Heat-Killed Lactobacilli Preparations Promote Healing in the Experimental Cutaneous Wounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Probiotics Preparations
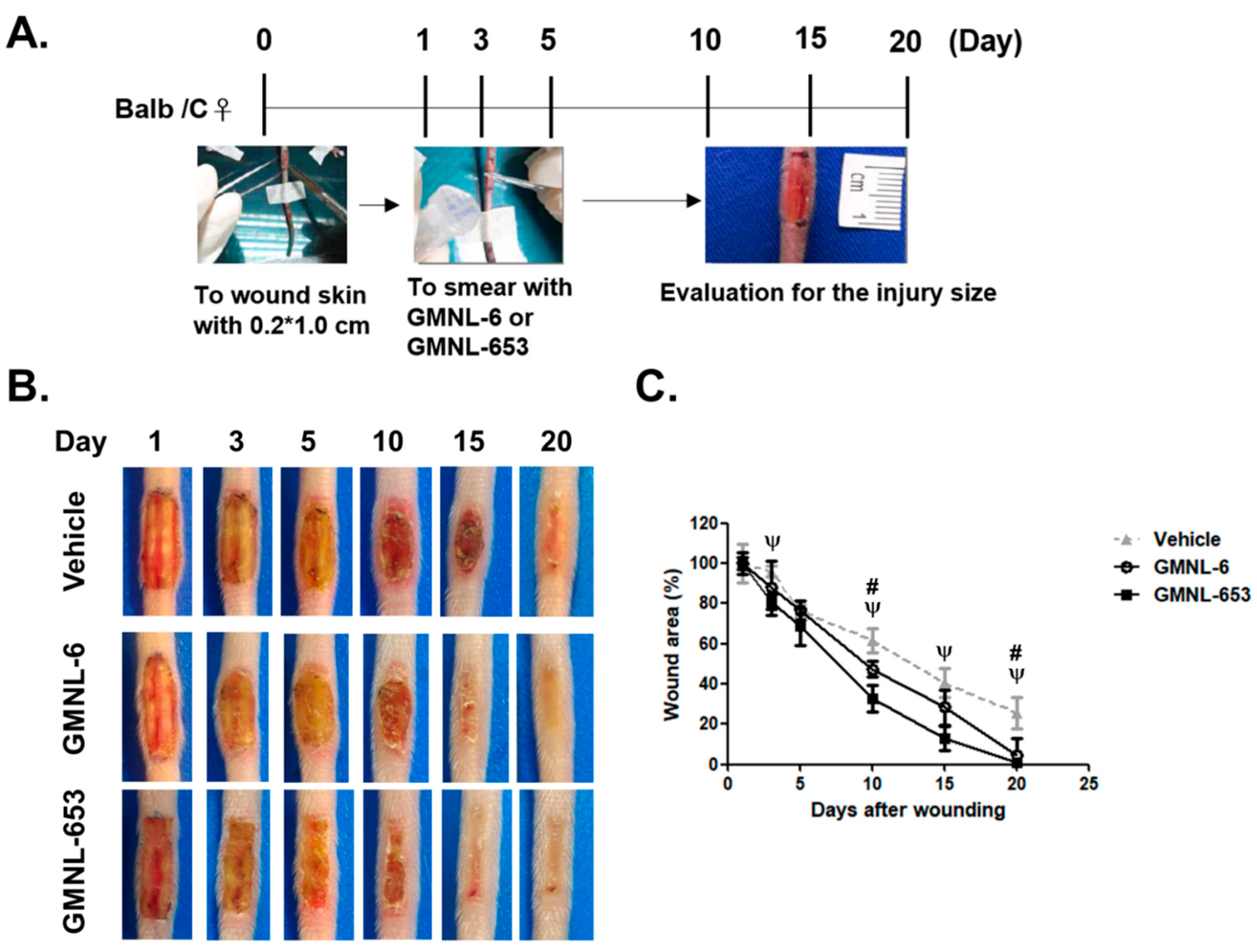
2.2. Tail Full-Thickness Wounding Surgery in a Mouse Model
2.3. Cell Culture
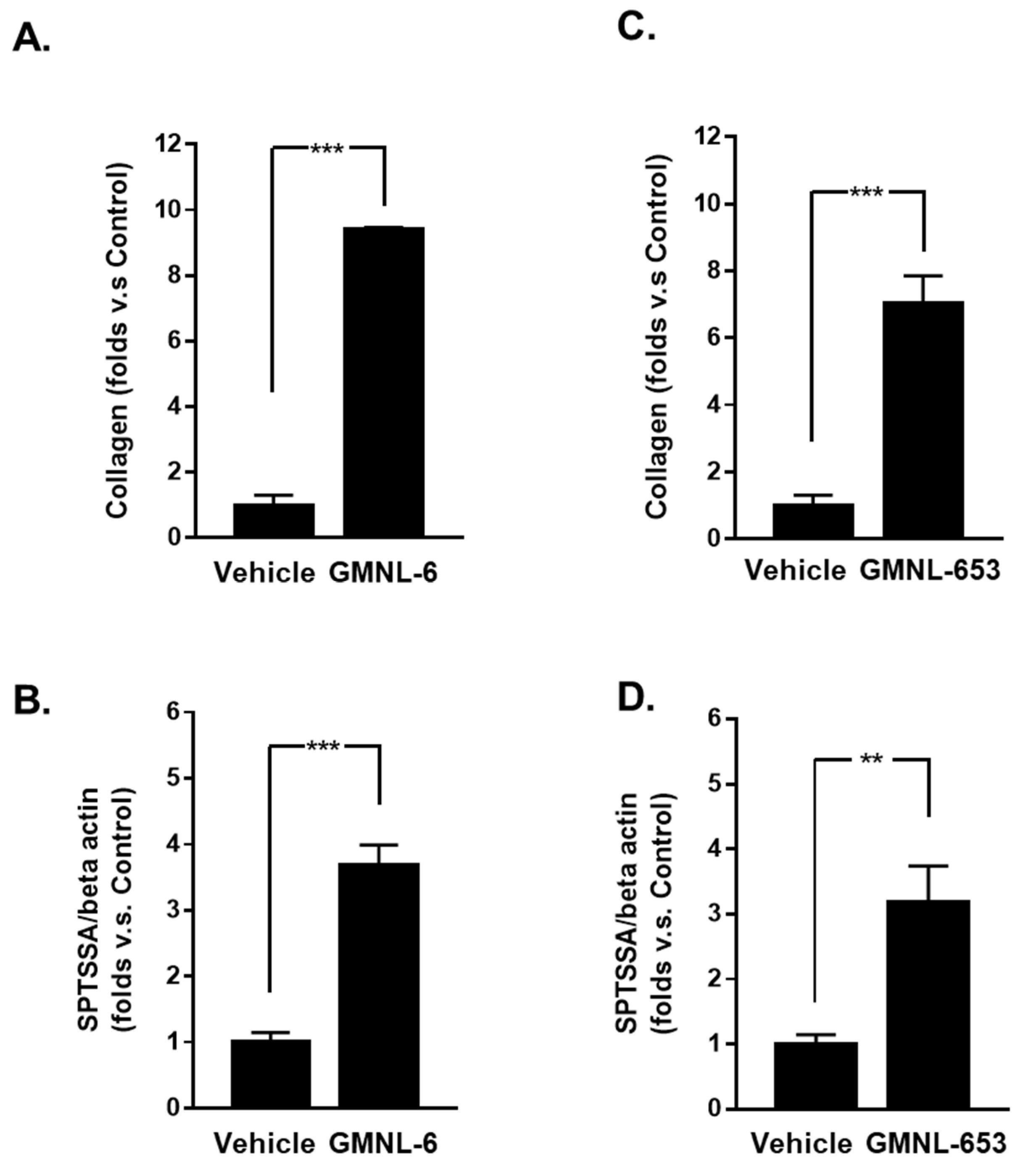
2.4. Synthesis Assay of Collagen
2.5. Detection of the Neuronal Amine Synthase Ability
2.6. Western Blot Analysis
2.7. Histological and Immunohistochemical Assessment
2.8. Masson’s Trichrome Staining
2.9. Statistical Analyses
3. Results
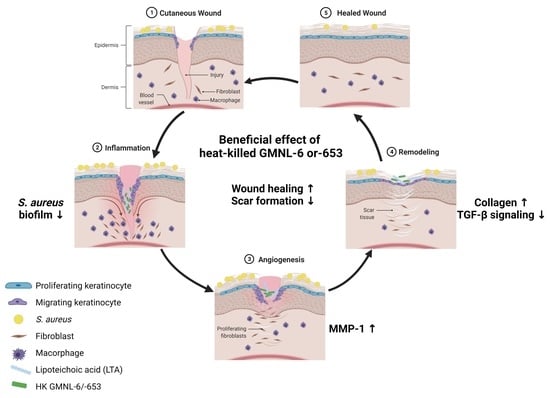
3.1. Heat-Killed Lactobacillus GMNL-6 or GMNL-653 Provides Beneficial Effects in Skin Repairing
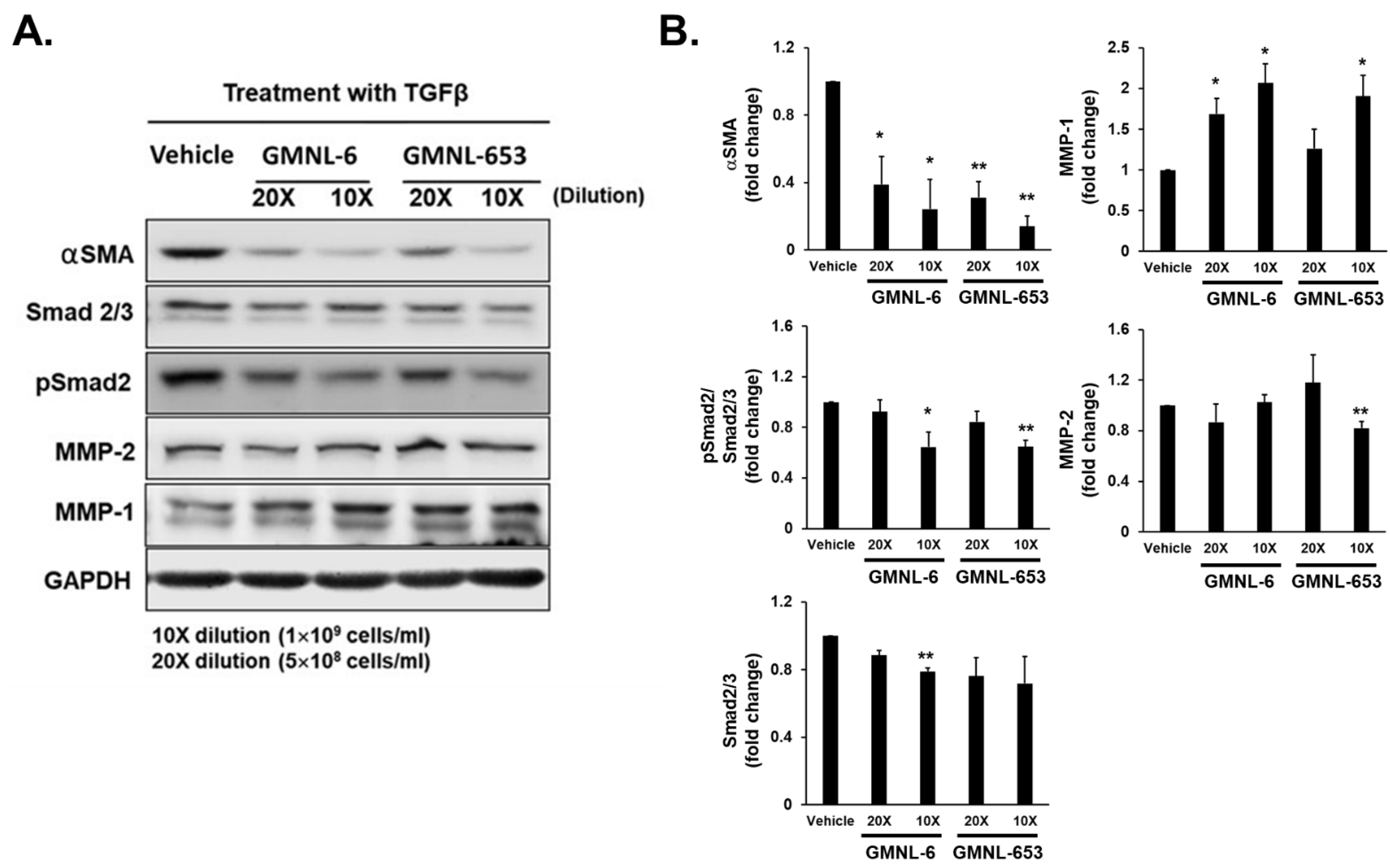
3.2. TGF-β/Smad Signaling Involved in the Heat-Killed Lactobacillus GMNL-6 or GMNL-653 Promoted Wound Healing Ability
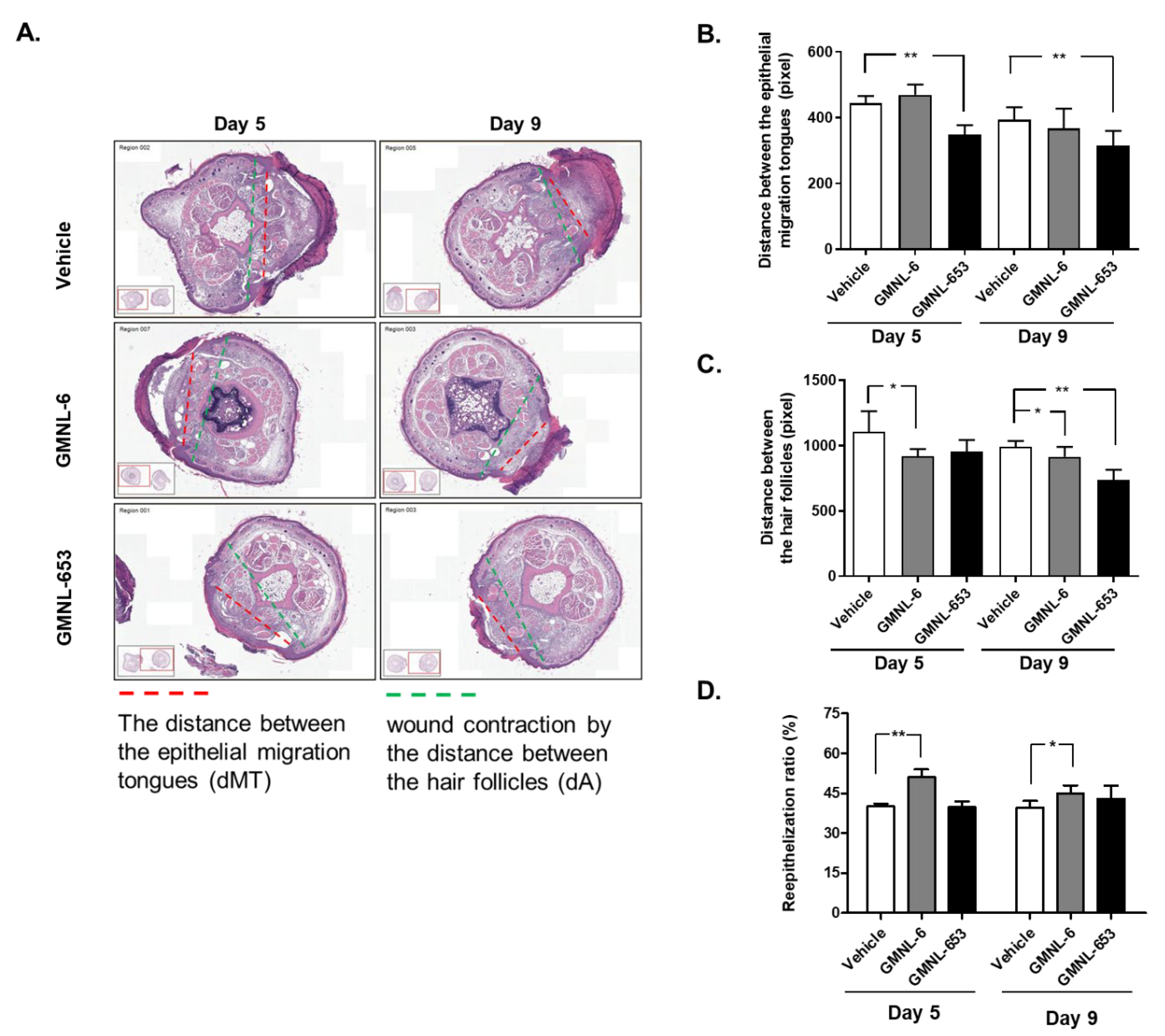
3.3. Heat-Killed Probiotics Preparations of GMNL-6 or GMNL-653 Exhibit Excellent Wound Healing Ability in the Mouse Model of Experimental Tail Wounds
3.4. Heat-Killed Probiotics GMNL-6 or GMNL-653 Preparations Provide Prominent Tail-Wound Healing Recovery Ability
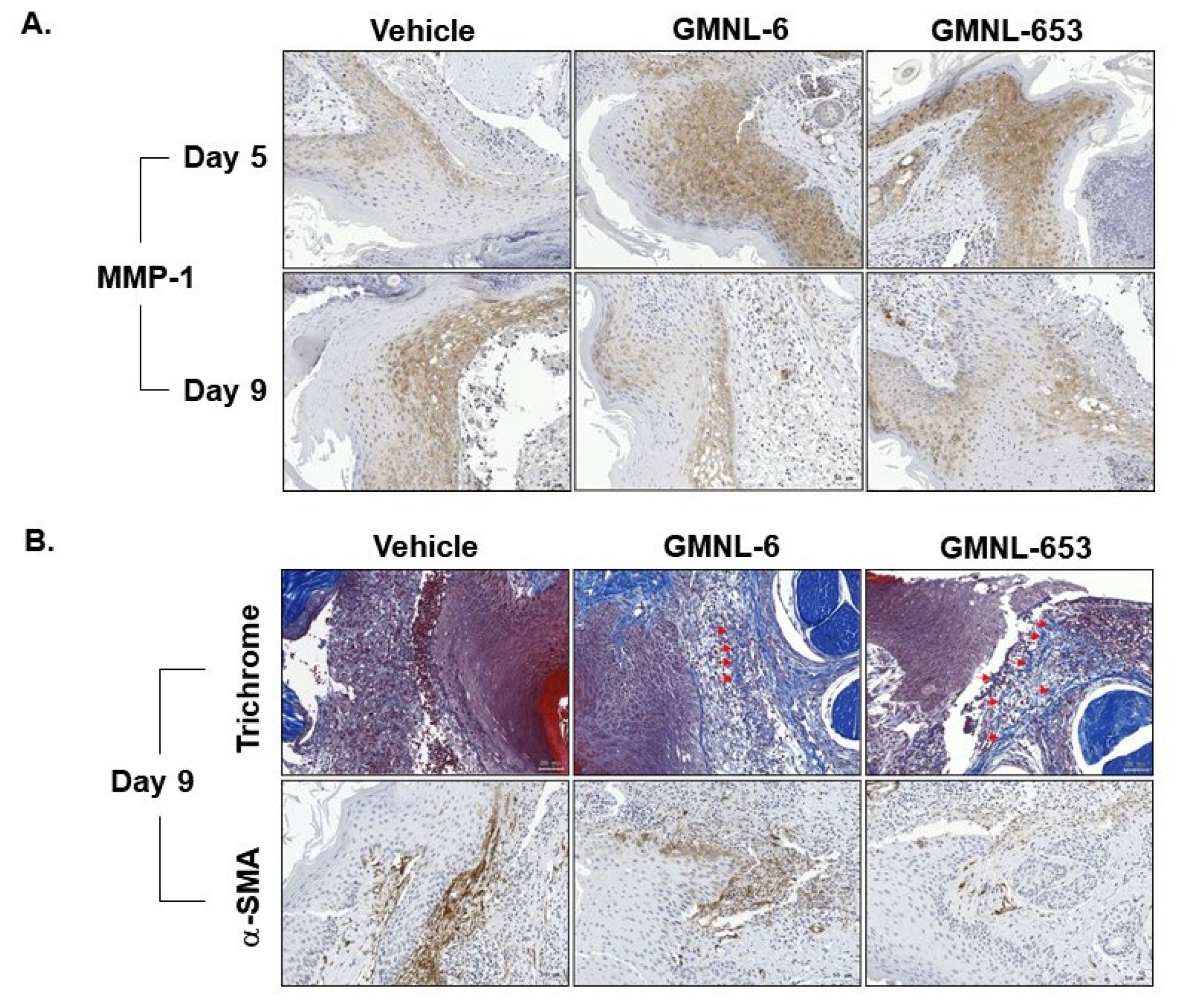
3.5. Heat-Killed Probiotics GMNL-6 or GMNL-653 Gel Preparations Increase MMP-1 Expression in the Early Stage (Day-5) and Decrease the αSMA Expression in the following Stage (Day-9) in the Tail-Wound Healing Mouse Model
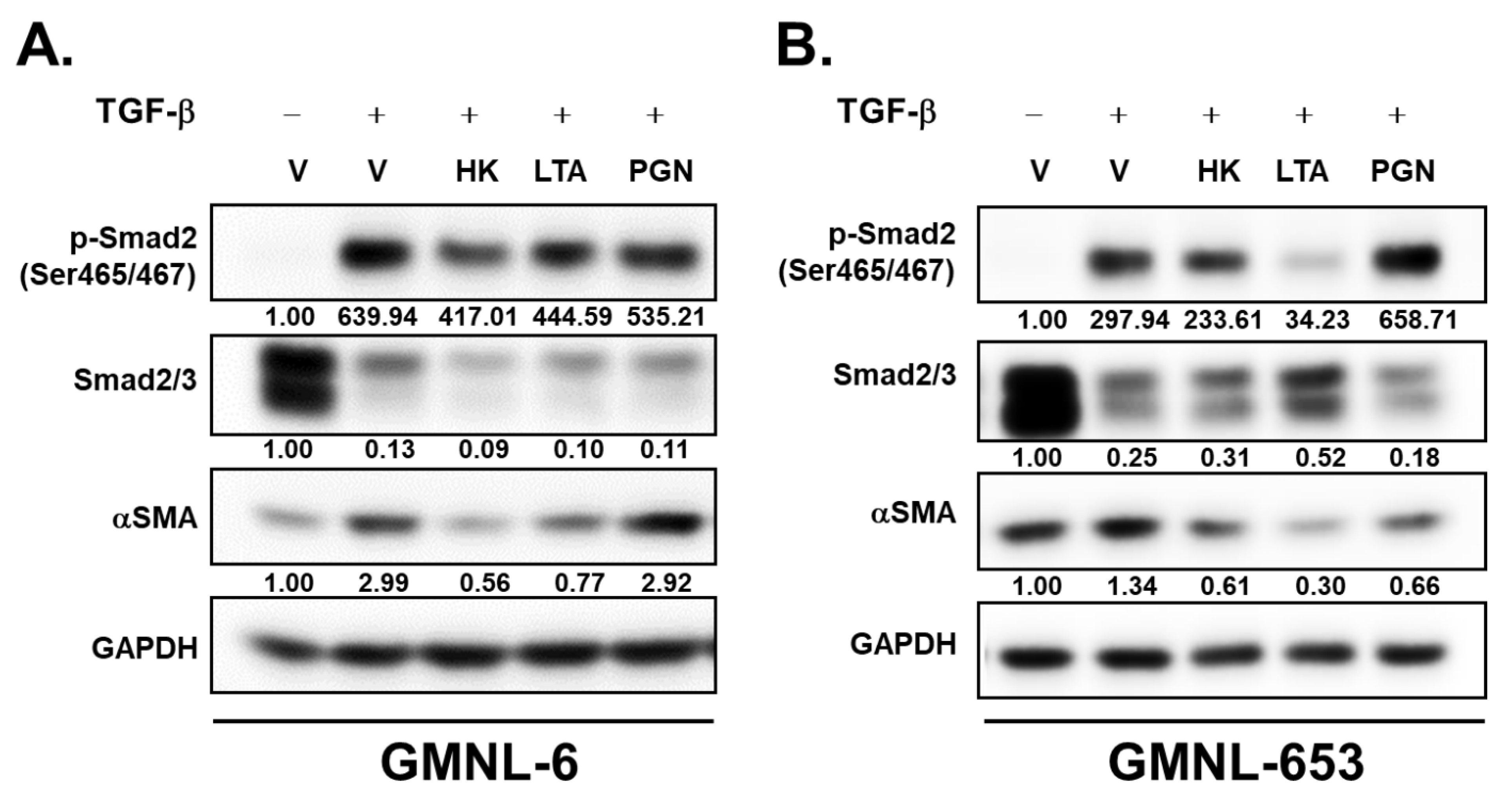
3.6. The Lipoteichoic Acid (LTA) from Cell Wall of GMNL-6 or GMNL-653 Cause the Similar Beneficial Effects to the TGF-β Induced Fibrosis of Hs68 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hernández-Chirlaque, C.; Aranda, C.J.; Ocón, B.; Capitán-Cañadas, F.; Ortega-González, M.; Carrero, J.J.; Suárez, M.D.; Zarzuelo, A.; de Medina, F.S.; Martínez-Augustin, O. Germ-free and antibiotic-treated mice are highly susceptible to epithelial injury in DSS colitis. J. Crohns Colitis 2016, 10, 1324–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hacini-Rachinel, F.; Gheit, H.; Le Luduec, J.B.; Dif, F.; Nancey, S.; Kaiserlian, D. Oral probiotic control skin inflammation by acting on both effector and regulatory T cells. PLoS ONE 2009, 4, e4903. [Google Scholar] [CrossRef] [Green Version]
- Qian, L.; Gao, R.; Huang, J.; Qin, H. Supplementation of triple viable probiotics combined with dietary intervention is associated with gut microbial improvement in humans on a high-fat diet. Exp. Ther. Med. 2019, 18, 2262–2270. [Google Scholar] [CrossRef]
- Tsai, Y.L.; Lin, T.L.; Chang, C.J.; Wu, T.R.; Lai, W.F.; Lu, C.C.; Lai, H.C. Probiotics, prebiotics and amelioration of diseases. J. Biomed. Sci. 2019, 26, 3. [Google Scholar] [CrossRef]
- Ljungh, A.; Wadström, T. Lactic acid bacteria as probiotics. Curr. Issues Intest. Microbiol. 2006, 7, 73–89. [Google Scholar]
- Hsieh, M.C.; Tsai, W.H.; Jheng, Y.P.; Su, S.L.; Wang, S.Y.; Lin, C.C.; Chen, Y.H.; Chang, W.W. The beneficial effects of Lactobacillus reuteri ADR-1 or ADR-3 consumption on type 2 diabetes mellitus: A randomized, double-blinded, placebo-controlled trial. Sci. Rep. 2018, 8, 16791. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Orgill, D.P.; Murphy, G.T. 4-The pathophysiologic basis for wound healing and cutaneous regeneration. In Biomaterials for Treating Skin Loss; Orgill, D.P., Blanco, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 25–57. [Google Scholar]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Pakyari, M.; Farrokhi, A.; Maharlooei, M.K.; Ghahary, A. Critical role of transforming growth factor beta in different phases of wound healing. Adv. Wound Care 2013, 2, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Lazarus, G.S.; Cooper, D.M.; Knighton, D.R.; Margolis, D.J.; Pecoraro, R.E.; Rodeheaver, G.; Robson, M.C. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch. Dermatol. 1994, 130, 489–493. [Google Scholar] [CrossRef] [Green Version]
- Ahn, C.; Salcido, R.S. Advances in wound photography and assessment methods. Adv. Skin Wound Care 2008, 21, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Loots, M.A.; Lamme, E.N.; Zeegelaar, J.; Mekkes, J.R.; Bos, J.D.; Middelkoop, E. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. J. Investig. Dermatol. 1998, 111, 850–857. [Google Scholar] [CrossRef] [Green Version]
- Gould, L.; Abadir, P.; Brem, H.; Carter, M.; Conner-Kerr, T.; Davidson, J.; DiPietro, L.; Falanga, V.; Fife, C.; Gardner, S.; et al. Chronic wound repair and healing in older adults: Current status and future research. Wound Repair Regen. 2015, 23, 427–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Finnson, K.W.; Arany, P.R.; Philip, A. Transforming growth factor beta signaling in cutaneous wound healing: Lessons learned from animal studies. Adv. Wound Care 2013, 2, 225–237. [Google Scholar] [CrossRef] [Green Version]
- Tarnuzzer, R.W.; Schultz, G.S. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen. 1996, 4, 321–325. [Google Scholar] [CrossRef]
- Patel, S.; Maheshwari, A.; Chandra, A. Biomarkers for wound healing and their evaluation. J. Wound Care 2016, 25, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Pastar, I.; Stojadinovic, O.; Krzyzanowska, A.; Barrientos, S.; Stuelten, C.; Zimmerman, K.; Blumenberg, M.; Brem, H.; Tomic-Canic, M. Attenuation of the transforming growth factor beta-signaling pathway in chronic venous ulcers. Mol. Med. 2010, 16, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Takeo, M.; Lee, W.; Ito, M. Wound healing and skin regeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a023267. [Google Scholar] [CrossRef] [PubMed]
- Qing, C. The molecular biology in wound healing & non-healing wound. Chin. J. Traumatol. 2017, 20, 189–193. [Google Scholar]
- Jutley, J.K.; Wood, E.J.; Cunliffe, W.J. Influence of retinoic acid and TGF-beta on dermal fibroblast proliferation and collagen production in monolayer cultures and dermal equivalents. Matrix 1993, 13, 235–241. [Google Scholar] [CrossRef]
- Knackstedt, R.; Knackstedt, T.; Gatherwright, J. The role of topical probiotics on wound healing: A review of animal and human studies. Int. Wound J. 2020, 17, 1687–1694. [Google Scholar] [CrossRef] [PubMed]
- Kothari, D.; Patel, S.; Kim, S.K. Probiotic supplements might not be universally-effective and safe: A review. Biomed. Pharmacother. 2019, 111, 537–547. [Google Scholar] [CrossRef]
- Gerharz, M.; Baranowsky, A.; Siebolts, U.; Eming, S.; Nischt, R.; Krieg, T.; Wickenhauser, C. Morphometric analysis of murine skin wound healing: Standardization of experimental procedures and impact of an advanced multitissue array technique. Wound Repair Regen. 2007, 15, 105–112. [Google Scholar] [CrossRef]
- Tsai, W.H.; Chou, C.H.; Chiang, Y.J.; Lin, C.G.; Lee, C.H. Regulatory effects of Lactobacillus plantarum-GMNL6 on human skin health by improving skin microbiome. Int. J. Med. Sci. 2021, 18, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.S.; Tager, A.M. Sphingolipid regulation of tissue fibrosis. Open Rheumatol. J. 2012, 6, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Bochaton-Piallat, M.L.; Gabbiani, G.; Hinz, B. The myofibroblast in wound healing and fibrosis: Answered and unanswered questions. F1000research 2016, 5, 752. [Google Scholar] [CrossRef] [Green Version]
- Rohani, M.G.; Parks, W.C. Matrix remodeling by MMPs during wound repair. Matrix Biol. 2015, 44, 113–121. [Google Scholar] [CrossRef]
- Grada, A.; Mervis, J.; Falanga, V. Research techniques made simple: Animal models of wound healing. J. Investig. Dermatol. 2018, 138, 2095–2105. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.C.; Tomita, S.; Kleerebezem, M.; Bron, P.A. The quest for probiotic effector molecules—unraveling strain specificity at the molecular level. Pharmacol. Res. 2013, 69, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health benefits of heat-killed (Tyndallized) probiotics: An overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falanga, V.; Schrayer, D.; Cha, J.; Butmarc, J.; Carson, P.; Roberts, A.B.; Kim, S.J. Full-thickness wounding of the mouse tail as a model for delayed wound healing: Accelerated wound closure in Smad3 knock-out mice. Wound Repair Regen. 2004, 12, 320–326. [Google Scholar] [CrossRef]
- Altoé, L.S.; Alves, R.S.; Sarandy, M.M.; Morais-Santos, M.; Novaes, R.D.; Gonçalves, R.V. Does antibiotic use accelerate or retard cutaneous repair? A systematic review in animal models. PLoS ONE 2019, 14, e0223511. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gu, W.; Masinde, G.; Hamilton-Ulland, M.; Xu, S.; Mohan, S.; Baylink, D.J. Genetic control of the rate of wound healing in mice. Heredity 2001, 86, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Pal-Ghosh, S.; Tadvalkar, G.; Jurjus, R.A.; Zieske, J.D.; Stepp, M.A. BALB/c and C57BL6 mouse strains vary in their ability to heal corneal epithelial debridement wounds. Exp. Eye Res. 2008, 87, 478–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.W.; Kang, S.S.; Woo, S.J.; Park, O.J.; Ahn, K.B.; Song, K.D.; Lee, H.K.; Yun, C.H.; Han, S.H. Lipoteichoic acid of probiotic Lactobacillus plantarum Attenuates Poly I:C-Induced IL-8 production in porcine intestinal epithelial cells. Front. Microbiol. 2017, 8, 1827. [Google Scholar] [CrossRef]
- Darby, I.A.; Laverdet, B.; Bonté, F.; Desmoulière, A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301–311. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.M.; Chen, L.; Bond, J.E.; Medina, M.A.; Ren, L.; Kokosis, G.; Selim, A.M.; Levinson, H. Myofibroblasts contribute to but are not necessary for wound contraction. Lab. Investig. 2015, 95, 1429–1438. [Google Scholar] [CrossRef] [Green Version]
- Desjardins-Park, H.E.; Foster, D.S.; Longaker, M.T. Fibroblasts and wound healing: An update. Regen. Med. 2018, 13, 491–495. [Google Scholar] [CrossRef] [Green Version]
- Rhoads, D.D.; Cox, S.B.; Rees, E.J.; Sun, Y.; Wolcott, R.D. Clinical identification of bacteria in human chronic wound infections: Culturing vs. 16S ribosomal DNA sequencing. BMC Infect. Dis. 2012, 12, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, S.; Santra, S.; Das, A.; Dixith, S.; Sinha, M.; Ghatak, S.; Ghosh, N.; Banerjee, P.; Khanna, S.; Mathew-Steiner, S.; et al. Staphylococcus aureus biofilm infection compromises wound healing by causing deficiencies in granulation tissue collagen. Ann. Surg. 2020, 271, 1174–1185. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A. Glucocorticoids inhibit wound healing: Novel mechanism of action. J. Investig. Dermatol. 2017, 137, 1012–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, L. Impaired wound healing in patients with diabetes. Nurs. Stand. 2001, 15, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 diabetes and its impact on the immune system. Curr. Diabetes Rev. 2020, 16, 442–449. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, W.-H.; Chou, C.-H.; Huang, T.-Y.; Wang, H.-L.; Chien, P.-J.; Chang, W.-W.; Lee, H.-T. Heat-Killed Lactobacilli Preparations Promote Healing in the Experimental Cutaneous Wounds. Cells 2021, 10, 3264. https://doi.org/10.3390/cells10113264
Tsai W-H, Chou C-H, Huang T-Y, Wang H-L, Chien P-J, Chang W-W, Lee H-T. Heat-Killed Lactobacilli Preparations Promote Healing in the Experimental Cutaneous Wounds. Cells. 2021; 10(11):3264. https://doi.org/10.3390/cells10113264
Chicago/Turabian StyleTsai, Wan-Hua, Chia-Hsuan Chou, Tsuei-Yin Huang, Hui-Ling Wang, Peng-Ju Chien, Wen-Wei Chang, and Hsueh-Te Lee. 2021. "Heat-Killed Lactobacilli Preparations Promote Healing in the Experimental Cutaneous Wounds" Cells 10, no. 11: 3264. https://doi.org/10.3390/cells10113264
APA StyleTsai, W.-H., Chou, C.-H., Huang, T.-Y., Wang, H.-L., Chien, P.-J., Chang, W.-W., & Lee, H.-T. (2021). Heat-Killed Lactobacilli Preparations Promote Healing in the Experimental Cutaneous Wounds. Cells, 10(11), 3264. https://doi.org/10.3390/cells10113264