Regenerating Damaged Myocardium: A Review of Stem-Cell Therapies for Heart Failure
Abstract
:1. Introduction
2. Somatic Stem and Progenitor Cells
2.1. Skeletal Myoblasts
2.2. Bone Marrow (BM)-Derived Cells
2.3. Adipose-Derived Stem Cells (ASCs)
2.4. Cardiac Progenitor Cells (CPCs)
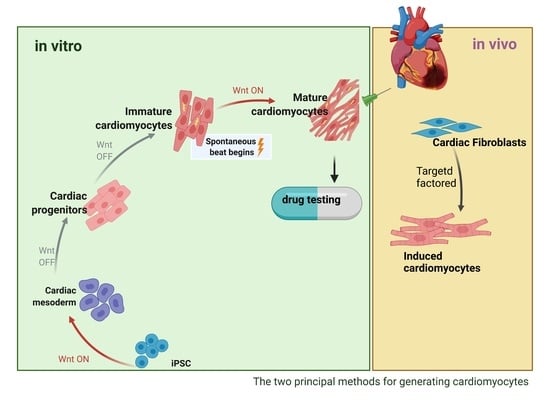
2.5. Embryonic Stem Cells (ESCs) and Induced Pluripotent Stem Cells (iPSCs)
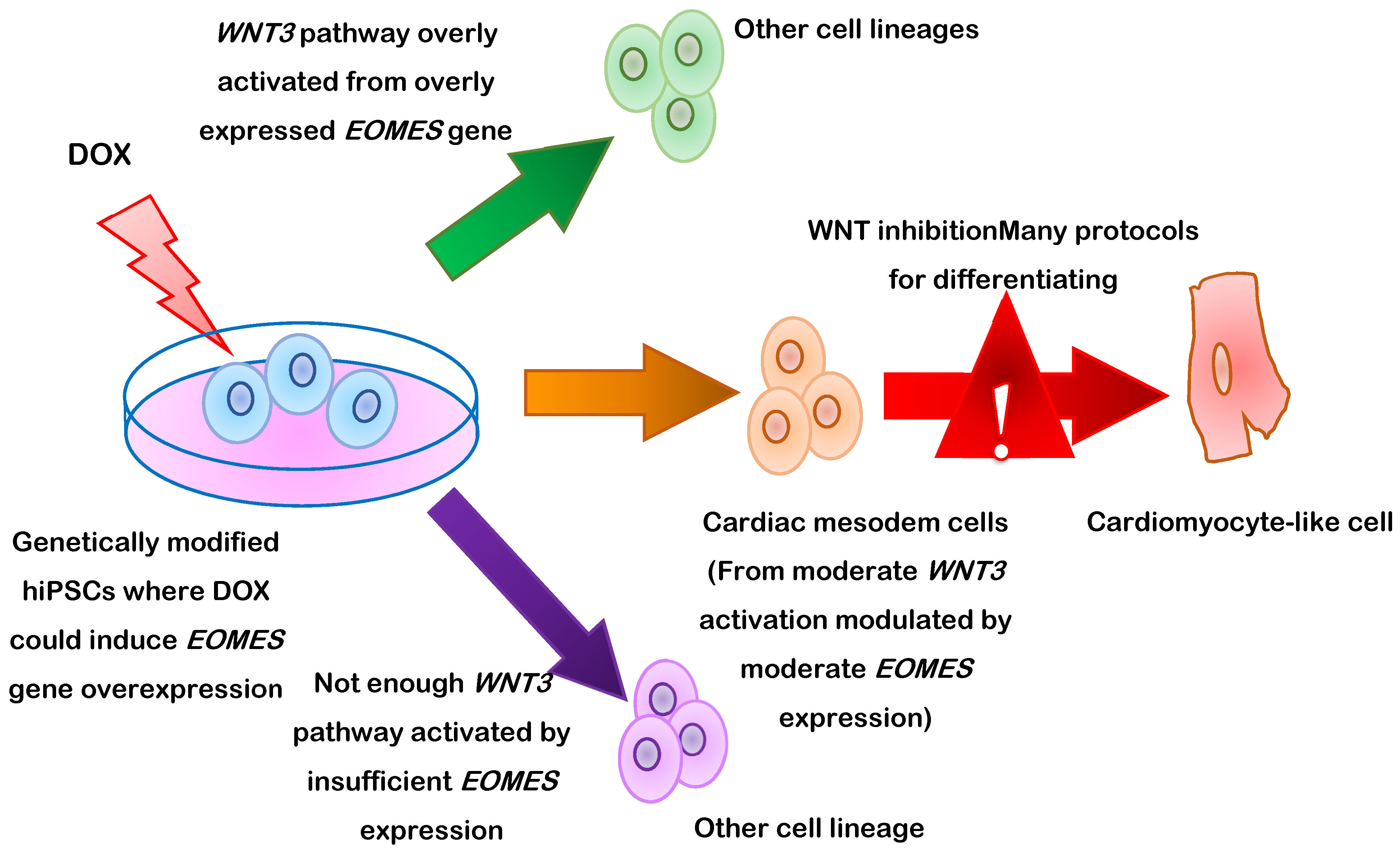
3. Differentiation of PSCs into Cardiomyocytes
4. Purification of Human iPSC-CMs
4.1. Lactate-Based Medium and Glucose Starvation
4.2. Positive or Negative Selection of Labeled Cells
4.3. CRISPR/Cas9-Mediated Integration of a Fluorescent Reporter
5. Confirmation of PSC-CM Identity and Functional Characterization
6. Promoting PSC-CM Maturation
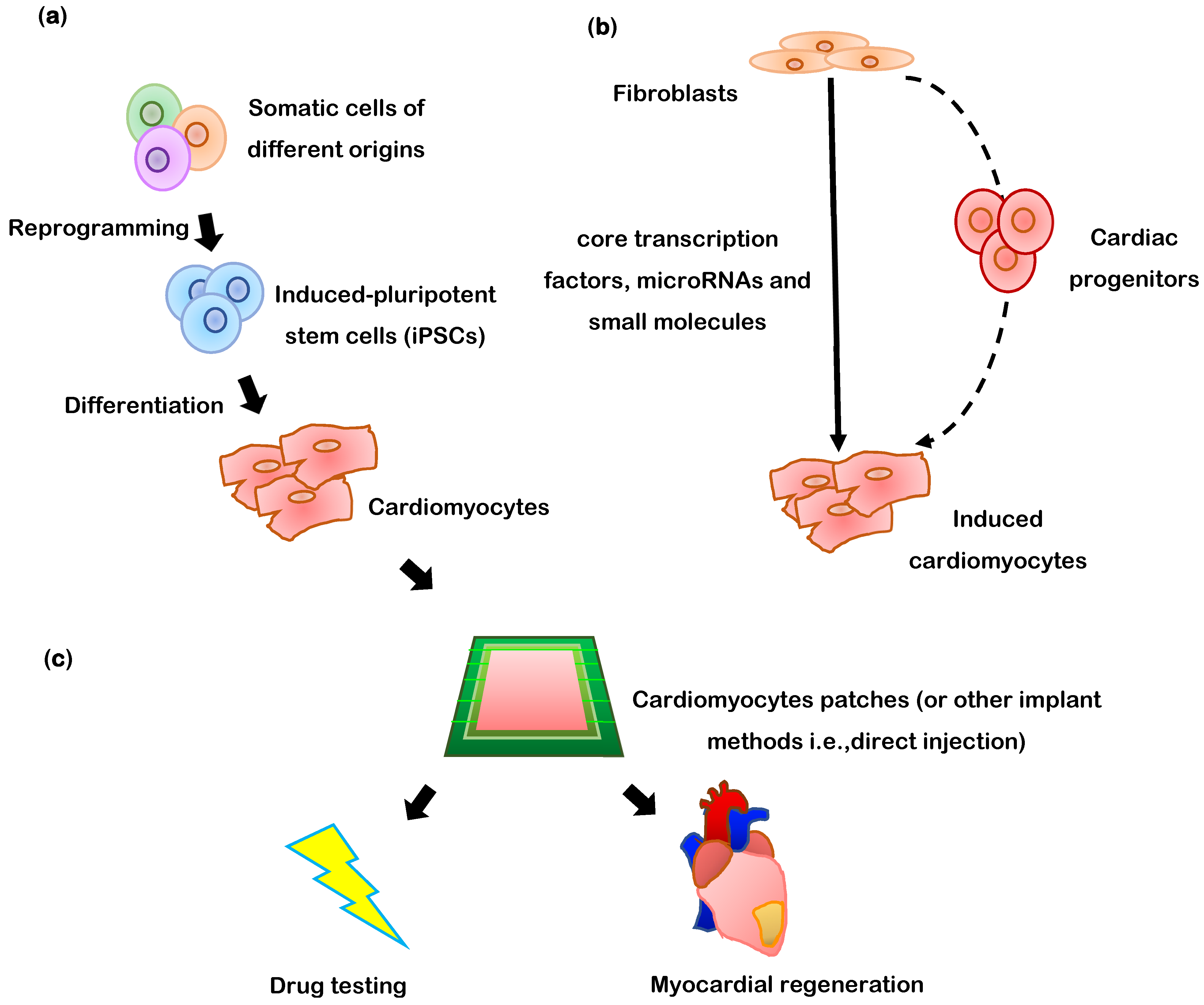
7. PSC-CMs for Myocardial Repair
8. Direct Transdifferentiation of Somatic Cells into Cardiomyocytes
9. Recent Clinical Trials
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 2020, 368, m1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, P.L.; David, R. Stem Cell Therapy in Heart Diseases—Cell Types, Mechanisms and Improvement Strategies. Cell. Physiol. Biochem. 2018, 48, 2607–2655. [Google Scholar] [CrossRef] [PubMed]
- Durrani, S.; Konoplyannikov, M.; Ashraf, M.; Haider, K.H. Skeletal myoblasts for cardiac repair. Regen. Med. 2010, 5, 919–932. [Google Scholar] [CrossRef] [Green Version]
- Dimmeler, S.; Zeiher, A.M. Cell therapy of acute myocardial infarction: Open questions. Cardiology 2009, 113, 155–160. [Google Scholar] [CrossRef]
- Houtgraaf, J.H.; den Dekker, W.K.; van Dalen, B.M.; Springeling, T.; de Jong, R.; van Geuns, R.J.; Geleijnse, M.L.; Fernandez-Aviles, F.; Zijlsta, F.; Serruys, P.W.; et al. First experience in humans using adipose tissue-derived regenerative cells in the treatment of patients with ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2012, 59, 539–540. [Google Scholar] [CrossRef] [Green Version]
- Bolli, R.; Chugh, A.R.; D’Amario, D.; Loughran, J.H.; Stoddard, M.F.; Ikram, S.; Beache, G.M.; Wagner, S.G.; Leri, A.; Hosoda, T.; et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): Initial results of a randomised phase 1 trial. Lancet 2011, 378, 1847–1857. [Google Scholar] [CrossRef] [Green Version]
- Nussbaum, J.; Minami, E.; Laflamme, M.A.; Virag, J.A.; Ware, C.B.; Masino, A.; Muskheli, V.; Pabon, L.; Reinecke, H.; Murry, C.E. Transplantation of undifferentiated murine embryonic stem cells in the heart: Teratoma formation and immune response. FASEB J. 2007, 21, 1345–1357. [Google Scholar] [CrossRef]
- Abou-Saleh, H.; Zouein, F.A.; El-Yazbi, A.; Sanoudou, D.; Raynaud, C.; Rao, C.; Pintus, G.; Dehaini, H.; Eid, A.H. The march of pluripotent stem cells in cardiovascular regenerative medicine. Stem. Cell Res. Ther. 2018, 9, 201. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef] [Green Version]
- Hagege, A.A.; Vilquin, J.-T.; Alheritiere, A.; Peyrard, S.; Duboc, D.; Abergel, E.; Messas, E.; Mousseaux, E.; Schwartz, K.; Desnos, M.; et al. Skeletal myoblast transplantation in ischemic heart failure: Long-term follow-up of the first phase I cohort of patients. Circulation 2006, 114, I-108–I-111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brazelton, T.R.; Keshet, G.I.; Blau, H.M. From marrow to brain: Expression of neuronal phenotypes in adult mice. Science 2000, 290, 1775–1779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leone, A.M.; Bonanno, G.; Abbate, A.; Rebuzzi, A.G.; Giovannini, S.; Lombardi, M.; Galiuto, L.; Liuzzo, G.; Andreotti, F.; Lanza, G.A.; et al. Mobilization of bone marrow-derived stem cells after myocardial infarction and left ventricular function. Eur. Heart J. 2005, 26, 1196–1204. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Latif, A.; Zuba-Surma, E.K.; Tleyjeh, I.M.; Hornung, C.A.; Dawn, B. Granulocyte colony-stimulating factor therapy for cardiac repair after acute myocardial infarction: A systematic review and meta-analysis of randomized controlled trials. Am. Heart J. 2008, 156, 216–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orlic, D.; Kajstura, J.; Chimenti, S.; Jakoniuk, I.; Anderson, S.M.; Li, B.; Pickel, J.; McKay, R.; Nadal-Ginard, B.; Bodine, D.M.; et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001, 410, 701–705. [Google Scholar] [CrossRef]
- Steinhoff, G.; Wolfien, M.; Kundt, G.; Börgermann, J.; David, R.; Garbade, J.; Große, J.; Haverich, A.; Hennig, H.; Kaminski, A.; et al. Cardiac Function Improvement and Bone Marrow Response: Outcome Analysis of the Randomized PERFECT Phase III Clinical Trial of Intramyocardial CD133(+) Application After Myocardial Infarction. EBioMedicine 2017, 22, 208–224. [Google Scholar] [CrossRef] [Green Version]
- Yerebakan, C.; Westphal, B.; Donndorf, P.; Glass, A.; Liebold, A.; Stamm, C.; Steinhoff, G. Impact of preoperative left ventricular function and time from infarction on the long-term benefits after intramyocardial CD133(+) bone marrow stem cell transplant. J. Thorac. Cardiovasc. Surg. 2011, 142, 1530–1539. [Google Scholar] [CrossRef] [Green Version]
- Karantalis, V.; Schulman, I.H.; Balkan, W.; Hare, J.M. Allogeneic cell therapy: A new paradigm in therapeutics. Circ. Res. 2015, 116, 12–15. [Google Scholar] [CrossRef]
- Mathiasen, A.B.; Jorgensen, E.; Helqvist, S.; Fischer-Nielsen, A.; Kofoed, K.F.; Haack-Sorensen, M.; Ekblond, A.; Kastrup, J. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: A randomized placebo-controlled trial (MSC-HF trial). Eur. Heart J. 2015, 36, 1744–1753. [Google Scholar] [CrossRef] [Green Version]
- Heldman, A.W.; Fishman, J.E.; Zambrano, J.P.; Trachtenberg, B.H.; Karantalis, V.; Mushtaq, M.; Williams, A.R.; Suncion, V.Y.; McNiece, I.K.; Ghersin, E.; et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: The TAC-HFT randomized trial. JAMA 2014, 311, 62–73. [Google Scholar] [CrossRef]
- Wang, J.A.; He, H.; Sun, Y.; Jiang, J.; Luo, R.H.; Fan, Y.Q.; Dong, L. A prospective, randomized, controlled trial of autologous mesenchymal stem cells transplantation for dilated cardiomyopathy. Zhonghua Xin Xue Guan Bing Za Zhi 2006, 34, 107–110. [Google Scholar] [PubMed]
- Perin, E.C.; Silva, G.V.; DeMaria, A.N.; Marroquin, O.C.; Huang, P.P.; Traverse, J.H.; Krum, H.; Skerrett, D.; Zheng, Y.; Willerson, J.T.; et al. A Phase II Dose-Escalation Study of Allogeneic Mesenchymal Precursor Cells in Patients With Ischemic or Nonischemic Heart Failure. Circ Res 2015, 117, 576–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, B.W.; Jung, S.H.; Das, S.; Lee, S.M.; Park, J.H.; Kim, H.; Hwang, J.W.; Lee, S.; Kim, H.J.; Kim, H.Y.; et al. In vivo priming of human mesenchymal stem cells with hepatocyte growth factor-engineered mesenchymal stem cells promotes therapeutic potential for cardiac repair. Sci. Adv. 2020, 6, eaay6994. [Google Scholar] [CrossRef] [Green Version]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef] [Green Version]
- Mildmay-White, A.; Khan, W. Cell Surface Markers on Adipose-Derived Stem Cells: A Systematic Review. Curr. Stem Cell Res. Ther. 2017, 12, 484–492. [Google Scholar] [CrossRef]
- Ghazanfari, R.; Zacharaki, D.; Li, H.; Lim, H.C.; Soneji, S.; Scheding, S. Human Primary Bone Marrow Mesenchymal Stromal Cells and Their in vitro Progenies Display Distinct Transcriptional Profile Signatures. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Perin, E.C.; Sanz-Ruiz, R.; Sanchez, P.L.; Lasso, J.; Perez-Cano, R.; Alonso-Farto, J.C.; Perez-David, E.; Fernandez-Santos, M.E.; Serruys, P.W.; Duckers, H.J.; et al. Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: The PRECISE Trial. Am. Heart J. 2014, 168, 88–95.e2. [Google Scholar] [CrossRef]
- Henry, T.D.; Pepine, C.J.; Lambert, C.R.; Traverse, J.H.; Schatz, R.; Costa, M.; Povsic, T.J.; David Anderson, R.; Willerson, J.T.; Kesten, S.; et al. The Athena trials: Autologous adipose-derived regenerative cells for refractory chronic myocardial ischemia with left ventricular dysfunction. Catheter. Cardiovasc. Interv. 2017, 89, 169–177. [Google Scholar] [CrossRef]
- Comella, K.; Parcero, J.; Bansal, H.; Perez, J.; Lopez, J.; Agrawal, A.; Ichim, T. Effects of the intramyocardial implantation of stromal vascular fraction in patients with chronic ischemic cardiomyopathy. J. Transl. Med. 2016, 14, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oyama, T.; Nagai, T.; Wada, H.; Naito, A.T.; Matsuura, K.; Iwanaga, K.; Takahashi, T.; Goto, M.; Mikami, Y.; Yasuda, N.; et al. Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo. J. Cell Biol. 2007, 176, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Genead, R.; Danielsson, C.; Andersson, A.B.; Corbascio, M.; Franco-Cereceda, A.; Sylven, C.; Grinnemo, K.H. Islet-1 cells are cardiac progenitors present during the entire lifespan: From the embryonic stage to adulthood. Stem. Cells Dev. 2010, 19, 1601–1615. [Google Scholar] [CrossRef] [PubMed]
- Uchida, S.; De Gaspari, P.; Kostin, S.; Jenniches, K.; Kilic, A.; Izumiya, Y.; Shiojima, I.; Grosse Kreymborg, K.; Renz, H.; Walsh, K.; et al. Sca1-derived cells are a source of myocardial renewal in the murine adult heart. Stem. Cell Rep. 2013, 1, 397–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, A.J.; Smith, R.R.; Matsushita, S.; Chakravarty, T.; Czer, L.S.; Burton, K.; Schwarz, E.R.; Davis, D.R.; Wang, Q.; Reinsmoen, N.L.; et al. Intrinsic cardiac origin of human cardiosphere-derived cells. Eur. Heart J. 2013, 34, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Malliaras, K.; Makkar, R.R.; Smith, R.R.; Cheng, K.; Wu, E.; Bonow, R.O.; Marban, L.; Mendizabal, A.; Cingolani, E.; Johnston, P.V.; et al. Intracoronary cardiosphere-derived cells after myocardial infarction: Evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction). J. Am. Coll. Cardiol. 2014, 63, 110–122. [Google Scholar] [CrossRef] [Green Version]
- Ishigami, S.; Ohtsuki, S.; Eitoku, T.; Ousaka, D.; Kondo, M.; Kurita, Y.; Hirai, K.; Fukushima, Y.; Baba, K.; Goto, T.; et al. Intracoronary Cardiac Progenitor Cells in Single Ventricle Physiology: The PERSEUS (Cardiac Progenitor Cell Infusion to Treat Univentricular Heart Disease) Randomized Phase 2 Trial. Circ. Res. 2017, 120, 1162–1173. [Google Scholar] [CrossRef]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behfar, A.; Zingman, L.V.; Hodgson, D.M.; Rauzier, J.M.; Kane, G.C.; Terzic, A.; Puceat, M. Stem cell differentiation requires a paracrine pathway in the heart. FASEB J. 2002, 16, 1558–1566. [Google Scholar] [CrossRef] [Green Version]
- Min, J.Y.; Yang, Y.; Converso, K.L.; Liu, L.; Huang, Q.; Morgan, J.P.; Xiao, Y.F. Transplantation of embryonic stem cells improves cardiac function in postinfarcted rats. J. Appl. Physiol. 2002, 92, 288–296. [Google Scholar] [CrossRef]
- Blin, G.; Stefanovic, S.; Neri, T.; Guillevic, O.; Brinon, B.; Bellamy, V.; Rucker-Martin, C.; Barbry, P.; Bel, A.; Bruneval, P.; et al. A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. J. Clin. Investig. 2010, 120, 1125–1139. [Google Scholar] [CrossRef] [Green Version]
- Shiba, Y.; Fernandes, S.; Zhu, W.Z.; Filice, D.; Muskheli, V.; Kim, J.; Palpant, N.J.; Gantz, J.; Moyes, K.W.; Reinecke, H.; et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature 2012, 489, 322–325. [Google Scholar] [CrossRef]
- Yeghiazarians, Y.; Gaur, M.; Zhang, Y.; Sievers, R.E.; Ritner, C.; Prasad, M.; Boyle, A.; Bernstein, H.S. Myocardial improvement with human embryonic stem cell-derived cardiomyocytes enriched by p38MAPK inhibition. Cytotherapy 2012, 14, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Chong, J.J.; Yang, X.; Don, C.W.; Minami, E.; Liu, Y.W.; Weyers, J.J.; Mahoney, W.M.; Van Biber, B.; Cook, S.M.; Palpant, N.J.; et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014, 510, 273–277. [Google Scholar] [CrossRef]
- Menasché, P.; Vanneaux, V.; Hagège, A.; Cholley, B.; Tachdjian, G.; Tosca, L.; Trouvin, J.-H.; Fabreguettes, J.-R.; Blons, H.; Al-Daccak, R.; et al. Abstract 14798: Human Embryonic Stem Cell-derived Cardiac Progenitors for Heart Failure. One-year Results of the ESCORT Trial. Circulaton 2017, 136, A14798. [Google Scholar]
- Robertson, J.A. Human embryonic stem cell research: Ethical and legal issues. Nat. Rev. Genet. 2001, 2, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Swijnenburg, R.J.; Tanaka, M.; Vogel, H.; Baker, J.; Kofidis, T.; Gunawan, F.; Lebl, D.R.; Caffarelli, A.D.; de Bruin, J.L.; Fedoseyeva, E.V.; et al. Embryonic stem cell immunogenicity increases upon differentiation after transplantation into ischemic myocardium. Circulation 2005, 112, I-166–I-172. [Google Scholar] [CrossRef] [PubMed]
- Martin, U. Genome stability of programmed stem cell products. Adv. Drug Deliv. Rev. 2017, 120, 108–117. [Google Scholar] [CrossRef]
- Miyagawa, S.; Sawa, Y. Building a new strategy for treating heart failure using Induced Pluripotent Stem Cells. J. Cardiol. 2018, 72, 445–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuerstenau-Sharp, M.; Zimmermann, M.E.; Stark, K.; Jentsch, N.; Klingenstein, M.; Drzymalski, M.; Wagner, S.; Maier, L.S.; Hehr, U.; Baessler, A.; et al. Generation of highly purified human cardiomyocytes from peripheral blood mononuclear cell-derived induced pluripotent stem cells. PLoS ONE 2015, 10, e0126596. [Google Scholar] [CrossRef] [Green Version]
- Mummery, C.L.; Zhang, J.; Ng, E.S.; Elliott, D.A.; Elefanty, A.G.; Kamp, T.J. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: A methods overview. Circ. Res. 2012, 111, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; de l’Hortet, A.C.; Soto-Gutierrez, A. Induced Pluripotent Stem Cell-Derived Endothelial Cells: Overview, Current Advances, Applications, and Future Directions. Am. J. Pathol. 2019, 189, 502–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamis, Y.; Silva, E.A.; Hewitt, K.J.; Brudno, Y.; Levenberg, S.; Mooney, D.J.; Garlick, J.A. Fibroblasts derived from human pluripotent stem cells activate angiogenic responses in vitro and in vivo. PLoS ONE 2013, 8, e83755. [Google Scholar] [CrossRef] [Green Version]
- Sobol, M.; Raykova, D.; Cavelier, L.; Khalfallah, A.; Schuster, J.; Dahl, N. Methods of Reprogramming to Induced Pluripotent Stem Cell Associated with Chromosomal Integrity and Delineation of a Chromosome 5q Candidate Region for Growth Advantage. Stem. Cells Dev. 2015, 24, 2032–2040. [Google Scholar] [CrossRef]
- Yoshihara, M.; Hayashizaki, Y.; Murakawa, Y. Genomic Instability of iPSCs: Challenges Towards Their Clinical Applications. Stem. Cell Rev. Rep. 2017, 13, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Steinle, H.; Weber, M.; Behring, A.; Mau-Holzmann, U.; von Ohle, C.; Popov, A.F.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Reprogramming of Urine-Derived Renal Epithelial Cells into iPSCs Using srRNA and Consecutive Differentiation into Beating Cardiomyocytes. Mol. Ther. Nucleic Acids 2019, 17, 907–921. [Google Scholar] [CrossRef] [Green Version]
- Kogut, I.; McCarthy, S.M.; Pavlova, M.; Astling, D.P.; Chen, X.; Jakimenko, A.; Jones, K.L.; Getahun, A.; Cambier, J.C.; Pasmooij, A.M.G.; et al. High-efficiency RNA-based reprogramming of human primary fibroblasts. Nat. Commun. 2018, 9, 745. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Malik, V.; Jauch, R. Reprogramming cells with synthetic proteins. Asian J. Androl. 2015, 17, 394–402. [Google Scholar] [CrossRef]
- Han, L.; Mich-Basso, J.; Kuhn, B. Generation of Human Induced Pluripotent Stem Cells and Differentiation into Cardiomyocytes. Methods Mol. Biol. 2021, 2158, 125–139. [Google Scholar] [CrossRef]
- Zhang, J.; Raval, K.K.; Lian, X.; Herman, A.M.; Wilson, G.F.; Barron, M.R.; Yu, J.; Palecek, S.P.; Thomson, J.A.; Kamp, T.J. Abstract 20724: Matrix-Promoted Efficient Cardiac Differentiation of Human iPS and ES Cells. Circulation 2010, 122, A20724. [Google Scholar]
- Sharma, A.; Li, G.; Rajarajan, K.; Hamaguchi, R.; Burridge, P.W.; Wu, S.M. Derivation of highly purified cardiomyocytes from human induced pluripotent stem cells using small molecule-modulated differentiation and subsequent glucose starvation. J. Vis. Exp. 2015, 97, 52628. [Google Scholar] [CrossRef] [PubMed]
- Pei, F.; Jiang, J.; Bai, S.; Cao, H.; Tian, L.; Zhao, Y.; Yang, C.; Dong, H.; Ma, Y. Chemical-defined and albumin-free generation of human atrial and ventricular myocytes from human pluripotent stem cells. Stem. Cell Res. 2017, 19, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Eschenhagen, T.; Bolli, R.; Braun, T.; Field, L.J.; Fleischmann, B.K.; Frisen, J.; Giacca, M.; Hare, J.M.; Houser, S.; Lee, R.T.; et al. Cardiomyocyte Regeneration: A Consensus Statement. Circulation 2017, 136, 680–686. [Google Scholar] [CrossRef]
- Christalla, P.; Hudson, J.E.; Zimmermann, W.H. The cardiogenic niche as a fundamental building block of engineered myocardium. Cells Tissues Organs 2012, 195, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [Green Version]
- Leitolis, A.; Robert, A.W.; Pereira, I.T.; Correa, A.; Stimamiglio, M.A. Cardiomyogenesis Modeling Using Pluripotent Stem Cells: The Role of Microenvironmental Signaling. Front. Cell Dev. Biol. 2019, 7, 164. [Google Scholar] [CrossRef] [Green Version]
- Oberwallner, B.; Brodarac, A.; Anic, P.; Saric, T.; Wassilew, K.; Neef, K.; Choi, Y.H.; Stamm, C. Human cardiac extracellular matrix supports myocardial lineage commitment of pluripotent stem cells. Eur. J. Cardiothorac. Surg. 2015, 47, 416–425, discussion 425. [Google Scholar] [CrossRef] [Green Version]
- Garreta, E.; de Onate, L.; Fernandez-Santos, M.E.; Oria, R.; Tarantino, C.; Climent, A.M.; Marco, A.; Samitier, M.; Martinez, E.; Valls-Margarit, M.; et al. Myocardial commitment from human pluripotent stem cells: Rapid production of human heart grafts. Biomaterials 2016, 98, 64–78. [Google Scholar] [CrossRef] [Green Version]
- Pfeiffer, M.J.; Quaranta, R.; Piccini, I.; Fell, J.; Rao, J.; Ropke, A.; Seebohm, G.; Greber, B. Cardiogenic programming of human pluripotent stem cells by dose-controlled activation of EOMES. Nat. Commun. 2018, 9, 440. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Soonpaa, M.H.; Adler, E.D.; Roepke, T.K.; Kattman, S.J.; Kennedy, M.; Henckaerts, E.; Bonham, K.; Abbott, G.W.; Linden, R.M.; et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature 2008, 453, 524–528. [Google Scholar] [CrossRef]
- Jha, R.; Li, D.; Wu, Q.; Ferguson, K.E.; Forghani, P.; Gibson, G.C.; Xu, C. A long non-coding RNA GATA6-AS1 adjacent to GATA6 is required for cardiomyocyte differentiation from human pluripotent stem cells. FASEB J. 2020, 34, 14336–14352. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, N.; Hu, Y.; Zhou, X.; Jiang, H.; Lin, Q.; Chen, R.; Liu, H.; Gu, Y.; Tong, C.; et al. Molecular Signatures and Networks of Cardiomyocyte Differentiation in Humans and Mice. Mol. Ther. Nucleic. Acids 2020, 21, 696–711. [Google Scholar] [CrossRef]
- Miltenyi, S.; Muller, W.; Weichel, W.; Radbruch, A. High gradient magnetic cell separation with MACS. Cytometry 1990, 11, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Galdos, F.X.; Darsha, A.K.; Paige, S.L.; Wu, S.M. Purification of Pluripotent Stem Cell-Derived Cardiomyocytes Using CRISPR/Cas9-Mediated Integration of Fluorescent Reporters. Methods Mol. Biol. 2021, 2158, 223–240. [Google Scholar] [CrossRef]
- Babiarz, J.E.; Ravon, M.; Sridhar, S.; Ravindran, P.; Swanson, B.; Bitter, H.; Weiser, T.; Chiao, E.; Certa, U.; Kolaja, K.L. Determination of the human cardiomyocyte mRNA and miRNA differentiation network by fine-scale profiling. Stem. Cells Dev. 2012, 21, 1956–1965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, M.L.; Graham, B.T.; Pabon, L.M.; Han, S.J.; Murry, C.E.; Sniadecki, N.J. Measuring the contractile forces of human induced pluripotent stem cell-derived cardiomyocytes with arrays of microposts. J. Biomech. Eng. 2014, 136, 051005. [Google Scholar] [CrossRef] [Green Version]
- Mannhardt, I.; Breckwoldt, K.; Letuffe-Breniere, D.; Schaaf, S.; Schulz, H.; Neuber, C.; Benzin, A.; Werner, T.; Eder, A.; Schulze, T.; et al. Human Engineered Heart Tissue: Analysis of Contractile Force. Stem. Cell Rep. 2016, 7, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Thavandiran, N.; Hale, C.; Blit, P.; Sandberg, M.L.; McElvain, M.E.; Gagliardi, M.; Sun, B.; Witty, A.; Graham, G.; Do, V.T.H.; et al. Functional arrays of human pluripotent stem cell-derived cardiac microtissues. Sci. Rep. 2020, 10, 6919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartman, M.E.; Dai, D.F.; Laflamme, M.A. Human pluripotent stem cells: Prospects and challenges as a source of cardiomyocytes for in vitro modeling and cell-based cardiac repair. Adv. Drug Deliv. Rev. 2016, 96, 3–17. [Google Scholar] [CrossRef] [Green Version]
- Barbuti, A.; Benzoni, P.; Campostrini, G.; Dell’Era, P. Human derived cardiomyocytes: A decade of knowledge after the discovery of induced pluripotent stem cells. Dev. Dyn. 2016, 245, 1145–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, S.; Zhao, D.; Wang, X.; Ni, X.; Fang, X.; Yu, M.; Ye, L.; Yang, J.; Wu, H.; Han, X.; et al. Retinoic acid promotes metabolic maturation of human Embryonic Stem Cell-derived Cardiomyocytes. Theranostics 2020, 10, 9686–9701. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y. Availability of a novel cardiotoxicity evaluation system using human induced pluripotent stem cell-derived atrial-like myocytes. Nihon Yakurigaku Zasshi 2020, 155, 303–308. [Google Scholar] [CrossRef]
- Giacomelli, E.; Meraviglia, V.; Campostrini, G.; Cochrane, A.; Cao, X.; van Helden, R.W.J.; Krotenberg Garcia, A.; Mircea, M.; Kostidis, S.; Davis, R.P.; et al. Human-iPSC-Derived Cardiac Stromal Cells Enhance Maturation in 3D Cardiac Microtissues and Reveal Non-cardiomyocyte Contributions to Heart Disease. Cell Stem Cell 2020, 26, 862–879.e11. [Google Scholar] [CrossRef]
- Zhu, R.; Blazeski, A.; Poon, E.; Costa, K.D.; Tung, L.; Boheler, K.R. Physical developmental cues for the maturation of human pluripotent stem cell-derived cardiomyocytes. Stem Cell Res. Ther. 2014, 5, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.J.; Kim, R.Y.; Park, B.W.; Lee, S.; Choi, S.W.; Park, J.H.; Choi, J.J.; Kim, S.W.; Jang, J.; Cho, D.W.; et al. Dual stem cell therapy synergistically improves cardiac function and vascular regeneration following myocardial infarction. Nat. Commun. 2019, 10, 3123. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhu, W.; Radisic, M.; Vunjak-Novakovic, G. Can We Engineer a Human Cardiac Patch for Therapy? Circ. Res. 2018, 123, 244–265. [Google Scholar] [CrossRef]
- Ye, L.; Chang, Y.H.; Xiong, Q.; Zhang, P.; Zhang, L.; Somasundaram, P.; Lepley, M.; Swingen, C.; Su, L.; Wendel, J.S.; et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell 2014, 15, 750–761. [Google Scholar] [CrossRef] [Green Version]
- Hattan, N.; Kawaguchi, H.; Ando, K.; Kuwabara, E.; Fujita, J.; Murata, M.; Suematsu, M.; Mori, H.; Fukuda, K. Purified cardiomyocytes from bone marrow mesenchymal stem cells produce stable intracardiac grafts in mice. Cardiovasc. Res. 2005, 65, 334–344. [Google Scholar] [CrossRef] [Green Version]
- Vuorenpaa, H.; Penttinen, K.; Heinonen, T.; Pekkanen-Mattila, M.; Sarkanen, J.R.; Ylikomi, T.; Aalto-Setala, K. Maturation of human pluripotent stem cell derived cardiomyocytes is improved in cardiovascular construct. Cytotechnology 2017, 69, 785–800. [Google Scholar] [CrossRef]
- Sun, X.; Wu, J.; Qiang, B.; Romagnuolo, R.; Gagliardi, M.; Keller, G.; Laflamme, M.A.; Li, R.K.; Nunes, S.S. Transplanted microvessels improve pluripotent stem cell-derived cardiomyocyte engraftment and cardiac function after infarction in rats. Sci. Transl. Med. 2020, 12, 2992. [Google Scholar] [CrossRef]
- Gao, L.; Gregorich, Z.R.; Zhu, W.; Mattapally, S.; Oduk, Y.; Lou, X.; Kannappan, R.; Borovjagin, A.V.; Walcott, G.P.; Pollard, A.E.; et al. Large Cardiac Muscle Patches Engineered From Human Induced-Pluripotent Stem Cell-Derived Cardiac Cells Improve Recovery From Myocardial Infarction in Swine. Circulation 2018, 137, 1712–1730. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Lei, A.; Tian, L.; Wang, X.; Correia, C.; Weiskittel, T.; Li, H.; Trounson, A.; Fu, Q.; Yao, K.; et al. Strategies for Genetically Engineering Hypoimmunogenic Universal Pluripotent Stem Cells. iScience 2020, 23, 101162. [Google Scholar] [CrossRef]
- Farjadian, S. The significance of HLA typing in transplantation. J. Nephropathol. 2012, 1, 160–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Yang, Z.; Zhao, Z.A.; Shen, Z. Direct reprogramming of fibroblasts into cardiomyocytes. Stem Cell Res. Ther. 2017, 8, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christoforou, N.; Chakraborty, S.; Kirkton, R.D.; Adler, A.F.; Addis, R.C.; Leong, K.W. Core Transcription Factors, MicroRNAs, and Small Molecules Drive Transdifferentiation of Human Fibroblasts Towards The Cardiac Cell Lineage. Sci. Rep. 2017, 7, 40285. [Google Scholar] [CrossRef] [Green Version]
- Wada, R.; Muraoka, N.; Inagawa, K.; Yamakawa, H.; Miyamoto, K.; Sadahiro, T.; Umei, T.; Kaneda, R.; Suzuki, T.; Kamiya, K.; et al. Induction of human cardiomyocyte-like cells from fibroblasts by defined factors. Proc. Natl. Acad. Sci. USA 2013, 110, 12667–12672. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.D.; Stone, N.R.; Liu, L.; Spencer, C.I.; Qian, L.; Hayashi, Y.; Delgado-Olguin, P.; Ding, S.; Bruneau, B.G.; Srivastava, D. Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state. Stem Cell Rep. 2013, 1, 235–247. [Google Scholar] [CrossRef] [Green Version]
- Nam, Y.J.; Song, K.; Luo, X.; Daniel, E.; Lambeth, K.; West, K.; Hill, J.A.; DiMaio, J.M.; Baker, L.A.; Bassel-Duby, R.; et al. Reprogramming of human fibroblasts toward a cardiac fate. Proc. Natl. Acad. Sci. USA 2013, 110, 5588–5593. [Google Scholar] [CrossRef] [Green Version]
- Cao, N.; Huang, Y.; Zheng, J.; Spencer, C.I.; Zhang, Y.; Fu, J.-D.; Nie, B.; Xie, M.; Zhang, M.; Wang, H.; et al. Conversion of human fibroblasts into functional cardiomyocytes by small molecules. Science 2016, 325, 1216–1220. [Google Scholar] [CrossRef] [Green Version]
- Song, K.; Luo, X.; Qi, X.; Tan, W.; Huang, G.N.; Acharya, A.; Smith, C.L.; Tallquist, M.D.; Neilson, E.G.; Hill, J.A.; et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nat. Commun. 2012, 485, 599–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, L.; Huang, Y.; Spencer, C.I.; Foley, A.; Vedantham, V.; Liu, L.; Conway, S.J.; Fu, J.D.; Srivastava, D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature 2012, 485, 593. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.R. Combination of Mesenchymal and C-kit+ Cardiac Stem Cells as Regenerative Therapy for Heart Failure. Available online: https://ClinicalTrials.gov/show/NCT02501811 (accessed on 19 August 2021).
- Kastrup, J. Allogeneic Stem Cell Therapy in Heart Failure. Available online: https://ClinicalTrials.gov/show/NCT03092284 (accessed on 19 August 2021).
- Marfella, R.; Sardu, C. Stem Cells Therapy in Advanced Heart Failure. Available online: https://ClinicalTrials.gov/show/NCT02871466 (accessed on 19 August 2021).
- Liu, Z. Multi-intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Heart Failure with Reduced Ejection Fraction (PRIME-HFrEF Study). Available online: https://ClinicalTrials.gov/show/NCT04992832 (accessed on 19 August 2021).
- Sawa, Y. Clinical Trial of Human (Allogeneic) iPS Cell-Derived Cardiomyocytes Sheet for Ischemic Cardiomyopathy; NCT04696328. 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT04696328 (accessed on 24 October 2021).
- Cyranoski, D. ‘Reprogrammed’ stem cells approved to mend human hearts for the first time. Nature 2018, 557, 619–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiji. Osaka University Transplants iPS Cell-Based Heart Cells in World’s First Clinical Trial. Available online: https://www.japantimes.co.jp/news/2020/01/28/national/science-health/osaka-university-transplants-ips-cell-based-heart-cells-worlds-first-clinical-trial/ (accessed on 24 October 2021).
| Cell Types | Skeletal Myoblasts | Bone Marrow-Derived Hematopoietic Stem Cells (Bm-Hscs) | Bone Marrow-Derived Endothelial Progenitor Cells (Bm-Epcs) | Bone Marrow-Derived Mesenchymal Stem Cells (Bm-Mscs) |
|---|---|---|---|---|
| origin | Autologous muscle biopsies (easy) | Autologous bone marrow/blood (easy) | Autologous bone marrow/blood (easy) | Autologous tissues (easy) |
| ethical concerns | Low | Low | Low | Low |
| tumorigenicity risk | Low | Low | Low | Low |
| cell quantity | Sufficient | Limited | Limited | Limited |
| differentiation potentials into cms | Cannot generate functional CMs | Limited potentials | Limited potentials | Limited potentials |
| growth | Rapid in vitro expansion | Rapid in vitro expansion | Rapid in vitro expansion | Rapid in vitro expansion |
| Resist ischemic conditions | Heterogenous cell population | Heterogenous cell population | Heterogenous cell population | |
| immunologic rejection risks | Low | Low | Low | Low |
| other advantages | - | Proved safe in clinical trials | Proved safe in clinical trials | - |
| - | Promote vasculogenesis Therapeutic secretome | - | ||
| other inconveniences | Ventricular arrhythmia hazard | Encourage inflammation | Ambiguous therapeutic results | |
| Cell Types | Adipose-Derived Stem Cells (ASCS) | Cardiac Stem Cells (cscs) and Cardiac Progenitor Cells (CPCS) | Embryonic Stem Cells (ESC) | Induced Pluripotent Stem Cell (IPSC) |
| origin | Autologous tissues (easy) | Autologous myocardial biopsies (invasive) | Inner cell mass of blastocysts from in vitro fecundation (non-autologous) | Reprogrammed from autologous cells (easy access) |
| ethical concerns | Low | Low | High | Low |
| tumorigenicity risk | Low | Low | High | High |
| cell quantity | Sufficient | Limited | Unlimited | Unlimited |
| differentiation potentials into cms | Limited potentials | Ambiguous results | Pluripotent differentiation potentials Generate CMs capable of integrating electromagnetically into the host myocardium | Pluripotent differentiation potentials Generate CMs capable of integrating electromagnetically into the host myocardium |
| growth | Rapid in vitro expansion | Insufficient cell characterization as CMs | Difficult to generate pure and mature cardiomyocytes in large quantities | Difficult to generate pure and mature cardiomyocytes in large quantities |
| Heterogenous cell population | Heterogenous cell population | Unavailability | Lack of standardized generation | |
| Low induction efficiency | ||||
| immunologic rejection risks | Low | Low | High risks require immunosuppression (non-autologous) | Low |
| other advantages | - | Proved safe in clinical trials | - | - |
| - | - | - | - | |
| other inconveniences | - | - | - | - |
| Original Cell | Dermal Fibroblast (DF) | Human Cardiac Fibroblast (HCF) | Embryonic Stem Cell (esc), Fetal Heart (FH), Neonatal Skin | Human Cardiac Fibroblast (HCF) | Human Foreskin Fibroblast (HFF) | Human Foreskin Fibroblast (HFF) |
|---|---|---|---|---|---|---|
| factors | ETS2 | GATA4 | GATA4 | GATA4 | GATA4 | CHIR99021 |
| MESP1 | MEF2C | MEF2C | MEF2C | HAND2 | A83-0 | |
| TBX5 | TBX5 | TBX5 | TBX5 | BIX01294 | ||
| MESP1 | ESRRG | MESP1 | MYOCD | AS8351 | ||
| MYOCD | MESP1 | MYOCD | miR-1 | SC1 | ||
| MYOCD | miR-133 | miR-133 | Y27632 | |||
| ZFPM2 | OAC2 | |||||
| SU16F | ||||||
| JNJ10198409 | ||||||
| markers (efficiency) | NKX2.5-tdTomato+ (30 colonies/plate of cardiac progenitor) | cTnT+ (5.9%) | α-MHC-mCherry+ (15.8%) | cTnT+ (27.8%) | cTnT+ (34.1%) | cTnT+ (6.6%) |
| α-actinin+ (5.5%) | α-MHC-mCherry+ & cTnT+ (13%) | α-actinin+ (8%) | ||||
| action potential | Negative | Positive | Positive | Not detected | Not detected | Positive |
| Ca2+ transient | Negative | Positive | Positive | Positive | Positive | Positive |
| beating | Negative | Positive | Not detected | Positive | Positive | Positive |
| Cells Type | Adult Cms | Ipsc-Cms | Transdifferentiated Cms |
|---|---|---|---|
| differentiation efficiency | - | >80% | ~60% expressing cTnT+ and α-actinin+ markers |
| size | Membrane capacitance 150 pF | Small size (membrane capacitance 18 pF), 1/10 size of adult CMs | Small size |
| nucleus | Bi- or multi-nuclear | Mononuclear | Mononuclear |
| morphology | Rod-shape | Circular shape Irregular shape | Spindle-shape |
| sarcomere | Highly organized | Better organized | Disarrayed |
| primary metabolic substrate | Fatty acid | Glucose | Glucose |
| markers | α-MHC+ | α-MHC+ | α-MHC+ |
| α-actinin+ | α-actinin+ | α-actinin+ | |
| Troponin T+ | Troponin T+ | Troponin T+ | |
| Ca2+ transient | Positive | Positive | Positive (few induced CMs) |
| electrophysiology | Resting membrane potential −90 mV (quicker action potential) | Resting membrane potential −60 mV (slower action potential) | Resting membrane potential −48 mV (slowest action potential) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, D.; Wu, H.; Pan, K.; Peng, H.; Wu, R. Regenerating Damaged Myocardium: A Review of Stem-Cell Therapies for Heart Failure. Cells 2021, 10, 3125. https://doi.org/10.3390/cells10113125
Fan D, Wu H, Pan K, Peng H, Wu R. Regenerating Damaged Myocardium: A Review of Stem-Cell Therapies for Heart Failure. Cells. 2021; 10(11):3125. https://doi.org/10.3390/cells10113125
Chicago/Turabian StyleFan, Dihan, Hanrong Wu, Kaichao Pan, Huashan Peng, and Rongxue Wu. 2021. "Regenerating Damaged Myocardium: A Review of Stem-Cell Therapies for Heart Failure" Cells 10, no. 11: 3125. https://doi.org/10.3390/cells10113125
APA StyleFan, D., Wu, H., Pan, K., Peng, H., & Wu, R. (2021). Regenerating Damaged Myocardium: A Review of Stem-Cell Therapies for Heart Failure. Cells, 10(11), 3125. https://doi.org/10.3390/cells10113125