52] ↓ CREB1 [87] | ||||
| Prolactin signaling pathway | 0.002 | - | 2.6 | ↓ miR-487a-3p [52], miR-136-5p [52], miR-543 [52], miR-889-3p [43] ↑SOCS4 [73] |
| Protein processing in endoplasmic reticulum | 0.002 | - | 2.6 | ↓ miR-219a-2-3p [34,52], miR-107 [56,57], miR-103a-3p [57], miR-30e-3p [49], miR-30a-3p [43], miR-195-5p [52,57], miR-16-5p [33,56,57], miR-15b-5p [47,57], miR-889-3p [43], miR-539-5p [43], miR-410-3p [52], miR-129-5p [52], miR-543 [52], miR-375 [43], miR-17-5p [34], miR-495-3p [52], miR-338-5p [60], miR-320a [43,44] ↑ HSPA4L [80] |
| Endocytosis | 0.004 | 0.002 | 2.4 | ↓ miR-298 [45], miR-539-5p [43], miR-18a-5p [34], miR-582-5p [43] ↑RAB5A [90] |
| ↑ miR-603 [65], miR-23a-3p [44,52], miR-3613-3p [51] ↓ DNAJC6 [80] | ||||
| ↑ miR-3613-3p [51], miR-23a-3p [44,52], miR-548 [34], miR-603 [65], miR-362-3p [52], miR-27a-3p [52,56], miR-146a-3p [27,56,67] ↓ DNM3 [80] | ||||
| AMPK signaling pathway | ↑ miR-142-3p [52] ↓ HGS [80] | |||
| AMPK signaling pathway | 0.005 | - | 2.3 | ↑ miR-425-5p [52,64], miR-155-5p [66,67], miR-27a-3p [52,56], miR-10a-5p [43], miR-362-3p [52], miR-374b-5p [34], miR-3613-3p [51], miR-200a-3p [52] ↓ CREB1 [87] |
| AMPK signaling pathway FoxO signaling pathway | ↑ miR-150-5p [52] ↓ PPP2R1A [80] | |||
| AMPK signaling pathway FoxO signaling pathway | 0.006 | - | 2.2 | ↓ miR-329-3p [52], miR-495-3p [52], miR-320a [43,44] ↑ CSNK1E [79] |
| AMPK signaling pathway FoxO signaling pathway | ↑ miR-550a-3p [34], miR-3613-3p [51], miR-23a-3p [44,52] ↓ CREBBP [87] | |||
| AMPK signaling pathway FoxO signaling pathway | ↑ miR-603 [65], miR-3613-3p [51] ↓ EP300 [87] | |||
| AMPK signaling pathway FoxO signaling pathway Adrenergic signaling in cardiomyocytes | ↑ miR-374b-5p [34], miR-3613-3p [51], miR-34c-3p [43,44,56] ↓ HOMER1 [80] | |||
| AMPK signaling pathway FoxO signaling pathway | 0.001 | - | 2.1 | ↑ miR-10a-5p [43], miR-425-5p [52,64], miR-374b-5p [34], miR-362-3p [52], miR-200a-3p [52], miR-155-5p [66,67], miR-27a-3p [52,56], miR-3613-3p [51] ↓ CREB1 [87] |
| Arrhythmogenic right ventricular cardiomyopathy (ARVC) | ↑ miR-150-5p [52] ↓ PPP2R1A [80] | |||
| Arrhythmogenic right ventricular cardiomyopathy (ARVC) Transcriptional mis-regulation in cancer | 0.008 | - | 2.1 | ↓ miR-320a [43,44], miR-543 [52], miR-582-5p [43], miR-889-3p [43], miR-410-3p [52], miR-539-5p [43], miR-30a-3p [43], miR-30e-3p [49], miR-329-3p [52], miR-298 [45], miR-338-5p [60] ↑ CREB1 [87] |
| Arrhythmogenic right ventricular cardiomyopathy (ARVC) Transcriptional mis-regulation in cancer | 0.009 | - | 2 | ↓ miR-15b-5p [47,57] ↑ H3F3A [80] |
| TGF-beta signaling pathway | 0.011 | - | 1.9 | ↑ miR-550a-3p [34], miR-3613-3p [51], miR-23a-3p [44,52] ↓ CREBBP [87] |
| ↑ miR-603 [65], miR-3613-3p [51] ↓ EP300 [87] | ||||
| ↑ miR-150-5p [52] ↓ PPP2R1A [80] | ||||
| Prostate cancer | 0.011 | - | 1.9 | ↑ miR-425-5p [52,64], miR-10a-5p [43], miR-200a-3p [52], miR-374b-5p [34], miR-362-3p [52], miR-27a-3p [52,56], miR-3613-3p [51], miR-155-5p [66,67] ↓ CREB1 [87] |
| ↑ miR-550a-3p [34], miR-23a-3p [44,52], miR-3613-3p [51] ↓ CREBBP [87] | ||||
| ↑ miR-603 [60], miR-3613-3p [46] ↓ EP300 [82] | ||||
| cAMP signaling pathway | 0.013 | - | 1.9 | ↑ miR-155-5p [61,62], miR-10a-5p [38], miR-200a-3p [47], miR-374b-5p [29], miR-27a-3p [47,51], miR-425-5p [47,50], miR-362-3p [47], miR-3613-3p [46] ↓ CREB1 [87] |
| ↑ miR-550a-3p [34], miR-23a-3p [44,52], miR-3613-3p [51] ↓ CREBBP [87] | ||||
| ↑ miR-603 [65], miR-3613-3p [51] ↓ EP300 [87] | ||||
| Cholinergic synapse | 0.015 | - | 1.8 | ↑ miR-155-5p [66,67], miR-10a-5p [43], miR-200a-3p [52], miR-374b-5p [34], miR-27a-3p [52,56], miR-425-5p [52,55], miR-362-3p [52], miR-3613-3p [51] ↓ CREB1 [87] |
| Amoebiasis | 0.020 | 0.004 | 1.7 | ↓ miR-18a-5p [34], miR-582-5p [43], miR-539-5p [43], miR-298 [45] ↑ RAB5A [90] |
| ↑ miR-23a-3p [44,52] ↓ ACTN2 [80] | ||||
| Gap junction | 0.021 | - | 1.7 | ↓ miR-539-5p [43], miR-664a-3p [49], miR-582-5p [43], miR-495-3p [52] ↑ GJA1 [80] |
| mRNA surveillance pathway | 0.024 | - | 1.6 | ↓ miR-410-3p [52], miR-129-5p [52], miR-582-5p [43], miR-769-5p [52], miR-889-3p [43], miR-128-3p [52], miR-320a [43,44], miR-495-3p [52] ↑ MSI2 [92] |
| ↑ miR-150-5p [52] ↓ PPP2R1A [80] | ||||
| Circadian rhythm | 0.025 | 0.001 | 1.6 | ↓ miR-136-5p [52] ↑ ARNTL [77] |
| ↓ miR-15b-5p [47,57], miR-195-5p [52,59], miR-16-5p [33,56,57], miR-889-3p [43], miR-543 [52], miR-338-5p [60], miR-29c-3p [53], miR-129-5p [52], miR-495-3p [52], miR-107 [56,57], miR-103a-3p [57] ↑ CLOCK [77] | ||||
| ↓ miR-329-3p [52], miR-495-5p [52] ↑ CSNK1E [79] | ||||
| ↑ miR-27a-3p [52,56], miR-10a-5p [43], miR-374b-5p [34], miR-155-5p [66,67], miR-200a-3p [52], miR-3613-3p [51], miR-362-3p [52], miR-425-5p [52,64] ↓ CREB1 [87] | ||||
| Insulin signaling pathway | 0.027 | - | 1.6 | ↓ miR-487a-3p [52], miR-136-5p [52], miR-543 [52], miR-889-3p [43] ↑ SOCS4 [73] |
| Bacterial invasion of epithelial cells | 0.352 | - | 1.5 | ↑ miR-603 [65], miR-23a-3p [44,52], miR-548 [34], miR-362-3p [52], miR-3613-3p [51], miR-27a-3p [52,56], miR-146a-3p [27,56,67] ↓ DNM3 [80] |
| cGMP-PKG signaling pathway | 0.035 | - | 1.4 | ↑ miR-155-5p [66,67], miR-10a-5p [43], miR-200a-3p [52], miR-374b-5p [34], miR-27a-3p [52,56], miR-425-5p [52,64], miR-362-3p [52], miR-3613-3p [51], ↓ CREB1 [87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tasker, R.; Rowlands, J.; Ahmed, Z.; Di Pietro, V. Co-Expression Network Analysis of Micro-RNAs and Proteins in the Alzheimer’s Brain: A Systematic Review of Studies in the Last 10 Years. Cells 2021, 10, 3479. https://doi.org/10.3390/cells10123479
Tasker R, Rowlands J, Ahmed Z, Di Pietro V. Co-Expression Network Analysis of Micro-RNAs and Proteins in the Alzheimer’s Brain: A Systematic Review of Studies in the Last 10 Years. Cells. 2021; 10(12):3479. https://doi.org/10.3390/cells10123479
Chicago/Turabian StyleTasker, Rachel, Joseph Rowlands, Zubair Ahmed, and Valentina Di Pietro. 2021. "Co-Expression Network Analysis of Micro-RNAs and Proteins in the Alzheimer’s Brain: A Systematic Review of Studies in the Last 10 Years" Cells 10, no. 12: 3479. https://doi.org/10.3390/cells10123479
APA StyleTasker, R., Rowlands, J., Ahmed, Z., & Di Pietro, V. (2021). Co-Expression Network Analysis of Micro-RNAs and Proteins in the Alzheimer’s Brain: A Systematic Review of Studies in the Last 10 Years. Cells, 10(12), 3479. https://doi.org/10.3390/cells10123479








