Roles of Mesenchymal Cells in the Lung: From Lung Development to Chronic Obstructive Pulmonary Disease
Abstract
1. Introduction
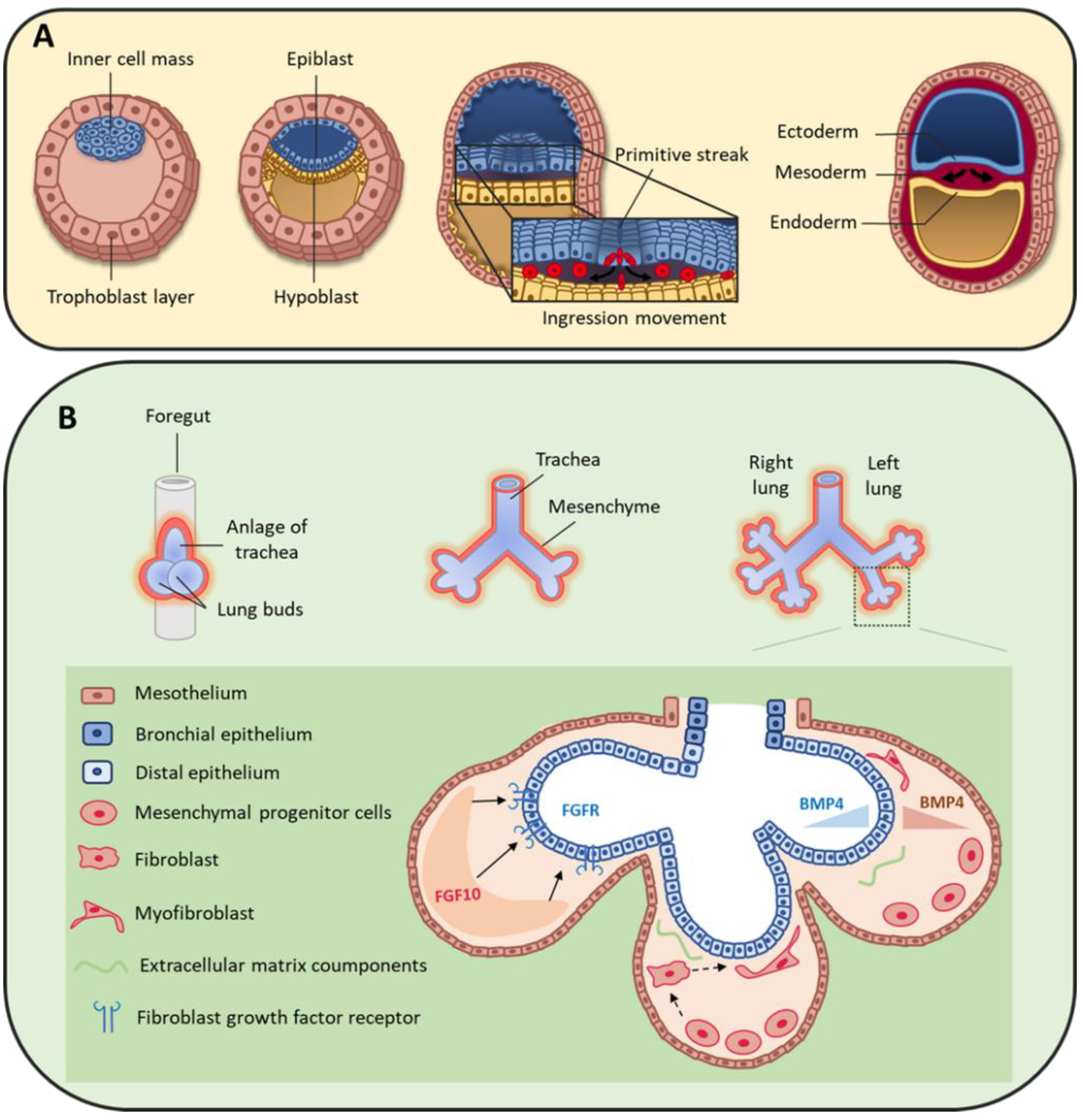
2. Origin of Pulmonary Mesenchymal Cells
3. Mesenchymal Cells during Lung Organogenesis
3.1. Peptide Growth Factors
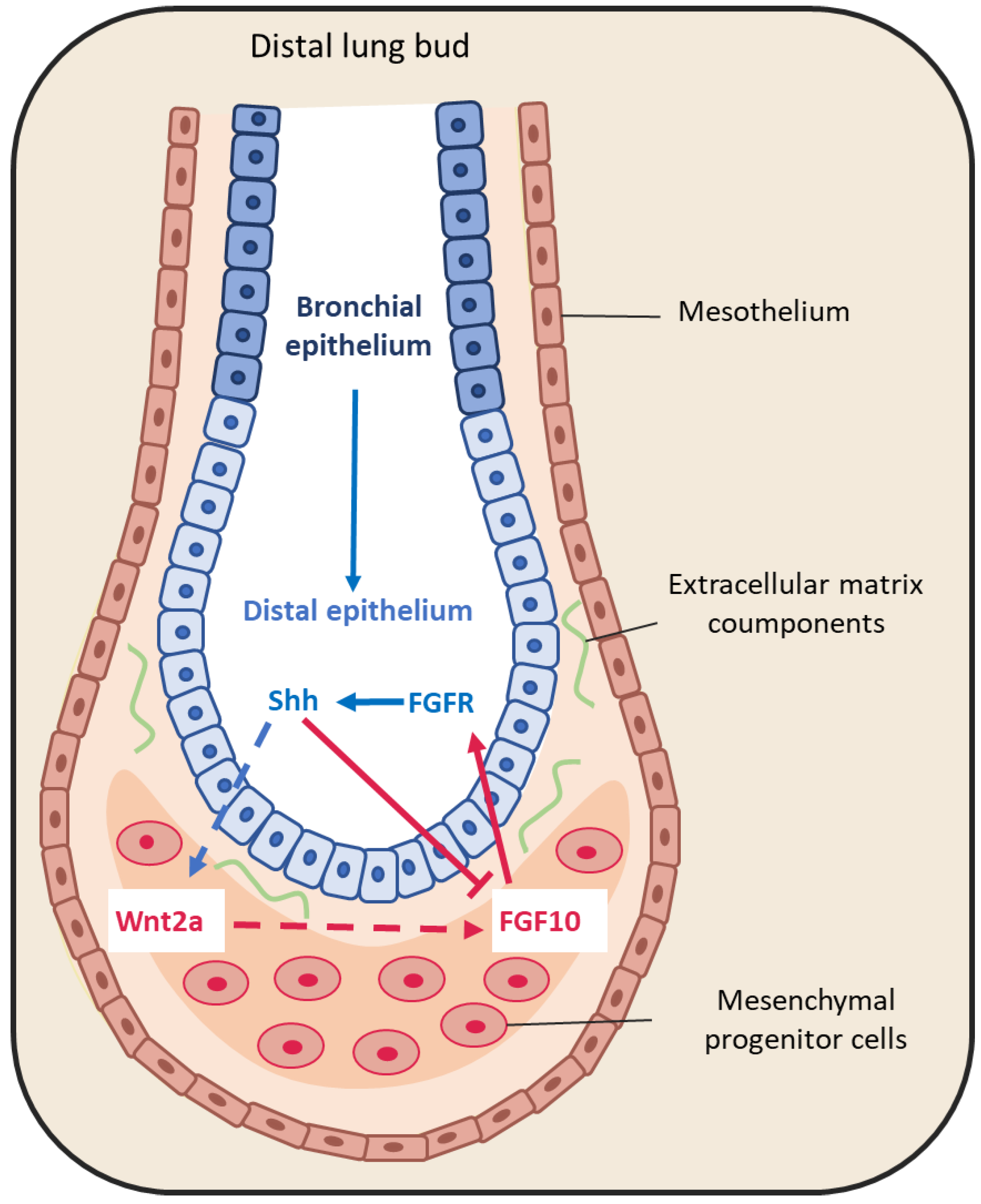
3.1.1. Fibroblast Growth Factors
3.1.2. Bone Morphogenic Protein 4 (BMP4)
3.1.3. Sonic Hedgehog
3.1.4. Epidermal Growth Factor (EGF)
3.1.5. Retinoic Acid (RA)
3.1.6. TGF-β
3.1.7. The Hippo Pathway
3.2. Extracellular Matrix Compounds
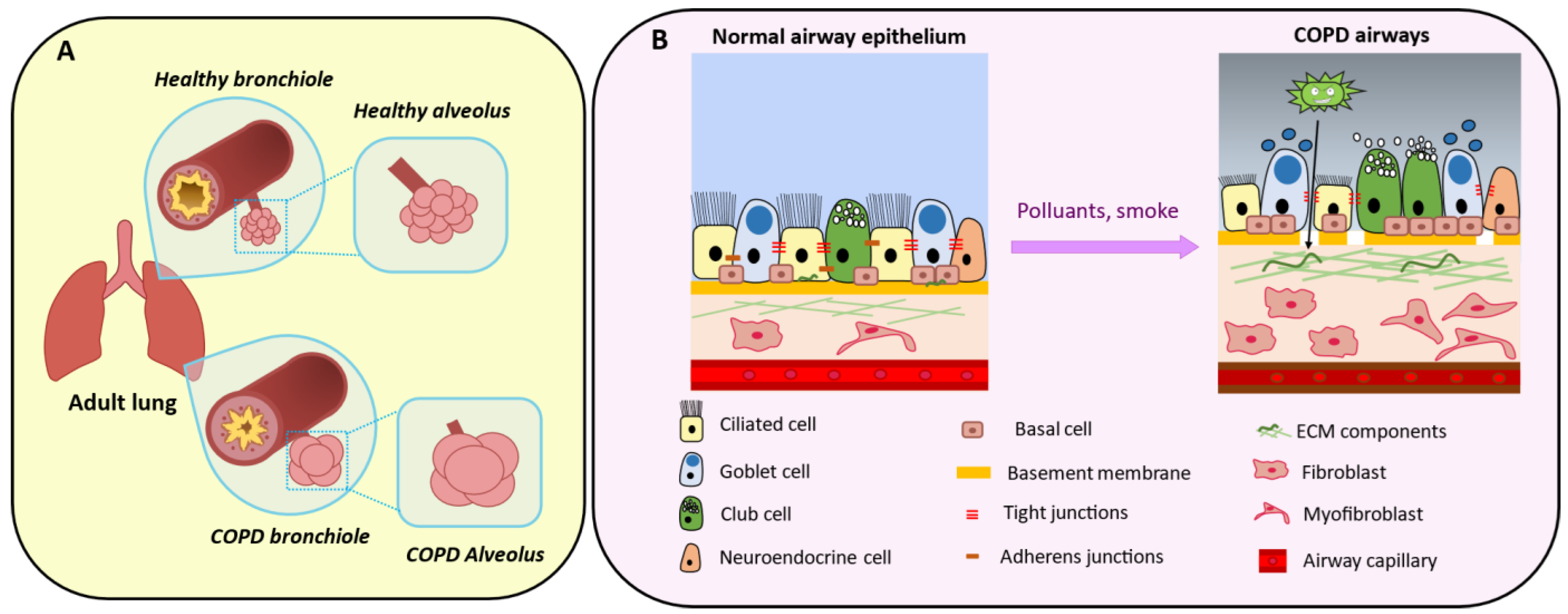
4. Physiological Roles of Mesenchymal Cells in Bronchioles
5. Mesenchymal Cells in Chronic Obstructive Pulmonary Disease
5.1. Genetic Contribution
5.2. The Epithelial–Mesenchymal Crosstalk
5.3. Peribronchiolar Fibrosis
5.4. Extracellular Matrix Composition
5.5. Epithelial–Mesenchymal Transition
5.6. Airway Smooth Muscle
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gohy, S.; Hupin, C.; Ladjemi, M.Z.; Hox, V.; Pilette, C. Key role of the epithelium in chronic upper airways diseases. Clin. Exp. Allergy 2020, 50, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Wittekindt, O.H. Tight junctions in pulmonary epithelia during lung inflammation. Pflug. Arch. 2017, 469, 135–147. [Google Scholar] [CrossRef]
- Powell, D.W. Barrier function of epithelia. Am. J. Physiol.-Gastrointest. Liver Physiol. 1981, 241, G275–G288. [Google Scholar] [CrossRef] [PubMed]
- Bustamante-Marin, X.M.; Ostrowski, L.E. Cilia and Mucociliary Clearance. Cold Spring Harb. Perspect. Biol. 2017, 9, a028241. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.M.; Chen, A.; Koh, P.W.; Deng, T.Z.; Sinha, R.; Tsai, J.M.; Barkal, A.A.; Shen, K.Y.; Jain, R.; Morganti, R.M.; et al. Mapping the Pairwise Choices Leading from Pluripotency to Human Bone, Heart, and Other Mesoderm Cell Types. Cell 2016, 166, 451–467. [Google Scholar] [CrossRef]
- Delorme, B.; Chateauvieux, S.; Charbord, P. The concept of mesenchymal stem cells. Regen. Med. 2006, 1, 497–509. [Google Scholar] [CrossRef]
- Bishop, A.E. Pulmonary epithelial stem cells. Cell Prolif. 2004, 37, 89–96. [Google Scholar] [CrossRef]
- Mammoto, A.; Mammoto, T. Vascular Niche in Lung Alveolar Development, Homeostasis, and Regeneration. Front. Bioeng. Biotechnol. 2019, 7, 318. [Google Scholar] [CrossRef]
- Hogan, B.L.M.; Barkauskas, C.E.; Chapman, H.A.; Epstein, J.A.; Jain, R.; Hsia, C.C.W.; Niklason, L.; Calle, E.; Le, A.; Randell, S.H.; et al. Repair and Regeneration of the Respiratory System: Complexity, Plasticity, and Mechanisms of Lung Stem Cell Function. Cell Stem Cell 2014, 15, 123–138. [Google Scholar] [CrossRef]
- McCulley, D.; Wienhold, M.; Sun, X. The Pulmonary Mesenchyme Directs Lung Development. Curr. Opin. Genet. Dev. 2015, 32, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Barron, L.; Gharib, S.A.; Duffield, J.S. Lung Pericytes and Resident Fibroblasts. Am. J. Pathol. 2016, 186, 2519–2531. [Google Scholar] [CrossRef]
- Lee, J.-H.; Tammela, T.; Hofree, M.; Choi, J.; Marjanovic, N.D.; Han, S.; Canner, D.; Wu, K.; Paschini, M.; Bhang, D.H.; et al. Anatomically and Functionally Distinct Lung Mesenchymal Populations Marked by Lgr5 and Lgr6. Cell 2017, 170, 1149–1163.e12. [Google Scholar] [CrossRef] [PubMed]
- Sveiven, S.N.; Nordgren, T.M. Lung-resident mesenchymal stromal cells are tissue-specific regulators of lung homeostasis. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2020, 319, L197–L210. [Google Scholar] [CrossRef]
- Soundararajan, M.; Kannan, S. Fibroblasts and Mesenchymal Stem Cells: Two Sides of the Same Coin? J. Cell. Physiol. 2018, 233, 9099–9109. [Google Scholar] [CrossRef]
- Hay, E.D. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev. Dyn. 2005, 233, 706–720. [Google Scholar] [CrossRef] [PubMed]
- da Silva Meirelles, L. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef]
- Rinn, J.L.; Bondre, C.; Gladstone, H.B.; Brown, P.O.; Chang, H.Y. Anatomic Demarcation by Positional Variation in Fibroblast Gene Expression Programs. PLoS Genet. 2006, 2, e119. [Google Scholar] [CrossRef]
- Spadafora, R.; Lu, J.; Khetani, R.S.; Zhang, C.; Iberg, A.; Li, H.; Shi, Y.; Lerou, P.H. Lung-Resident Mesenchymal Stromal Cells Reveal Transcriptional Dynamics of Lung Development in Preterm Infants. Am. J. Respir. Crit. Care Med. 2018, 198, 961–964. [Google Scholar] [CrossRef]
- Abreu, S.C.; Rolandsson Enes, S.; Dearborn, J.; Goodwin, M.; Coffey, A.; Borg, Z.D.; dos Santos, C.C.; Wargo, M.J.; Cruz, F.F.; Loi, R.; et al. Lung inflammatory environments differentially alter mesenchymal stromal cell behavior. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 317, L823–L831. [Google Scholar] [CrossRef] [PubMed]
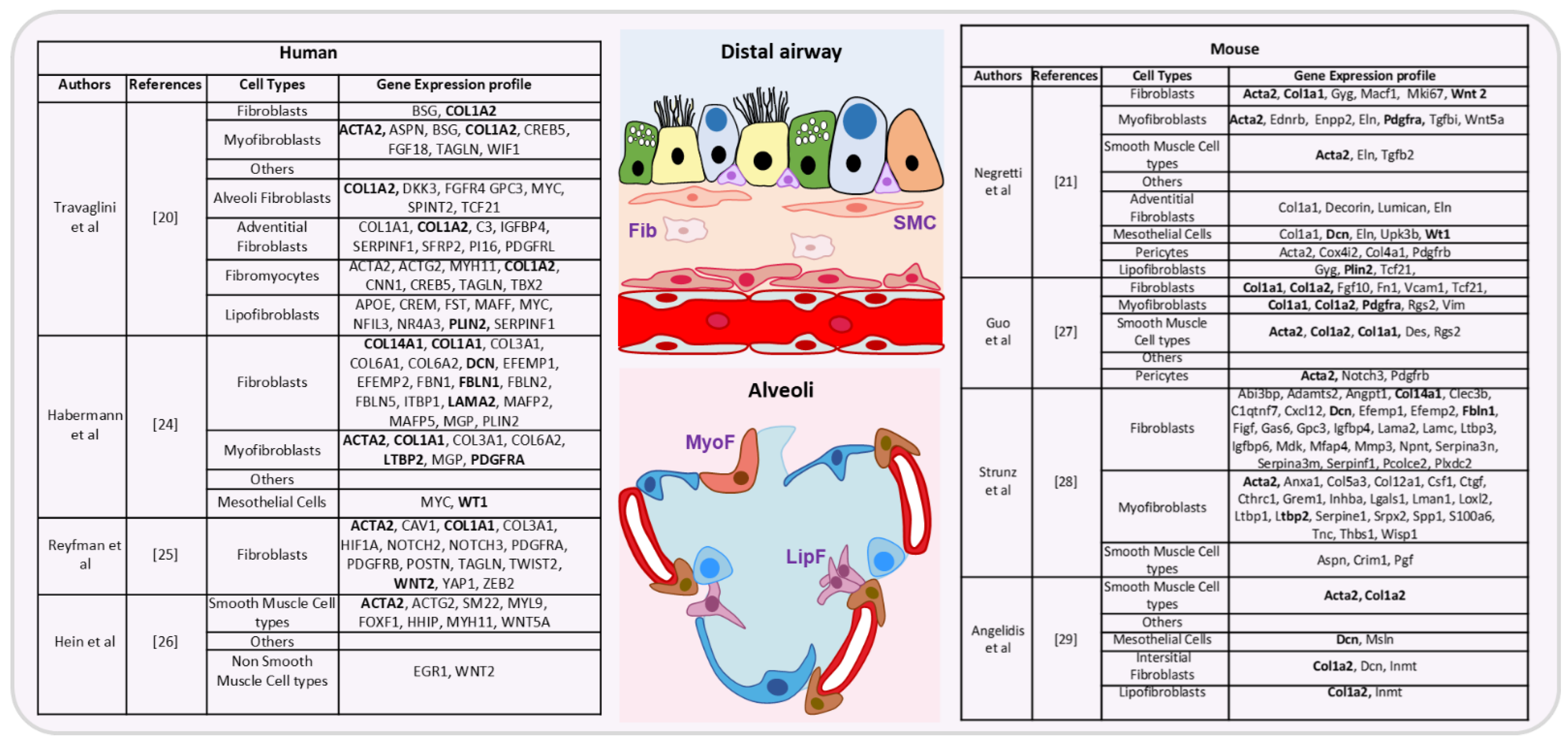
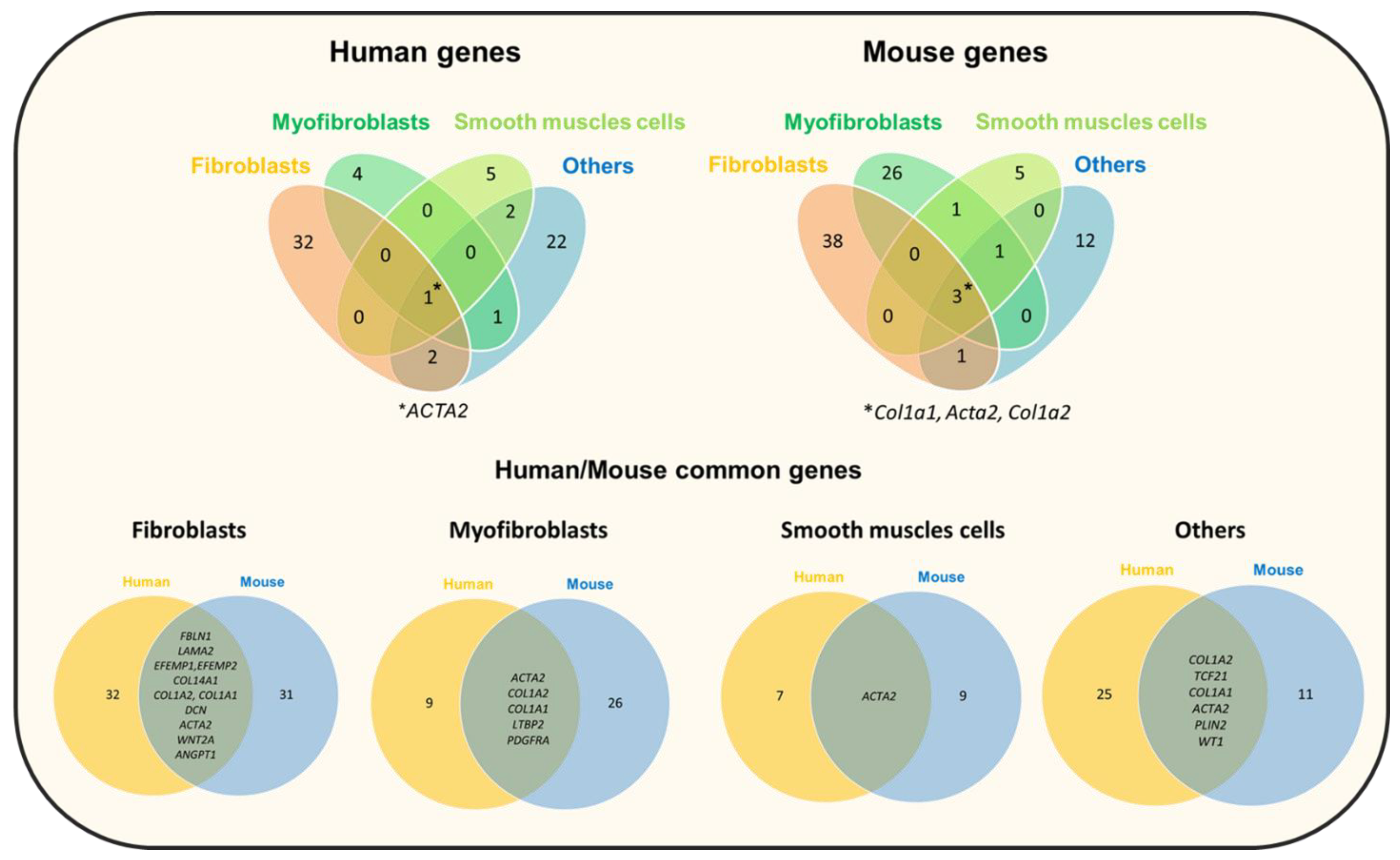
- Travaglini, K.J.; Nabhan, A.N.; Penland, L.; Sinha, R.; Gillich, A.; Sit, R.V.; Chang, S.; Conley, S.D.; Mori, Y.; Seita, J.; et al. A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature 2020, 587, 619–625. [Google Scholar] [CrossRef]
- Negretti, N.M.; Plosa, E.J.; Benjamin, J.T.; Schuler, B.A.; Christian, A.; Jetter, C.; Gulleman, P.; Taylor, C.J.; Nichols, D.; Matlock, K.; et al. A Single Cell Atlas of Lung Development. bioRxiv 2021. [Google Scholar] [CrossRef]
- Slavkin, H.C.; Snead, M.L.; Zeichner-David, M.; Jaskoll, T.F.; Smith, B.T. Concepts of epithelial-mesenchymal interactions during development: Tooth and lung organogenesis. J. Cell. Biochem. 1984, 26, 117–125. [Google Scholar] [CrossRef]
- Agha, E.E.; Herold, S.; Alam, D.A.; Quantius, J.; MacKenzie, B.; Carraro, G.; Moiseenko, A.; Chao, C.-M.; Minoo, P.; Seeger, W.; et al. Fgf10-positive cells represent a progenitor cell population during lung development and postnatally. Development 2014, 141, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Habermann, A.C.; Gutierrez, A.J.; Bui, L.T.; Yahn, S.L.; Winters, N.I.; Calvi, C.L.; Peter, L.; Chung, M.-I.; Taylor, C.J.; Jetter, C.; et al. Single-Cell RNA Sequencing Reveals Profibrotic Roles of Distinct Epithelial and Mesenchymal Lineages in Pulmonary Fibrosis. Sci. Adv. 2020, 6, eaba1972. [Google Scholar] [CrossRef] [PubMed]
- Reyfman, P.A.; Walter, J.M.; Joshi, N.; Anekalla, K.R.; McQuattie-Pimentel, A.C.; Chiu, S.; Fernandez, R.; Akbarpour, M.; Chen, C.-I.; Ren, Z.; et al. Single-Cell Transcriptomic Analysis of Human Lung Provides Insights into the Pathobiology of Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2019, 199, 1517–1536. [Google Scholar] [CrossRef]
- Hein, R.F.C.; Wu, J.H.; Tsai, Y.-H.; Wu, A.; Miller, A.J.; Holloway, E.M.; Frum, T.; Conchola, A.S.; Szenker-Ravi, E.; Reversade, B.; et al. R-SPONDIN2+ Mesenchymal Cells Form the Bud Tip Progenitor Niche During Human Lung Development. bioRxiv 2021. [Google Scholar] [CrossRef]
- Guo, M.; Du, Y.; Gokey, J.J.; Ray, S.; Bell, S.M.; Adam, M.; Sudha, P.; Perl, A.K.; Deshmukh, H.; Potter, S.S.; et al. Single Cell RNA Analysis Identifies Cellular Heterogeneity and Adaptive Responses of the Lung at Birth. Nat. Commun. 2019, 10, 37. [Google Scholar] [CrossRef]
- Strunz, M.; Simon, L.M.; Ansari, M.; Kathiriya, J.J.; Angelidis, I.; Mayr, C.H.; Tsidiridis, G.; Lange, M.; Mattner, L.F.; Yee, M.; et al. Alveolar regeneration through a Krt8+ transitional stem cell state that persists in human lung fibrosis. Nat. Commun. 2020, 11, 3559. [Google Scholar] [CrossRef]
- Angelidis, I. An Atlas of the Aging Lung Mapped by Single Cell Transcriptomics and Deep Tissue Proteomics. Nat. Commun. 2019, 10, 963. [Google Scholar] [CrossRef]
- Solnica-Krezel, L.; Sepich, D.S. Gastrulation: Making and Shaping Germ Layers. Annu. Rev. Cell Dev. Biol. 2012, 28, 687–717. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.; Nieto, M.A. Epithelial-Mesenchymal Transitions in Development and Disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Douarin, N.M.L.; Creuzet, S.; Couly, G.; Dupin, E. Neural crest cell plasticity and its limits. Development 2004, 131, 4637–4650. [Google Scholar] [CrossRef] [PubMed]
- Takashima, Y.; Era, T.; Nakao, K.; Kondo, S.; Kasuga, M.; Smith, A.G.; Nishikawa, S.-I. Neuroepithelial Cells Supply an Initial Transient Wave of MSC Differentiation. Cell 2007, 129, 1377–1388. [Google Scholar] [CrossRef]
- Que, J.; Wilm, B.; Hasegawa, H.; Wang, F.; Bader, D.; Hogan, B.L.M. Mesothelium contributes to vascular smooth muscle and mesenchyme during lung development. Proc. Natl. Acad. Sci. USA 2008, 105, 16626–16630. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Wang, F.; Ornitz, D.M. Mesothelial- and epithelial-derived FGF9 have distinct functions in the regulation of lung development. Development 2011, 138, 3169–3177. [Google Scholar] [CrossRef]
- Batra, H.; Antony, V.B. The pleural mesothelium in development and disease. Front. Physiol. 2014, 5, 284. [Google Scholar] [CrossRef]
- Schittny, J.C. Development of the lung. Cell Tissue Res. 2017, 367, 427–444. [Google Scholar] [CrossRef]
- Lange, P.; Celli, B.; Agustí, A.; Boje Jensen, G.; Divo, M.; Faner, R.; Guerra, S.; Marott, J.L.; Martinez, F.D.; Martinez-Camblor, P.; et al. Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2015, 373, 111–122. [Google Scholar] [CrossRef]
- Nogawa, H.; Ito, T. Branching Morphogenesis of Embryonic Mouse Lung Epithelium in Mesenchyme-Free Culture. Development 1995, 121, 1015–1022. [Google Scholar] [CrossRef]
- Warburton, D.; Seth, R.; Shum, L.; Horcher, P.G.; Hall, F.L.; Werb, Z.; Slavkin, H.C. Epigenetic role of epidermal growth factor expression and signalling in embryonic mouse lung morphogenesis. Dev. Biol. 1992, 149, 123–133. [Google Scholar] [CrossRef]
- Warburton, D.; El-Hashash, A.; Carraro, G.; Tiozzo, C.; Sala, F.; Rogers, O.; De Langhe, S.; Kemp, P.J.; Riccardi, D.; Torday, J.; et al. Lung Organogenesis. Curr. Top. Dev. Biol. 2010, 90, 73–158. [Google Scholar] [CrossRef]
- Koopmans, T.; Rinkevich, Y. Mesothelial to mesenchyme transition as a major developmental and pathological player in trunk organs and their cavities. Commun. Biol. 2018, 1, 1–14. [Google Scholar] [CrossRef]
- Ariza, L.; Carmona, R.; Cañete, A.; Cano, E.; Muñoz-Chápuli, R. Coelomic epithelium-derived cells in visceral morphogenesis. Dev. Dyn. 2016, 245, 307–322. [Google Scholar] [CrossRef] [PubMed]
- White, A.C.; Xu, J.; Yin, Y.; Smith, C.; Schmid, G.; Ornitz, D.M. FGF9 and SHH signaling coordinate lung growth and development through regulation of distinct mesenchymal domains. Development 2006, 133, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Ornitz, D.M. FGF9 and FGF10 activate distinct signaling pathways to direct lung epithelial specification and branching. Sci. Signal. 2020, 13, 621. [Google Scholar] [CrossRef]
- Nakamura, K.T.; McCray, P.B. Fetal airway smooth-muscle contractility and lung development. A player in the band or just someone in the audience? Am. J. Respir. Cell Mol. Biol. 2000, 23, 3–6. [Google Scholar] [CrossRef]
- Fayon, M.; Andrieux, A.; Bara, I.; Rebola, M.; Labbé, A.; Marthan, R.; Berger, P. An Age-Wise Comparison of Human Airway Smooth Muscle Proliferative Capacity. PLoS ONE 2015, 10, e0122446. [Google Scholar] [CrossRef]
- Bokka, K.K.; Jesudason, E.C.; Lozoya, O.A.; Guilak, F.; Warburton, D.; Lubkin, S.R. Morphogenetic Implications of Peristalsis-Driven Fluid Flow in the Embryonic Lung. PLoS ONE 2015, 10, e0132015. [Google Scholar] [CrossRef]
- Nelson, C.M.; Gleghorn, J.P.; Pang, M.-F.; Jaslove, J.M.; Goodwin, K.; Varner, V.D.; Miller, E.; Radisky, D.C.; Stone, H.A. Microfluidic chest cavities reveal that transmural pressure controls the rate of lung development. Development 2017, 144, 4328–4335. [Google Scholar] [CrossRef] [PubMed]
- Ornitz, D.M.; Itoh, N. Fibroblast Growth Factors. Genome Biol. 2001, 2, 3005.1–3005.12. [Google Scholar] [CrossRef]
- Shannon, J.M.; Hyatt, B.A. Epithelial-Mesenchymal Interactions in the Developing Lung. Annu. Rev. Physiol. 2004, 66, 625–645. [Google Scholar] [CrossRef]
- Bellusci, S.; Grindley, J.; Emoto, H.; Itoh, N.; Hogan, B.L. Fibroblast Growth Factor 10 (FGF10) and Branching Morphogenesis in the Embryonic Mouse Lung. Development 1997, 124, 4867–4878. [Google Scholar] [CrossRef]
- Park, W.Y.; Miranda, B.; Lebeche, D.; Hashimoto, G.; Cardoso, W.V. FGF-10 Is a Chemotactic Factor for Distal Epithelial Buds during Lung Development. Dev. Biol. 1998, 201, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.; Izvolsky, K.I.; Qian, J.; Cardoso, W.V. Identification of FGF10 Targets in the Embryonic Lung Epithelium during Bud Morphogenesis. J. Biol. Chem. 2005, 280, 4834–4841. [Google Scholar] [CrossRef]
- Sekine, K.; Ohuchi, H.; Fujiwara, M.; Yamasaki, M.; Yoshizawa, T.; Sato, T.; Yagishita, N.; Matsui, D.; Koga, Y.; Itoh, N.; et al. Fgf10 is essential for limb and lung formation. Nat. Genet. 1999, 21, 138–141. [Google Scholar] [CrossRef]
- Min, H.; Danilenko, D.M.; Scully, S.A.; Bolon, B.; Ring, B.D.; Tarpley, J.E.; DeRose, M.; Simonet, W.S. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 1998, 12, 3156–3161. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, S.; Heiner, M.; Vazquez-Armendariz, A.I.; Wilhelm, J.; Herold, S.; Chen, C.; Zhang, J.S.; Bellusci, S. Characterization in mice of the resident mesenchymal niche maintaining AT2 stem cell proliferation in homeostasis and disease. Stem Cells 2021, 39, 1382–1394. [Google Scholar] [CrossRef] [PubMed]
- Colvin, J.S.; Green, R.P.; Schmahl, J.; Capel, B.; Ornitz, D.M. Male-to-Female Sex Reversal in Mice Lacking Fibroblast Growth Factor 9. Cell 2001, 104, 875–889. [Google Scholar] [CrossRef]
- Winnier, G.; Blessing, M.; Labosky, P.A.; Hogan, B.L. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995, 9, 2105–2116. [Google Scholar] [CrossRef]
- Weaver, M.; Dunn, N.R.; Hogan, B.L. Bmp4 and Fgf10 Play Opposing Roles during Lung Bud Morphogenesis. Development 2000, 127, 2695–2704. [Google Scholar] [CrossRef]
- Weaver, M.; Batts, L.; Hogan, B.L.M. Tissue interactions pattern the mesenchyme of the embryonic mouse lung. Dev. Biol. 2003, 258, 169–184. [Google Scholar] [CrossRef]
- Bellusci, S.; Henderson, R.; Winnier, G.; Oikawa, T.; Hogan, B.L. Evidence from Normal Expression and Targeted Misexpression That Bone Morphogenetic Protein (Bmp-4) Plays a Role in Mouse Embryonic Lung Morphogenesis. Development 1996, 122, 1693–1702. [Google Scholar] [CrossRef]
- Herriges, M.; Morrisey, E.E. Lung development: Orchestrating the generation and regeneration of a complex organ. Development 2014, 141, 502–513. [Google Scholar] [CrossRef]
- Eblaghie, M.C.; Reedy, M.; Oliver, T.; Mishina, Y.; Hogan, B.L.M. Evidence that autocrine signaling through Bmpr1a regulates the proliferation, survival and morphogenetic behavior of distal lung epithelial cells. Dev. Biol. 2006, 291, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhao, J.; Anderson, K.D.; Warburton, D. Gremlin negatively modulates BMP-4 induction of embryonic mouse lung branching morphogenesis. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2001, 280, L1030–L1039. [Google Scholar] [CrossRef] [PubMed]
- Hirashima, T.; Iwasa, Y.; Morishita, Y. Mechanisms for split localization of Fgf10 expression in early lung development. Dev. Dyn. 2009, 238, 2813–2822. [Google Scholar] [CrossRef]
- Menshykau, D.; Kraemer, C.; Iber, D. Branch Mode Selection during Early Lung Development. PLoS Comput. Biol. 2012, 8, e1002377. [Google Scholar] [CrossRef]
- Carballo, G.B.; Honorato, J.R.; de Lopes, G.P.F.; de Sampaio e Spohr, T.C.L. A highlight on Sonic hedgehog pathway. Cell Commun. Signal. 2018, 16, 11. [Google Scholar] [CrossRef]
- Litingtung, Y.; Lei, L.; Westphal, H.; Chiang, C. Sonic hedgehog is essential to foregut development. Nat. Genet. 1998, 20, 58–61. [Google Scholar] [CrossRef]
- Cardoso, W.V.; Lü, J. Regulation of early lung morphogenesis: Questions, facts and controversies. Development 2006, 133, 1611–1624. [Google Scholar] [CrossRef]
- Pepicelli, C.V.; Lewis, P.M.; McMahon, A.P. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr. Biol. 1998, 8, 1083–1086. [Google Scholar] [CrossRef]
- Peng, T.; Tian, Y.; Boogerd, C.J.; Lu, M.M.; Kadzik, R.S.; Stewart, K.M.; Evans, S.M.; Morrisey, E.E. Coordination of heart and lung co-development by a multipotent cardiopulmonary progenitor. Nature 2013, 500, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, P.J.; Berger, J.E.; Meneses, J.; Phung, Y.; Pedersen, R.A.; Werb, Z.; Derynck, R. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature 1995, 376, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, P.J.; Warburton, D.; Bu, D.; Zhao, J.-S.; Berger, J.E.; Minoo, P.; Koivisto, T.; Allen, L.; Dobbs, L.; Werb, Z.; et al. Impaired Lung Branching Morphogenesis in the Absence of Functional EGF Receptor. Dev. Biol. 1997, 186, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Seth, R.; Shum, L.; Wu, F.; Wuenschell, C.; Hall, F.L.; Slavkin, H.C.; Warburton, D. Role of Epidermal Growth Factor Expression in Early Mouse Embryo Lung Branching Morphogenesis in Culture: Antisense Oligodeoxynucleotide Inhibitory Strategy. Dev. Biol. 1993, 158, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Schuger, L.; Varani, J.; Mitra, R.; Gilbride, K. Retinoic acid stimulates mouse lung development by a mechanism involving epithelial-mesenchymal interaction and regulation of epidermal growth factor receptors. Dev. Biol. 1993, 159, 462–473. [Google Scholar] [CrossRef]
- Mark, M.; Ghyselinck, N.B.; Chambon, P. Function of retinoid nuclear receptors: Lessons from Genetic and Pharmacological Dissections of the Retinoic Acid Signaling Pathway During Mouse Embryogenesis. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 451–480. [Google Scholar] [CrossRef]
- Mendelsohn, C.; Lohnes, D.; Décimo, D.; Lufkin, T.; LeMeur, M.; Chambon, P.; Mark, M. Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development 1994, 120, 2749–2771. [Google Scholar] [CrossRef]
- Fernandes-Silva, H.; Araújo-Silva, H.; Correia-Pinto, J.; Moura, R.S. Retinoic Acid: A Key Regulator of Lung Development. Biomolecules 2020, 10, 152. [Google Scholar] [CrossRef]
- Chen, F.; Desai, T.J.; Qian, J.; Niederreither, K.; Lü, J.; Cardoso, W.V. Inhibition of Tgfβ signaling by endogenous retinoic acid is essential for primary lung bud induction. Development 2007, 134, 2969–2979. [Google Scholar] [CrossRef]
- Kahata, K.; Dadras, M.S.; Moustakas, A. TGF-β Family Signaling in Epithelial Differentiation and Epithelial–Mesenchymal Transition. Cold Spring Harb. Perspect. Biol. 2018, 10, a022194. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Horie, M.; Nagase, T. TGF-β Signaling in Lung Health and Disease. Int. J. Mol. Sci. 2018, 19, 2460. [Google Scholar] [CrossRef] [PubMed]
- Bragg, A.D.; Moses, H.L.; Serra, R. Signaling to the epithelium is not sufficient to mediate all of the effects of transforming growth factor β and bone morphogenetic protein 4 on murine embryonic lung development. Mech. Dev. 2001, 109, 13–26. [Google Scholar] [CrossRef]
- Warburton, D.; Bellusci, S.; De Langhe, S.; Del Moral, P.-M.; Fleury, V.; Mailleux, A.; Tefft, D.; Unbekandt, M.; Wang, K.; Shi, W. Molecular Mechanisms of Early Lung Specification and Branching Morphogenesis. Pediatr. Res. 2005, 57, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Kaartinen, V.; Voncken, J.W.; Shuler, C.; Warburton, D.; Bu, D.; Heisterkamp, N.; Groffen, J. Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nat. Genet. 1995, 11, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhuang, F.; Liu, Y.-H.; Xu, B.; del Moral, P.; Deng, W.; Chai, Y.; Kolb, M.; Gauldie, J.; Warburton, D.; et al. TGF-β receptor II in epithelia versus mesenchyme plays distinct roles in the developing lung. Eur. Respir. J. 2008, 32, 285–295. [Google Scholar] [CrossRef]
- Xing, Y.; Li, C.; Hu, L.; Tiozzo, C.; Li, M.; Chai, Y.; Bellusci, S.; Anderson, S.; Minoo, P. Mechanisms of TGFβ Inhibition of Lung Endodermal Morphogenesis: The role of TβRII, Smads, Nkx2.1 and Pten. Dev. Biol. 2008, 320, 340–350. [Google Scholar] [CrossRef]
- Edgar, B.A. From Cell Structure to Transcription: Hippo Forges a New Path. Cell 2006, 124, 267–273. [Google Scholar] [CrossRef]
- Meng, Z.; Moroishi, T.; Guan, K.-L. Mechanisms of Hippo pathway regulation. Genes Dev. 2016, 30, 1–17. [Google Scholar] [CrossRef]
- Makita, R.; Uchijima, Y.; Nishiyama, K.; Amano, T.; Chen, Q.; Takeuchi, T.; Mitani, A.; Nagase, T.; Yatomi, Y.; Aburatani, H.; et al. Multiple renal cysts, urinary concentration defects, and pulmonary emphysematous changes in mice lacking TAZ. Am. J. Physiol. Ren. Physiol. 2008, 294, F542–F553. [Google Scholar] [CrossRef] [PubMed]
- Isago, H.; Mitani, A.; Mikami, Y.; Horie, M.; Urushiyama, H.; Hamamoto, R.; Terasaki, Y.; Nagase, T. Epithelial Expression of YAP and TAZ Is Sequentially Required in Lung Development. Am. J. Respir. Cell Mol. Biol. 2020, 62, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Little, D.R.; Lynch, A.M.; Yan, Y.; Akiyama, H.; Kimura, S.; Chen, J. Differential chromatin binding of the lung lineage transcription factor NKX2-1 resolves opposing murine alveolar cell fates in vivo. Nat. Commun. 2021, 12, 2509. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, J.E.; Mori, M.; Szymaniak, A.D.; Varelas, X.; Cardoso, W.V. The Hippo Pathway Effector Yap Controls Patterning and Differentiation of Airway Epithelial Progenitors. Dev. Cell 2014, 30, 137–150. [Google Scholar] [CrossRef]
- Zhao, R.; Fallon, T.R.; Saladi, S.V.; Pardo-Saganta, A.; Villoria, J.; Mou, H.; Vinarsky, V.; Gonzalez-Celeiro, M.; Nunna, N.; Hariri, L.P.; et al. Yap Tunes Airway Epithelial Size and Architecture by Regulating the Identity, Maintenance, and Self-renewal of Stem Cells. Dev. Cell 2014, 30, 151–165. [Google Scholar] [CrossRef]
- Volckaert, T.; Yuan, T.; Chao, C.-M.; Bell, H.; Sitaula, A.; Szimmtenings, L.; El Agha, E.; Chanda, D.; Majka, S.; Bellusci, S.; et al. Fgf10-Hippo epithelial mesenchymal crosstalk maintains and recruits lung basal stem cells. Dev. Cell 2017, 43, 48–59.e5. [Google Scholar] [CrossRef]
- McGowan, S.E. Extracellular matrix and the regulation of lung development and repair1. FASEB J. 1992, 6, 2895–2904. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Guzmán, C.; San Martin, S.; Pereda, J. Proteoglycan and collagen expression during human air conducting system development. Eur. J. Histochem. 2012, 56, e29. [Google Scholar] [CrossRef]
- Zhao, J.; Sime, P.J.; Bringas, P.; Gauldie, J.; Warburton, D. Adenovirus-mediated decorin gene transfer prevents TGF-β-induced inhibition of lung morphogenesis. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1999, 277, L412–L422. [Google Scholar] [CrossRef] [PubMed]
- Izvolsky, K.I.; Shoykhet, D.; Yang, Y.; Yu, Q.; Nugent, M.A.; Cardoso, W.V. Heparan sulfate–FGF10 interactions during lung morphogenesis. Dev. Biol. 2003, 258, 185–200. [Google Scholar] [CrossRef]
- Kotton, D.N.; Morrisey, E.E. Lung regeneration: Mechanisms, applications and emerging stem cell populations. Nat. Med. 2014, 20, 822–832. [Google Scholar] [CrossRef]
- Rock, J.R.; Onaitis, M.W.; Rawlins, E.L.; Lu, Y.; Clark, C.P.; Xue, Y.; Randell, S.H.; Hogan, B.L.M. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. USA 2009, 106, 12771–12775. [Google Scholar] [CrossRef] [PubMed]
- Rawlins, E.L.; Okubo, T.; Xue, Y.; Brass, D.M.; Auten, R.L.; Hasegawa, H.; Wang, F.; Hogan, B.L.M. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 2009, 4, 525–534. [Google Scholar] [CrossRef]
- Tata, P.R.; Mou, H.; Pardo-Saganta, A.; Zhao, R.; Prabhu, M.; Prabhu, M.; Law, B.M.; Vinarsky, V.; Cho, J.L.; Breton, S.; et al. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature 2013, 503, 218–223. [Google Scholar] [CrossRef]
- Lafkas, D.; Shelton, A.; Chiu, C.; de Leon Boenig, G.; Chen, Y.; Stawicki, S.S.; Siltanen, C.; Reichelt, M.; Zhou, M.; Wu, X.; et al. Therapeutic antibodies reveal Notch control of transdifferentiation in the adult lung. Nature 2015, 528, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Frank, D.B.; Kadzik, R.S.; Morley, M.P.; Rathi, K.S.; Wang, T.; Zhou, S.; Cheng, L.; Lu, M.M.; Morrisey, E.E. Hedgehog actively maintains adult lung quiescence and regulates repair and regeneration. Nature 2015, 526, 578–582. [Google Scholar] [CrossRef]
- Fang, S.; Zhang, S.; Dai, H.; Hu, X.; Li, C.; Xing, Y. The role of pulmonary mesenchymal cells in airway epithelium regeneration during injury repair. Stem Cell Res. Ther. 2019, 10, 366. [Google Scholar] [CrossRef]
- Zepp, J.A.; Zacharias, W.J.; Frank, D.B.; Cavanaugh, C.A.; Zhou, S.; Morley, M.P.; Morrisey, E.E. Distinct Mesenchymal Lineages and Niches Promote Epithelial Self-Renewal and Myofibrogenesis in the Lung. Cell 2017, 170, 1134–1148.e10. [Google Scholar] [CrossRef]
- Barnes, J.L.; Gorin, Y. Myofibroblast differentiation during fibrosis: Role of NAD(P)H oxidases. Kidney Int. 2011, 79, 944–956. [Google Scholar] [CrossRef] [PubMed]
- El Agha, E.; Kramann, R.; Schneider, R.K.; Li, X.; Seeger, W.; Humphreys, B.D.; Bellusci, S. Mesenchymal Stem Cells in Fibrotic Disease. Cell Stem Cell 2017, 21, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Buechler, M.B.; Pradhan, R.N.; Krishnamurty, A.T.; Cox, C.; Calviello, A.K.; Wang, A.W.; Yang, Y.A.; Tam, L.; Caothien, R.; Roose-Girma, M.; et al. Cross-tissue organization of the fibroblast lineage. Nature 2021, 593, 575–579. [Google Scholar] [CrossRef]
- Yu, K.; Xu, J.; Liu, Z.; Sosic, D.; Shao, J.; Olson, E.N.; Towler, D.A.; Ornitz, D.M. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development 2003, 130, 3063–3074. [Google Scholar] [CrossRef]
- Harrell, C.R.; Jankovic, M.G.; Fellabaum, C.; Volarevic, A.; Djonov, V.; Arsenijevic, A.; Volarevic, V. Molecular mechanisms responsible for anti-inflammatory and immunosuppressive effects of mesenchymal stem cell-derived factors. In Tissue Engineering and Regenerative Medicine; Pham, P.V., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 187–206. [Google Scholar]
- Wolters, P.J.; Collard, H.R.; Jones, K.D. Pathogenesis of Idiopathic Pulmonary Fibrosis. Annu. Rev. Pathol. 2014, 9, 157–179. [Google Scholar] [CrossRef] [PubMed]
- Gohy, S.T.; Hupin, C.; Pilette, C.; Ladjemi, M.Z. Chronic inflammatory airway diseases: The central role of the epithelium revisited. Clin. Exp. Allergy 2016, 46, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.F.; Watz, H. Chronic obstructive pulmonary disease. Lancet 2017, 389, 1931–1940. [Google Scholar] [CrossRef]
- Barnes, P.J. Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2000, 343, 1969–1971. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Burney, P.G.J.; Silverman, E.K.; Celli, B.R.; Vestbo, J.; Wedzicha, J.A.; Wouters, E.F.M. Chronic obstructive pulmonary disease. Nat. Rev. Dis. Primers 2015, 1, 15076. [Google Scholar] [CrossRef] [PubMed]
- Celli, B.R.; Wedzicha, J.A. Update on Clinical Aspects of Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2019, 381, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Perez-Padilla, R.; Thirion-Romero, I.; Guzman, N. Underdiagnosis of chronic obstructive pulmonary disease: Should smokers be offered routine spirometry tests? Expert Rev. Respir. Med. 2018, 12, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.F.; Hurst, J.R.; Suissa, S. Cardiovascular disease and COPD: Dangerous liaisons? Eur. Respir. Rev. 2018, 27, 180057. [Google Scholar] [CrossRef]
- Lee, A.L.; Goldstein, R.S. Gastroesophageal reflux disease in COPD: Links and risks. Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 1935–1949. [Google Scholar] [CrossRef]
- Lahousse, L.; Tiemeier, H.; Ikram, M.A.; Brusselle, G.G. Chronic obstructive pulmonary disease and cerebrovascular disease: A comprehensive review. Respir. Med. 2015, 109, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Gea, J.; Sancho-Muñoz, A.; Chalela, R. Nutritional status and muscle dysfunction in chronic respiratory diseases: Stable phase versus acute exacerbations. J. Thorac. Dis. 2018, 10, S1332–S1354. [Google Scholar] [CrossRef]
- Jaitovich, A.; Barreiro, E. Skeletal Muscle Dysfunction in Chronic Obstructive Pulmonary Disease. What We Know and Can Do for Our Patients. Am. J. Respir. Crit. Care Med. 2018, 198, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.C.; Wrobel, J.P. Epidemiology and clinical impact of major comorbidities in patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 871–888. [Google Scholar] [CrossRef]
- Anderson, D.O. Smoking And Respiratory Disease. Am. J. Public Health Nations Health 1964, 54, 1856–1863. [Google Scholar] [CrossRef]
- Fletcher, C.; Peto, R. The natural history of chronic airflow obstruction. Br. Med. J. 1977, 1, 1645–1648. [Google Scholar] [CrossRef]
- Laniado-Laborín, R. Smoking and Chronic Obstructive Pulmonary Disease (COPD). Parallel Epidemics of the 21st Century. Int. J. Environ. Res. Public Health 2009, 6, 209–224. [Google Scholar] [CrossRef]
- Fullerton, D.G.; Bruce, N.; Gordon, S.B. Indoor air pollution from biomass fuel smoke is a major health concern in the developing world. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Salvi, S.S.; Barnes, P.J. Chronic obstructive pulmonary disease in non-smokers. Lancet 2009, 374, 733–743. [Google Scholar] [CrossRef]
- Jiang, X.-Q.; Mei, X.-D.; Feng, D. Air pollution and chronic airway diseases: What should people know and do? J. Thorac. Dis. 2016, 8, E31–E40. [Google Scholar] [CrossRef]
- Cohen, R.; Patel, A.; Green, F. Lung Disease Caused by Exposure to Coal Mine and Silica Dust. Semin. Respir. Crit. Care Med. 2008, 29, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.D. Early-Life Origins of Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2016, 375, 871–878. [Google Scholar] [CrossRef]
- Wilk, J.B.; Chen, T.-H.; Gottlieb, D.J.; Walter, R.E.; Nagle, M.W.; Brandler, B.J.; Myers, R.H.; Borecki, I.B.; Silverman, E.K.; Weiss, S.T.; et al. A genome-wide association study of pulmonary function measures in the Framingham Heart Study. PLoS Genet. 2009, 5, e1000429. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.G.; Ge, D.; Zhu, G.; Kong, X.; Shianna, K.V.; Need, A.C.; Feng, S.; Hersh, C.P.; Bakke, P.; Gulsvik, A.; et al. A genome-wide association study in chronic obstructive pulmonary disease (COPD): Identification of two major susceptibility loci. PLoS Genet. 2009, 5, e1000421. [Google Scholar] [CrossRef] [PubMed]
- Repapi, E.; Sayers, I.; Wain, L.V.; Burton, P.R.; Johnson, T.; Obeidat, M.; Zhao, J.H.; Ramasamy, A.; Zhai, G.; Vitart, V.; et al. Genome-wide association study identifies five loci associated with lung function. Nat. Genet. 2010, 42, 36–44. [Google Scholar] [CrossRef]
- Soler Artigas, M.; Loth, D.W.; Wain, L.V.; Gharib, S.A.; Obeidat, M.; Tang, W.; Zhai, G.; Zhao, J.H.; Smith, A.V.; Huffman, J.E.; et al. Genome-wide association and large-scale follow up identifies 16 new loci influencing lung function. Nat. Genet. 2011, 43, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Chuang, P.-T.; McMahon, A.P. Vertebrate Hedgehog signalling modulated by induction of a Hedgehog-binding protein. Nature 1999, 397, 617–621. [Google Scholar] [CrossRef]
- Smith, B.M.; Traboulsi, H.; Austin, J.H.M.; Manichaikul, A.; Hoffman, E.A.; Bleecker, E.R.; Cardoso, W.V.; Cooper, C.; Couper, D.J.; Dashnaw, S.M.; et al. Human airway branch variation and chronic obstructive pulmonary disease. Proc. Natl. Acad. Sci. USA. 2018, 115, E974–E981. [Google Scholar] [CrossRef]
- Agusti, A.; Faner, R. Lung function trajectories in health and disease. Lancet Respir. Med. 2019, 7, 358–364. [Google Scholar] [CrossRef]
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef]
- Shaykhiev, R.; Crystal, R.G. Early Events in the Pathogenesis of Chronic Obstructive Pulmonary Disease. Smoking-induced Reprogramming of Airway Epithelial Basal Progenitor Cells. Ann. Am. Thorac. Soc. 2014, 11, S252–S258. [Google Scholar] [CrossRef]
- Baraldo, S.; Turato, G.; Badin, C.; Bazzan, E.; Beghé, B.; Zuin, R.; Calabrese, F.; Casoni, G.; Maestrelli, P.; Papi, A.; et al. Neutrophilic infiltration within the airway smooth muscle in patients with COPD. Thorax 2004, 59, 308–312. [Google Scholar] [CrossRef]
- Leopold, P.L.; O’Mahony, M.J.; Lian, X.J.; Tilley, A.E.; Harvey, B.-G.; Crystal, R.G. Smoking Is Associated with Shortened Airway Cilia. PLoS ONE 2009, 4, e8157. [Google Scholar] [CrossRef]
- Mahmood, M.Q.; Sohal, S.S.; Shukla, S.D.; Ward, C.; Hardikar, A.; Noor, W.D.; Muller, H.K.; Knight, D.A.; Walters, E.H. Epithelial mesenchymal transition in smokers: Large versus small airways and relation to airflow obstruction. Int. J. Chronic Obs. Pulm. Dis. 2015, 10, 1515–1524. [Google Scholar] [CrossRef]
- Higham, A.; Quinn, A.M.; Cançado, J.E.D.; Singh, D. The pathology of small airways disease in COPD: Historical aspects and future directions. Respir. Res. 2019, 20, 1–11. [Google Scholar] [CrossRef]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef]
- Osei, E.T.; Hackett, T.-L. Epithelial-mesenchymal crosstalk in COPD: An update from in vitro model studies. Int. J. Biochem. Cell Biol. 2020, 125, 105775. [Google Scholar] [CrossRef]
- Hogg, J.C. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet 2004, 364, 709–721. [Google Scholar] [CrossRef]
- McDonough, J.E.; Yuan, R.; Suzuki, M.; Seyednejad, N.; Elliott, W.M.; Sanchez, P.G.; Wright, A.C.; Gefter, W.B.; Litzky, L.; Coxson, H.O.; et al. Small-Airway Obstruction and Emphysema in Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2011, 365, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Small airway fibrosis in COPD. Int. J. Biochem. Cell Biol. 2019, 116, 105598. [Google Scholar] [CrossRef] [PubMed]
- Araya, J.; Cambier, S.; Markovics, J.A.; Wolters, P.; Jablons, D.; Hill, A.; Finkbeiner, W.; Jones, K.; Broaddus, V.C.; Sheppard, D.; et al. Squamous metaplasia amplifies pathologic epithelial-mesenchymal interactions in COPD patients. J. Clin. Investig. 2007, 117, 3551–3562. [Google Scholar] [CrossRef] [PubMed]
- de Boer, W.I.; van Schadewijk, A.; Sont, J.K.; Sharma, H.S.; Stolk, J.; Hiemstra, P.S.; van Krieken, J.H. Transforming growth factor beta1 and recruitment of macrophages and mast cells in airways in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1998, 158, 1951–1957. [Google Scholar] [CrossRef] [PubMed]
- Gohy, S.T.; Hupin, C.; Fregimilicka, C.; Detry, B.R.; Bouzin, C.; Gaide Chevronay, H.; Lecocq, M.; Weynand, B.; Ladjemi, M.Z.; Pierreux, C.E.; et al. Imprinting of the COPD airway epithelium for dedifferentiation and mesenchymal transition. Eur. Respir. J. 2015, 45, 1258–1272. [Google Scholar] [CrossRef]
- Eapen, M.S.; Lu, W.; Hackett, T.L.; Singhera, G.K.; Mahmood, M.Q.; Hardikar, A.; Ward, C.; Walters, E.H.; Sohal, S.S. Increased myofibroblasts in the small airways, and relationship to remodelling and functional changes in smokers and COPD patients: Potential role of epithelial-mesenchymal transition. ERJ Open Res. 2021, 7, 00876-2020. [Google Scholar] [CrossRef]
- Karakioulaki, M.; Papakonstantinou, E.; Stolz, D. Extracellular matrix remodelling in COPD. Eur. Respir. Rev. 2020, 29, 190124. [Google Scholar] [CrossRef]
- Annoni, R.; Lanças, T.; Tanigawa, R.Y.; Matsushita, M.D.M.; Fernezlian, S.D.M.; Bruno, A.; da Silva, L.F.F.; Roughley, P.J.; Battaglia, S.; Dolhnikoff, M.; et al. Extracellular matrix composition in COPD. Eur. Respir. J. 2012, 40, 1362–1373. [Google Scholar] [CrossRef]
- Eurlings, I.M.; Dentener, M.A.; Cleutjens, J.P.; Peutz, C.J.; Rohde, G.G.; Wouters, E.F.; Reynaert, N.L. Similar matrix alterations in alveolar and small airway walls of COPD patients. BMC Pulm. Med. 2014, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Bidan, C.M.; Veldsink, A.C.; Meurs, H.; Gosens, R. Airway and Extracellular Matrix Mechanics in COPD. Front. Physiol. 2015, 6, 346. [Google Scholar] [CrossRef]
- Hedström, U.; Hallgren, O.; Öberg, L.; DeMicco, A.; Vaarala, O.; Westergren-Thorsson, G.; Zhou, X. Bronchial extracellular matrix from COPD patients induces altered gene expression in repopulated primary human bronchial epithelial cells. Sci. Rep. 2018, 8, 3502. [Google Scholar] [CrossRef]
- Milara, J.; Peiró, T.; Serrano, A.; Cortijo, J. Epithelial to mesenchymal transition is increased in patients with COPD and induced by cigarette smoke. Thorax 2013, 68, 410–420. [Google Scholar] [CrossRef]
- Nieto, M.A.; Cano, A. The epithelial–mesenchymal transition under control: Global programs to regulate epithelial plasticity. Semin. Cancer Biol. 2012, 22, 361–368. [Google Scholar] [CrossRef]
- Kalluri, R.; Neilson, E.G. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Investig. 2003, 112, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, M.; Neilson, E.G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Investig. 2009, 119, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Dedhar, S.; Kalluri, R.; Thompson, E.W. The epithelial–mesenchymal transition: New insights in signaling, development, and disease. J. Cell Biol. 2006, 172, 973–981. [Google Scholar] [CrossRef]
- Nieto, M.A.; Huang, R.Y.-J.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef]
- Soltani, A.; Sohal, S.S.; Reid, D.; Weston, S.; Wood-Baker, R.; Walters, E.H. Vessel-Associated Transforming Growth Factor-Beta1 (TGF-β1) Is Increased in the Bronchial Reticular Basement Membrane in COPD and Normal Smokers. PLoS ONE 2012, 7, e39736. [Google Scholar] [CrossRef][Green Version]
- Eapen, M.S.; Sharma, P.; Gaikwad, A.V.; Lu, W.; Myers, S.; Hansbro, P.M.; Sohal, S.S. Epithelial–mesenchymal transition is driven by transcriptional and post transcriptional modulations in COPD: Implications for disease progression and new therapeutics. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 1603–1610. [Google Scholar] [CrossRef]
- Baarsma, H.A.; Spanjer, A.I.R.; Haitsma, G.; Engelbertink, L.H.J.M.; Meurs, H.; Jonker, M.R.; Timens, W.; Postma, D.S.; Kerstjens, H.A.M.; Gosens, R. Activation of WNT/β-Catenin Signaling in Pulmonary Fibroblasts by TGF-β1 Is Increased in Chronic Obstructive Pulmonary Disease. PLoS ONE 2011, 6, e25450. [Google Scholar] [CrossRef]
- Sohal, S.S.; Reid, D.; Soltani, A.; Ward, C.; Weston, S.; Muller, H.K.; Wood-Baker, R.; Walters, E.H. Evaluation of epithelial mesenchymal transition in patients with chronic obstructive pulmonary disease. Respir. Res. 2011, 12, 130. [Google Scholar] [CrossRef]
- Heijink, I.H.; Brandenburg, S.M.; Postma, D.S.; van Oosterhout, A.J.M. Cigarette smoke impairs airway epithelial barrier function and cell-cell contact recovery. Eur. Respir. J. 2012, 39, 419–428. [Google Scholar] [CrossRef]
- Sohal, S.S.; Reid, D.; Soltani, A.; Ward, C.; Weston, S.; Muller, H.K.; Wood-Baker, R.; Walters, E.H. Reticular basement membrane fragmentation and potential epithelial mesenchymal transition is exaggerated in the airways of smokers with chronic obstructive pulmonary disease. Respirology 2010, 15, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.R.; Roth, M.; Tamm, M.; Hughes, M.; Ge, Q.; King, G.; Burgess, J.K.; Black, J.L. Airway smooth muscle cell proliferation is increased in asthma. Am. J. Respir. Crit. Care Med. 2001, 164, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Trian, T.; Benard, G.; Begueret, H.; Rossignol, R.; Girodet, P.-O.; Ghosh, D.; Ousova, O.; Vernejoux, J.-M.; Marthan, R.; Tunon-de-Lara, J.-M.; et al. Bronchial smooth muscle remodeling involves calcium-dependent enhanced mitochondrial biogenesis in asthma. J. Exp. Med. 2007, 204, 3173–3181. [Google Scholar] [CrossRef] [PubMed]
- Saetta, M.; Di Stefano, A.; Turato, G.; Facchini, F.M.; Corbino, L.; Mapp, C.E.; Maestrelli, P.; Ciaccia, A.; Fabbri, L.M. CD8+ T-lymphocytes in peripheral airways of smokers with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1998, 157, 822–826. [Google Scholar] [CrossRef]
- Antunes, M.A.; Abreu, S.C.; Cruz, F.F.; Teixeira, A.C.; Lopes-Pacheco, M.; Bandeira, E.; Olsen, P.C.; Diaz, B.L.; Takyia, C.M.; Freitas, I.P.; et al. Effects of different mesenchymal stromal cell sources and delivery routes in experimental emphysema. Respir. Res. 2014, 15, 1–14. [Google Scholar] [CrossRef]
- Gu, W.; Song, L.; Li, X.-M.; Wang, D.; Guo, X.-J.; Xu, W.-G. Mesenchymal stem cells alleviate airway inflammation and emphysema in COPD through down-regulation of cyclooxygenase-2 via p38 and ERK MAPK pathways. Sci. Rep. 2015, 5, 8733. [Google Scholar] [CrossRef]
- Weiss, D.J.; Casaburi, R.; Flannery, R.; LeRoux-Williams, M.; Tashkin, D.P. A Placebo-Controlled, Randomized Trial of Mesenchymal Stem Cells in COPD. Chest 2013, 143, 1590–1598. [Google Scholar] [CrossRef]
- Le Thi Bich, P.; Nguyen Thi, H.; Dang Ngo Chau, H.; Phan Van, T.; Do, Q.; Dong Khac, H.; Le Van, D.; Nguyen Huy, L.; Mai Cong, K.; Ta Ba, T.; et al. Allogeneic umbilical cord-derived mesenchymal stem cell transplantation for treating chronic obstructive pulmonary disease: A pilot clinical study. Stem Cell Res. Ther. 2020, 11, 60. [Google Scholar] [CrossRef]
- Antunes, M.A.; Lapa e Silva, J.R.; Rocco, P.R. Mesenchymal stromal cell therapy in COPD: From bench to bedside. Int. J. Chronic Obs. Pulm. Dis. 2017, 12, 3017–3027. [Google Scholar] [CrossRef]
- Broekman, W.; Khedoe, P.P.S.J.; Schepers, K.; Roelofs, H.; Stolk, J.; Hiemstra, P.S. Mesenchymal stromal cells: A novel therapy for the treatment of chronic obstructive pulmonary disease? Thorax 2018, 73, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Ikonomou, L.; Wagner, D.E.; Turner, L.; Weiss, D.J. Translating Basic Research into Safe and Effective Cell-based Treatments for Respiratory Diseases. Ann. ATS 2019, 16, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Najar, M.; Johanne, M.P.; Jean Pierre, P.; Fahmi, H. Novel Insights for Improving the Therapeutic Safety and Efficiency of Mesenchymal Stromal Cells. World J. Stem Cells 2020, 12, 1474–1491. [Google Scholar] [CrossRef] [PubMed]
- Paris, G.C.; Azevedo, A.A.; Ferreira, A.L.; Azevedo, Y.M.A.; Rainho, M.A.; Oliveira, G.P.; Silva, K.R.; Cortez, E.A.C.; Stumbo, A.C.; Carvalho, S.N.; et al. Therapeutic potential of mesenchymal stem cells in multiple organs affected by COVID-19. Life Sci. 2021, 278, 119510. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasri, A.; Foisset, F.; Ahmed, E.; Lahmar, Z.; Vachier, I.; Jorgensen, C.; Assou, S.; Bourdin, A.; De Vos, J. Roles of Mesenchymal Cells in the Lung: From Lung Development to Chronic Obstructive Pulmonary Disease. Cells 2021, 10, 3467. https://doi.org/10.3390/cells10123467
Nasri A, Foisset F, Ahmed E, Lahmar Z, Vachier I, Jorgensen C, Assou S, Bourdin A, De Vos J. Roles of Mesenchymal Cells in the Lung: From Lung Development to Chronic Obstructive Pulmonary Disease. Cells. 2021; 10(12):3467. https://doi.org/10.3390/cells10123467
Chicago/Turabian StyleNasri, Amel, Florent Foisset, Engi Ahmed, Zakaria Lahmar, Isabelle Vachier, Christian Jorgensen, Said Assou, Arnaud Bourdin, and John De Vos. 2021. "Roles of Mesenchymal Cells in the Lung: From Lung Development to Chronic Obstructive Pulmonary Disease" Cells 10, no. 12: 3467. https://doi.org/10.3390/cells10123467
APA StyleNasri, A., Foisset, F., Ahmed, E., Lahmar, Z., Vachier, I., Jorgensen, C., Assou, S., Bourdin, A., & De Vos, J. (2021). Roles of Mesenchymal Cells in the Lung: From Lung Development to Chronic Obstructive Pulmonary Disease. Cells, 10(12), 3467. https://doi.org/10.3390/cells10123467







