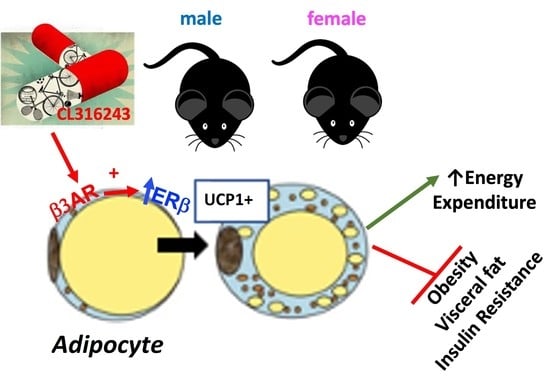
White Adipose Tissue Depots Respond to Chronic Beta-3 Adrenergic Receptor Activation in a Sexually Dimorphic and Depot Divergent Manner
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Animals and Experimental Design
2.3. Body Composition and Tissue Weights
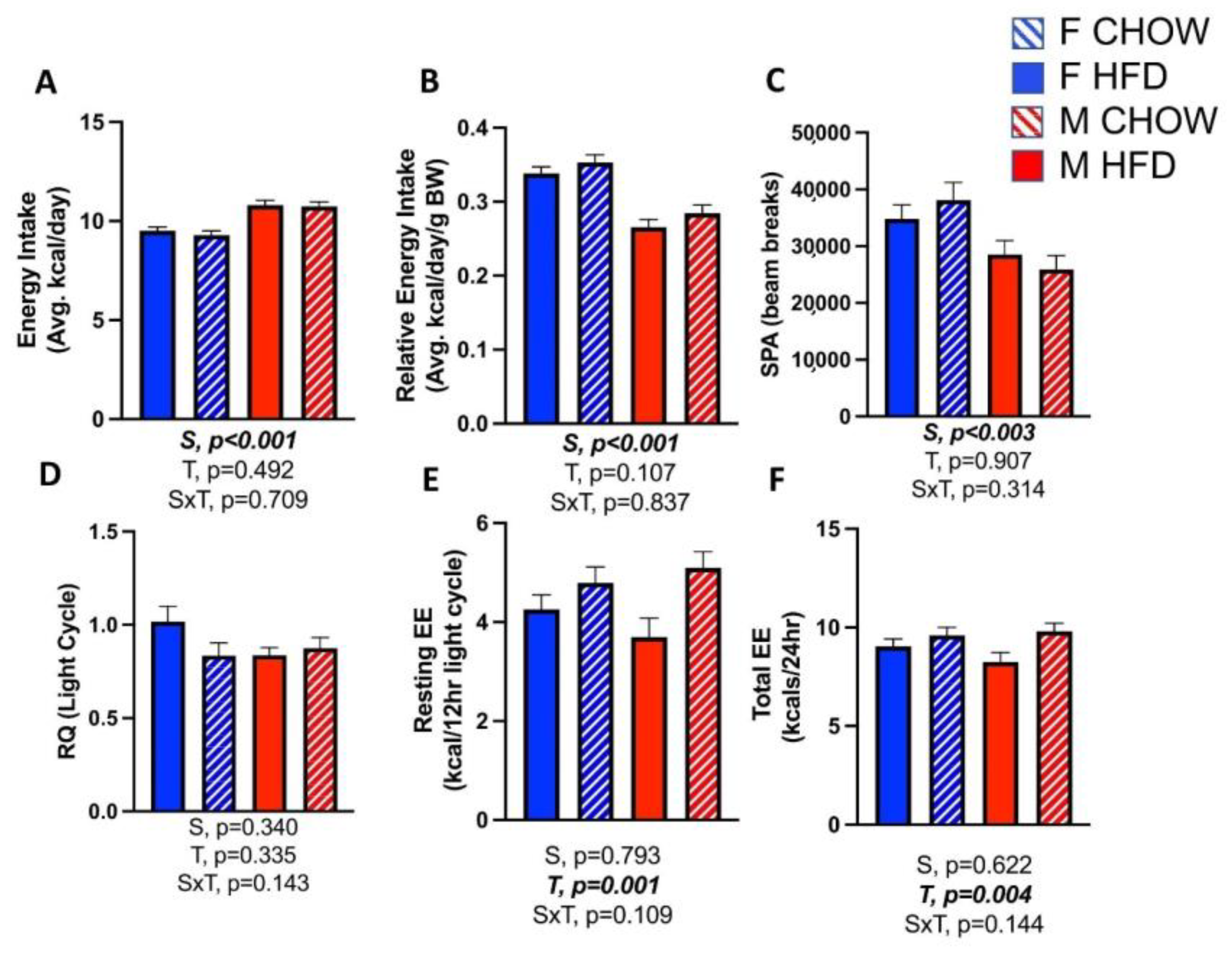
2.4. Energy Intake and Expenditure Assessments
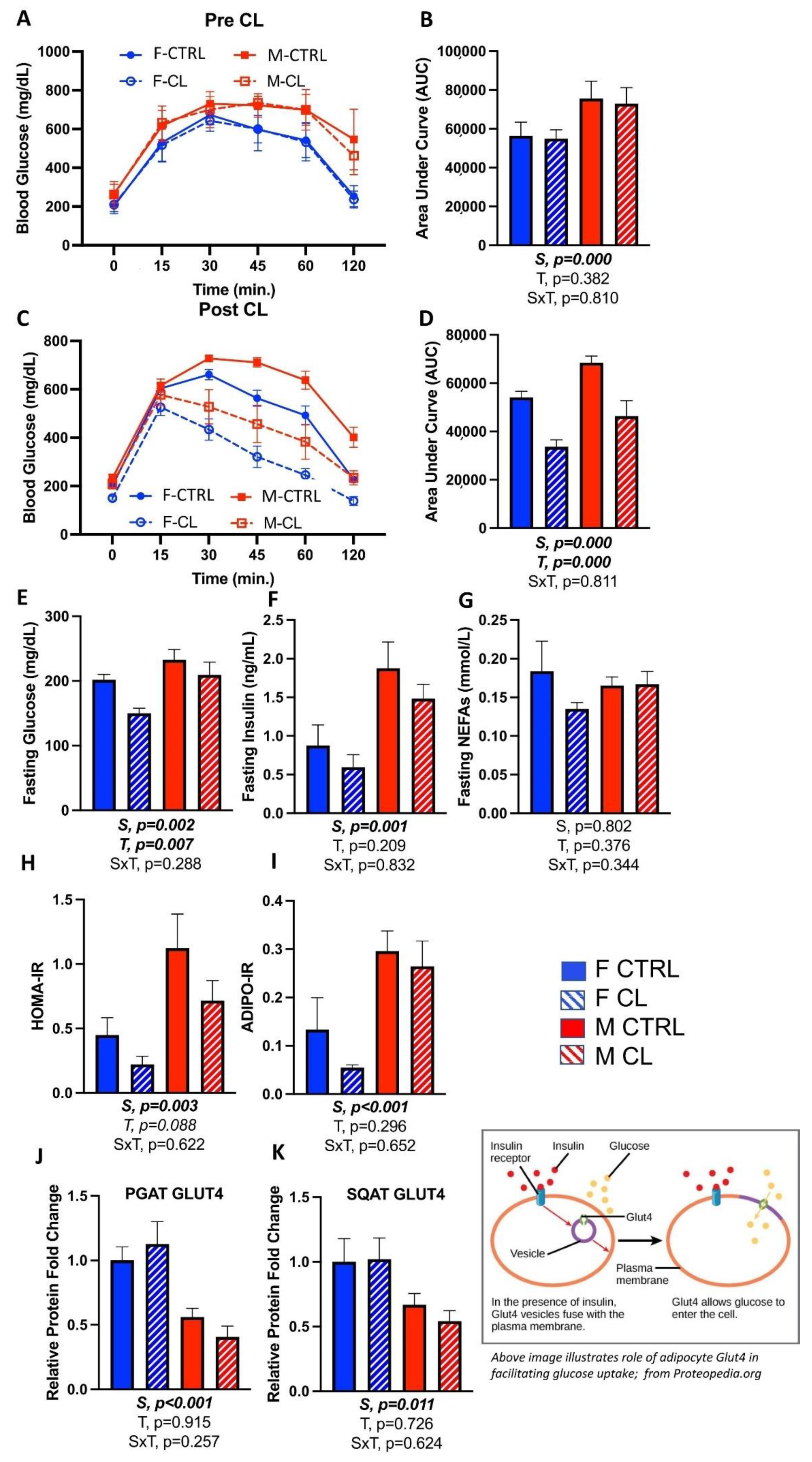
2.5. Glucose Tolerance Testing (GTT)
2.6. Fasting Blood Parameters
2.7. Protein Isolation and Western Blotting
2.8. RNA Extraction and Quantitative Real-Time RT-PCR
2.9. Adipocyte Cell Size Quantification
2.10. Statistical Analysis
3. Results
3.1. Sex Differences in HFD-Induced Obesity
3.2. Effects of CL on Body Composition
3.3. Effects of CL on Energy Expenditure
3.4. Effects of CL on Glucose Tolerance and Adipose Tissue Insulin Sensitivity
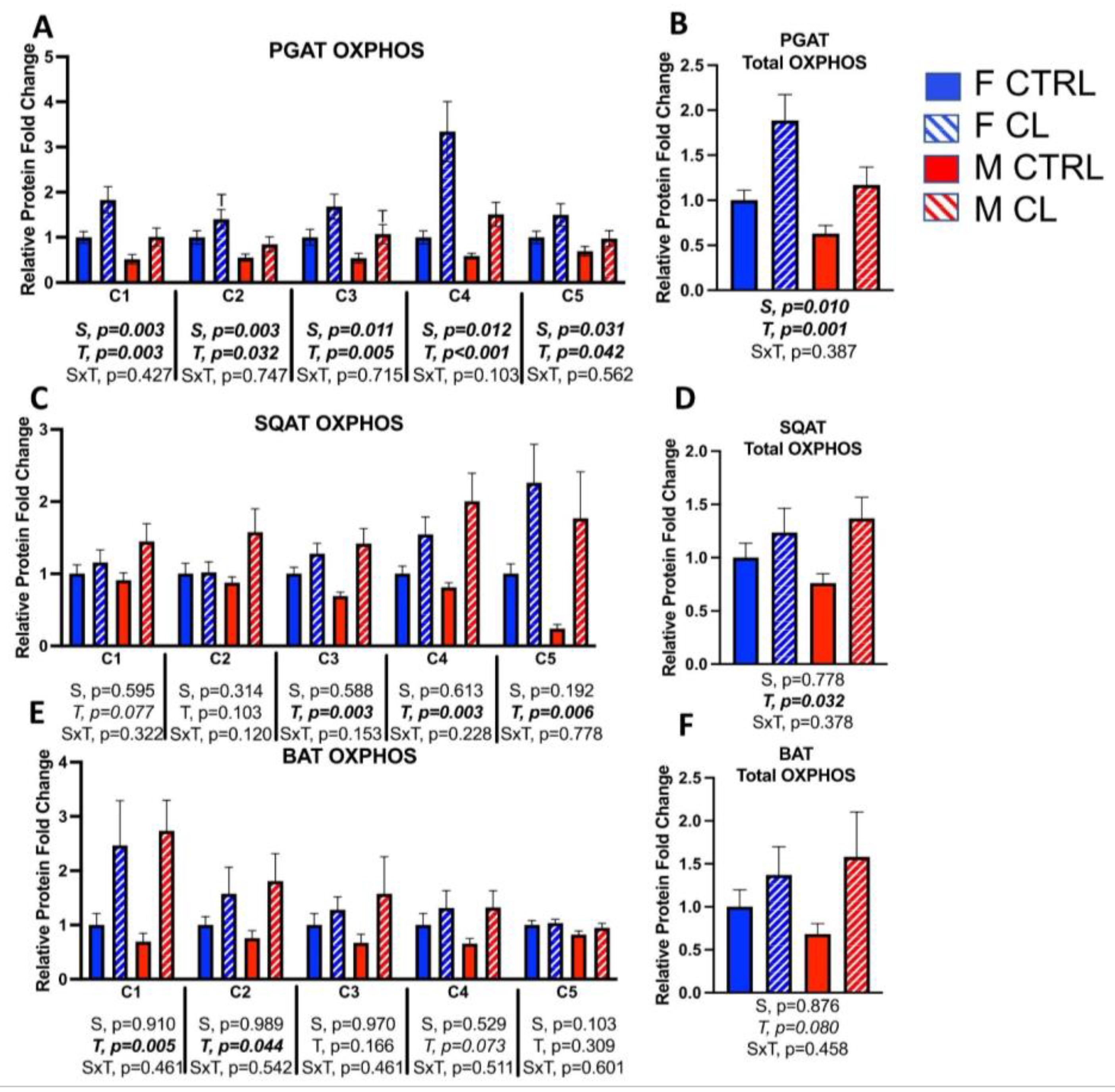
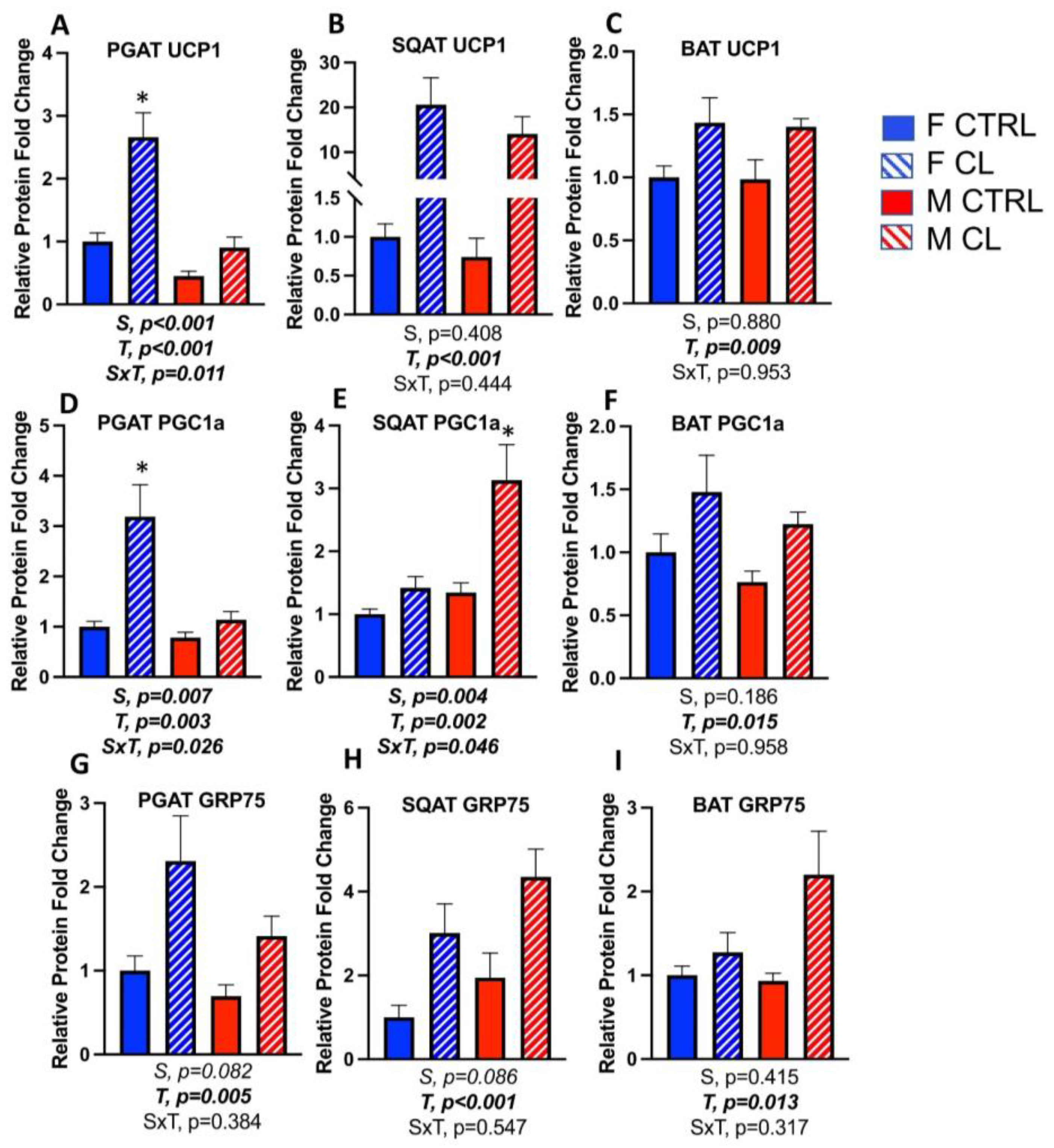
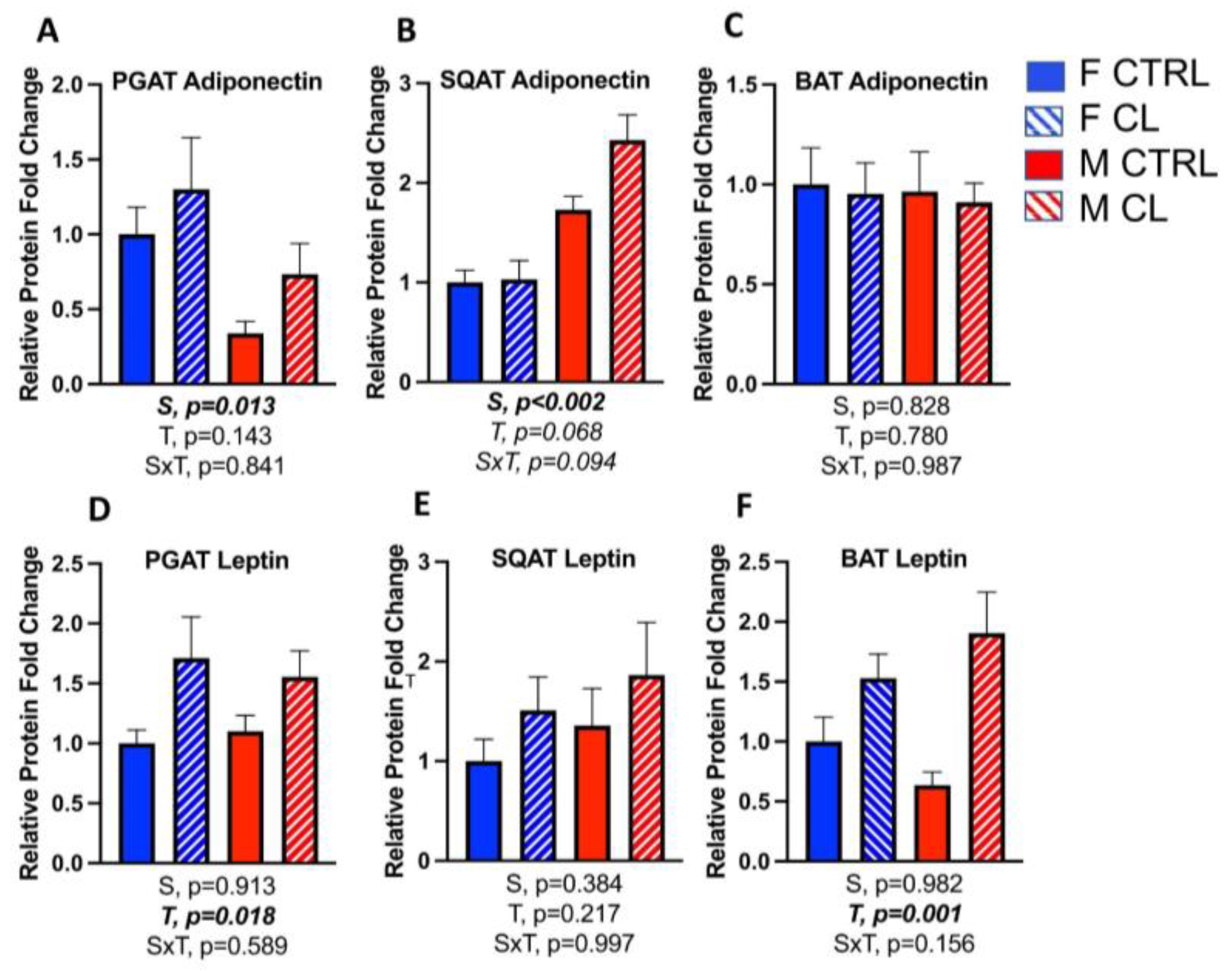
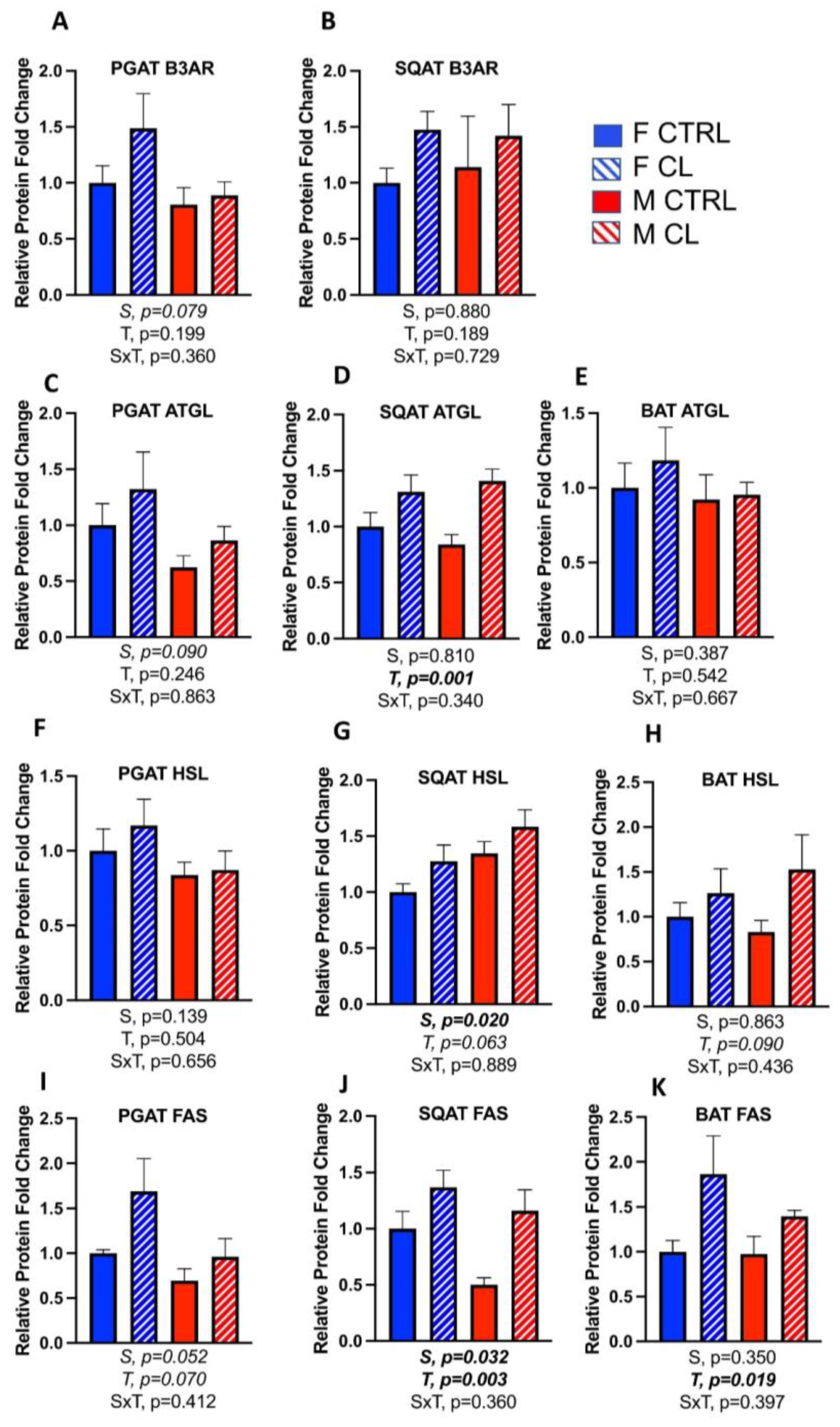
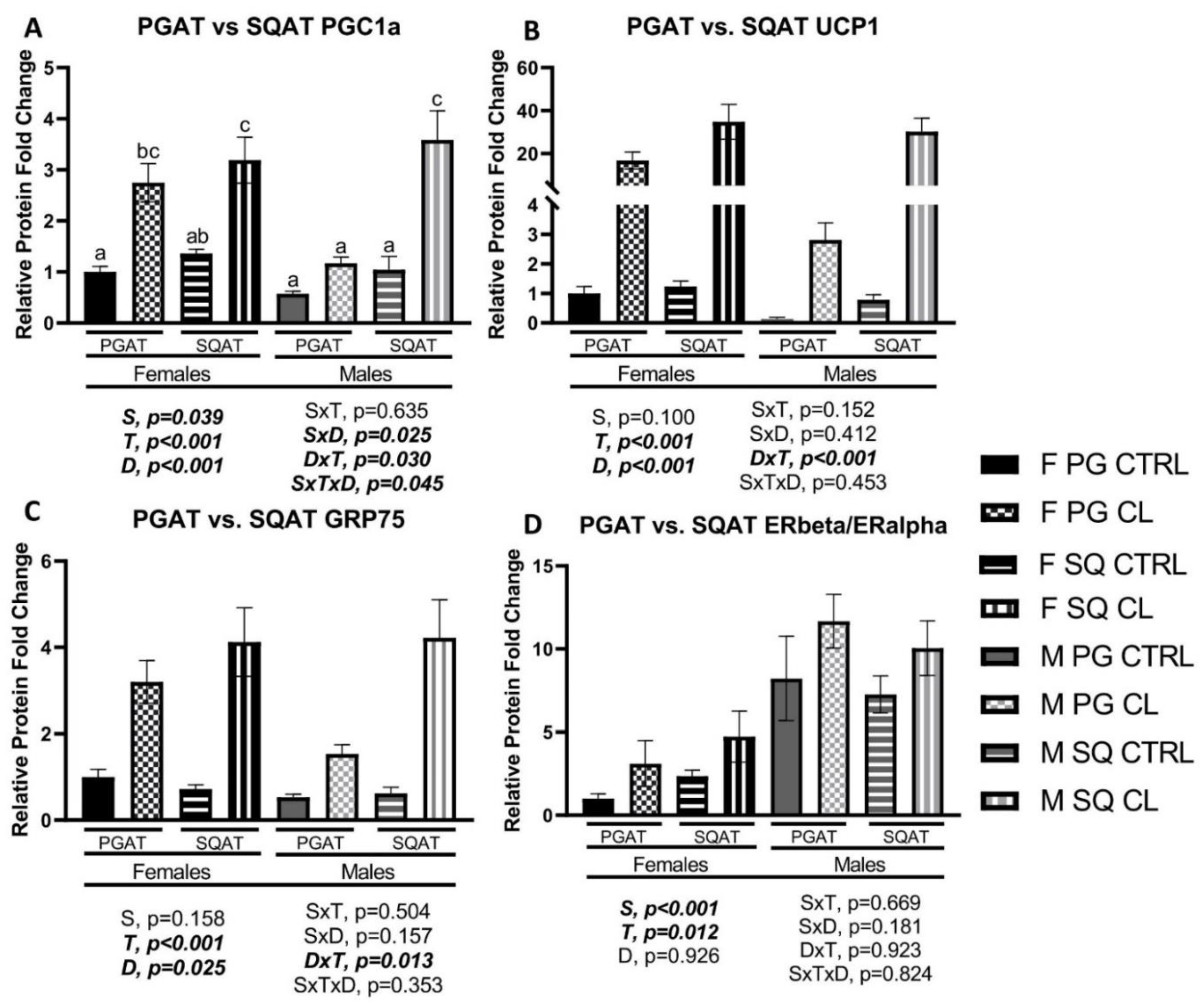
3.5. Sex- and Depot-Specific Effects of CL on WAT Browning and Adipokine Expression
3.6. Sex- and Depot-Specific Effects of CL on Adipose Tissue AMPK Activation
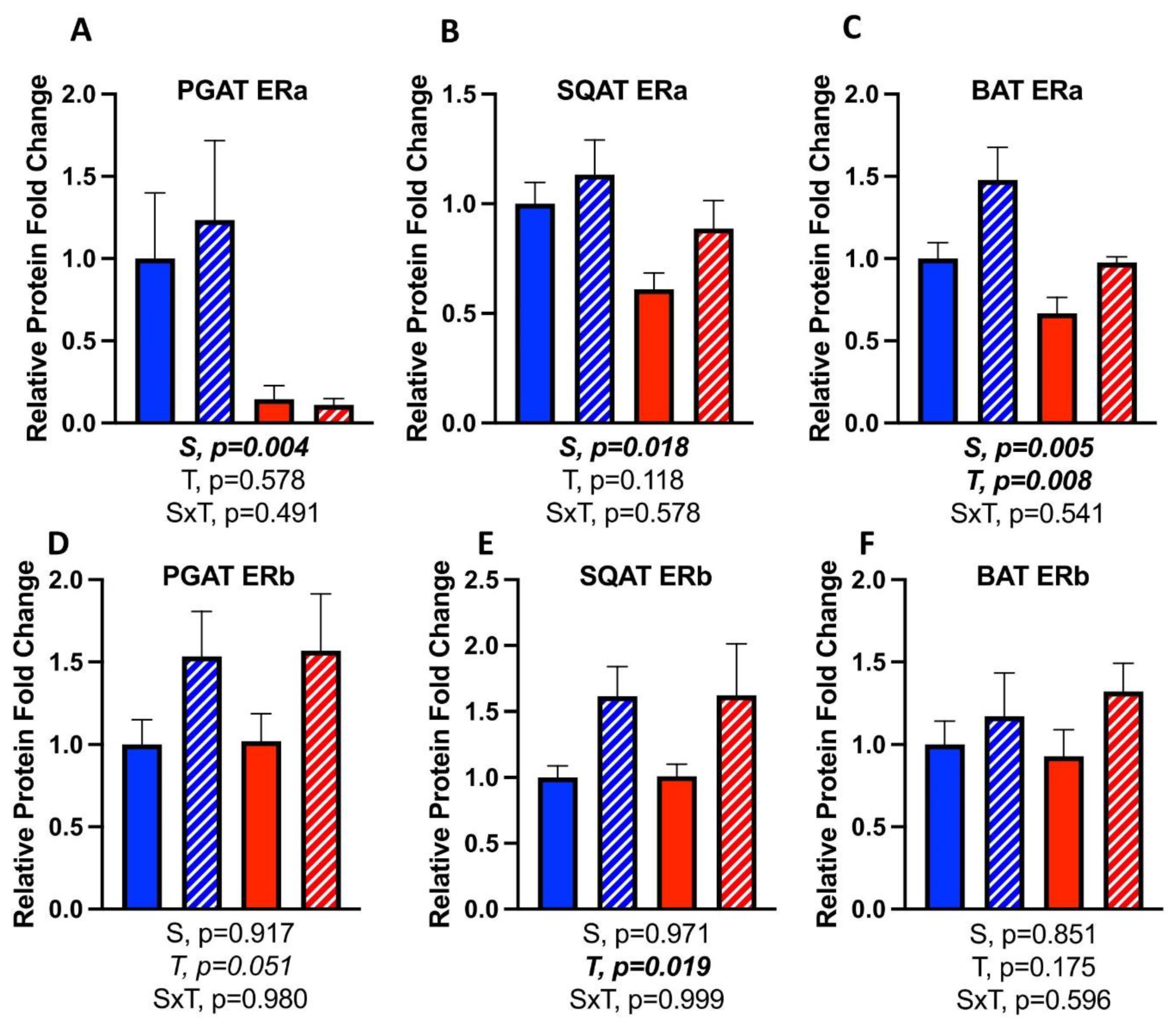
3.7. Effects of CL on WAT Depot-Specific Estrogen Receptor (ER) Expression
3.8. Between-Depot Comparisions of CL Responsiveness
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varghese, M.; Griffin, C.; McKernan, K.; Eter, L.; Lanzetta, N.; Agarwal, D.; Abrishami, S.; Singer, K. Sex Differences in Inflammatory Responses to Adipose Tissue Lipolysis in Diet-Induced Obesity. Endocrinology 2019, 160, 293–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auro, K.; Joensuu, A.; Fischer, K.; Kettunen, J.; Salo, P.; Mattsson, H.; Niironen, M.; Kaprio, J.; Eriksson, J.G.; Lehtimäki, T.; et al. A Metabolic View on Menopause and Ageing. Nat. Commun. 2014, 5, 4708. [Google Scholar] [CrossRef] [Green Version]
- Kuk, J.L.; Ardern, C.I. Age and Sex Differences in the Clustering of Metabolic Syndrome Factors. Diabetes Care 2010, 33, 2457–2461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendelsohn, M.E.; Karas, R.H. Molecular and Cellular Basis of Cardiovascular Gender Differences. Science 2005, 308, 1583–1587. [Google Scholar] [CrossRef] [Green Version]
- Alkhouli, M.; Nanjundappa, A.; Annie, F.; Bates, M.C.; Bhatt, D.L. Sex Differences in Case Fatality Rate of COVID-19: Insights From a Multinational Registry. Mayo Clin. Proc. 2020, 95, 1613–1620. [Google Scholar] [CrossRef]
- Chen, J.-Q.; Cammarata, P.R.; Baines, C.P.; Yager, J.D. Regulation of Mitochondrial Respiratory Chain Biogenesis by Estrogens/Estrogen Receptors and Physiological, Pathological and Pharmacological Implications. Biochim. Biophys. Acta 2009, 1793, 1540–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clegg, D.; Hevener, A.L.; Moreau, K.L.; Morselli, E.; Criollo, A.; Van Pelt, R.E.; Vieira-Potter, V.J. Sex Hormones and Cardiometabolic Health: Role of Estrogen and Estrogen Receptors. Endocrinology 2017, 158, 1095–1105. [Google Scholar] [CrossRef]
- Davis, K.E.; Neinast, M.D.; Sun, K.; Skiles, W.M.; Bills, J.D.; Zehr, J.A.; Zeve, D.; Hahner, L.D.; Cox, D.W.; Gent, L.M.; et al. The Sexually Dimorphic Role of Adipose and Adipocyte Estrogen Receptors in Modulating Adipose Tissue Expansion, Inflammation, and Fibrosis. Mol. Metab. 2013, 2, 227–242. [Google Scholar] [CrossRef]
- Porter, J.W.; Barnas, J.L.; Welly, R.; Spencer, N.; Pitt, J.; Vieira-Potter, V.J.; Kanaley, J.A. Age, Sex, and Depot-Specific Differences in Adipose Tissue Estrogen Receptors in Individuals with Obesity. Obesity 2020, 28, 1698–1707. [Google Scholar] [CrossRef]
- Dieudonné, M.N.; Leneveu, M.C.; Giudicelli, Y.; Pecquery, R. Evidence for Functional Estrogen Receptors Alpha and Beta in Human Adipose Cells: Regional Specificities and Regulation by Estrogens. Am. J. Physiol. Cell Physiol. 2004, 286, C655–C661. [Google Scholar] [CrossRef]
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen Receptors Alpha (ERα) and Beta (ERβ): Subtype-Selective Ligands and Clinical Potential. Steroids 2014, 90, 13–29. [Google Scholar] [CrossRef] [Green Version]
- Klöting, N.; Fasshauer, M.; Dietrich, A.; Kovacs, P.; Schön, M.R.; Kern, M.; Stumvoll, M.; Blüher, M. Insulin-Sensitive Obesity. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E506–E515. [Google Scholar] [CrossRef] [PubMed]
- Lomonaco, R.; Ortiz-Lopez, C.; Orsak, B.; Webb, A.; Hardies, J.; Darland, C.; Finch, J.; Gastaldelli, A.; Harrison, S.; Tio, F.; et al. Effect of Adipose Tissue Insulin Resistance on Metabolic Parameters and Liver Histology in Obese Patients with Nonalcoholic Fatty Liver Disease. Hepatology 2012, 55, 1389–1397. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Gaggini, M.; DeFronzo, R.A. Role of Adipose Tissue Insulin Resistance in the Natural History of Type 2 Diabetes: Results From the San Antonio Metabolism Study. Diabetes 2017, 66, 815–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernasochi, G.B.; Bell, J.R.; Simpson, E.R.; Delbridge, L.M.D.; Boon, W.C. Impact of Estrogens on the Regulation of White, Beige, and Brown Adipose Tissue Depots. Compr. Physiol. 2019, 9, 457–475. [Google Scholar] [CrossRef]
- das Graças Abeles, E.; de Souza Cordeiro, L.M.; de Sousa Martins, A.; Pesquero, J.L.; dos Reis, A.M.; Andrade, S.P.; Botion, L.M. Estrogen Therapy Attenuates Adiposity Markers in Spontaneously Hypertensive Rats. Metabolism 2012, 61, 1100–1107. [Google Scholar] [CrossRef]
- D’Eon, T.M.; Souza, S.C.; Aronovitz, M.; Obin, M.S.; Fried, S.K.; Greenberg, A.S. Estrogen Regulation of Adiposity and Fuel Partitioning evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J. Biol. Chem. 2005, 280, 35983–35991. [Google Scholar] [CrossRef] [Green Version]
- Simpson, E.R.; Mahendroo, M.S.; Means, G.D.; Kilgore, M.W.; Hinshelwood, M.M.; Graham-Lorence, S.; Amarneh, B.; Ito, Y.; Fisher, C.R.; Michael, M.D.; et al. Aromatase Cytochrome P450, The Enzyme Responsible for Estrogen Biosynthesis*. Endocr. Rev. 1994, 15, 342–355. [Google Scholar] [CrossRef]
- Simpson, E.R. Sources of Estrogen and Their Importance. J. Steroid Biochem. Mol. Biol. 2003, 86, 225–230. [Google Scholar] [CrossRef]
- Kim, S.-N.; Jung, Y.-S.; Kwon, H.-J.; Seong, J.K.; Granneman, J.G.; Lee, Y.-H. Sex Differences in Sympathetic Innervation and Browning of White Adipose Tissue of Mice. Biol. Sex Differ. 2016, 7, 67. [Google Scholar] [CrossRef] [Green Version]
- Haas, B.; Schlinkert, P.; Mayer, P.; Eckstein, N. Targeting Adipose Tissue. Diabetol. Metab. Syndr. 2012, 4, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klöting, N.; Blüher, M. Adipocyte Dysfunction, Inflammation and Metabolic Syndrome. Rev. Endocr. Metab. Disord. 2014, 15, 277–287. [Google Scholar] [CrossRef]
- Winn, N.C.; Grunewald, Z.I.; Gastecki, M.L.; Woodford, M.L.; Welly, R.J.; Clookey, S.L.; Ball, J.R.; Gaines, T.L.; Karasseva, N.G.; Kanaley, J.A.; et al. Deletion of UCP1 Enhances Ex Vivo Aortic Vasomotor Function in Female but Not Male Mice despite Similar Susceptibility to Metabolic Dysfunction. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E402–E412. [Google Scholar] [CrossRef] [Green Version]
- Clookey, S.L.; Welly, R.J.; Zidon, T.M.; Gastecki, M.L.; Woodford, M.L.; Grunewald, Z.I.; Winn, N.C.; Eaton, D.; Karasseva, N.G.; Sacks, H.S.; et al. Increased Susceptibility to OVX-Associated Metabolic Dysfunction in UCP1-Null Mice. J. Endocrinol. 2018, 239, 107–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moschinger, M.; Hilse, K.E.; Rupprecht, A.; Zeitz, U.; Erben, R.G.; Rülicke, T.; Pohl, E.E. Age-Related Sex Differences in the Expression of Important Disease-Linked Mitochondrial Proteins in Mice. Biol. Sex Differ. 2019, 10, 56. [Google Scholar] [CrossRef] [Green Version]
- Norheim, F.; Hasin-Brumshtein, Y.; Vergnes, L.; Krishnan, K.C.; Pan, C.; Seldin, M.M.; Hui, S.T.; Mehrabian, M.; Zhou, Z.; Gupta, S.; et al. Gene-by-Sex Interactions in Mitochondrial Functions and Cardio-Metabolic Traits. Cell Metab. 2019, 29, 932–949.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clookey, S.L.; Welly, R.J.; Shay, D.; Woodford, M.L.; Fritsche, K.L.; Rector, R.S.; Padilla, J.; Lubahn, D.B.; Vieira-Potter, V.J. Beta 3 Adrenergic Receptor Activation Rescues Metabolic Dysfunction in Female Estrogen Receptor Alpha-Null Mice. Front. Physiol. 2019, 10, 9. [Google Scholar] [CrossRef]
- Uenoyama, Y.; Inoue, N.; Nakamura, S.; Tsukamura, H. Kisspeptin Neurons and Estrogen–Estrogen Receptor α Signaling: Unraveling the Mystery of Steroid Feedback System Regulating Mammalian Reproduction. Int. J. Mol. Sci. 2021, 22, 9229. [Google Scholar] [CrossRef]
- Clusan, L.; Le Goff, P.; Flouriot, G.; Pakdel, F. A Closer Look at Estrogen Receptor Mutations in Breast Cancer and Their Implications for Estrogen and Antiestrogen Responses. Int. J. Mol. Sci. 2021, 22, 756. [Google Scholar] [CrossRef] [PubMed]
- Zidon, T.M.; Padilla, J.; Fritsche, K.L.; Welly, R.J.; McCabe, L.T.; Stricklin, O.E.; Frank, A.; Park, Y.; Clegg, D.J.; Lubahn, D.B.; et al. Effects of ERβ and ERα on OVX-Induced Changes in Adiposity and Insulin Resistance. J. Endocrinol. 2020, 245, 165–178. [Google Scholar] [CrossRef]
- MacPherson, R.E.K.; Castellani, L.; Beaudoin, M.-S.; Wright, D.C. Evidence for Fatty Acids Mediating CL 316,243-Induced Reductions in Blood Glucose in Mice. Am. J. Physiol.-Endocrinol. Metab. 2014, 307, E563–E570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, T.; Sakane, N.; Wakabayashi, Y.; Umekawa, T.; Kondo, M. Anti-Obesity Effect of CL 316,243, a Highly Specific Β3-Adrenoceptor Agonist, in Mice with Monosodium-l-Glutamate-Induced Obesity. Eur. J. Endocrinol. 1994, 131, 97–102. [Google Scholar] [CrossRef]
- Ghorbani, M.; Himms-Hagen, J. Appearance of Brown Adipocytes in White Adipose Tissue during CL 316,243-Induced Reversal of Obesity and Diabetes in Zucker Fa/Fa Rats. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 1997, 21, 465–475. [Google Scholar] [CrossRef] [Green Version]
- Komai, A.M.; Musovic, S.; Peris, E.; Alrifaiy, A.; El Hachmane, M.F.; Johansson, M.; Wernstedt Asterholm, I.; Olofsson, C.S. White Adipocyte Adiponectin Exocytosis Is Stimulated via Β3-Adrenergic Signaling and Activation of Epac1: Catecholamine Resistance in Obesity and Type 2 Diabetes. Diabetes 2016, 65, 3301–3313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, W.; Okamatsu-Ogura, Y.; Matsuoka, S.; Tsubota, A.; Kimura, K. Impaired Adrenergic Agonist-Dependent Beige Adipocyte Induction in Obese Mice. J. Vet. Med. Sci. 2019, 81, 799–807. [Google Scholar] [CrossRef] [Green Version]
- Cero, C.; Lea, H.J.; Zhu, K.Y.; Cypess, A.M. 2020-P: SS3-Adrenergic Receptors Regulate Lipolysis and Thermogenesis in Human Brown/Beige Adipocytes. Diabetes 2020, 69 (Suppl. 1). [Google Scholar] [CrossRef]
- Kaisanlahti, A.; Glumoff, T. Browning of White Fat: Agents and Implications for Beige Adipose Tissue to Type 2 Diabetes. J. Physiol. Biochem. 2019, 75, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winn, N.C.; Vieira-Potter, V.J.; Gastecki, M.L.; Welly, R.J.; Scroggins, R.J.; Zidon, T.M.; Gaines, T.L.; Woodford, M.L.; Karasseva, N.G.; Kanaley, J.A.; et al. Loss of UCP1 Exacerbates Western Diet-Induced Glycemic Dysregulation Independent of Changes in Body Weight in Female Mice. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2017, 312, R74–R84. [Google Scholar] [CrossRef]
- Aldiss, P.; Betts, J.; Sale, C.; Pope, M.; Budge, H.; Symonds, M.E. Exercise-Induced ‘Browning’ of Adipose Tissues. Metabolism 2018, 81, 63–70. [Google Scholar] [CrossRef] [Green Version]
- De Matteis, R.; Lucertini, F.; Guescini, M.; Polidori, E.; Zeppa, S.; Stocchi, V.; Cinti, S.; Cuppini, R. Exercise as a New Physiological Stimulus for Brown Adipose Tissue Activity. Nutr. Metab. Cardiovasc. Dis. NMCD 2013, 23, 582–590. [Google Scholar] [CrossRef]
- Mabire, L.; Mani, R.; Liu, L.; Mulligan, H.; Baxter, D. The Influence of Age, Sex and Body Mass Index on the Effectiveness of Brisk Walking for Obesity Management in Adults: A Systematic Review and Meta-Analysis. J. Phys. Act. Health 2017, 14, 389–407. [Google Scholar] [CrossRef]
- Beavers, K.M.; Neiberg, R.H.; Kritchevsky, S.B.; Nicklas, B.J.; Kitzman, D.W.; Messier, S.P.; Rejeski, W.J.; Ard, J.D.; Beavers, D.P. Association of Sex or Race With the Effect of Weight Loss on Physical Function. JAMA Netw. Open 2020, 3, e2014631. [Google Scholar] [CrossRef]
- Klinge, C.M. Estrogens Regulate Life and Death in Mitochondria. J. Bioenerg. Biomembr. 2017, 49, 307–324. [Google Scholar] [CrossRef]
- Jia, G.; Aroor, A.R.; Sowers, J.R. Estrogen and Mitochondria Function in Cardiorenal Metabolic Syndrome. Prog. Mol. Biol. Transl. Sci. 2014, 127, 229–249. [Google Scholar] [CrossRef] [Green Version]
- Sergi, D.; Naumovski, N.; Heilbronn, L.K.; Abeywardena, M.; O’Callaghan, N.; Lionetti, L.; Luscombe-Marsh, N. Mitochondrial (Dys)Function and Insulin Resistance: From Pathophysiological Molecular Mechanisms to the Impact of Diet. Front. Physiol. 2019, 10, 532. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.T.; Lolli, F.; Thomas, J.D.; Kola, B.; Korbonits, M. Measurement of AMP-Activated Protein Kinase Activity and Expression in Response to Ghrelin. Methods Enzymol. 2012, 514, 271–287. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, K.A.; Valentine, R.J.; Sudit, B.S.; Allen, K.; Dagon, Y.; Kahn, B.B.; Ruderman, N.B.; Saha, A.K. PKD1 Inhibits AMPKα2 through Phosphorylation of Serine 491 and Impairs Insulin Signaling in Skeletal Muscle Cells. J. Biol. Chem. 2016, 291, 5664–5675. [Google Scholar] [CrossRef] [Green Version]
- Padilla, J.; Jenkins, N.T.; Roberts, M.D.; Arce-Esquivel, A.A.; Martin, J.S.; Laughlin, M.H.; Booth, F.W. Differential Changes in Vascular MRNA Levels between Rat Iliac and Renal Arteries Produced by Cessation of Voluntary Running. Exp. Physiol. 2013, 98, 337–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roseguini, B.T.; Mehmet Soylu, S.; Whyte, J.J.; Yang, H.T.; Newcomer, S.; Laughlin, M.H. Intermittent Pneumatic Leg Compressions Acutely Upregulate VEGF and MCP-1 Expression in Skeletal Muscle. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H1991–H2000. [Google Scholar] [CrossRef] [PubMed]
- Wainright, K.S.; Fleming, N.J.; Rowles, J.L.; Welly, R.J.; Zidon, T.M.; Park, Y.-M.; Gaines, T.L.; Scroggins, R.J.; Anderson-Baucum, E.K.; Hasty, A.H.; et al. Retention of Sedentary Obese Visceral White Adipose Tissue Phenotype with Intermittent Physical Activity despite Reduced Adiposity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R594–R602. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, U.; Christensen, B.; Nielsen, T.S.; Pedersen, S.B.; Ørskov, L.; Lund, S.; Møller, N.; Jessen, N. GLUT4 and UBC9 Protein Expression Is Reduced in Muscle from Type 2 Diabetic Patients with Severe Insulin Resistance. PLoS ONE 2011, 6, e27854. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, U.S.; Waldén, T.B.; Carlsson, P.-O.; Jansson, L.; Phillipson, M. Female Mice Are Protected against High-Fat Diet Induced Metabolic Syndrome and Increase the Regulatory T Cell Population in Adipose Tissue. PLoS ONE 2012, 7, e46057. [Google Scholar] [CrossRef]
- Boucher, J.M.; Ryzhova, L.; Harrington, A.; Davis-Knowlton, J.; Turner, J.E.; Cooper, E.; Maridas, D.; Ryzhov, S.; Rosen, C.; Vary Calvin, P.H.; et al. Pathological Conversion of Mouse Perivascular Adipose Tissue by Notch Activation. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2227–2243. [Google Scholar] [CrossRef] [PubMed]
- Tramunt, B.; Smati, S.; Grandgeorge, N.; Lenfant, F.; Arnal, J.-F.; Montagner, A.; Gourdy, P. Sex Differences in Metabolic Regulation and Diabetes Susceptibility. Diabetologia 2020, 63, 453–461. [Google Scholar] [CrossRef] [Green Version]
- Kotani, K.; Tokunaga, K.; Fujioka, S.; Kobatake, T.; Keno, Y.; Yoshida, S.; Shimomura, I.; Tarui, S.; Matsuzawa, Y. Sexual Dimorphism of Age-Related Changes in Whole-Body Fat Distribution in the Obese. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 1994, 18, 202–207. [Google Scholar]
- Himms-Hagen, J.; Cui, J.; Danforth, E., Jr.; Taatjes, D.J.; Lang, S.S.; Waters, B.L.; Claus, T.H. Effect of CL-316,243, a Thermogenic Beta 3-Agonist, on Energy Balance and Brown and White Adipose Tissues in Rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1994, 266, R1371–R1382. [Google Scholar] [CrossRef]
- Goossens, G.H.; Jocken, J.W.E.; Blaak, E.E. Sexual Dimorphism in Cardiometabolic Health: The Role of Adipose Tissue, Muscle and Liver. Nat. Rev. Endocrinol. 2021, 17, 47–66. [Google Scholar] [CrossRef]
- Bargut, T.C.L.; Souza-Mello, V.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Browning of White Adipose Tissue: Lessons from Experimental Models. Horm. Mol. Biol. Clin. Investig. 2017, 31. [Google Scholar] [CrossRef]
- Jornayvaz, F.R.; Shulman, G.I. Regulation of Mitochondrial Biogenesis. Essays Biochem. 2010, 47. [Google Scholar] [CrossRef] [Green Version]
- Jia, R.; Luo, X.-Q.; Wang, G.; Lin, C.-X.; Qiao, H.; Wang, N.; Yao, T.; Barclay, J.L.; Whitehead, J.P.; Luo, X.; et al. Characterization of Cold-Induced Remodelling Reveals Depot-Specific Differences across and within Brown and White Adipose Tissues in Mice. Acta Physiol. Oxf. Engl. 2016, 217, 311–324. [Google Scholar] [CrossRef] [Green Version]
- Honrath, B.; Culmsee, C.; Dolga, A.M. One Protein, Different Cell Fate: The Differential Outcome of Depleting GRP75 during Oxidative Stress in Neurons. Cell Death Dis. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Patergnani, S.; Suski, J.M.; Agnoletto, C.; Bononi, A.; Bonora, M.; De Marchi, E.; Giorgi, C.; Marchi, S.; Missiroli, S.; Poletti, F.; et al. Calcium Signaling around Mitochondria Associated Membranes (MAMs). Cell Commun. Signal. CCS 2011, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Szabadkai, G.; Bianchi, K.; Várnai, P.; De Stefani, D.; Wieckowski, M.R.; Cavagna, D.; Nagy, A.I.; Balla, T.; Rizzuto, R. Chaperone-Mediated Coupling of Endoplasmic Reticulum and Mitochondrial Ca2+ Channels. J. Cell Biol. 2006, 175, 901–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, I.-S.; Jeong, Y.J.; Jeong, S.H.; Kim, J.E.; Han, J.; Kim, T.-H.; Jang, S.-W. Modulation of Mitochondrial ERβ Expression Inhibits Triple-Negative Breast Cancer Tumor Progression by Activating Mitochondrial Function. Cell. Physiol. Biochem. 2019, 52, 468–485. [Google Scholar]
- Hui, X.; Gu, P.; Zhang, J.; Nie, T.; Pan, Y.; Wu, D.; Feng, T.; Zhong, C.; Wang, Y.; Lam, K.S.L.; et al. Adiponectin Enhances Cold-Induced Browning of Subcutaneous Adipose Tissue via Promoting M2 Macrophage Proliferation. Cell Metab. 2015, 22, 279–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mottillo, E.P.; Balasubramanian, P.; Lee, Y.-H.; Weng, C.; Kershaw, E.E.; Granneman, J.G. Coupling of Lipolysis and de Novo Lipogenesis in Brown, Beige, and White Adipose Tissues during Chronic Β3-Adrenergic Receptor Activation. J. Lipid Res. 2014, 55, 2276–2286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Zhang, L.; Li, B.; Jiang, H.; Duan, Y.; Xie, Z.; Shuai, L.; Li, J.; Li, J. AMP-Activated Protein Kinase (AMPK) Regulates Energy Metabolism through Modulating Thermogenesis in Adipose Tissue. Front. Physiol. 2018, 9, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadian, M.; Abbott, M.J.; Tang, T.; Hudak, C.S.S.; Kim, Y.; Bruss, M.; Hellerstein, M.K.; Lee, H.-Y.; Samuel, V.T.; Shulman, G.I.; et al. Desnutrin/ATGL Is Regulated by AMPK and Is Required for a Brown Adipose Phenotype. Cell Metab. 2011, 13, 739–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacPherson, R.E.K.; Dragos, S.M.; Ramos, S.; Sutton, C.; Frendo-Cumbo, S.; Castellani, L.; Watt, M.J.; Perry, C.G.R.; Mutch, D.M.; Wright, D.C. Reduced ATGL-Mediated Lipolysis Attenuates β-Adrenergic-Induced AMPK Signaling, but Not the Induction of PKA-Targeted Genes, in Adipocytes and Adipose Tissue. Am. J. Physiol.-Cell Physiol. 2016, 311, C269–C276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohsaka, Y.; Nishino, H.; Nomura, Y. Adipose Cells Induce Phospho-Thr-172 AMPK Production by Epinephrine or CL316243 in Mouse 3T3-L1 Adipocytes or MAPK Activation and G Protein-Associated PI3K Responses Induced by CL316243 or Aluminum Fluoride in Rat White Adipocytes. Folia Biol. 2014, 60, 168–179. [Google Scholar]
- Pulinilkunnil, T.; He, H.; Kong, D.; Asakura, K.; Peroni, O.D.; Lee, A.; Kahn, B.B. Adrenergic Regulation of AMP-Activated Protein Kinase in Brown Adipose Tissue in Vivo. J. Biol. Chem. 2011, 286, 8798–8809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clart, L.M.; Welly, R.J.; Queathem, E.D.; Rector, R.S.; Padilla, J.; Baines, C.P.; Kanaley, J.A.; Lubahn, D.B.; Vieira-Potter, V.J. Role of ERβ in Adipocyte Metabolic Response to Wheel Running Following Ovariectomy. J. Endocrinol. 2021, 1. [Google Scholar] [CrossRef]
- Winn, N.C.; Jurrissen, T.J.; Grunewald, Z.I.; Cunningham, R.P.; Woodford, M.L.; Kanaley, J.A.; Lubahn, D.B.; Manrique-Acevedo, C.; Rector, R.S.; Vieira-Potter, V.J.; et al. Estrogen Receptor-α Signaling Maintains Immunometabolic Function in Males and Is Obligatory for Exercise-Induced Amelioration of Nonalcoholic Fatty Liver. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E156–E167. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Q.; Delannoy, M.; Cooke, C.; Yager, J.D. Mitochondrial Localization of ERalpha and ERbeta in Human MCF7 Cells. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E1011–E1022. [Google Scholar] [CrossRef]
- Burstein, S.R.; Kim, H.J.; Fels, J.A.; Qian, L.; Zhang, S.; Zhou, P.; Starkov, A.A.; Iadecola, C.; Manfredi, G. Estrogen Receptor Beta Modulates Permeability Transition in Brain Mitochondria. Biochim. Biophys. Acta Bioenerg. 2018, 1859, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.-L.; Lee, Y.-C.; Tzeng, C.-R.; Wang, Y.-P.; Chang, H.-Y.; Lin, Y.-F.; Kao, S.-H. Mitochondrial Translocation of Estrogen Receptor β Affords Resistance to Oxidative Insult-Induced Apoptosis and Contributes to the Pathogenesis of Endometriosis. Free Radic. Biol. Med. 2019, 134, 359–373. [Google Scholar] [CrossRef]
- Hsieh, Y.-C.; Yu, H.-P.; Suzuki, T.; Choudhry, M.A.; Schwacha, M.G.; Bland, K.I.; Chaudry, I.H. Upregulation of Mitochondrial Respiratory Complex IV by Estrogen Receptor-β Is Critical for Inhibiting Mitochondrial Apoptotic Signaling and Restoring Cardiac Functions Following Trauma–Hemorrhage. J. Mol. Cell. Cardiol. 2006, 41, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, S.; Tran, Q.T.; Harvey, I.; Smallwood, H.S.; Thiyagarajan, T.; Banerjee, S.; Johnson, D.L.; Dalton, J.T.; Sullivan, R.D.; Miller, D.D.; et al. Pharmacologic Activation of Estrogen Receptor β Increases Mitochondrial Function, Energy Expenditure, and Brown Adipose Tissue. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 266–281. [Google Scholar] [CrossRef] [Green Version]



| Table 1A: Female qRT-PCR Data Table | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PGAT CTRL | PGAT CL | SQAT CTRL | SQAT CL | BAT CTRL | BAT CL | ||||||||||
| Gene | Mean | SEM | Mean | SEM | p Value | Mean | SEM | Mean | SEM | p Value | Mean | SEM | Mean | SEM | p Value |
| Ppargc1a | 1 | ±0.16 | 7.5 | ±1.98 | 0.005 | 1 | ±0.43 | 24.7 | ±21.27 | 0.181 | 1 | ±0.19 | 1.5 | ±0.47 | 0.392 |
| Ucp1 | 1 | ±0.62 | 6.4 | ±2.07 | 0.026 | 1 | ±0.55 | 331.1 | ±283.96 | 0.239 | 1 | ±0.15 | 0.9 | ±0.21 | 0.586 |
| Hspa9 | 1 | ±0.27 | 3.1 | ±0.97 | 0.057 | 1 | ±0.4 | 207.5 | ±179.42 | 0.202 | 1 | ±0.16 | 0.6 | ±0.19 | 0.146 |
| Cidea | 1 | ±0.78 | 9940.0 | ±4346.72 | 0.092 | 1 | ±0.37 | 1.8 | ±0.50 | 0.297 | 1 | ±0.12 | 8.3 | ±7.10 | 0.444 |
| Prdm16 | 1 | ±0.18 | 608.8 | ±483.46 | 0.283 | 1 | ±0.4 | 88.4 | ±59.81 | 0.117 | 1 | ±0.21 | 0.8 | ±0.23 | 0.563 |
| Lipe | 1 | ±0.33 | 3.5 | ±1.26 | 0.117 | 1 | ±0.21 | 1.0 | ±0.26 | 0.932 | 1 | ±0.17 | 1.0 | ±0.35 | 0.898 |
| Fasn | 1 | ±0.24 | 2.1 | ±0.61 | 0.117 | 1 | ±0.22 | 2.5 | ±1.05 | 0.237 | 1 | ±0.28 | 0.9 | ±0.34 | 0.875 |
| Adipoq | 1 | ±0.11 | 1.7 | ±0.19 | 0.067 | 1 | ±0.12 | 7.6 | ±5.83 | 0.342 | 1 | ±0.08 | 0.5 | ±0.17 | 0.042 |
| Lep | 1 | ±0.23 | 0.7 | ±0.21 | 0.329 | 1 | ±0.53 | 40.7 | ±14.78 | 0.029 | 1 | ±0.23 | 0.4 | ±0.13 | 0.058 |
| Esr1 | 1 | ±0.13 | 1.7 | ±0.22 | 0.013 | 1 | ±0.14 | 3053.6 | ±1365.46 | 0.061 | 1 | ±0.22 | 1.5 | ±0.21 | 0.148 |
| Esr2 | 1 | ±0.38 | 2.6 | ±0.79 | 0.091 | 1 | ±0.19 | 0.2 | ±0.09 | 0.005 | 1 | ±0.34 | 4.5 | ±1.40 | 0.044 |
| Table 1B: Male qRT-PCR Data Table | |||||||||||||||
| PGAT CTRL | PGAT CL | SQAT CTRL | SQAT CL | BAT CTRL | BAT CL | ||||||||||
| Gene | Mean | SEM | Mean | SEM | p Value | Mean | SEM | Mean | SEM | p Value | Mean | SEM | Mean | SEM | p Value |
| Ppargc1a | 1 | ±0.87 | 0.8 | ±0.67 | 0.853 | 1 | ±0.89 | 3.7 | ±2.29 | 0.35 | 1 | ±0.44 | 2559.3 | ±2392.59 | 0.369 |
| Ucp1 | 1 | ±0.34 | 52.8 | ±17.48 | 0.012 | 1 | ±0.47 | 7.0 | ±3.29 | 0.096 | 1 | ±0.59 | 4.0 | ±2.38 | 0.281 |
| Hspa9 | 1 | ±0.22 | 2.0 | ±0.54 | 0.114 | 1 | ±0.3 | 2.3 | ±0.87 | 0.208 | 1 | ±0.47 | 22.7 | ±19.84 | 0.359 |
| Cidea | 1 | ±0.7 | 2.7 | ±0.89 | 0.186 | 1 | ±0.55 | 0.2 | ±0.08 | 0.125 | 1 | ±0.49 | 141.7 | ±79.44 | 0.16 |
| Prdm16 | 1 | ±0.4 | 2.3 | ±0.75 | 0.191 | 1 | ±0.23 | 160.6 | ±110.49 | 0.179 | 1 | ±0.85 | 334.4 | ±312.72 | 0.336 |
| Lipe | 1 | ±0.25 | 6.2 | ±1.93 | 0.028 | 1 | ±0.2 | 2.6 | ±0.62 | 0.092 | 1 | ±0.53 | 3.8 | ±1.56 | 0.148 |
| Fasn | 1 | ±0.17 | 2.8 | ±0.81 | 0.051 | 1 | ±0.19 | 2.5 | ±0.46 | 0.032 | 1 | ±0.66 | 7.1 | ±5.06 | 0.288 |
| Adipoq | 1 | ±0.21 | 2.4 | ±0.75 | 0.109 | 1 | ±0.15 | 2.2 | ±0.39 | 0.056 | 1 | ±0.58 | 3477.8 | ±3249.14 | 0.408 |
| Lep | 1 | ±0.22 | 1.0 | ±0.23 | 0.989 | 1 | ±0.41 | 2.1 | ±0.45 | 0.131 | 1 | ±0.84 | 337.4 | ±315.61 | 0.336 |
| Esr1 | 1 | ±0.16 | 1.6 | ±0.36 | 0.159 | 1 | ±0.25 | 1045.7 | ±663.33 | 0.207 | 1 | ±0.35 | 1.9 | ±0.26 | 0.092 |
| Esr2 | 1 | ±0.2 | 1.8 | ±0.41 | 0.111 | 1 | ±0.33 | 13.77 | ±12.05 | 0.432 | 1 | ±0.43 | 7.4 | ±3.39 | 0.091 |
| PGAT | SQAT | BAT | |
|---|---|---|---|
| Overall effect of CL on fat pad weight and mean adipocyte size |  ⇓ ⇓ |  ⇔ ⇔ |  ⇓ ⇓ |
| Adipose tissue-specific effects of CL: | |||
| Mitochondrial OX PHOS content |  ⇑ ⇑ | ⇑ | ⇑ |
| Mitochondrial biogenesis |  ⇑⇑ ⇑⇑ |  ⇑⇑ ⇑⇑ | ⇑ |
| Mitochondrial uncoupling |  ⇑⇑ ⇑⇑ | ⇑ | ⇑ |
| Mitochondrial GRP75 induction |  ⇑ ⇑ |  ⇑ ⇑ | ⇑ |
| AMPK total protein content |  |  | ⇑ |
| Net AMPK activity | ⇑ | ⇔ | |
| Adiponectin expression |  |  | ⇔ |
| Leptin expression | ⇑ | ⇔ | ⇑ |
| GLUT4 expression |  ⇔ ⇔ |  ⇔ ⇔ | |
| Lipogenesis protein (FAS) induction |  ⇑ ⇑ |  ⇑ ⇑ | ⇑ |
| Lipolysis protein (ATGL) induction | ⇔ | ⇑ | ⇔ |
| ERβ protein induction | ⇑ | ⇑ | ⇔ |
| ERα protein induction |  ⇔ ⇔ |  ⇔ ⇔ |  ⇑ ⇑ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Queathem, E.D.; Welly, R.J.; Clart, L.M.; Rowles, C.C.; Timmons, H.; Fitzgerald, M.; Eichen, P.A.; Lubahn, D.B.; Vieira-Potter, V.J. White Adipose Tissue Depots Respond to Chronic Beta-3 Adrenergic Receptor Activation in a Sexually Dimorphic and Depot Divergent Manner. Cells 2021, 10, 3453. https://doi.org/10.3390/cells10123453
Queathem ED, Welly RJ, Clart LM, Rowles CC, Timmons H, Fitzgerald M, Eichen PA, Lubahn DB, Vieira-Potter VJ. White Adipose Tissue Depots Respond to Chronic Beta-3 Adrenergic Receptor Activation in a Sexually Dimorphic and Depot Divergent Manner. Cells. 2021; 10(12):3453. https://doi.org/10.3390/cells10123453
Chicago/Turabian StyleQueathem, Eric D., Rebecca J. Welly, Laura M. Clart, Candace C. Rowles, Hunter Timmons, Maggie Fitzgerald, Peggy A. Eichen, Dennis B. Lubahn, and Victoria J. Vieira-Potter. 2021. "White Adipose Tissue Depots Respond to Chronic Beta-3 Adrenergic Receptor Activation in a Sexually Dimorphic and Depot Divergent Manner" Cells 10, no. 12: 3453. https://doi.org/10.3390/cells10123453
APA StyleQueathem, E. D., Welly, R. J., Clart, L. M., Rowles, C. C., Timmons, H., Fitzgerald, M., Eichen, P. A., Lubahn, D. B., & Vieira-Potter, V. J. (2021). White Adipose Tissue Depots Respond to Chronic Beta-3 Adrenergic Receptor Activation in a Sexually Dimorphic and Depot Divergent Manner. Cells, 10(12), 3453. https://doi.org/10.3390/cells10123453