A Novel Megakaryocyte Subpopulation Poised to Exert the Function of HSC Niche as Possible Driver of Myelofibrosis
Abstract
:1. Introduction
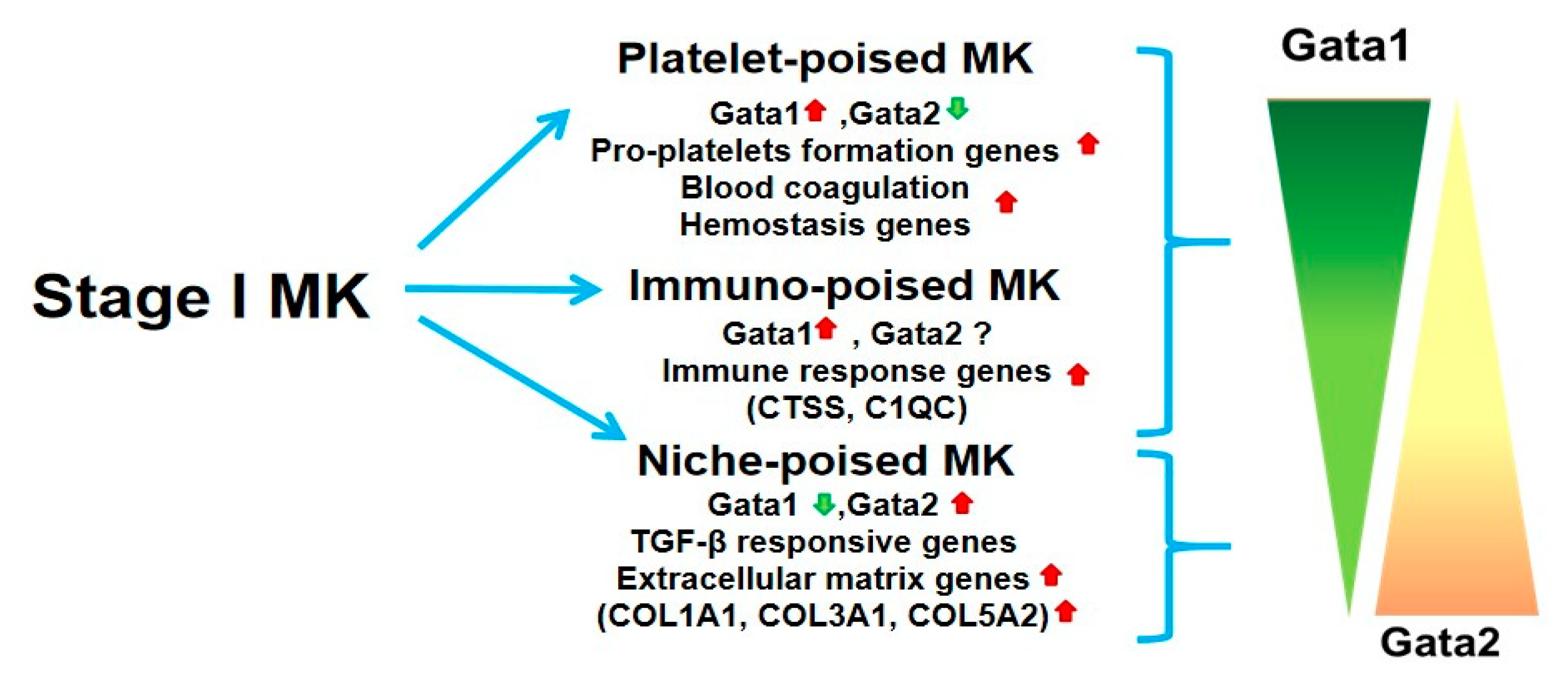
2. Novel Insights into the Megakaryocyte Differentiation Pathway Have Identified a Novel Population of Cells Poised to Exert Niche Functions Which May Represent Drivers for Myelofibrosis
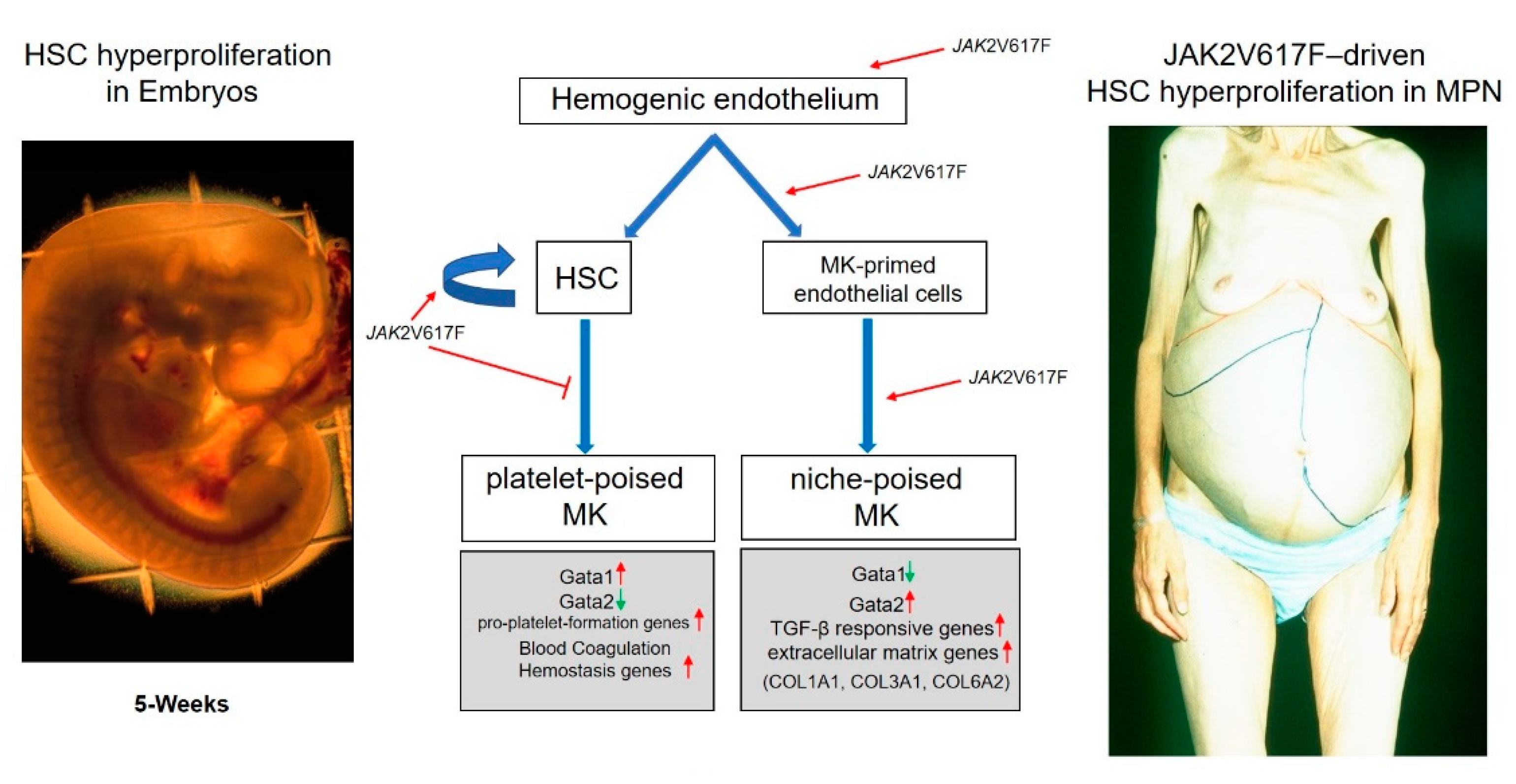
3. Endothelial Cells (EC), a New Source of Megakaryocyte Precursors Which May Be Responsible to Generate the Megakaryocytes That Drive Myelofibrosis
4. Conclusions
Funding
Conflicts of Interest
References
- Tefferi, A. Primary myelofibrosis: 2021 update on diagnosis, risk-stratification and management. Am. J. Hematol. 2021, 96, 145–162. [Google Scholar] [CrossRef]
- Mascarenhas, J.; Hoffman, R. A comprehensive review and analysis of the effect of ruxolitinib therapy on the survival of patients with myelofibrosis. Blood 2013, 121, 4832–4837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zucker-Franklin, D. Ultrastructural studies of hematopoietic elements in relation to the myelofibrosis-osteosclerosis syndrome, megakaryocytes and platelets (MMM or MOS). Adv. Biosci. 1975, 16, 127–143. [Google Scholar]
- Vannucchi, A.M.; Pancrazzi, A.; Guglielmelli, P.; Di Lollop, S.; Bogani, C.; Baroni, G.; Bianchi, L.; Migliaccio, A.R.; Bosi, A.; Paoletti, F. Abnormalities of GATA-1 in megakaryocytes from patients with idiopathic myelofibrosis. Am. J. Pathol. 2005, 167, 849–858. [Google Scholar] [CrossRef] [Green Version]
- Gilles, L.; Arslan, A.D.; Marinaccio, C.; Wen, Q.J.; Arya, P.; McNulty, M.; Yang, Q.; Zhao, J.C.; Konstantinoff, K.; Lasho, T.; et al. Downregulation of GATA1 drives impaired hematopoiesis in primary myelofibrosis. J. Clin. Investig. 2017, 127, 1316–1320. [Google Scholar] [CrossRef]
- Shivdasani, R.A. Molecular and Transcriptional Regulation of Megakaryocyte Differentiation. Stem Cells 2001, 19, 397–407. [Google Scholar] [CrossRef]
- Shivdasani, R.A.; Fujiwara, Y.; McDevitt, M.A.; Orkin, S.H. A lineage-selective knockout establishes the critical role of transcription factor GATA-1 in megakaryocyte growth and platelet development. EMBO J. 1997, 16, 3965–3973. [Google Scholar] [CrossRef] [Green Version]
- Vyas, P.; Ault, K.; Jackson, C.W.; Orkin, S.H.; Shivdasani, R.A. Consequences of GATA-1 deficiency in megakaryocytes and platelets. Blood 1999, 93, 2867–2875. [Google Scholar] [CrossRef]
- Vannucchi, A.M.; Bianchi, L.; Cellai, C.; Paoletti, F.; Rana, R.A.; Lorenzini, R.; Migliaccio, G.; Migliaccio, A.R. Development of myelofibrosis in mice genetically impaired for GATA-1 expression (GATA-1low mice). Blood 2002, 100, 1123–1132. [Google Scholar] [CrossRef]
- Schmitt, A.; Jouault, H.; Guichard, J.; Wendling, F.; Drouin, A.; Cramer, E.M. Pathologic interaction between megakaryocytes and polymorphonuclear leukocytes in myelofibrosis. Blood 2000, 96, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Le Bousse-Kerdilès, M.C.; Chevillard, S.; Charpentier, A.; Romquin, N.; Clay, D.; Smadja-Joffe, F.; Praloran, V.; Dupriez, B.; Demory, J.L.; Jasmin, C.; et al. Differential expression of transforming growth factor-beta, basic fibroblast growth factor, and their receptors in CD34+ hematopoietic progenitor cells from patients with myelofibrosis and myeloid metaplasia. Blood 1996, 88, 4534–4546. [Google Scholar] [CrossRef] [Green Version]
- Campanelli, R.; Rosti, V.; Villani, L.; Castagno, M.; Moretti, E.; Bonetti, E.; Bergamaschi, G.; Balduini, A.; Barosi, G.; Massa, M. Evaluation of the bioactive and total transforming growth factor β1 levels in primary myelofibrosis. Cytokine 2011, 53, 100–106. [Google Scholar] [CrossRef]
- Zingariello, M.; Martelli, F.; Ciaffoni, F.; Masiello, F.; Ghinassi, B.; Amore, E.D.; Massa, M.; Barosi, G.; Sancillo, L.; Li, X.; et al. Characterization of the TGF-β1 signaling abnormalities in the Gata1 low mouse model of myelo fi brosis. Blood J. Am. Soc. Hematol. 2019, 121, 3345–3364. [Google Scholar] [CrossRef]
- Ling, T.; Crispino, J.D.; Zingariello, M.; Martelli, F.; Migliaccio, A.R. GATA1 insufficiencies in primary myelofibrosis and other hematopoietic disorders: Consequences for therapy. Expert Rev. Hematol. 2018, 11, 169–184. [Google Scholar] [CrossRef]
- Eliades, A.; Papadantonakis, N.; Bhupatiraju, A.; Burridge, K.A.; Johnston-Cox, H.A.; Migliaccio, A.R.; Crispino, J.D.; Lucero, H.A.; Trackman, P.C.; Ravid, K. Control of megakaryocyte expansion and bone marrow fibrosis by lysyl oxidase. J. Biol. Chem. 2011, 286, 27630–27638. [Google Scholar] [CrossRef] [Green Version]
- Jeremy Wen, Q.; Yang, Q.; Goldenson, B.; Malinge, S.; Lasho, T.; Schneider, R.K.; Breyfogle, L.J.; Schultz, R.; Gilles, L.; Koppikar, P.; et al. Targeting megakaryocytic-induced fibrosis in myeloproliferative neoplasms by AURKA inhibition. Nat. Med. 2015, 21, 1473–1480. [Google Scholar] [CrossRef]
- Eran, Z.; Zingariello, M.; Bochicchio, M.T.; Bardelli, C.; Migliaccio, A.R. Novel strategies for the treatment of myelofibrosis driven by recent advances in understanding the role of the microenvironment in its etiology. F1000Research 2019, 8, 1662. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; He, J.; Xu, C.; Chen, X.; Yang, H.; Shi, S.; Liu, C.; Zeng, Y.; Wu, D.; Bai, Z.; et al. Decoding Human Megakaryocyte Development. Cell Stem Cell 2021, 28, 535–549e8. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.K.; Villacorta-Martin, C.; Hon, S.; Rock, J.R.; Murphy, G.J. Lung megakaryocytes display distinct transcriptional and phenotypic properties. Blood Adv. 2020, 4, 6204–6217. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Jin, C.; Si, J.; Lei, Y.; Chen, K.; Cui, Y.; Liu, Z.; Liu, J.; Zhao, M.; Zhang, X.; et al. Single-Cell Analysis of Ploidy and Transcriptome Reveals Functional and Spatial Divergency in Murine Megakaryopoiesis. Blood 2021. [CrossRef] [PubMed]
- Pariser, D.N.; Hilt, Z.T.; Ture, S.K.; Blick-Nitko, S.K.; Looney, M.R.; Cleary, S.J.; Roman-Pagan, E.; Saunders, J.; Georas, S.N.; Veazey, J.; et al. Lung megakaryocytes are immune modulatory cells. J. Clin. Investig. 2021, 131, e1373776. [Google Scholar] [CrossRef] [PubMed]
- Boilard, E.; Machlus, K.R. Location is everything when it comes to megakaryocyte function. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef]
- Lefrançais, E.; Ortiz-Muñoz, G.; Caudrillier, A.; Mallavia, B.; Liu, F.; Sayah, D.M.; Thornton, E.E.; Headley, M.B.; David, T.; Coughlin, S.R.; et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 2017, 544, 105–109. [Google Scholar] [CrossRef]
- Sun, S.; Jin, C.; Li, Y.; Si, J.; Cui, Y.; Rondina, M.T.; Tang, F.; Wang, Q. Transcriptional and Spatial Heterogeneity of Mouse Megakaryocytes at Single-Cell Resolution. Blood 2019, 134, 275. [Google Scholar] [CrossRef]
- Liu, C.; Huang, B.; Wang, H.; Zhou, J. The heterogeneity of megakaryocytes and platelets and implications for ex vivo platelet generation. Stem Cells Transl Med. 2021, 1–7. [Google Scholar] [CrossRef]
- Verstovsek, S.; Manshouri, T.; Pilling, D.; Bueso-Ramos, C.E.; Newberry, K.J.; Prijic, S.; Knez, L.; Bozinovic, K.; Harris, D.M.; Spaeth, E.L.; et al. Role of neoplastic monocyte-derived fibrocytes in primary myelofibrosis. J. Exp. Med. 2016, 213, 1723–1740. [Google Scholar] [CrossRef]
- Abbonante, V.; Di Buduo, C.A.; Gruppi, C.; Malara, A.; Gianelli, U.; Celesti, G.; Anselmo, A.; Laghi, L.; Vercellino, M.; Visai, L.; et al. Thrombopoietin/TGF-β1 Loop Regulates Megakaryocyte Extracellular Matrix Component Synthesis. Stem Cells 2016, 34, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Malara, A.; Currao, M.; Gruppi, C.; Celesti, G.; Viarengo, G.; Buracchi, C.; Laghi, L.; Kaplan, D.L.; Balduini, A. Megakaryocytes contribute to the bone marrow-matrix environment by expressing fibronectin, type IV collagen, and laminin. Stem Cells 2014, 32, 926–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manshouri, T.; Verstovsek, S.; Harris, D.M.; Veletic, I.; Zhang, X.; Post, S.M.; Bueso-Ramos, C.E.; Estrov, Z. Primary myelofibrosis marrow-derived CD14+/CD34- monocytes induce myelofibrosis-like phenotype in immunodeficient mice and give rise to megakaryocytes. PLoS ONE 2019, 14, e0222912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzarini, M.; Verachi, P.; Martelli, F.; Migliaccio, A.R. Role of β1 integrin in thrombocytopoiesis. Fac. Rev. 2021, 10. [Google Scholar] [CrossRef]
- Smith, B.W.; Murphy, G.J. Stem cells, megakaryocytes, and platelets. Curr. Opin. Hematol. 2014, 21, 430–437. [Google Scholar] [CrossRef]
- Malara, A.; Abbonante, V.; Zingariello, M.; Migliaccio, A.; Balduini, A. Megakaryocyte contribution to bone marrow fibrosis: Many arrows in the quiver. Mediterr J. Hematol Infect. Dis 2018, 10, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Sozer, S.; Fiel, M.I.; Schiano, T.; Xu, M.; Mascarenhas, J.; Hoffman, R. The presence of JAK2V617F mutation in the liver endothelial cells of patients with Budd-Chiari syndrome. Blood 2009, 113, 5246–5249. [Google Scholar] [CrossRef]
- Rosti, V.; Villani, L.; Riboni, R.; Poletto, V.; Bonetti, E.; Tozzi, L.; Bergamaschi, G.; Catarsi, P.; Dallera, E.; Novara, F.; et al. Spleen endothelial cells from patients with myelofibrosis harbor the JAK2V617F mutation. Blood 2013, 121, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.S.; Zhang, Y.; Kaushansky, K.; Zhan, H. JAK2V617F -bearing vascular niche enhances malignant hematopoietic regeneration following radiation injury. Haematologica 2018, 103, 1160–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, H.; Ma, Y.; Lin, C.H.S.; Kaushansky, K. JAK2(V617F)-mutant megakaryocytes contribute to hematopoietic stem/progenitor cell expansion in a model of murine myeloproliferation. Leukemia 2016, 30, 2332–2341. [Google Scholar] [CrossRef] [Green Version]
- Woods, B.; Chen, W.; Chiu, S.; Marinaccio, C.; Fu, C.; Gu, L.; Bulic, M.; Yang, Q.; Zouak, A.; Jia, S.; et al. Activation of JAK/STAT Signaling in Megakaryocytes Sustains Myeloproliferation In Vivo. Clin. Cancer Res. 2019, 25, 5901–5912. [Google Scholar] [CrossRef]
- Adamson, J.W.; Fialkow, P.J.; Murphy, S.; Prchal, J.F.; Steinmann, L. Polycythemia Vera: Stem-Cell and Probable Clonal Origin of the Disease. N Engl J. Med. 1976, 295, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Dzierzak, E.; Bigas, A. Blood Development: Hematopoietic Stem Cell Dependence and Independence. Cell Stem Cell 2018, 22, 639–651. [Google Scholar] [CrossRef] [Green Version]
- Tavian, M.; Hallais, M.F.; Péault, B. Emergence of intraembryonic hematopoietic precursors in the pre-liver human embryo. Development 1999, 126, 793–803. [Google Scholar] [CrossRef]
- Zambidis, E.T.; Peault, B.; Park, T.S.; Bunz, F.; Civin, C.I. Hematopoietic differentiation of human embryonic stem cells progresses through sequential hematoendothelial, primitive, and definitive stages resembling human yolk sac development. Blood 2005, 106, 860–870. [Google Scholar] [CrossRef] [Green Version]
- Zahr, A.A.; Salama, M.E.; Carreau, N.; Tremblay, D.; Verstovsek, S.; Mesa, R.; Hoffman, R.; Mascarenhas, J. Bone marrow fibrosis in myelofibrosis: Pathogenesis, prognosis and targeted strategies. Haematologica 2016, 101, 660–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, M.; Sternberg, A.; Hall, G.; Thomas, A.; Smith, O.; O’Marcaigh, A.; Wynn, R.; Stevens, R.; Addison, M.; King, D.; et al. Natural history of GATA1 mutations in Down syndrome. Blood 2004, 103, 2480–2489. [Google Scholar] [CrossRef]
- Li, Z.; Godinho, F.J.; Klusmann, J.-H.; Garriga-Canut, M.; Yu, C.; Orkin, S.H. Developmental stage–selective effect of somatically mutated leukemogenic transcription factor GATA1. Nat. Genet. 2005, 37, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.; Lee, J.; Moore, L.; Baxter, E.J.; Hewinson, J.; Dawson, K.J.; Menzies, A.; Godfrey, A.L.; Green, A.R.; Campbell, P.J.; et al. Phylogenetic reconstruction of myeloproliferative neoplasm reveals very early origins and lifelong evolution. bioRxiv 2020. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migliaccio, A.R. A Novel Megakaryocyte Subpopulation Poised to Exert the Function of HSC Niche as Possible Driver of Myelofibrosis. Cells 2021, 10, 3302. https://doi.org/10.3390/cells10123302
Migliaccio AR. A Novel Megakaryocyte Subpopulation Poised to Exert the Function of HSC Niche as Possible Driver of Myelofibrosis. Cells. 2021; 10(12):3302. https://doi.org/10.3390/cells10123302
Chicago/Turabian StyleMigliaccio, Anna Rita. 2021. "A Novel Megakaryocyte Subpopulation Poised to Exert the Function of HSC Niche as Possible Driver of Myelofibrosis" Cells 10, no. 12: 3302. https://doi.org/10.3390/cells10123302
APA StyleMigliaccio, A. R. (2021). A Novel Megakaryocyte Subpopulation Poised to Exert the Function of HSC Niche as Possible Driver of Myelofibrosis. Cells, 10(12), 3302. https://doi.org/10.3390/cells10123302




