The Role of Serum Th1, Th2, and Th17 Cytokines in Patients with Alopecia Areata: Clinical Implications
Abstract
1. Introduction
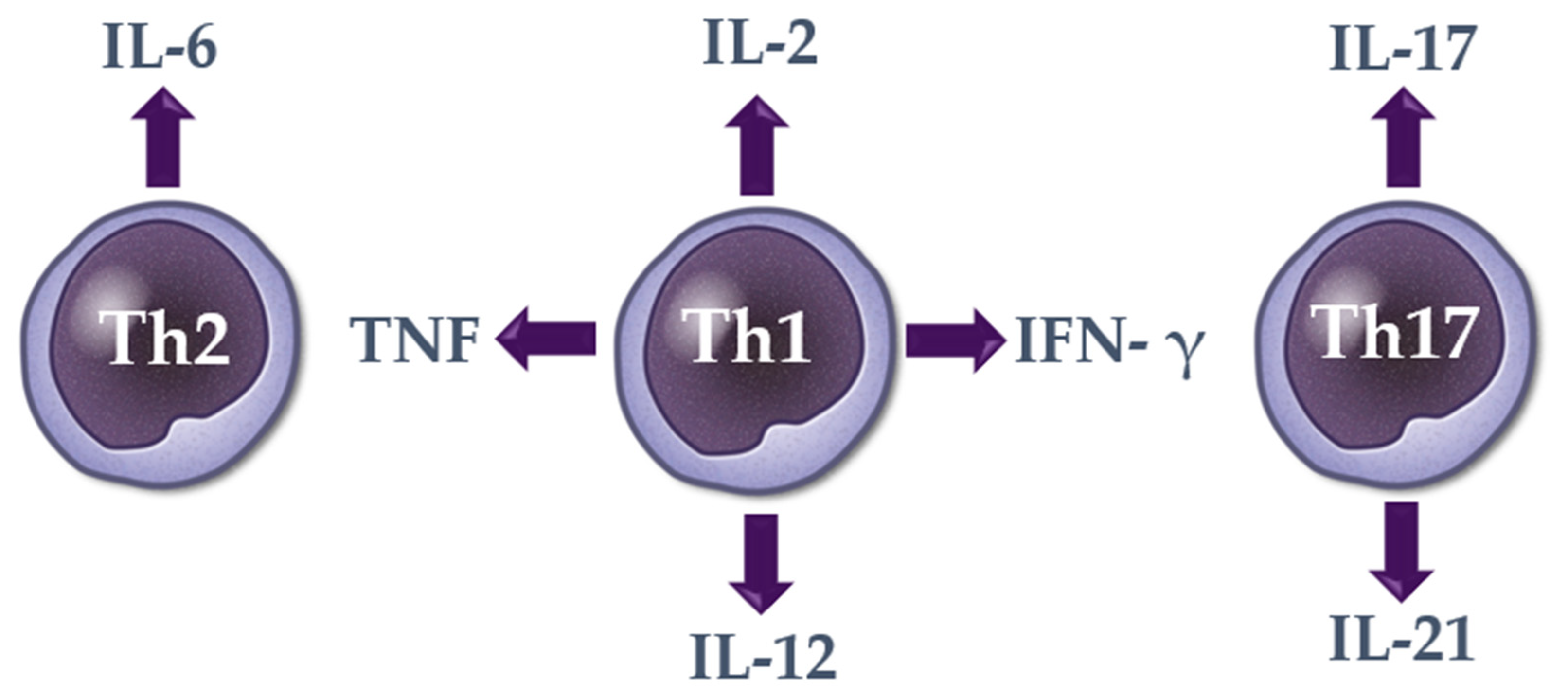
2. Cytokines in Alopecia Areata
2.1. Th1 Cytokines
2.1.1. Interleukin 2 (IL-2)
2.1.2. Interferon Gamma (IFN-γ)
2.1.3. Tumor Necrosis Factor (TNF)
2.1.4. Interleukin 12 (IL-12)
2.1.5. Interleukin 18 (IL-18)
2.2. Th2 Cytokines
2.2.1. Interleukin 4 (IL-4)
2.2.2. Interleukin 5 (IL-5)
2.2.3. Interleukin 6 (IL-6)
2.2.4. Interleukin 9 (IL-9)
2.2.5. Interleukin 10 (IL-10)
2.2.6. Interleukin 13 (IL-13)
2.2.7. Interleukin 17E (IL-17E)
2.2.8. Interleukin 31 (IL-31)
2.2.9. Interleukin 33 (IL-33)
2.3. Th17 Cytokines
2.3.1. Interleukin 17 (IL-17)
2.3.2. Interleukin 17F (IL-17F)
2.3.3. Interleukin 21 (IL-21)
2.3.4. Interleukin 22 (IL-22)
2.3.5. Interleukin 23 (IL-23)
2.3.6. Transforming Growth Factor Beta (TGF- β)
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Alkhalifah, A. Alopecia areata update. Dermatol. Clin. 2013, 31, 93–108. [Google Scholar] [CrossRef]
- Safavi, K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch. Dermatol. 1992, 128, 702. [Google Scholar] [CrossRef]
- Mirzoyev, S.A.; Schrum, A.G.; Davis, M.D.P.; Torgerson, R.R. Lifetime incidence risk of alopecia areata estimated at 2.1% by Rochester Epidemiology Project, 1990–2009. J. Investig. Dermatol. 2014, 134, 1141–1142. [Google Scholar] [CrossRef]
- Vano-Galvan, S.; Saceda-Corralo, D.; Blume-Peytavi, U.; Cucchia, J.; Dlova, N.C.; Gavazzoni Dias, M.F.R.; Grimalt, R.; Guzman-Sanchez, D.; Harries, M.; Ho, A.; et al. Frequency of the Types of Alopecia at Twenty-Two Specialist Hair Clinics: A Multicenter Study. Ski. Appendage Disord. 2019, 5, 309–315. [Google Scholar] [CrossRef]
- Waśkiel-Burnat, A.; Rakowska, A.; Sikora, M.; Olszewska, M.; Rudnicka, L. Alopecia areata predictive score: A new trichoscopy-based tool to predict treatment outcome in patients with patchy alopecia areata. J. Cosmet. Dermatol. 2020, 19, 746–751. [Google Scholar] [CrossRef]
- Meah, N.; Wall, D.; York, K.; Bhoyrul, B.; Bokhari, L.; Sigall, D.A.; Bergfeld, W.F.; Betz, R.C.; Blume-Peytavi, U.; Callender, V.; et al. The Alopecia Areata Consensus of Experts (ACE) study: Results of an international expert opinion on treatments for alopecia areata. J. Am. Acad. Dermatol. 2020, 83, 123–130. [Google Scholar] [CrossRef]
- Cranwell, W.C.; Lai, V.W.; Photiou, L.; Meah, N.; Wall, D.; Rathnayake, D.; Joseph, S.; Chitreddy, V.; Gunatheesan, S.; Sindhu, K.; et al. Treatment of alopecia areata: An Australian expert consensus statement. Australas J. Dermatol. 2019, 60, 163–170. [Google Scholar] [CrossRef]
- Malik, K.; Guttman-Yassky, E. Cytokine Targeted Therapeutics for Alopecia Areata: Lessons from Atopic Dermatitis and Other Inflammatory Skin Diseases. J. Investig. Dermatol. Symp. Proc. 2018, 19, S62–S64. [Google Scholar] [CrossRef]
- Żeberkiewicz, M.; Rudnicka, L.; Malejczyk, J. Immunology of alopecia areata. Cent. Eur. J. Immunol. 2020, 45, 325–333. [Google Scholar] [CrossRef]
- Simakou, T.; Butcher, J.P.; Reid, S.; Henriquez, F.L. Alopecia areata: A multifactorial autoimmune condition. J. Autoimmun. 2019, 98, 74–85. [Google Scholar] [CrossRef]
- Kasumagić-Halilovic, E.; Cavaljuga, S.; Ovcina-Kurtovic, N.; Zecevic, L. Serum Levels of Interleukin-2 in Patients with Alopecia Areata: Relationship with Clinical Type and Duration of the Disease. Ski. Appendage Disord. 2018, 4, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Gregoriou, S.; Papafragkaki, D.; Kontochristopoulos, G.; Rallis, E.; Kalogeromitros, D.; Rigopoulos, D. Cytokines and other mediators in alopecia areata. Mediat. Inflamm. 2010, 2010, 928030. [Google Scholar] [CrossRef] [PubMed]
- Giordano, C.N.; Sinha, A.A. Cytokine pathways and interactions in alopecia areata. Eur. J. Dermatol. 2013, 23, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, F.; Drake, L.A.; Senna, M.M.; Rezaei, N. Alopecia areata: A review of disease pathogenesis. Br. J. Dermatol. 2018, 179, 1033–1048. [Google Scholar] [CrossRef] [PubMed]
- Symington, F.W. Lymphotoxin, tumor necrosis factor, and gamma interferon are cytostatic for normal human keratinocytes. J. Investig. Dermatol. 1989, 92, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Philpott, M.P.; Sanders, D.A.; Bowen, J.; Kealey, T. Effects of interleukins, colony-stimulating factor and tumour necrosis factor on human hair follicle growth in vitro: A possible role for interleukin-1 and tumour necrosis factor-alpha in alopecia areata. Br. J. Dermatol. 1996, 135, 942–948. [Google Scholar] [CrossRef]
- Suárez-Fariñas, M.; Ungar, B.; Noda, S.; Shroff, A.; Mansouri, Y.; Fuentes-Duculan, J.; Czernik, A.; Zheng, X.; Estrada, Y.D.; Xu, H.; et al. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J. Allergy Clin. Immunol. 2015, 136, 1277–1287. [Google Scholar] [CrossRef]
- Kageyama, R.; Ito, T.; Hanai, S.; Morishita, N.; Nakazawa, S.; Fujiyama, T.; Honda, T.; Tokura, Y. Immunological Properties of Atopic Dermatitis-Associated Alopecia Areata. Int. J. Mol. Sci. 2021, 22, 2618. [Google Scholar] [CrossRef]
- Marks, D.H.; Senna, M.M. A Potential Role for IL-4 and IL-13 in an Alopecia Areata-Like Phenotype: A Clinical Perspective. J. Investig. Dermatol. Symp. Proc. 2020, 20, S58–S59. [Google Scholar] [CrossRef]
- Hohl, T.M. 6-Cell-Mediated Defense against Infection. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases (Eighth Edition); Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Saunders: Philadelphia, PA, USA, 2015; pp. 50–69.e56. [Google Scholar]
- Rudnicka, L.; Waśkiel-Burnat, A. Systemic aspects of alopecia areata Comment to the article by Lai and Sinclair. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e214–e215. [Google Scholar] [CrossRef]
- Tembhre, M.K.; Sharma, V.K. T-helper and regulatory T-cell cytokines in the peripheral blood of patients with active alopecia areata. Br. J. Dermatol. 2013, 169, 543–548. [Google Scholar] [CrossRef]
- Teraki, Y.; Imanishi, K.; Shiohara, T. Cytokines in alopecia areata: Contrasting cytokine profiles in localized form and extensive form (alopecia universalis). Acta Dermatol. Venereol. 1996, 76, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Alzolibani, A.A.; Rasheed, Z.; Bin Saif, G.; Al-Dhubaibi, M.S.; Al Robaee, A.A. Altered expression of intracellular Toll-like receptors in peripheral blood mononuclear cells from patients with alopecia areata. BBA Clin. 2016, 5, 134–142. [Google Scholar] [CrossRef]
- Gautam, R.K.; Singh, Y.; Gupta, A.; Arora, P.; Khurana, A.; Chitkara, A. The profile of cytokines (IL-2, IFN-γ, IL-4, IL-10, IL-17A, and IL-23) in active alopecia areata. J. Cosmet. Dermatol. 2020, 19, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Barahmani, N.; Lopez, A.; Babu, D.; Hernandez, M.; Donley, S.E.; Duvic, M. Serum T helper 1 cytokine levels are greater in patients with alopecia areata regardless of severity or atopy. Clin. Exp. Dermatol. 2010, 35, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Aşkın, Ö.; Yücesoy, S.N.; Coşkun, E.; Engin, B.; Serdaroğlu, S. Evaluation of the level of serum Interleukins (IL-2, IL-4, IL-15 andIL-17) and its relationship with disease severity in patients with alopecia areata. Bras. Dermatol. 2021, 96, 551–557. [Google Scholar] [CrossRef]
- Loh, S.H.; Moon, H.N.; Lew, B.L.; Sim, W.Y. Role of T helper 17 cells and T regulatory cells in alopecia areata: Comparison of lesion and serum cytokine between controls and patients. J. Eur. Acad. Dermatol. Venereol. 2017, 2, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Giri, J.G.; Kumaki, S.; Ahdieh, M.; Friend, D.J.; Loomis, A.; Shanebeck, K.; DuBose, R.; Cosman, D.; Park, L.S.; Anderson, D.M. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. Embo. J. 1995, 14, 3654–3663. [Google Scholar] [CrossRef]
- El Aziz Ragab, M.A.; Hassan, E.M.; El Niely, D.; Mohamed, M.M. Serum level of interleukin-15 in active alopecia areata patients and its relation to age, sex, and disease severity. Postepy Dermatol. Alergol. 2020, 37, 904–908. [Google Scholar] [CrossRef]
- Tabara, K.; Kozłowska, M.; Jędrowiak, A.; Bienias, W.; Kaszuba, A. Serum concentrations of selected proinflammatory cytokines in children with alopecia areata. Adv. Dermatol. Allergol./Postępy Dermatol. i Alergol. 2019, 36, 63–69. [Google Scholar] [CrossRef]
- Song, T.; Pavel, A.B.; Wen, H.C.; Malik, K.; Estrada, Y.; Gonzalez, J.; Hashim, P.W.; Nia, J.K.; Baum, D.; Kimmel, G.; et al. An integrated model of alopecia areata biomarkers highlights both T(H)1 and T(H)2 upregulation. J. Allergy Clin. Immunol. 2018, 142, 1631–1634.e1613. [Google Scholar] [CrossRef]
- Arca, E.; Muşabak, U.; Akar, A.; Erbil, A.H.; Taştan, H.B. Interferon-gamma in alopecia areata. Eur. J. Dermatol. 2004, 14, 33–36. [Google Scholar]
- Gong, Y.; Zhao, Y.; Zhang, X.; Qi, S.; Li, S.; Ye, Y.; Yang, J.; Caulloo, S.; McElwee, K.J.; Zhang, X. Serum level of IL-4 predicts response to topical immunotherapy with diphenylcyclopropenone in alopecia areata. Exp. Dermatol. 2020, 29, 231–238. [Google Scholar] [CrossRef]
- Tomaszewska, K.; Kozłowska, M.; Kaszuba, A.; Lesiak, A.; Narbutt, J.; Zalewska-Janowska, A. Increased Serum Levels of IFN-γ, IL-1β, and IL-6 in Patients with Alopecia Areata and Nonsegmental Vitiligo. Oxid. Med. Cell Longev. 2020, 2020, 5693572. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Luo, L.; Li, L.; He, X.; Lu, W.; Sha, X.; Mao, Y. Diphenylcyclopropenone plays an effective therapeutic role by up-regulating the TSLP/OX40L/IL-13 pathway in severe alopecia areata. Exp. Dermatol. 2021, 30, 278–283. [Google Scholar] [CrossRef]
- Ma, X.; Chen, S.; Jin, W.; Gao, Y. Th1/Th2 PB balance and CD200 expression of patients with active severe alopecia areata. Exp. Ther. Med. 2017, 13, 2883–2887. [Google Scholar] [CrossRef][Green Version]
- Manimaran, R.P.; Ramassamy, S.; Rajappa, M.; Chandrashekar, L. Therapeutic outcome of diphencyprone and its correlation with serum cytokine profile in alopecia areata. J. Dermatol. Treat. 2020, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kasumagic-Halilovic, E.; Prohic, A.; Karamehic, J. Serum concentrations of interferon-gamma (IFN-g) in patients with alopecia areata: Correlation with clinical type and duration of the disease. Med. Arh. 2010, 64, 212–214. [Google Scholar] [PubMed]
- Omar, S.I.; Hamza, A.M.; Eldabah, N.; Habiba, D.A. IFN-α and TNF-α serum levels and their association with disease severity in Egyptian children and adults with alopecia areata. Int. J. Dermatol. 2021, 60, 1397–1404. [Google Scholar] [CrossRef]
- Cho, W.I.; Seo, S.J.; Kim, M.N.; Hong, C.K.; Ro, B.I. Circulating levels of IFN-γ, IL-10 and IL-16 in patients with alopecia areata. Korean J. Dermatol. 2006, 44, 399–404. [Google Scholar]
- Sadeghi, S.; Sanati, M.H.; Taghizadeh, M.; Mansouri, P.; Jadali, Z. Study of Th1/Th2 balance in peripheral blood mononuclear cells of patients with alopecia areata. Acta Microbiol. Immunol. Hung. 2015, 62, 275–285. [Google Scholar] [CrossRef][Green Version]
- Zöller, M.; McElwee, K.J.; Vitacolonna, M.; Hoffmann, R. The progressive state, in contrast to the stable or regressive state of alopecia areata, is reflected in peripheral blood mononuclear cells. Exp. Dermatol. 2004, 13, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, K.; Arakawa, S.; Hatano, Y. In vivo levels of IL-4, IL-10, TGF-beta1 and IFN-gamma mRNA of the peripheral blood mononuclear cells in patients with alopecia areata in comparison to those in patients with atopic dermatitis. Arch. Dermatol. Res. 2007, 298, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006, 25, 409–416. [Google Scholar] [CrossRef]
- Bilgic, O.; Sivrikaya, A.; Unlu, A.; Altinyazar, H.C. Serum cytokine and chemokine profiles in patients with alopecia areata. J. Dermatol. Treat. 2016, 27, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Atwa, M.A.; Youssef, N.; Bayoumy, N.M. T-helper 17 cytokines (interleukins 17, 21, 22, and 6, and tumor necrosis factor-alpha) in patients with alopecia areata: Association with clinical type and severity. Int. J. Dermatol. 2016, 55, 666–672. [Google Scholar] [CrossRef]
- Kasumagić-Halilović, E. Serum concentration of tumor necrosis-alpha (TNF-α) in patients with alopecia universalis. Serumske Konc. Fakt. nekroze tumora-alfa kod pacijenata sa univerzalnom alopeciom. Med. J. 2017, 23, 115–118. [Google Scholar]
- Bain, K.A.; McDonald, E.; Moffat, F.; Tutino, M.; Castelino, M.; Barton, A.; Cavanagh, J.; Ijaz, U.Z.; Siebert, S.; McInnes, I.B.; et al. Alopecia areata is characterized by dysregulation in systemic type 17 and type 2 cytokines, which may contribute to disease-associated psychological morbidity. Br. J. Dermatol. 2020, 182, 130–137. [Google Scholar] [CrossRef]
- Rossi, A.; Cantisani, C.; Carlesimo, M.; Scarnò, M.; Scali, E.; Mari, E.; Garelli, V.; Maxia, C.; Calvieri, S. Serum concentrations of IL-2, IL-6, IL-12 and TNF-α in patients with alopecia areata. Int. J. Immunopathol. Pharm. 2012, 25, 781–788. [Google Scholar] [CrossRef]
- Vignali, D.A.A.; Kuchroo, V.K. IL-12 family cytokines: Immunological playmakers. Nat. Immunol. 2012, 13, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Attia, E.A.; El Shennawy, D.; Sefin, A. Serum Interleukin-4 and Total Immunoglobulin E in Nonatopic Alopecia Areata Patients and HLA-DRB1 Typing. Dermatol. Res. Pr. 2010, 2010, 503587. [Google Scholar] [CrossRef]
- Wawrocki, S.; Druszczynska, M.; Kowalewicz-Kulbat, M.; Rudnicka, W. Interleukin 18 (IL-18) as a target for immune intervention. Acta Biochim. Pol. 2016, 63, 59–63. [Google Scholar] [CrossRef]
- Lee, D.; Hong, S.K.; Park, S.W.; Hur, D.Y.; Shon, J.H.; Shin, J.G.; Hwang, S.W.; Sung, H.S. Serum levels of IL-18 and sIL-2R in patients with alopecia areata receiving combined therapy with oral cyclosporine and steroids. Exp. Dermatol. 2010, 19, 145–147. [Google Scholar] [CrossRef]
- Skaria, T.; Burgener, J.; Bachli, E.; Schoedon, G. IL-4 Causes Hyperpermeability of Vascular Endothelial Cells through Wnt5A Signaling. PLoS ONE 2016, 11, e0156002. [Google Scholar] [CrossRef]
- Hassani, M.; Koenderman, L. Immunological and hematological effects of IL-5(Rα)-targeted therapy: An overview. Allergy 2018, 73, 1979–1988. [Google Scholar] [CrossRef]
- Jones, B.E.; Maerz, M.D.; Buckner, J.H. IL-6: A cytokine at the crossroads of autoimmunity. Curr. Opin. Immunol. 2018, 55, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Marlow, G.J.; van Gent, D.; Ferguson, L.R. Why interleukin-10 supplementation does not work in Crohn’s disease patients. World J. Gastroenterol. 2013, 19, 3931–3941. [Google Scholar] [CrossRef]
- Rojas-Zuleta, W.G.; Sanchez, E. IL-9: Function, Sources, and Detection. Methods Mol. Biol. 2017, 1585, 21–35. [Google Scholar] [CrossRef]
- Martínez-Reyes, C.P.; Gómez-Arauz, A.Y.; Torres-Castro, I.; Manjarrez-Reyna, A.N.; Palomera, L.F.; Olivos-García, A.; Mendoza-Tenorio, E.; Sánchez-Medina, G.A.; Islas-Andrade, S.; Melendez-Mier, G.; et al. Serum Levels of Interleukin-13 Increase in Subjects with Insulin Resistance but Do Not Correlate with Markers of Low-Grade Systemic Inflammation. J. Diabetes Res. 2018, 2018, 7209872. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. IL-13 Effector Functions. Annu. Rev. Immunol. 2003, 21, 425–456. [Google Scholar] [CrossRef] [PubMed]
- Takamori, A.; Nambu, A.; Sato, K.; Yamaguchi, S.; Matsuda, K.; Numata, T.; Sugawara, T.; Yoshizaki, T.; Arae, K.; Morita, H.; et al. IL-31 is crucial for induction of pruritus, but not inflammation, in contact hypersensitivity. Sci. Rep. 2018, 8, 6639. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, E.; Ventura-Spagnolo, E.; Casciaro, M.; Navarra, M.; Gangemi, S. IL-33/IL-31 Axis: A Potential Inflammatory Pathway. Mediat. Inflamm. 2018, 2018, 3858032. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Kochumon, S.; Al-Ozairi, E.; Tuomilehto, J.; Ahmad, R. Association between Adipose Tissue Interleukin-33 and Immunometabolic Markers in Individuals with Varying Degrees of Glycemia. Dis. Markers 2019, 2019, 7901062. [Google Scholar] [CrossRef]
- Kuwabara, T.; Ishikawa, F.; Kondo, M.; Kakiuchi, T. The Role of IL-17 and Related Cytokines in Inflammatory Autoimmune Diseases. Mediat. Inflamm 2017, 2017, 3908061. [Google Scholar] [CrossRef] [PubMed]
- El-Morsy, E.H.; Eid, A.A.; Ghoneim, H.; Al-Tameemi, K.A. Serum level of interleukin-17A in patients with alopecia areata and its relationship to age. Int. J. Dermatol. 2016, 55, 869–874. [Google Scholar] [CrossRef]
- Elela, M.A.; Gawdat, H.I.; Hegazy, R.A.; Fawzy, M.M.; Abdel Hay, R.M.; Saadi, D.; Shaker, O. B cell activating factor and T-helper 17 cells: Possible synergistic culprits in the pathogenesis of Alopecia Areata. Arch. Dermatol. Res. 2016, 308, 115–121. [Google Scholar] [CrossRef]
- Hatif, S.T.; Hussein, T.A.; Muhieldden, A.A.R. Evaluation of il17-a serum levels in iraqi patients with alopecia areata and alopecia universalis. Biochem. Cell Arch. 2020, 20, 6419–6425. [Google Scholar]
- Morsy, H.; Maher, R.; Negm, D. Correlation between serum IL-17A level and SALT score in patients with alopecia areata before and after NB-UVB therapy. J. Cosmet. Dermatol. 2018, 17, 533–537. [Google Scholar] [CrossRef]
- Chang, S.H.; Dong, C. IL-17F: Regulation, signaling and function in inflammation. Cytokine 2009, 46, 7–11. [Google Scholar] [CrossRef]
- Leonard, W.J.; Wan, C.-K. IL-21 Signaling in Immunity. F1000Research 2016, 5, F1000 Faculty Rev-1224. [Google Scholar] [CrossRef]
- Xuan, X.; Zhang, L.; Tian, C.; Wu, T.; Ye, H.; Cao, J.; Chen, F.; Liang, Y.; Yang, H.; Huang, C. Interleukin-22 and connective tissue diseases: Emerging role in pathogenesis and therapy. Cell Biosci. 2021, 11, 2. [Google Scholar] [CrossRef]
- Umezawa, N.; Kawahata, K.; Mizoguchi, F.; Kimura, N.; Yoshihashi-Nakazato, Y.; Miyasaka, N.; Kohsaka, H. Interleukin-23 as a therapeutic target for inflammatory myopathy. Sci. Rep. 2018, 8, 5498. [Google Scholar] [CrossRef] [PubMed]
- Sanjabi, S.; Zenewicz, L.A.; Kamanaka, M.; Flavell, R.A. Anti-inflammatory and pro-inflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunity. Curr. Opin. Pharmacol. 2009, 9, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Conic, R.R.Z.; Chu, S.; Tamashunas, N.L.; Damiani, G.; Bergfeld, W. Prevalence of cardiac and metabolic diseases among patients with alopecia areata. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e128–e129. [Google Scholar] [CrossRef] [PubMed]
| Cytokine | An Increased Serum Level (Number of Patients with Alopecia Areata) | A Decreased Serum Level (Number of Patients with Alopecia Areata) | Comparable to Healthy Controls (Number of Patients with Alopecia Areata) |
|---|---|---|---|
| IL-2 | Teraki et al., 1996 (14), Barahmani et al., 2010 (269), Tembhre et al., 2013 (51) Alzolibani et al., 2016 (25), Kasumagić-Halilovic et al., 2018 (60), Gautam et al., 2020 (40), Aşkın et al., 2021 (61) | Tabara et al., 2019 (42) | Gong et al., 2020 (33) |
| IFN-γ | Omar et al., 2021 (72), Teraki et al., 1996 (14), Arca et al., 2004 (40), Barahmani et al., 2010 (269), Kasumagic-Halilovic et al., 2010 (60), Tembhre et al., 2013 (51), Ma et al., 2017 (100), Song et al., 2018 (30), Tabara et al., 2019 (42), Gong et al., 2020 (33), Manimaran et al., 2020 (33), Tomaszewska et al., 2020 (30), Gong et al., 2021 (33) | - | Loh et al., 2018 (55), Bain et al., 2020 (39) |
| TNF | Omar et al., 2021 (72), Alzolibani et al., 2016 (25), Atwa et al., 2016 (47), Bilgic et al., 2016 (40), Kasumagic-Halilovic et al., 2011 (60), Loh et al., 2018 (55), Bain et al., 2020 (39) | - | Barahmani et al., 2010 (269) |
| IL-12 | Barahmani et al., 2010 (269), Gong et al., 2020 (33) | - | - |
| IL-18 | Lee et al., 2010 (21) | - | Chodorowska et al., 2007 (14), Barahmani et al., 2010 (269), Gong et al., 2020 (33) |
| Cytokine | An Increased Serum Level (Number of Patients with Alopecia Areata) | A Decreased Serum Level (Number of Patients with Alopecia Areata) | Comparable to Healthy Controls (Number of Patients with Alopecia Areata) |
|---|---|---|---|
| IL-4 | Teraki et al., 1996 (14), Attia et al., 2010 (54), Manimaran et al., 2020 (33), Aşkın et al., 2021 (61) | Gautam et al., 2020 (40) | Barahmani et al., 2010 (269), Alzolibani et al., 2016 (25), Gong et al., 2020 (33) |
| IL-5 | - | Gong et al., 2020 (33), Gong et al., 2021 (33) | Alzolibani et al., 2016 (25) |
| IL-6 | Barahmani et al., 2010 (269), Atwa et al., 2016 (47), Bilgic et al., 2016 (40), Tabara et al., 2019 (42), Bain et al., 2020 (39), Tomaszewska et al., 2020 (30) | - | Ataseven et al., 2011 (43) |
| IL-9 | Manimaran et al., 2020 (33) | - | Barahmani et al., 2010 (269) |
| IL-10 | Cho et al., 2006 (21), Barahmani et al., 2010 (269), Bain et al., 2020 (39), Gautam et al., 2020 (40) | Ma et al., 2017 (100) | Ataseven et al., 2011 (43), Tembhre et al., 2013 (51), Gong et al., 2020 (33) |
| IL-13 | Tembhre et al., 2013 (51), Song et al., 2018 (30), Manimaran et al., 2020 (33) | Loh et al., 2018 (55) | Barahmani et al., 2010, Gong et al., 2020 |
| IL-17E (IL-25) | Bain et al., 2020 (39) | - | - |
| IL-31 | Bain et al., 2020 (39) | - | - |
| IL-33 | Bain et al., 2020 (39) | - | - |
| Cytokine | An Increased Serum Level (Number of Patients with Alopecia Areata) | A Decreased Serum Level (Number of Patients with Alopecia Areata) | Comparable to Healthy Controls (Number of Patients with Alopecia Areata) |
|---|---|---|---|
| IL-17 | Tembhre et al., 2013 (51), Alzolibani et al., 2016 (25), Atwa et al., 2016 (47) El-Morsy et al., 2016 (39), Elela et al., 2016 (40), Loh et al., 2018 (55), Tabara et al., 2019 (42), Bain et al., 2020 (39), Gautam et al., 2020 (40), Hatif et al., 2020 (58), Manimaran et al., 2020 (33) | - | Morsy et al., 2018 (20), Gong et al., 2020 (33), Aşkın et al., 2021 (61) |
| IL-17F | Bain et al., 2020 (39) | - | - |
| IL-21 | Atwa et al., 2016 (47), Bain et al., 2020 (39) | - | - |
| IL-22 | Atwa et al., 2016 (47) | - | Loh et al., 2018 (55), Gong et al., 2020 (33) |
| IL-23 | Bilgic et al., 2016 (40), Bain et al., 2020 (39) | - | Loh et al., 2018 (55), Gautam et al., 2020 (40) |
| TGF-β | Loh et al., 2018 (55), Manimaran et al., 2020 (33) | Tembhre et al., 2013 (51), Alzolibani et al., 2016 (25) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waśkiel-Burnat, A.; Osińska, M.; Salińska, A.; Blicharz, L.; Goldust, M.; Olszewska, M.; Rudnicka, L. The Role of Serum Th1, Th2, and Th17 Cytokines in Patients with Alopecia Areata: Clinical Implications. Cells 2021, 10, 3397. https://doi.org/10.3390/cells10123397
Waśkiel-Burnat A, Osińska M, Salińska A, Blicharz L, Goldust M, Olszewska M, Rudnicka L. The Role of Serum Th1, Th2, and Th17 Cytokines in Patients with Alopecia Areata: Clinical Implications. Cells. 2021; 10(12):3397. https://doi.org/10.3390/cells10123397
Chicago/Turabian StyleWaśkiel-Burnat, Anna, Marta Osińska, Anna Salińska, Leszek Blicharz, Mohamad Goldust, Małgorzata Olszewska, and Lidia Rudnicka. 2021. "The Role of Serum Th1, Th2, and Th17 Cytokines in Patients with Alopecia Areata: Clinical Implications" Cells 10, no. 12: 3397. https://doi.org/10.3390/cells10123397
APA StyleWaśkiel-Burnat, A., Osińska, M., Salińska, A., Blicharz, L., Goldust, M., Olszewska, M., & Rudnicka, L. (2021). The Role of Serum Th1, Th2, and Th17 Cytokines in Patients with Alopecia Areata: Clinical Implications. Cells, 10(12), 3397. https://doi.org/10.3390/cells10123397









