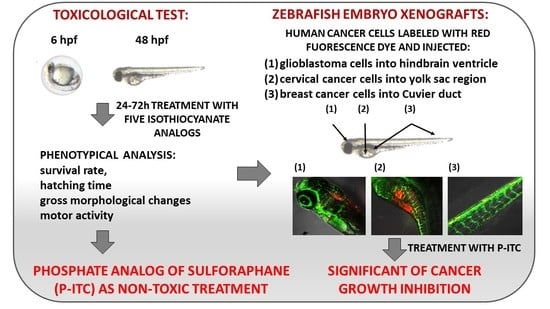
The Anti-Tumoral Potential of Phosphonate Analog of Sulforaphane in Zebrafish Xenograft Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry
2.2. Zebrafish Maintenance
2.3. Cell Culture
2.4. Embryotoxic Test of Isothiocyanates in Zebrafish
2.5. Microinjection of Human Tumor Cells into Zebrafish Embryos
2.6. Xenograft Exposure Assay
2.7. Statistical Analysis
3. Results
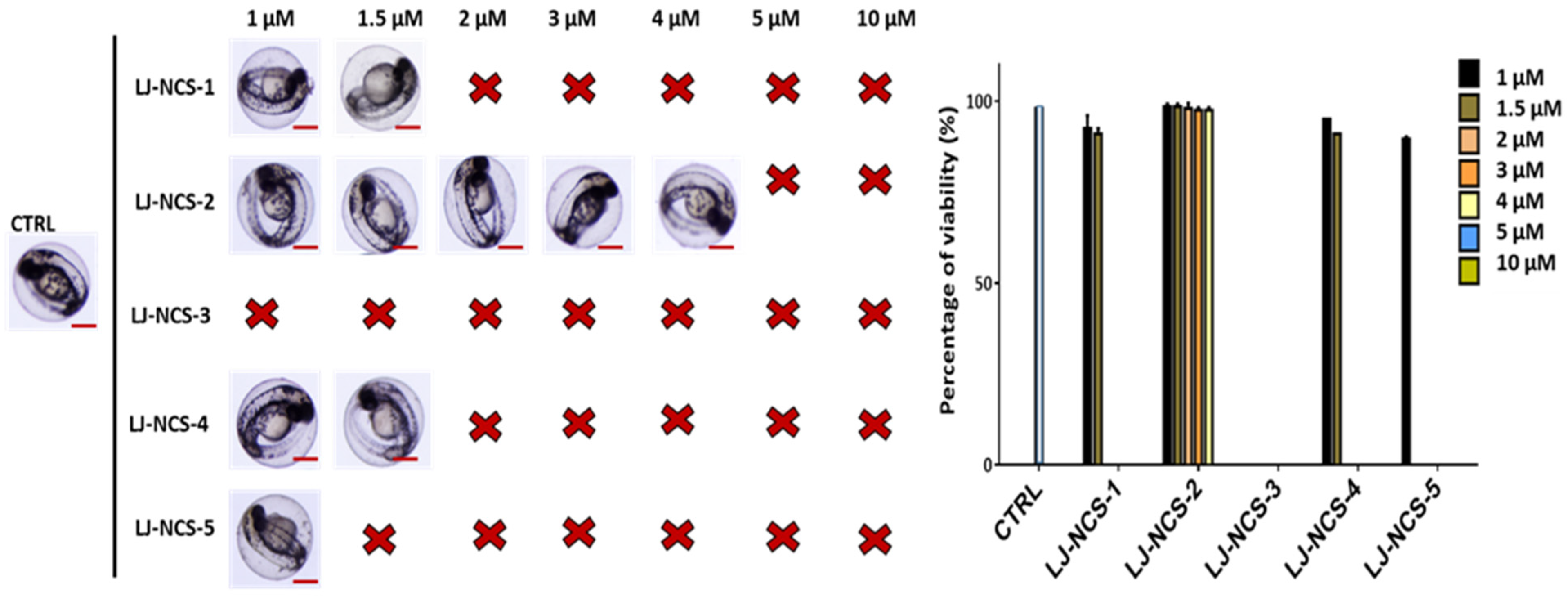
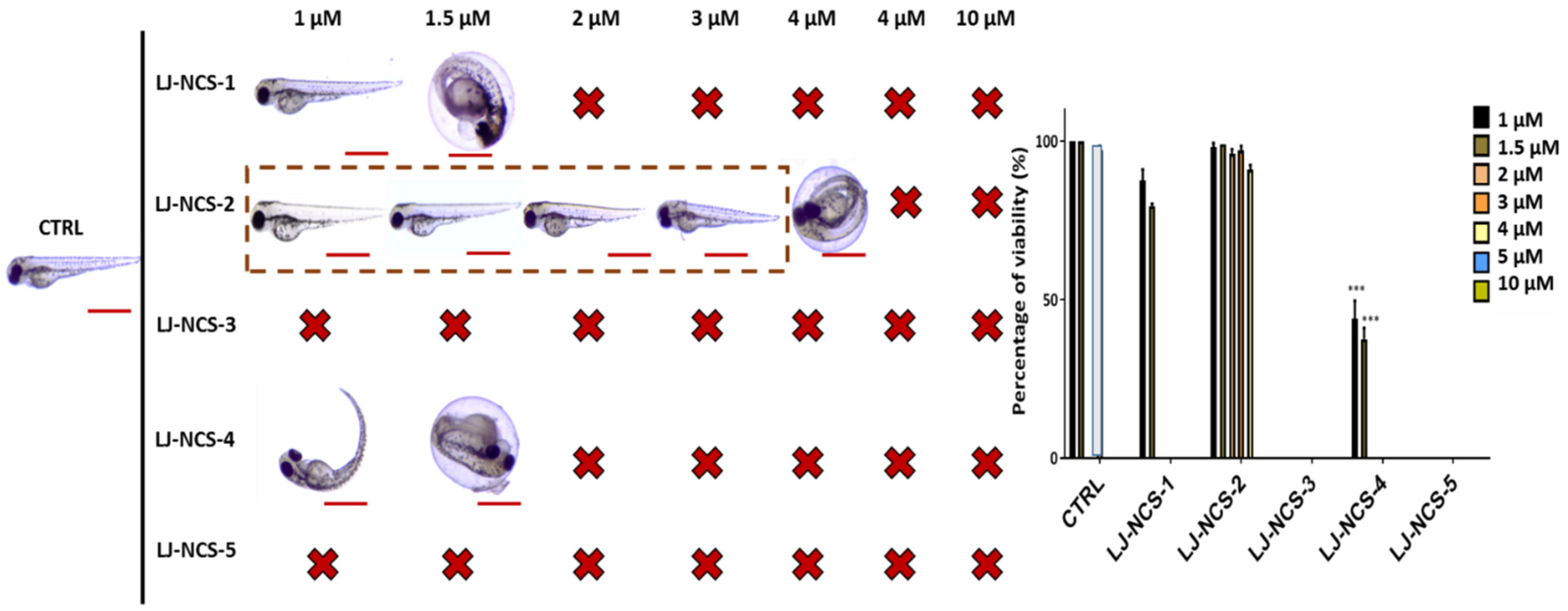
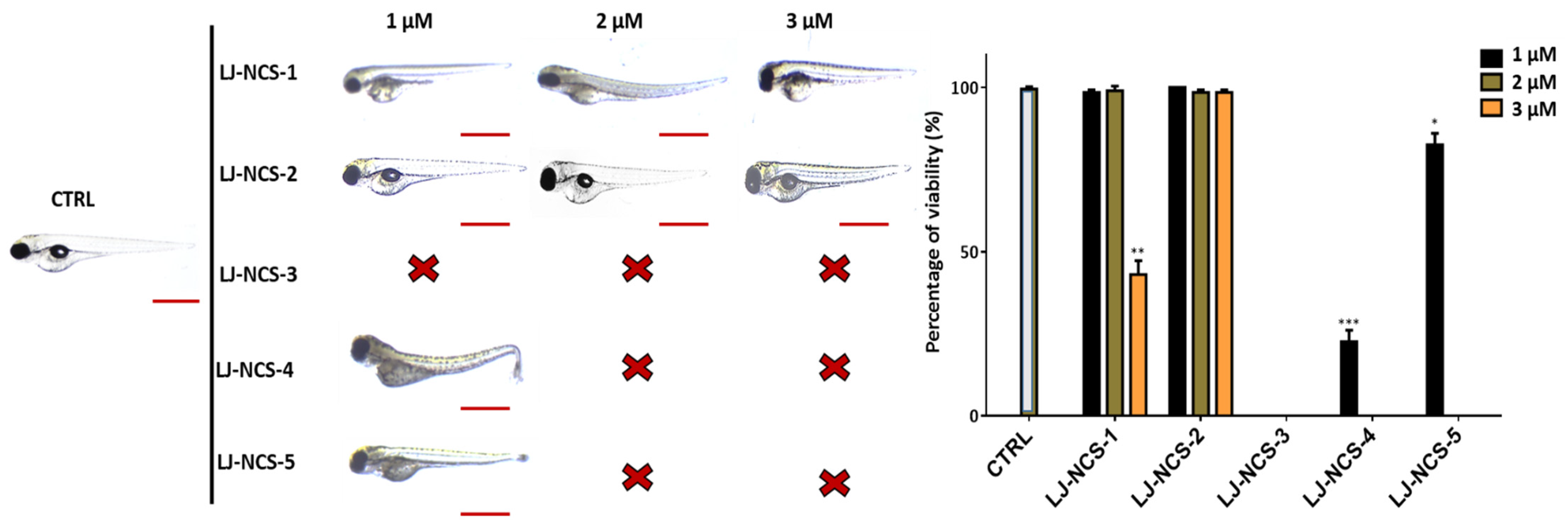
3.1. Structurally Diverse Isothiocyanates in 1–10 µM Concentration Rate Showed a Different Toxic Effect in Zebrafish Embryos
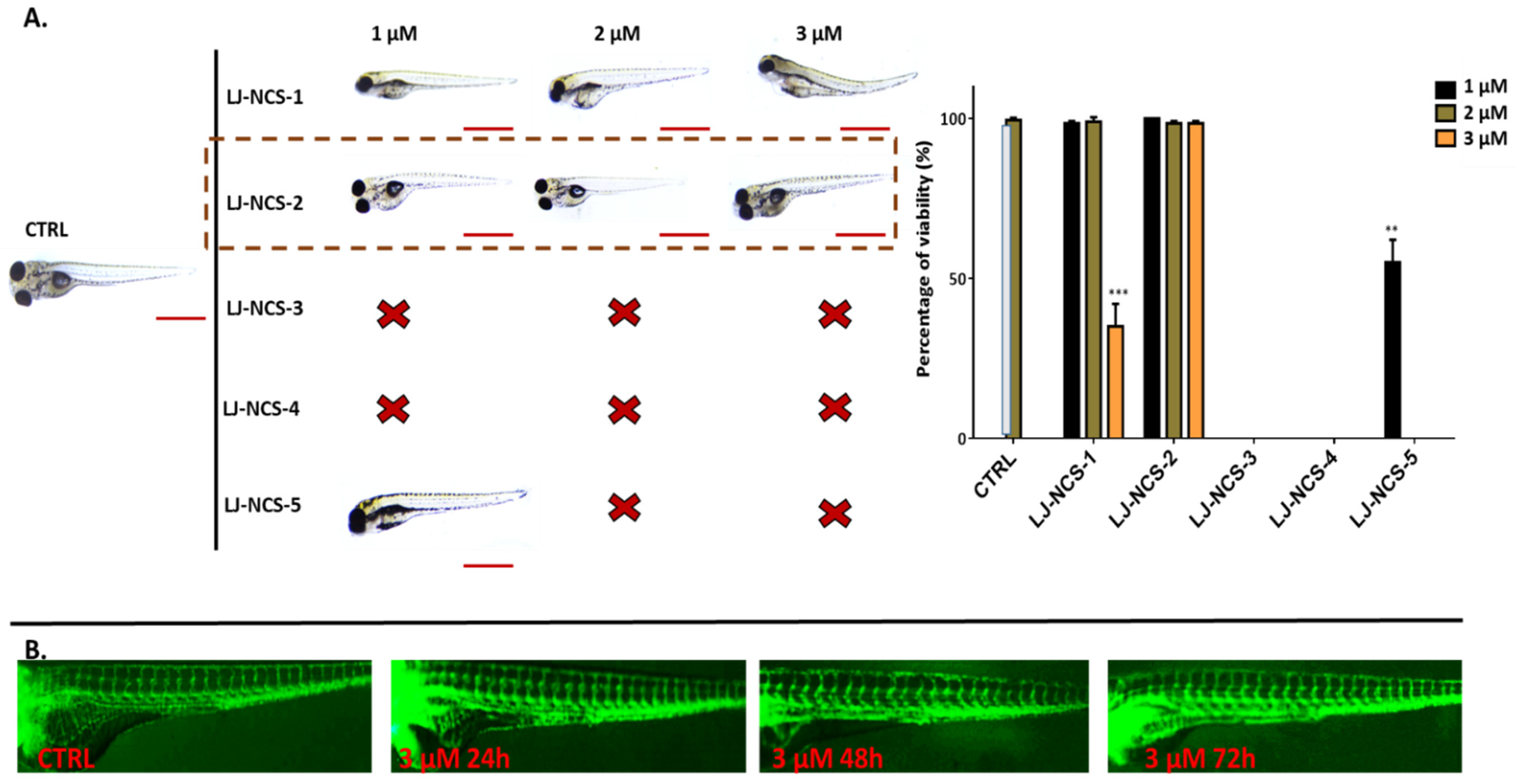
3.2. Phosphonate Analog of Sulforaphane as the Safest Treatment for Zebrafish Embryos at Different Stages of Development
3.3. Phosphonate Analog of Sulforaphane Significantly Reduces the Human Cancer Cells Growth in Zebrafish Xenograft Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masahiko, I.; Masakazu, H.; Nobuko, F.; Tomohiro, K.; Yasujiro, M. Glucosinolate metabolism, functionality and breeding for the improvement of Brassicaceae vegetables. Breed. Sci. 2014, 64. [Google Scholar] [CrossRef] [Green Version]
- Lung Cheung, K.; Kong, A.N. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J. 2010, 12. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, F.; Paredes-Gonzalez, X.; Kong, A.N.T. Dietary Glucosinolates Sulforaphane, Phenethyl Isothiocyanate, Indole-3-Carbinol/3,3’-Diindolylmethane: Anti-Oxidative Stress/Inflammation, Nrf2, Epigenetics/Epigenomics and In Vivo Cancer Chemopreventive Efficacy. Curr. Pharmacol. Rep. 2015, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Zhou, Q.; Xu, K. Are isothiocyanates potential anti-cancer drugs? Acta Pharmacol. Sin. 2009, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hecht, S.S. Chemoprevention of lung cancer by isothiocyanates. Adv. Exp. Med. Biol. 1996, 401. [Google Scholar] [CrossRef]
- Matsui, T.A.; Murata, H.; Sakabe, T.; Sowa, Y.; Horie, N.; Nakanishi, R.; Sakai, T.; Kubo, T. Sulforaphane induces cell cycle arrest and apoptosis in murine osteosarcoma cells in vitro and inhibits tumor growth in vivo. Oncol. Rep. 2007, 18, 1263–1268. [Google Scholar] [CrossRef] [Green Version]
- Sehrawat, A.; Singh, S.V. Benzyl isothiocyanate inhibits epithelial-mesenchymal transition in cultured and xenografted human breast cancer cells. Cancer Prev. Res. 2011, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Psurski, M.; Janczewski, Ł.; Świtalska, M.; Gajda, A.; Goszczyński, T.M.; Oleksyszyn, J.; Wietrzyk, J.; Gajda, T. Novel phosphonate analogs of sulforaphane: Synthesis, in vitro and in vivo anticancer activity. Eur. J. Med. Chem. 2017, 132, 63–80. [Google Scholar] [CrossRef]
- Lütken, C.D.; Fiehn, A.M.K.; Federspiel, B.; Achiam, M.P. Impact of isolated tumor cells in regional lymph nodes in adeno-and squamous cell carcinoma of the esophagus and the esophagogastric junction-A systematic review. Pathol. Res. Pract. 2019, 215. [Google Scholar] [CrossRef]
- Zhu, M.; Li, W.; Dong, X.; Chen, Y.; Lu, Y.; Lin, B.; Guo, J.; Li, M. Benzyl-isothiocyanate Induces Apoptosis and Inhibits Migration and Invasion of Hepatocellular Carcinoma Cells in vitro. J. Cancer 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Li, Y.; Wang, X.Q.; Wang, X.-Q.; Meng, Y.; Zhang, Q.; Zhu, J.-Y.; Chen, J.-Q.; Cao, W.-S.; Wang, X.-Q.; et al. Phenethyl isothiocyanate inhibits colorectal cancer stem cells by suppressing Wnt/β-catenin pathway. Phytother. Res. PTR 2018, 32. [Google Scholar] [CrossRef]
- Abbaoui, B.; Lucas, C.R.; Riedl, K.M.; Clinton, S.K.; Mortazavi, A. Cruciferous Vegetables, Isothiocyanates, and Bladder Cancer Prevention. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Janczewski, Ł.; Psurski, M.; Świtalska, M.; Gajda, A.; Goszczyński, T.M.; Oleksyszyn, J.; Wietrzyk, J.; Gajda, T. Design, Synthesis, and Evaluation of ω-(Isothiocyanato)alkylphosphinates and Phosphine Oxides as Antiproliferative Agents. ChemMedChem 2018, 13, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Palliyaguru, D.L.; Yuan, J.M.; Kensler, T.W.; Fahey, J.W. Isothiocyanates: Translating the Power of Plants to People. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, S.M.; Filho, S.A.V.; Nogueira-Machado, J.A.; Caligiorne, R.B. The anti-oxidant properties of isothiocyanates: A review. Recent Pat. Endocr. Metab. Immune Drug Discov. 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Burčul, F.; Mekinić, I.G.; Radan, M.; Rolli, P.; Blažević, I. Isothiocyanates: Cholinesterase inhibiting, antioxidant, and anti-inflammatory activity. J. Enzym. Inhib. Med. Chem. 2018, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romeo, L.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. Isothiocyanates: An Overview of Their Antimicrobial Activity against Human Infections. Molecules 2018, 23, 624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latronico, T.; Larocca, M.; Milella, S.; Fasano, S.; Rossano, R.; Liuzzi, G.M. Neuroprotective potential of isothiocyanates in an in vitro model of neuroinflammation. Inflammopharmacology 2021, 29. [Google Scholar] [CrossRef]
- Citi, V.; Corvino, A.; Fiorino, F.; Frecentese, F.; Magli, E.; Perissutti, E.; Santagada, V.; Brogi, S.; Flori, L.; Gorica, E.; et al. Structure-activity relationships study of isothiocyanates for H 2 S releasing properties: 3-Pyridyl-isothiocyanate as a new promising cardioprotective agent. J. Adv. Res. 2020, 27. [Google Scholar] [CrossRef] [PubMed]
- Hason, M.; Bartůněk, P. Zebrafish Models of Cancer-New Insights on Modeling Human Cancer in a Non-Mammalian Vertebrate. Genes 2019, 10, 935. [Google Scholar] [CrossRef] [Green Version]
- Chahardehi, A.M.; Arsad, H.; Lim, V. Zebrafish as a Successful Animal Model for Screening Toxicity of Medicinal Plants. Plants 2020, 9, 1345. [Google Scholar] [CrossRef] [PubMed]
- Busquet, F.; Strecker, R.; Rawlings, J.M.; Belanger, S.E.; Braunbeck, T.; Carr, G.J.; Cenijn, P.; Fochtman, P.; Gourmelon, A.; Hübler, N.; et al. OECD validation study to assess intra- and inter-laboratory reproducibility of the zebrafish embryo toxicity test for acute aquatic toxicity testing. Regul. Toxicol. Pharmacol. RTP 2014, 69. [Google Scholar] [CrossRef]
- Hill, D.; Chen, L.; Snaar-Jagalska, E.; Chaudhry, B. Embryonic zebrafish xenograft assay of human cancer metastasis. F1000Research 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Glasgow, E.; Agarwal, S. Zebrafish Xenografts for Drug Discovery and Personalized Medicine. Trends Cancer 2020, 6. [Google Scholar] [CrossRef]
- Gupta, P.; Wright, S.E.; Kim, S.H.; Srivastava, S.K. Phenethyl isothiocyanate: A comprehensive review of anti-cancer mechanisms. Biochim. Biophys. Acta 2014, 1846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janczewski, Ł.; Kręgiel, D.; Kolesińska, B. Synthesis of Isothiocyanates Using DMT/NMM/TsO—As a New Desulfurization Reagent. Molecules 2021, 26, 2740. [Google Scholar] [CrossRef]
- Mays, J.R.; Roska, R.L.W.; Sarfaraz, S.; Mukhtar, H.; Rajski, S.R. Identification, synthesis, and enzymology of non-natural glucosinolate chemopreventive candidates. Chembiochem Eur. J. Chem. Biol. 2008, 9. [Google Scholar] [CrossRef]
- Janczewski, Ł.; Gajda, A.; Gajda, T. Direct, Microwave-Assisted Synthesis of Isothiocyanates. Eur. J. Org. Chem. 2019, 2528–2532. [Google Scholar] [CrossRef]
- Janczewski, Ł.; Gajda, A.; Braszczyńska, J.; Gajda, T. Microwave-assisted synthesis of dialkyl ω-azidoalkylphosphonates. Synth. Commun. 2016, 46, 1625–1633. [Google Scholar] [CrossRef]
- Berens, E.B.; Sharif, G.M.; Wellstein, A.; Glasgow, E. Testing the Vascular Invasive Ability of Cancer Cells in Zebrafish (Danio Rerio). J. Vis. Exp. JoVE 2016, e55007. [Google Scholar] [CrossRef]
- Wehmas, L.C.; Tanguay, R.L.; Punnoose, A.; Greenwood, J.A. Developing a Novel Embryo-Larval Zebrafish Xenograft Assay to Prioritize Human Glioblastoma Therapeutics. Zebrafish 2016, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, I.J.; Weiss, F.U.; Vlecken, D.H.; Nitsche, C.; Bakkers, J.; Lagendijk, A.K.; Partecke, L.I.; Heidecke, C.L.; Lerch, M.M.; Bagowski, C.P. Metastatic behaviour of primary human tumours in a zebrafish xenotransplantation model. BMC Cancer 2009, 9. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Liu, S.; Cui, C.; Dijke, P. Invasive Behavior of Human Breast Cancer Cells in Embryonic Zebrafish. J. Vis. Exp. JoVE 2017, e55459. [Google Scholar] [CrossRef]
- Mitsiogianni, M.; Koutsidis, G.; Mavroudis, N.; Trafalis, D.T.; Botaitis, S.; Franco, R.; Zoumpourlis, V.; Amery, T.; Galanis, A.; Pappa, A.; et al. The Role of Isothiocyanates as Cancer Chemo-Preventive, Chemo-Therapeutic and Anti-Melanoma Agents. Antioxidants 2019, 8, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morse, M.A.; Eklind, K.I.; Amin, S.G.; Hecht, S.S.; Chung, F.L. Effects of alkyl chain length on the inhibition of NNK-induced lung neoplasia in A/J mice by arylalkyl isothiocyanates. Carcinogenesis 1989, 10. [Google Scholar] [CrossRef]
- Lange, T.S.; Horan, T.C.; Kim, K.K.; Singh, A.P.; Vorsa, N.; Brard, L.; Moore, R.G.; Singh, R.K. Cytotoxic properties of Adamantyl isothiocyanate and potential in vivo metabolite adamantyl-N-acetylcystein in gynecological cancer cells. Chem. Biol. Drug Des. 2012, 79. [Google Scholar] [CrossRef]
- Xiao, D.; Singh, S.V. Phenethyl isothiocyanate inhibits angiogenesis in vitro and ex vivo. Cancer Res. 2007, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, K.W.W.; Po, W.W.; Sohn, W.D.; Kim, H.J. Benzyl Isothiocyanate Induces Apoptosis via Reactive Oxygen Species-Initiated Mitochondrial Dysfunction and DR4 and DR5 Death Receptor Activation in Gastric Adenocarcinoma Cells. Biomolecules 2019, 9, 839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satyan, K.S.; Swamy, N.; Dizon, D.S.; Singh, R.; Granai, C.O.; Brard, L. Phenethyl isothiocyanate (PEITC) inhibits growth of ovarian cancer cells by inducing apoptosis: Role of caspase and MAPK activation. Gynecol. Oncol. 2006, 103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yao, S.; Li, J. Vegetable-derived isothiocyanates: Anti-proliferative activity and mechanism of action. Proc. Nutr. Soc. 2006, 65. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Loganathan, S.; Humphreys, I.; Srivastava, S.K. Benzyl isothiocyanate-induced DNA damage causes G2/M cell cycle arrest and apoptosis in human pancreatic cancer cells. J. Nutr. 2006, 136. [Google Scholar] [CrossRef] [Green Version]
- Cornet, C.; Calzolari, S.; Miñana-Prieto, R.; Dyballa, S.; van Doornmalen, E.; Rutjes, H.; Savy, T.; D’Amico, D.; Terriente, J. ZeGlobalTox: An Innovative Approach to Address Organ Drug Toxicity Using Zebrafish. Int. J. Mol. Sci. 2017, 18, 864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerlai, R. High-throughput behavioral screens: The first step towards finding genes involved in vertebrate brain function using zebrafish. Molecules 2010, 15, 2609. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, M.; Egmond, R. The use of the zebrafish (Danio rerio) embryo for the acute toxicity testing of surfactants, as a possible alternative to the acute fish test. Altern. Lab. Anim. ATLA 2010, 38. [Google Scholar] [CrossRef]
- Amatruda, J.F.; Shepard, J.L.; Stern, H.M.; Zon, L.I. Zebrafish as a cancer model system. Cancer Cell 2002, 1. [Google Scholar] [CrossRef] [Green Version]
- Stern, H.M.; Zon, L.I. Cancer genetics and drug discovery in the zebrafish. Nat. Rev. Cancer 2003, 3. [Google Scholar] [CrossRef]
- Cassar, S.; Adatto, I.; Freeman, J.L.; Gamse, J.T.; Iturria, I.; Lawrence, C.; Muriana, A.; Peterson, R.T.; van Cruchten, S.; Zon, L.I. Use of Zebrafish in Drug Discovery Toxicology. Chem. Res. Toxicol. 2020, 33. [Google Scholar] [CrossRef] [Green Version]
- Haldi, M.; Ton, C.; Seng, W.L.; McGrath, P. Human melanoma cells transplanted into zebrafish proliferate, migrate, produce melanin, form masses and stimulate angiogenesis in zebrafish. Angiogenesis 2006, 9. [Google Scholar] [CrossRef]
- Danilova, N.; Hohman, V.S.; Sacher, F.; Ota, T.; Willett, C.E.; Steiner, L.A. T cells and the thymus in developing zebrafish. Dev. Comp. Immunol. 2004, 28. [Google Scholar] [CrossRef]
- Rao, C.V. Benzyl isothiocyanate: Double trouble for breast cancer cells. Cancer Prev. Res. 2013, 6. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, K.; Hussein, U.K.; Anupalli, R.; Barnett, R.; Bachaboina, L.; Scalici, J.; Rocconi, R.P.; Owen, L.B.; Piazza, G.A.; Palle, K. Allyl isothiocyanate induces replication-associated DNA damage response in NSCLC cells and sensitizes to ionizing radiation. Oncotarget 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Rajan, T.S.; De Nicola, G.R.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. Anticancer activity of glucomoringin isothiocyanate in human malignant astrocytoma cells. Fitoterapia 2016, 110. [Google Scholar] [CrossRef]
- Kang, L.; Ding, L.; Wang, Z.Y. Isothiocyanates repress estrogen receptor alpha expression in breast cancer cells. Oncol. Rep. 2009, 21, 185–192. [Google Scholar]
- Kalkunte, S.; Swamy, N.; Dizon, D.S.; Brard, L. Benzyl isothiocyanate (BITC) induces apoptosis in ovarian cancer cells in vitro. J. Exp. Ther. Oncol. 2006, 5, 287–300. [Google Scholar]
- Liu, X.; Takano, C.; Shimizu, T.; Yokobe, S.; Abe-Kanoh, N.; Zhu, B.; Nakamura, T.; Munemasa, S.; Murata, Y.; Nakamura, Y. Inhibition of phosphatidylinositide 3-kinase ameliorates antiproliferation by benzyl isothiocyanate in human colon Cancer Cells. Biochem. Biophys. Res. Commun. 2017, 491. [Google Scholar] [CrossRef]
- Wang, Y.; Dacosta, C.; Wang, W.; Zhou, Z.; Liu, M.; Bao, Y. Synergy between sulforaphane and selenium in protection against oxidative damage in colonic CCD841 cells. Nutr. Res. 2015, 35. [Google Scholar] [CrossRef] [Green Version]
- Kaminski, B.M.; Weigert, A.; Brüne, B.; Schumacher, M.; Wenzel, U.; Steinhilber, D.; Stein, J.; Ulrich, S. Sulforaphane potentiates oxaliplatin-induced cell growth inhibition in colorectal cancer cells via induction of different modes of cell death. Cancer Chemother. Pharmacol. 2011, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.-S.; Lin, J.-J.; Lin, C.-C.; Lien, J.-C.; Peng, S.-F.; Fan, M.-J.; Hsu, F.-T.; Chung, J.-G. Benzyl isothiocyanate inhibits human brain glioblastoma multiforme GBM 8401 cell xenograft tumor in nude mice in vivo. Environ. Toxicol. 2018, 33. [Google Scholar] [CrossRef]
- Nyein, C.M.; Zhong, X.; Lu, J.; Luo, H.; Wang, J.; Rapposelli, S.; Li, M.; Ou-yang, Y.; Pi, R.; He, X. Synthesis and anti-glioblastoma effects of artemisinin-isothiocyanate derivatives. RSC Adv. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, T.; Nishino, H.; Iwashima, A. Isothiocyanates inhibit cell cycle progression of HeLa cells at G2/M phase. Anti-Cancer Drugs 1993, 4. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Srivastava, S.K. Antitumor activity of phenethyl isothiocyanate in HER2-positive breast cancer models. BMC Med. 2012, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayed, A.; Bracci, M.; Ciarapica, V.; Malavolta, M.; Provinciali, M.; Pieragostini, E.; Gaetani, S.; Monaco, F.; Lucarini, G.; Rapisarda, V. Allyl Isothiocyanate Exhibits No Anticancer Activity in MDA-MB-231 Breast Cancer Cells. Int. J. Mol. Sci. 2018, 19, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]

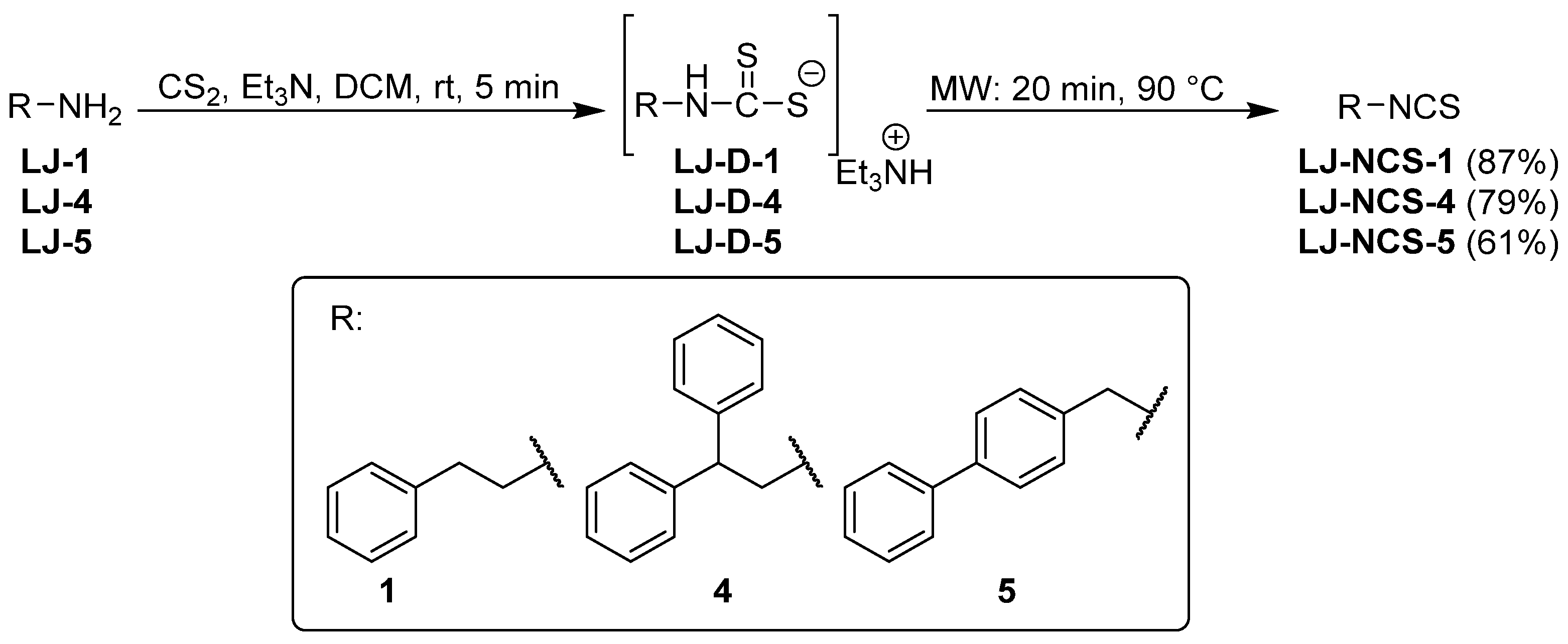
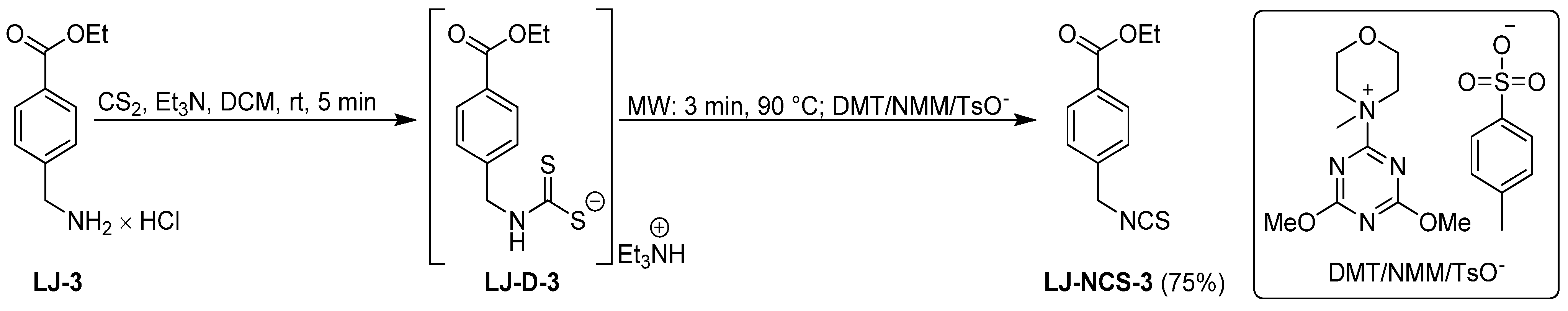
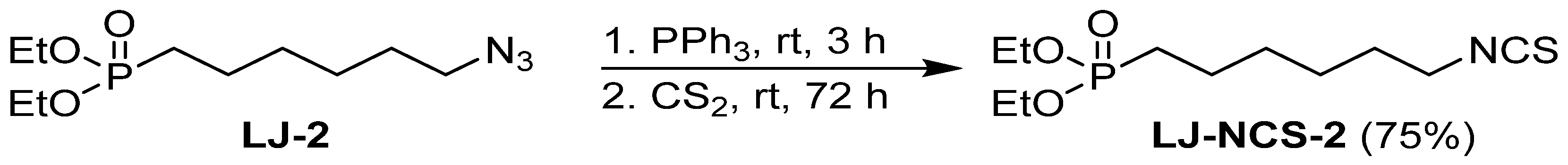



| Symbol | Formula | Information | |
|---|---|---|---|
| LJ-NCS-1 | 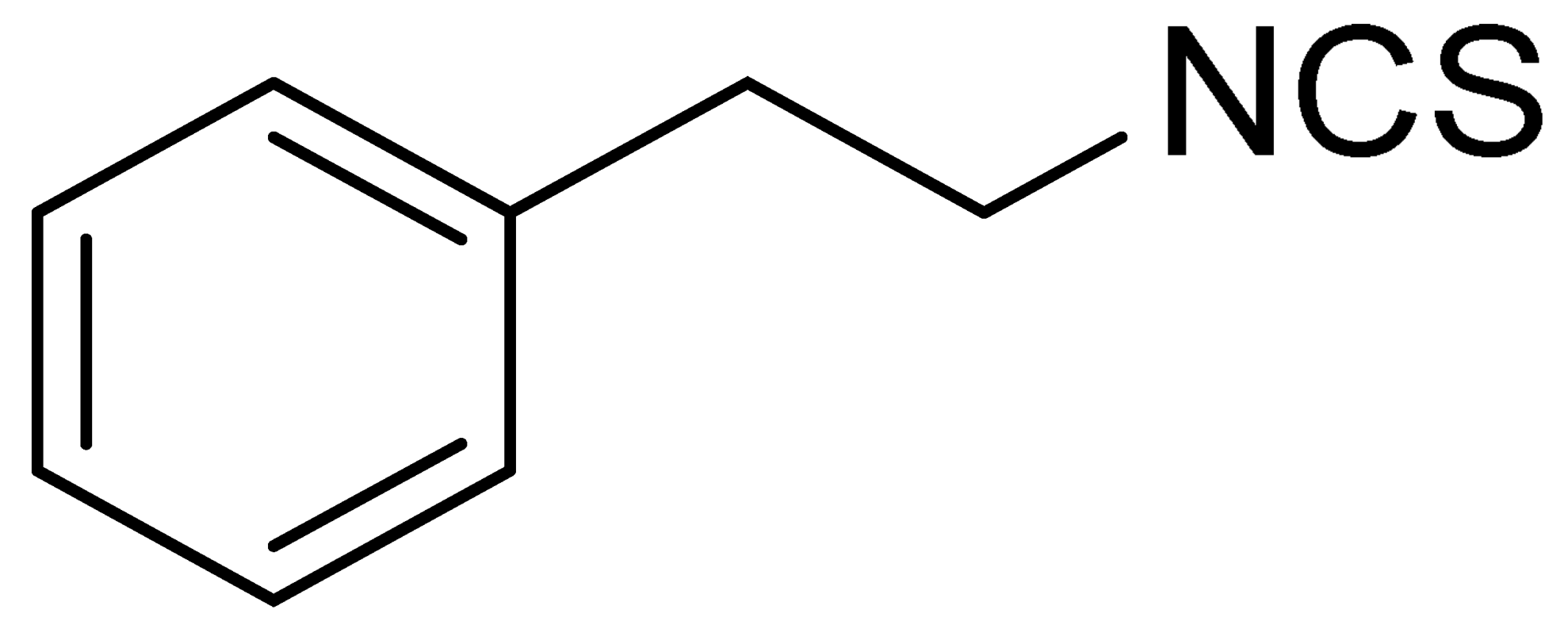 | C9H9NS | Phenethyl isothiocyanate; natural isothiocyanate with high biological activity [25] |
| LJ-NCS-2 | 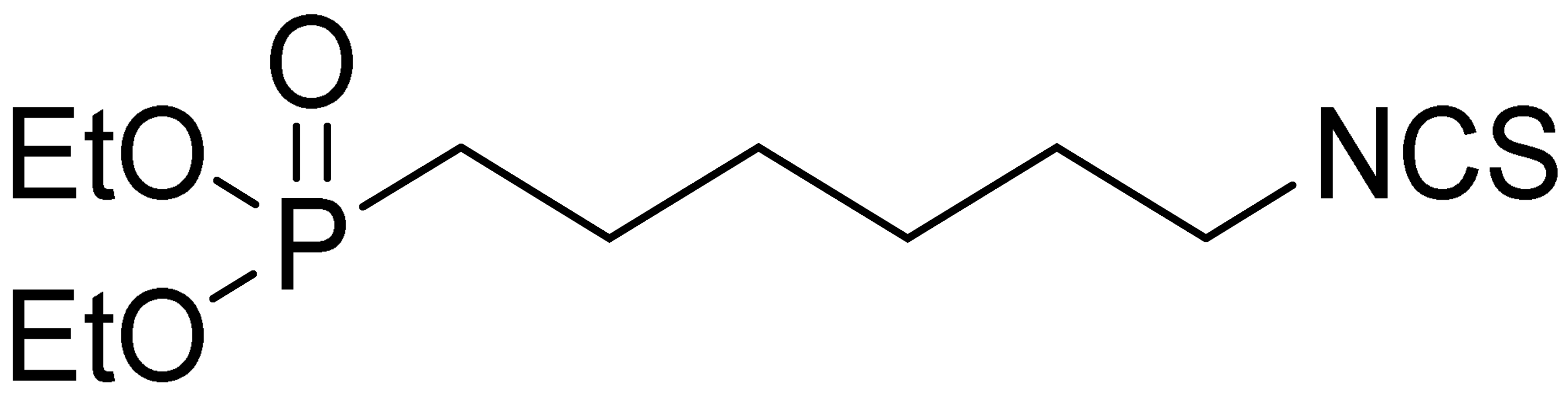 | C11H22NO3PS | Phosphonate analogs of sulforaphane; synthetic isothiocyanate with anti-tumorigenic/anti-proliferative activity in vitro and in vivo [8] |
| LJ-NCS-3 |  | C11H11NO2S | A substance derived from 4-aminomethylbenzoic acid with hemostatic and antibacterial properties [26] |
| LJ-NCS-4 | 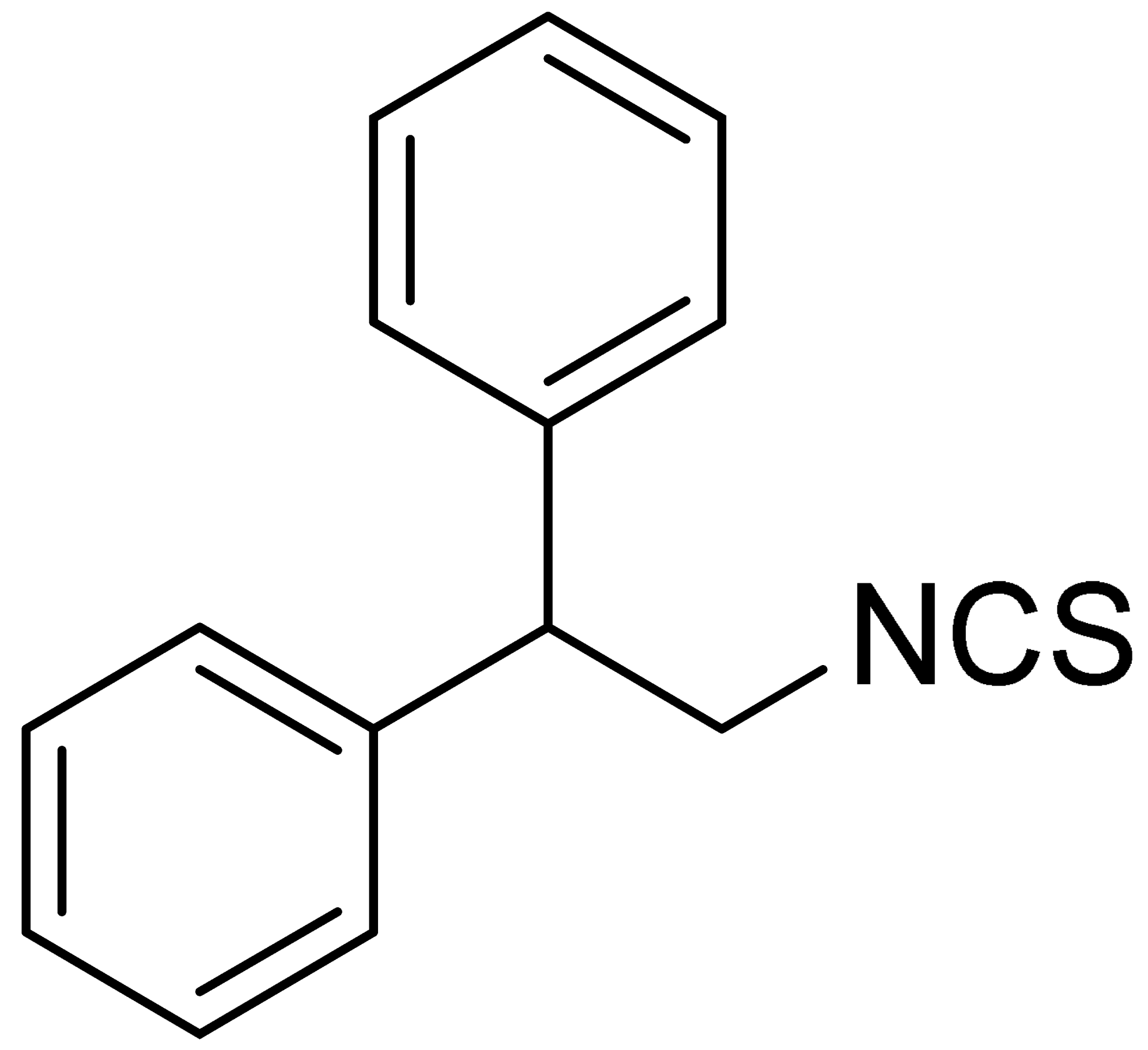 | C15H13NS | A substance containing two aromatic rings with the potential to being anti- cancerogenic agent [27] |
| LJ-NCS-5 | 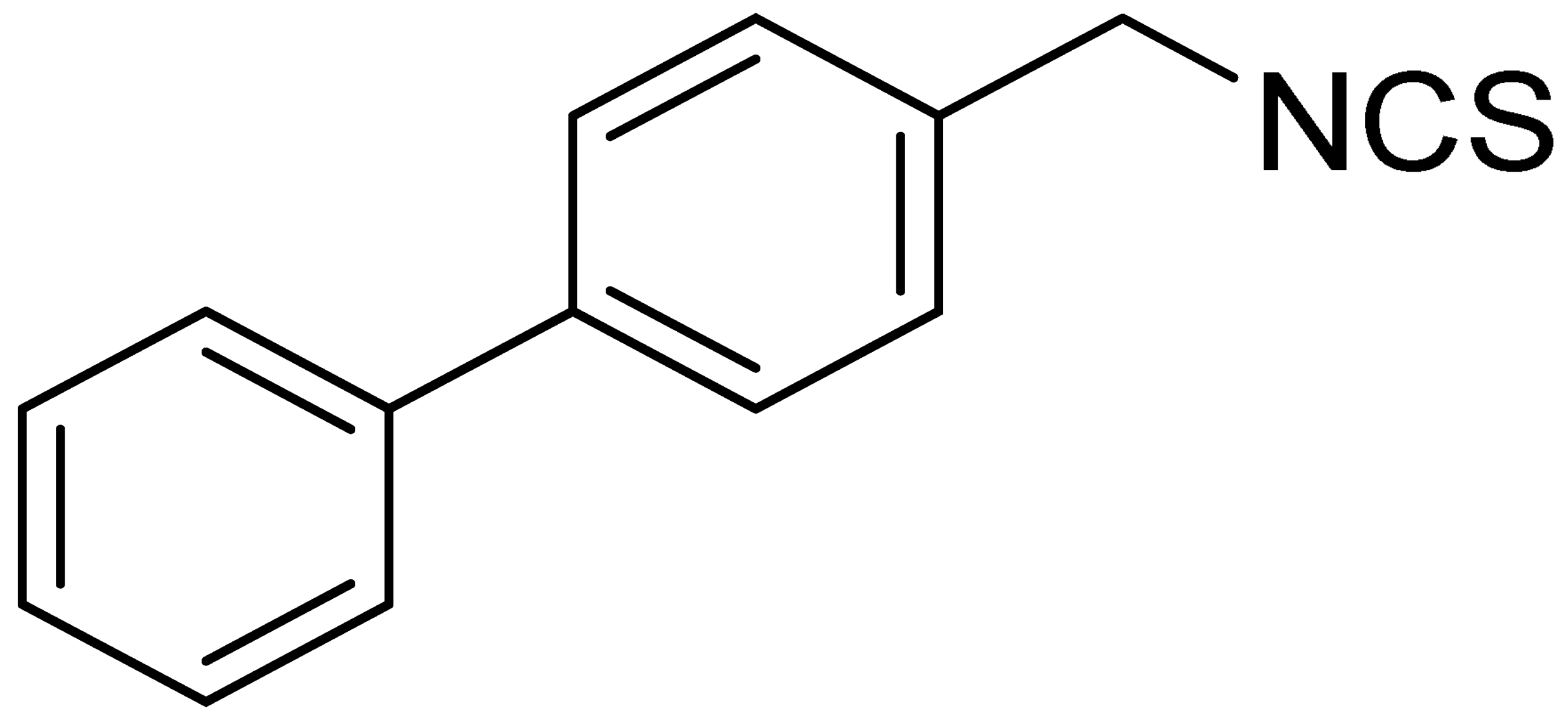 | C14H11NS | A substance containing two aromatic rings with the potential to being anti- cancerogenic agent [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudzinska-Radecka, M.; Janczewski, Ł.; Gajda, A.; Godlewska, M.; Chmielewska-Krzesinska, M.; Wasowicz, K.; Podlasz, P. The Anti-Tumoral Potential of Phosphonate Analog of Sulforaphane in Zebrafish Xenograft Model. Cells 2021, 10, 3219. https://doi.org/10.3390/cells10113219
Rudzinska-Radecka M, Janczewski Ł, Gajda A, Godlewska M, Chmielewska-Krzesinska M, Wasowicz K, Podlasz P. The Anti-Tumoral Potential of Phosphonate Analog of Sulforaphane in Zebrafish Xenograft Model. Cells. 2021; 10(11):3219. https://doi.org/10.3390/cells10113219
Chicago/Turabian StyleRudzinska-Radecka, Magdalena, Łukasz Janczewski, Anna Gajda, Marlena Godlewska, Malgorzata Chmielewska-Krzesinska, Krzysztof Wasowicz, and Piotr Podlasz. 2021. "The Anti-Tumoral Potential of Phosphonate Analog of Sulforaphane in Zebrafish Xenograft Model" Cells 10, no. 11: 3219. https://doi.org/10.3390/cells10113219
APA StyleRudzinska-Radecka, M., Janczewski, Ł., Gajda, A., Godlewska, M., Chmielewska-Krzesinska, M., Wasowicz, K., & Podlasz, P. (2021). The Anti-Tumoral Potential of Phosphonate Analog of Sulforaphane in Zebrafish Xenograft Model. Cells, 10(11), 3219. https://doi.org/10.3390/cells10113219