Trans-Ned 19-Mediated Antagonism of Nicotinic Acid Adenine Nucleotide—Mediated Calcium Signaling Regulates Th17 Cell Plasticity in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Reagents
2.3. Cytokines
2.4. In Vivo Trans-Ned 19 Administration
2.5. Anti-CD3 Antibody Treatment
2.6. Isolation of Cells from the Small Intestine of the Mouse
2.7. Isolation of CD4+ T Cells from Murine Spleen and Lymph Nodes
2.8. Imaging of Global Ca2+ Signalling in Primary T Cells from Mice
2.9. Seahorse Metabolic Flux Measurement
2.10. Proliferation Assay
2.11. Flow Cytometric Analysis
2.12. In Vitro Differentiation of CD4+ T Cells
2.13. In Vitro Suppression Assay
2.14. In Vitro Cell Death Assay
2.15. Statistical Analysis
3. Results
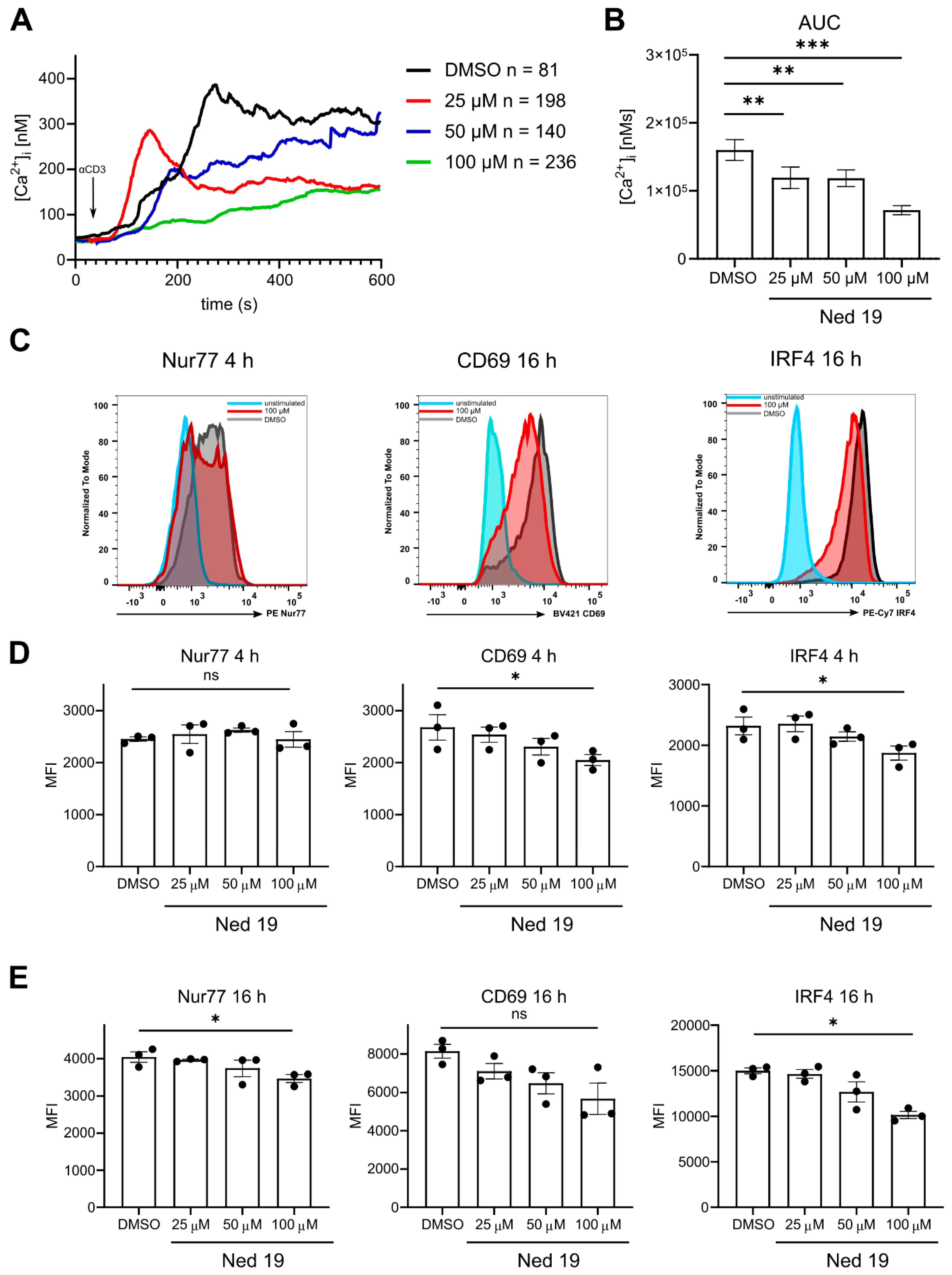
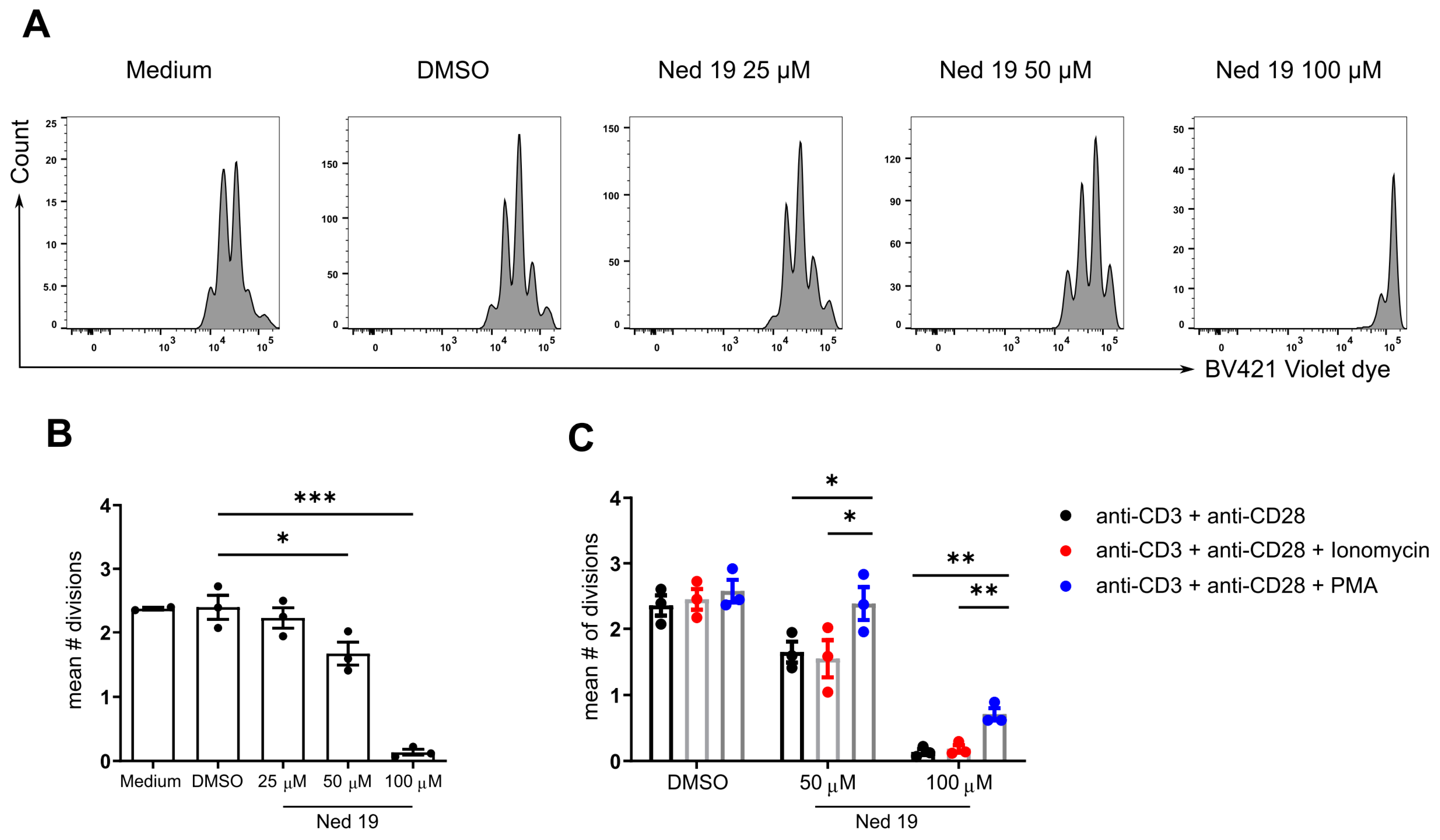
3.1. Antagonism of NAADP by Means of Trans-Ned 19 Inhibits Ca2+ Signaling upon TCR Stimulation, and Activation and Proliferation of CD4+ T Cells
3.2. NAADP Inhibition Promotes the Differentiation of Th1 and Th17 Cells and It Inhibits the Differentiation of Foxp3+ Regulatory T Cells In Vitro
3.3. NAADP Inhibition with Trans-Ned 19 Promotes Production of IL-10 by In Vitro Differentiated Effector T Cells and Foxp3+ T Regulatory Cells and Increases Their Suppressive Capacity
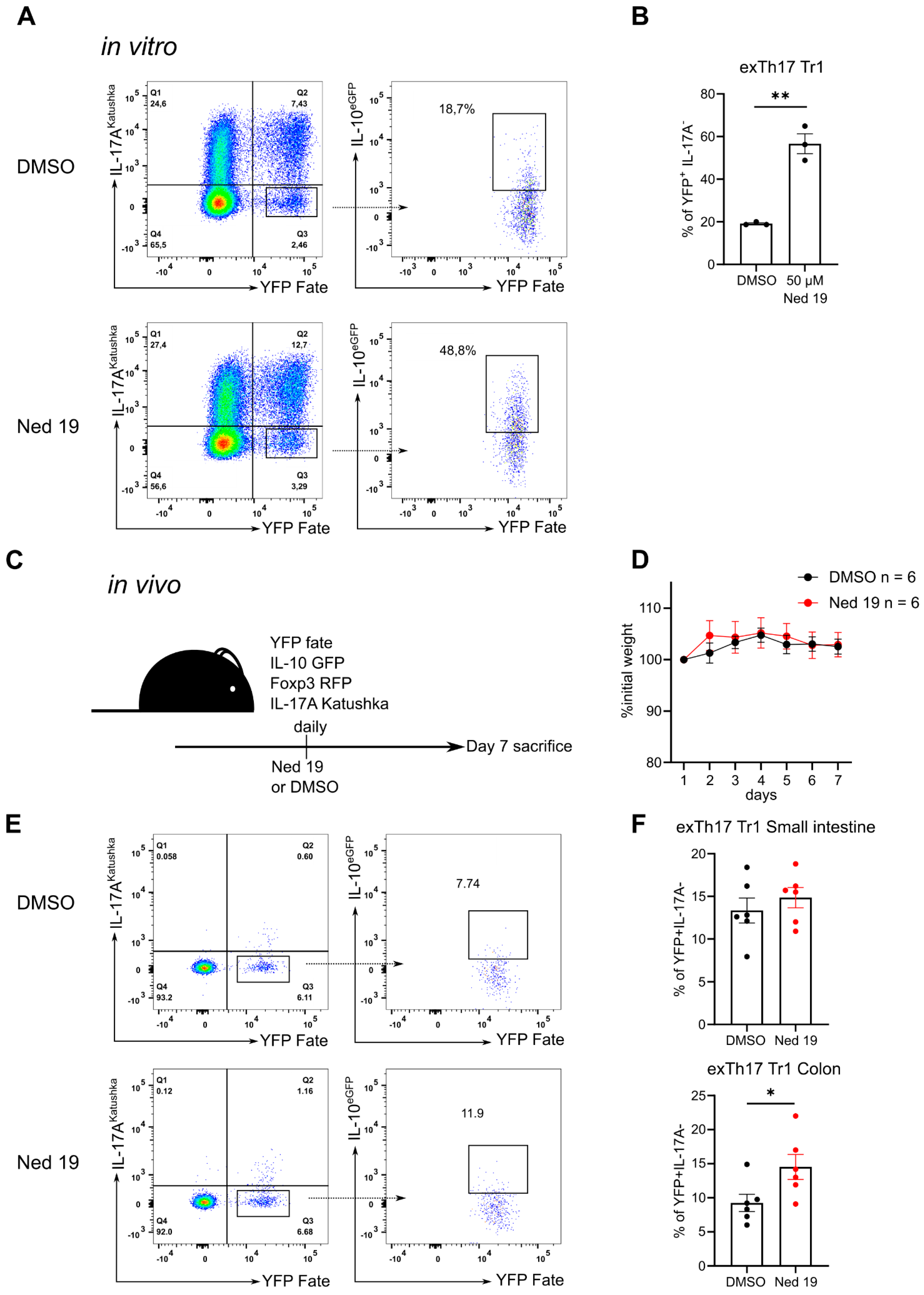
3.4. NAADP Inhibition Promotes the Transdifferentiation of Th17 Cells into T Regulatory Type 1 Cells In Vitro and In Vivo
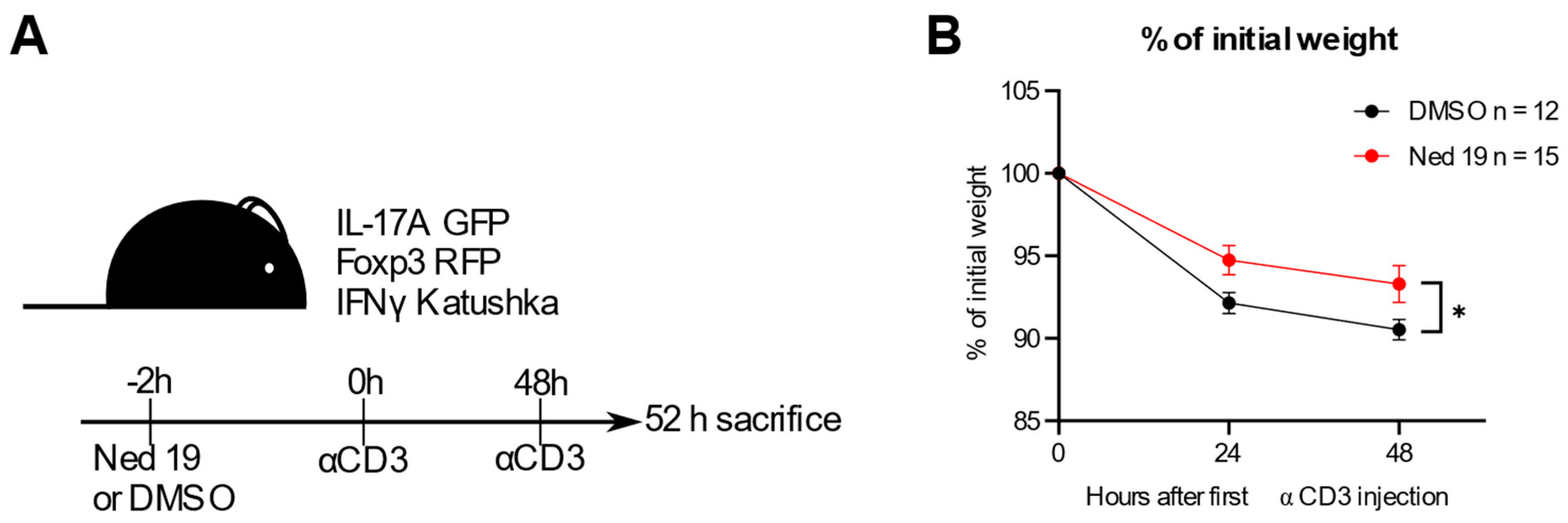
3.5. NAADP Inhibition In Vivo Ameliorates Disease in the Anti-CD3 Induced Intestinal Inflammation Mouse Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trebak, M.; Kinet, J.P. Calcium signalling in T cells. Nat. Rev. Immunol. 2019, 19, 154–169. [Google Scholar] [CrossRef]
- Ernst, I.M.A.; Fliegert, R.; Guse, A.H. Adenine dinucleotide second messengers and T-lymphocyte calcium signaling. Front. Immunol. 2013, 4, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; McCarl, C.A.; Khalil, S.; Lüthy, K.; Feske, S. T-cell-specific deletion of STIM1 and STIM2 protects mice from EAE by impairing the effector functions of Th1 and Th17 cells. Eur. J. Immunol. 2010, 40, 3028–3042. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, U.; Shaw, P.J.; Kozhaya, L.; Subramanian, R.; Gaida, K.; Unutmaz, D.; McBride, H.J.; Feske, S. Selective ORAI1 Inhibition Ameliorates Autoimmune Central Nervous System Inflammation by Suppressing Effector but Not Regulatory T Cell Function. J. Immunol. 2016, 196, 573–585. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, U.; Kahlfuss, S.; Yang, J.; Ivanova, E.; Koralov, S.B.; Feske, S. Calcium Signaling Controls Pathogenic Th17 Cell-Mediated Inflammation by Regulating Mitochondrial Function. Cell Metab. 2019, 29, 1104–1118.e6. [Google Scholar] [CrossRef]
- Lee, H.C.; Aarhus, R. A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose. J. Biol. Chem. 1995, 270, 2152–2157. [Google Scholar] [CrossRef] [Green Version]
- Guse, A.H.; Diercks, B.P. Integration of nicotinic acid adenine dinucleotide phosphate (NAADP)-dependent calcium signalling. J. Physiol. 2018, 596, 2735–2743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, I.M.A.; Guse, A.H. Ca2+ microdomains in T-lymphocytes. Front. Oncol. 2017, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Gasser, A.; Bruhn, S.; Guse, A.H. Second messenger function of nicotinic acid adenine dinucleotide phosphate revealed by an improved enzymatic cycling assay. J. Biol. Chem. 2006, 281, 16906–16913. [Google Scholar] [CrossRef] [Green Version]
- Dammermann, W.; Guse, A.H. Functional ryanodine receptor expression is required for NAADP-mediated local Ca2+ signaling in T-lymphocytes. J. Biol. Chem. 2005, 280, 21394–21399. [Google Scholar] [CrossRef] [Green Version]
- Steen, M.; Kirchberger, T.; Guse, A.H. NAADP mobilizes calcium from the endoplasmic reticular Ca2+ store in T-lymphocytes. J. Biol. Chem. 2007, 282, 18864–18871. [Google Scholar] [CrossRef] [Green Version]
- Wolf, I.M.A.; Diercks, B.P.; Gattkowski, E.; Czarniak, F.; Kempski, J.; Werner, R.; Schetelig, D.; Mittrücker, H.W.; Schumacher, V.; Von Osten, M.; et al. Frontrunners of T cell activation: Initial, localized Ca2+ signals mediated by NAADP and the type 1 ryanodine receptor. Sci. Signal. 2015, 8, ra102. [Google Scholar] [CrossRef]
- Calcraft, P.J.; Ruas, M.; Pan, Z.; Cheng, X.; Arredouani, A.; Hao, X.; Tang, J.; Rietdorf, K.; Teboul, L.; Chuang, K.T.; et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 2009, 459, 596–600. [Google Scholar] [CrossRef] [Green Version]
- Lin-Moshier, Y.; Walseth, T.F.; Churamani, D.; Davidson, S.M.; Slama, J.T.; Hooper, R.; Brailoiu, E.; Patel, S.; Marchant, J.S. Photoaffinity labeling of nicotinic acid adenine dinucleotide phosphate (NAADP) targets in mammalian cells. J. Biol. Chem. 2012, 287, 2296–2307. [Google Scholar] [CrossRef] [Green Version]
- Roggenkamp, H.G.; Khansahib, I.; Hernandez, C.L.C.; Zhang, Y.; Lodygin, D.; Krüger, A.; Gu, F.; Möckl, F.; Löhndorf, A.; Wolters, V.; et al. HN1L/JPT2: A signaling protein that connects NAADP generation to Ca2+ microdomain formation. Sci. Signal. 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Gunaratne, G.S.; Brailoiu, E.; He, S.; Unterwald, E.M.; Patel, S.; Slama, J.T.; Walseth, T.F.; Marchant, J.S. Essential requirement for JPT2 in NAADP-evoked Ca2+ signaling. Sci. Signal. 2021, 14, 5605. [Google Scholar] [CrossRef]
- Dammermann, W.; Zhang, B.; Nebel, M.; Cordiglieri, C.; Odoardi, F.; Kirchberger, T.; Kawakami, N.; Dowden, J.; Schmid, F.; Dornmair, K.; et al. NAADP-mediated Ca2+ signaling via type 1 ryanodine receptor in T cells revealed by a synthetic NAADP antagonist. Proc. Natl. Acad. Sci. USA 2009, 106, 10678–10683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, R.A.; Camick, C.; Wiles, K.; Walseth, T.F.; Slama, J.T.; Bhattacharya, S.; Giovannucci, D.R.; Wall, K.A. Nicotinic acid adenine dinucleotide phosphate plays a critical role in naive and effector murine t cells but not natural regulatory t cells. J. Biol. Chem. 2016, 291, 4503–4522. [Google Scholar] [CrossRef] [Green Version]
- Naylor, E.; Arredouani, A.; Vasudevan, S.R.; Lewis, A.M.; Parkesh, R.; Mizote, A.; Rosen, D.; Thomas, J.M.; Izumi, M.; Ganesan, A.; et al. Identification of a chemical probe for NAADP by virtual screening. Nat. Chem. Biol. 2009, 5, 220–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordiglieri, C.; Odoardi, F.; Zhang, B.; Nebel, M.; Kawakami, N.; Klinkert, W.E.F.; Lodygin, D.; Lühder, F.; Breunig, E.; Schild, D.; et al. Nicotinic acid adenine dinucleotide phosphate-mediated calcium signalling in effector T cells regulates autoimmunity of the central nervous system. Brain 2010, 133, 1930–1943. [Google Scholar] [CrossRef]
- Gagliani, N.; Huber, S. Basic aspects of T helper cell differentiation. Methods Mol. Biol. 2017, 1514, 19–30. [Google Scholar] [CrossRef]
- Gagliani, N.; Amezcua Vesely, M.C.; Iseppon, A.; Brockmann, L.; Xu, H.; Palm, N.W.; De Zoete, M.R.; Licona-Limón, P.; Paiva, R.S.; Ching, T.; et al. TH17 cells transdifferentiate into regulatory T cells uring resolution of inflammation. Nature 2015, 523, 221–225. [Google Scholar] [CrossRef]
- Zhou, L.; Chong, M.M.W.; Littman, D.R. Plasticity of CD4+ T Cell Lineage Differentiation. Immunity 2009, 30, 646–655. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.K.; Turner, H.; Maynard, C.L.; Oliver, J.R.; Chen, D.; Elson, C.O.; Weaver, C.T. Late Developmental Plasticity in the T Helper 17 Lineage. Immunity 2009, 30, 92–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamanaka, M.; Kim, S.T.; Wan, Y.Y.; Sutterwala, F.S.; Lara-Tejero, M.; Galán, J.E.; Harhaj, E.; Flavell, R.A. Expression of Interleukin-10 in Intestinal Lymphocytes Detected by an Interleukin-10 Reporter Knockin tiger Mouse. Immunity 2006, 25, 941–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esplugues, E.; Huber, S.; Gagliani, N.; Hauser, A.E.; Town, T.; Wan, Y.Y.; O’Connor, W.; Rongvaux, A.; Van Rooijen, N.; Haberman, A.M.; et al. Control of TH17 cells occurs in the small intestine. Nature 2011, 475, 514–518. [Google Scholar] [CrossRef] [Green Version]
- Wan, Y.Y.; Flavell, R.A. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc. Natl. Acad. Sci. USA 2005, 102, 5126–5131. [Google Scholar] [CrossRef] [Green Version]
- Favia, A.; Pafumi, I.; Desideri, M.; Padula, F.; Montesano, C.; Passeri, D.; Nicoletti, C.; Orlandi, A.; Del Bufalo, D.; Sergi, M.; et al. NAADP-Dependent Ca2+ Signaling Controls Melanoma Progression, Metastatic Dissemination and Neoangiogenesis. Sci. Rep. 2016, 6, 18925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashouri, J.F.; Weiss, A. Endogenous Nur77 Is a Specific Indicator of Antigen Receptor Signaling in Human T and B Cells. J. Immunol. 2017, 198, 657–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Man, K.; Miasari, M.; Shi, W.; Xin, A.; Henstridge, D.C.; Preston, S.; Pellegrini, M.; Belz, G.T.; Smyth, G.K.; Febbraio, M.A.; et al. The transcription factor IRF4 is essential for TCR affinity-mediated metabolic programming and clonal expansion of T cells. Nat. Immunol. 2013, 14, 1155–1165. [Google Scholar] [CrossRef]
- Buck, M.D.; O’Sullivan, D.; Pearce, E.L. T cell metabolism drives immunity. J. Exp. Med. 2015, 212, 1345–1360. [Google Scholar] [CrossRef] [Green Version]
- Nebel, M.; Schwoerer, A.P.; Warszta, D.; Siebrands, C.C.; Limbrock, A.C.; Swarbrick, J.M.; Fliegert, R.; Weber, K.; Bruhn, S.; Hohenegger, M.; et al. Nicotinic acid adenine dinucleotide phosphate (NAADP)-mediated calcium signaling and arrhythmias in the heart evoked by β-adrenergic stimulation. J. Biol. Chem. 2013, 288, 16017–16030. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Watt, J.M.; Cordiglieri, C.; Dammermann, W.; Mahon, M.F.; Flügel, A.; Guse, A.H.; Potter, B.V.L. Small Molecule Antagonists of NAADP-Induced Ca2+ Release in T-Lymphocytes Suggest Potential Therapeutic Agents for Autoimmune Disease. Sci. Rep. 2018, 8, 1–17. [Google Scholar] [CrossRef]
- Brockmann, L.; Soukou, S.; Steglich, B.; Czarnewski, P.; Zhao, L.; Wende, S.; Bedke, T.; Ergen, C.; Manthey, C.; Agalioti, T.; et al. Molecular and functional heterogeneity of IL-10-producing CD4+ T cells. Nat. Commun. 2018, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Gagliani, N.; Huber, S.; Flavell, R.A. The intestine: Where amazing things happen. Cell Res. 2012, 22, 277–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, S.; Gagliani, N.; Esplugues, E.; O’Connor, W., Jr.; Huber, F.J.; Chaudhry, A.; Kamanaka, M.; Kobayashi, Y.; Booth, C.J.; Rudensky, A.Y.; et al. Th17 Cells Express Interleukin-10 Receptor and Are Controlled by Foxp3− and Foxp3+ Regulatory CD4+ T Cells in an Interleukin-10-Dependent Manner. Immunity 2011, 34, 554–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreschi, I.; Bruzzone, S.; Melone, L.; De Flora, A.; Zocchi, E. NAADP+ synthesis from cADPRP and nicotinic acid by ADP-ribosyl cyclases. Biochem. Biophys. Res. Commun. 2006, 345, 573–580. [Google Scholar] [CrossRef]
- Aarhus, R.; Graeff, R.M.; Dickey, D.M.; Walseth, T.F.; Lee, H.C. ADP-ribosyl cyclase and CD38 catalyze the synthesis of a calcium-mobilizing metabolite from NADP. J. Biol. Chem. 1995, 270, 30327–30333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, F.; Bruhn, S.; Weber, K.; Mittrücker, H.W.; Guse, A.H. CD38: A NAADP degrading enzyme. FEBS Lett. 2011, 585, 3544–3548. [Google Scholar] [CrossRef] [Green Version]
- Billington, R.A.; Thuring, J.W.; Conway, S.J.; Packman, L.; Holmes, A.B.; Genazzani, A.A. Production and characterization of reduced NAADP (nicotinic acid-adenine dinucleotide phosphate). Biochem. J. 2004, 378, 275. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, S.; Meijles, D.N.; Pagano, P.J. NADPH oxidases: Key modulators in aging and age-related cardiovascular diseases? Clin. Sci. 2016, 130, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigutto, S.; Hoste, C.; Grasberger, H.; Milenkovic, M.; Communi, D.; Dumont, J.E.; Corvilain, B.; Miot, F.; de Deken, X. Activation of dual oxidases Duox1 and Duox2: Differential regulation mediated by cAMP-dependent protein kinase and protein kinase C-dependent phosphorylation. J. Biol. Chem. 2009, 284, 6725–6734. [Google Scholar] [CrossRef] [Green Version]
- Cibrián, D.; Sánchez-Madrid, F. CD69: From activation marker to metabolic gatekeeper. Eur. J. Immunol. 2017, 47, 946–953. [Google Scholar] [CrossRef]
- Mahnke, J.; Schumacher, V.; Ahrens, S.; Käding, N.; Feldhoff, L.M.; Huber, M.; Rupp, J.; Raczkowski, F.; Mittrücker, H.W. Interferon Regulatory Factor 4 controls T H1 cell effector function and metabolism. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuyama, T.; Grossman, A.; Mittrücker, H.-W.; Siderovski, D.P.; Kiefer, F.; Kawakami, T.; Richardson, C.D.; Taniguchi, T.; Yoshinaga, S.K.; Mak, T.W. Molecular cloning of LSIRF, a lymphoid-specific member of the interferon regulatory factor family that binds the interferon-stimulated response element (ISRE). Nucleic Acids Res. 1995, 23, 2127–2136. [Google Scholar] [CrossRef] [Green Version]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawrocki, M.; Lory, N.; Bedke, T.; Stumme, F.; Diercks, B.-P.; Guse, A.H.; Meier, C.; Gagliani, N.; Mittrücker, H.-W.; Huber, S. Trans-Ned 19-Mediated Antagonism of Nicotinic Acid Adenine Nucleotide—Mediated Calcium Signaling Regulates Th17 Cell Plasticity in Mice. Cells 2021, 10, 3039. https://doi.org/10.3390/cells10113039
Nawrocki M, Lory N, Bedke T, Stumme F, Diercks B-P, Guse AH, Meier C, Gagliani N, Mittrücker H-W, Huber S. Trans-Ned 19-Mediated Antagonism of Nicotinic Acid Adenine Nucleotide—Mediated Calcium Signaling Regulates Th17 Cell Plasticity in Mice. Cells. 2021; 10(11):3039. https://doi.org/10.3390/cells10113039
Chicago/Turabian StyleNawrocki, Mikołaj, Niels Lory, Tanja Bedke, Friederike Stumme, Björn-Phillip Diercks, Andreas H. Guse, Chris Meier, Nicola Gagliani, Hans-Willi Mittrücker, and Samuel Huber. 2021. "Trans-Ned 19-Mediated Antagonism of Nicotinic Acid Adenine Nucleotide—Mediated Calcium Signaling Regulates Th17 Cell Plasticity in Mice" Cells 10, no. 11: 3039. https://doi.org/10.3390/cells10113039
APA StyleNawrocki, M., Lory, N., Bedke, T., Stumme, F., Diercks, B.-P., Guse, A. H., Meier, C., Gagliani, N., Mittrücker, H.-W., & Huber, S. (2021). Trans-Ned 19-Mediated Antagonism of Nicotinic Acid Adenine Nucleotide—Mediated Calcium Signaling Regulates Th17 Cell Plasticity in Mice. Cells, 10(11), 3039. https://doi.org/10.3390/cells10113039






