Precisely Monomeric Linear RNAs of Viroids Belonging to Pospiviroid and Hostuviroid Genera Are Infectious Regardless of Transcription Initiation Site and 5′-Terminal Structure
Abstract
1. Introduction
2. Materials and Methods
2.1. Viroid Sources
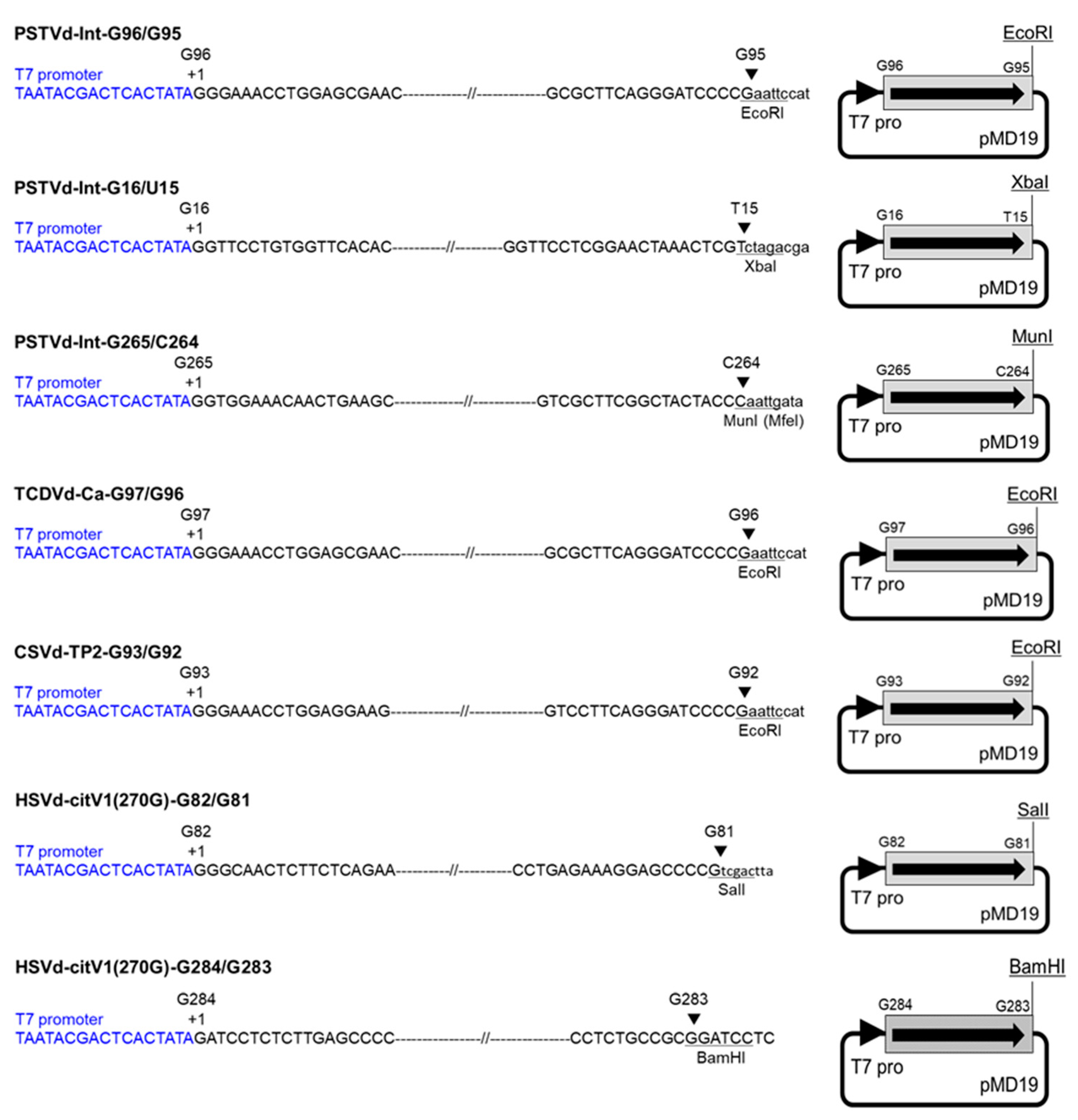
2.2. Infectious Monomeric Viroid cDNA Clones
2.3. Infectious Dimeric Viroid RNA Transcripts Derived from Dimeric cDNA Clones
2.4. In Vitro Synthesis of Monomeric Viroid RNA Transcripts
2.5. Modification of 5′-Terminus of Monomeric Viroid RNA Transcripts
2.6. Inoculation to Plants and Evaluation of Infectivity of Inoculum Nucleic Acids
2.7. Detection of Viroids from Inoculated Plants by RT-PCR
2.8. Northern Blot Hybridization
3. Results
3.1. Precisely Monomeric Viroid RNA Transcripts Are Infectious to Host Plants
3.2. Transcription Initiation at the Pcessing/Ligation Site Is Preferable but Not Necessary for the Production of Infectious Viroid RNA
3.3. Precisely Monomeric Viroid RNAs Are Infectious Regardless of 5′-Terminal Structures
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Serra, P.; Messmer, A.; Sanderson, D.; James, D.; Flores, R. Apple hammerhead viroid-like RNA is a bona fide viroid: Autonomous replication and structural features support its inclusion as a new member in the genus Pelamoviroid. Virus Res. 2018, 249, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Bojić, T.; Beeharry, Y.; Zhang, D.J.; Pelchat, M. Tomato RNA polymerase II interacts with the rod-like conformation of the left terminal domain of the potato spindle tuber viroid positive RNA genome. J. Gen. Virol. 2012, 93, 1591–1600. [Google Scholar] [CrossRef]
- Kolonko, N.; Bannach, O.; Aschermann, K.; Hu, K.H.; Moors, M.; Schmitz, M.; Steger, G.; Riesner, D. Transcription of potato spindle tuber viroid by RNA polymerase II starts in the left terminal loop. Virology 2006, 347, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qu, J.; Ji, S.; Wallace, A.J.; Wu, J.; Li, Y.; Gopalan, V.; Ding, B. A land plant-specific transcription factor directly enhances transcription of a pathogenic noncoding RNA template by DNA-dependent RNA polymerase II. Plant Cell 2016, 28, 1094–1107. [Google Scholar] [CrossRef]
- Gas, M.E.; Hernández, C.; Flores, R.; Daròs, J.A. Processing of nuclear viroids in vivo: An interplay between RNA conformations. PLoS Pathog. 2007, 3, e182. [Google Scholar] [CrossRef]
- Gas, M.E.; Molina-Serrano, D.; Hernández, C.; Flores, R.; Daròs, J.A. Monomeric linear RNA of Citrus exocortis viroid resulting from processing in vivo has 5′-phosphomonoester and 3′-hydroxyl termini: Implications for the RNase and RNA ligase involved in replication. J. Virol. 2008, 82, 10321–10325. [Google Scholar] [CrossRef] [PubMed]
- Nohales, M.A.; Flores, R.; Darὸs, J.A. Viroid RNA redirects host DNA ligase 1 to act as an RNA ligase. Proc. Natl. Acad. Sci. USA 2012, 109, 13805–13810. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Daròs, J.A.; Hernández, C. Avsunviroidae family: Viroids containing hammerhead ribozymes. Adv. Virus Res. 2000, 55, 271–323. [Google Scholar] [PubMed]
- Flores, R.; Minoia, S.; López-Carrasco, A.; Delgado, S.; Martínez de Alba, Á.E.; Kalantidis, K. Viroid replication. In Viroids and Satellites; Hadidi, A., Flores, R., Randles, J.W., Palukaitis, P., Eds.; Academic Press: London, UK, 2017; pp. 71–81. [Google Scholar]
- Nohales, M.A.; Molina-Serrano, D.; Flores, R.; Darὸs, J.A. Involvement of the chloroplastic isoform of tRNA ligase in the replication of viroids belonging to the family Avsunviroidae. J. Virol. 2012, 86, 8269–8276. [Google Scholar] [CrossRef]
- Côté, F.; Perreault, J.P. Peach latent mosaic viroid is locked by a 2′,5′-phosphodiester bond produced by in vitro self-ligation. J. Mol. Biol. 1997, 273, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Cress, D.E.; Kiefer, M.C.; Owens, R.A. Construction of infectious potato spindle tuber viroid cDNA clones. Nucleic Acids Res. 1983, 11, 6821–6835. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tabler, M.; Sänger, H.L. Cloned single- and double-stranded DNA copies of potato spindle tuber viroid (PSTV) RNA and co-inoculated subgenomic DNA fragments are infectious. EMBO J. 1984, 3, 3055–3062. [Google Scholar] [CrossRef]
- Tabler, M.; Sänger, H.L. Infectivity studies on different potato spindle tuber viroid (PSTV) RNAs synthesized in vitro with the SP6 transcription system. EMBO J. 1985, 4, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Ohno, T.; Ishikawa, M.; Takamatsu, N.; Meshi, T.; Okada, Y.; Sano, T.; Shikata, E. In vitro synthesis of infectious RNA molecules from cloned hop stunt viroid complementary DNA. Proc. Jpn. Acad. Ser. B 1983, 59, 251–254. [Google Scholar] [CrossRef][Green Version]
- Ishikawa, M.; Meshi, T.; Ohno, T.; Okada, Y.; Sano, T.; Ueda, I.; Shikata, E. A revised replication cycle for viroids: The role of longer than unit length RNA in viroid replication. Mol. Gen. Genet. 1984, 196, 421–428. [Google Scholar] [CrossRef]
- Meshi, T.; Ishikawa, M.; Ohno, T.; Okada, Y.; Sano, T.; Ueda, I.; Shikata, E. Double-stranded cDNAs of hop stunt viroid are infectious. J. Biochem. 1984, 95, 1521–1524. [Google Scholar] [CrossRef]
- Meshi, T.; Ishikawa, M.; Watanabe, Y.; Yamaya, J.; Okada, Y.; Sano, T.; Shikata, E. The sequence necessary for the infectivity of hop stunt viroid cDNA clones. Mol. Gen. Genet. 1985, 200, 199–206. [Google Scholar] [CrossRef]
- Hashimoto, J.; Machida, Y. The sequence in the potato spindle tuber viroid required for its cDNA to be infective: A putative processing site in viroid replication. J. Gen. Appl. Microbiol. 1985, 31, 551–561. [Google Scholar] [CrossRef]
- Visvader, J.E.; Forster, A.C.; Symons, R.H. Infectivity and in vitro mutagenesis of monomeric cDNA clones of citrus exocortis viroid indicates the site of processing of viroid precursors. Nucleic Acids Res. 1985, 13, 5843–5856. [Google Scholar] [CrossRef] [PubMed]
- Baumstark, T.; Schröder, A.R.W.; Riesner, D. Viroid processing: Switch from cleavage to ligation is driven by a change from a tetraloop to a loop E conformation. EMBO J. 1997, 16, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Rakowski, A.G.; Symons, R.H. Infectivity of linear monomeric transcripts of citrus exocortis viroid: Terminal sequence requirements for processing. Virology 1994, 203, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.Y.; Cho, I.S.; Choi, G.S.; Choi, S.K. Construction of infectious cDNA clone of a Chrysanthemum stunt viroid Korean isolate. Plant Pathol. J. 2014, 30, 68–74. [Google Scholar] [CrossRef]
- Rigden, J.E.; Rezaian, M.A. In vitro synthesis of an infectious viroid: Analysis of the infectivity of monomeric linear CEV. Virology 1992, 186, 201–206. [Google Scholar] [CrossRef]
- López-Carrasco, A.; Ballesteros, C.; Sentandreu, V.; Delgado, S.; Gago-Zachert, S.; Flores, R.; Sanjuán, R. Different rates of spontaneous mutation of chloroplastic and nuclear viroids as determined by high-fidelity ultra-deep sequencing. PLOS Pathog. 2017, 13, e1006547. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, Y.; Penmetcha, K.K.R. In vitro-transcribed Chrysanthemum stunt viroid RNA is infectious to chrysanthemum and other plants. Phytopathology 2009, 99, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Steyn, C.; Cook, G.; Burger, J.T.; Maree, H.J. Construction and application of infectious citrus viroids for biological indexing. J. Cit. Pathol. 2016. Available online: iocv_journalcitruspathology_37092 (accessed on 28 October 2021). [CrossRef]
- Gross, H.J.; Domdey, H.; Lossow, C.; Jank, P.; Raba, M.; Alberty, H.; Sänger, H.L. Nucleotide sequence and secondary structure of potato spindle tuber viroid. Nature 1978, 273, 203–208. [Google Scholar] [CrossRef]
- Singh, R.P.; Nie, X.; Singh, M. Tomato chlorotic dwarf viroid: An evolutionary link in the origin of pospiviroids. J. Gen. Virol. 1999, 80, 2823–2828. [Google Scholar] [CrossRef]
- Li, S.; Hataya, T.; Furuta, K.; Horita, H.; Sano, T.; Shikata, E. Occurrence of chrysanthemum stunt disease in Hokkaido and detection of chrysanthemum stunt viroid by electrophoresis and hybridization. Ann. Rept. Plant Prot. North Jpn. 1997, 48, 113–117. [Google Scholar]
- Sano, T.; Hataya, T.; Shikata, E. Complete nucleotide sequence of a viroid isolated from Etrog citron, a new member of hop stunt viroid group. Nucleic Acids Res. 1988, 16, 347. [Google Scholar] [CrossRef]
- Li, X.; Hataya, T. Construction and characterization of an infectious cDNA clone of potato virus S developed from selected populations that survived genetic bottlenecks. Virology J. 2019, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, K.; Hataya, T.; Uyeda, I. A simple, rapid method of nucleic acid extraction without tissue homogenization for detecting viroids by hybridization and RT-PCR. J. Virol. Methods 1999, 77, 47–58. [Google Scholar] [CrossRef]
- Hataya, T. Duplex reverse transcription-polymerase chain reaction system to detect Potato spindle tuber viroid using an internal control mRNA and a non-infectious positive control RNA. J. Gen. Plant Pathol. 2009, 75, 167–172. [Google Scholar] [CrossRef]
- Thompson, J.R.; Wetzel, S.; Klerks, M.; Vašková, D.; Schoen, C.D.; Špak, J.; Jelkmann, W. Multiplex RT-PCR detection of four aphid-borne strawberry viruses in Fragaria spp. in combination with a plant mRNA specific internal control. J. Virol. Methods 2003, 111, 85–93. [Google Scholar] [CrossRef]
- Naoi, T.; Kitabayashi, S.; Kasai, A.; Sugawara, K.; Adkar-Purushothama, C.R.; Senda, M.; Hataya, T.; Sano, T. Suppression of RNA-dependent RNA polymerase 6 in tomatoes allows potato spindle tuber viroid to invade basal part but not apical part including pluripotent stem cells of shoot apical meristem. PLoS ONE 2020, 15, e0236481. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Onodera, S.; Sano, T.; Yoshida, K.; Wang, G.; Shikata, E. Gene diagnosis of viroids: Comparisons of return-PAGE and hybridization using DIG-labeled DNA and RNA probes for practical diagnosis of hop stunt, citrus exocortis and apple scar skin viroids in their natural host plants. Ann. Phytopathol. Soc. Jpn. 1995, 61, 381–390. [Google Scholar] [CrossRef]
- Owens, R.A.; Erbe, E.; Hadidi, A.; Steere, R.L.; Diener, T.O. Separation and infectivity of circular and linear forms of potato spindle tuber viroid. Proc. Natl. Acad. Sci. USA 1977, 74, 3859–3863. [Google Scholar] [CrossRef]
- Palukaitis, P.; Zaitlin, M. The nature and biological significance of linear potato spindle tuber viroid molecules. Virology 1987, 157, 199–210. [Google Scholar] [CrossRef]
- Palukaitis, P.; Symons, R.H. Purification and characterization of the circular and linear forms of chrysanthemum stunt viroid. J. Gen. Virol. 1980, 46, 477–489. [Google Scholar] [CrossRef]
- Dissanayaka Mudiyanselage, S.D.; Wang, Y. Evidence supporting that RNA polymerase II catalyzes de novo transcription using potato spindle tuber viroid circular RNA templates. Viruses 2020, 12, 371. [Google Scholar] [CrossRef]
- Takagi, T.; Taylor, G.S.; Kusakabe, T.; Charbonneau, H.; Buratowski, S. A protein tyrosine phosphatase-like protein from baculovirus has RNA 5′-triphosphatase activities. Proc. Natl. Acad. Sci. USA 1998, 95, 9808–9812. [Google Scholar] [CrossRef]
- Deana, A.; Celesnik, H.; Belasco, J.G. The bacterial enzyme RppH triggers messenger RNA degradation by 5′ pyrophosphate removal. Nature 2008, 451, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Desphande, T.; Takagi, T.; Hao, L.; Buratowski, S.; Charbonneau, H. Human PIR1 of the protein-tyrosine phosphatase superfamily has RNA 5′-triphophatase and diphosphatase activities. J. Biol. Chem. 1999, 274, 16590–16594. [Google Scholar]
- Yuan, Y.; Li, D.M.; Sun, H. PIR1, a novel phosphatase that exhibits high affinity to RNA∙ribonucleoprotein complexes. J. Biol. Chem. 1998, 273, 20347–20353. [Google Scholar] [CrossRef] [PubMed]
- Chaves, D.A.; Dai, H.; Li, L.; Moresco, J.J.; Oh, M.E.; Conte, D., Jr.; Yates, J.R., III; Mello, C.C.; Gu, W. The RNA phosphatase PIR-1 regulates endogenous small RNA pathways in C. elegans. Mol. Cell 2021, 81, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Branch, A.D.; Robertson, H.D.; Greer, C.; Gegenheimer, P.; Peebles, C.; Abelson, J. Cell-free circularization of viroid progeny RNA by an RNA ligase from wheat germ. Science 1982, 217, 1147–1149. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, P.A.; Hu, Y.; Owens, R.A. Precisely full length, circularizable, complementary RNA: An infectious form of potato spindle tuber viroid. Proc. Natl. Acad. Sci. USA 1998, 95, 6560–6565. [Google Scholar] [CrossRef]
- Genschik, P.; Billy, E.; Swianiewicz, M.; Filipowicz, W. The human RNA 3′-terminal phosphate cyclase is a member of a new family of proteins conserved in Eucarya, Bacteria and Archaea. EMBO J. 1997, 16, 2955–2967. [Google Scholar] [CrossRef] [PubMed]

| Viroid | Inoculum Nucleic Acid | Inoculum Quantity (µg) | Assay Plant and Days Post-Inoculation (dpi) | ||
|---|---|---|---|---|---|
| 2 | 0.4 | 0.08 | |||
| Potato spindle tuber viroid (PSTVd) | 1U RNA(G96/G95) | 3/3 + 3/3 1 | 3/3 + 3/3 | 3/3 + 3/3 | “Rutgers,”2 42 dpi |
| 1U RNA(G265/C264) | 3/3 | ‒ | ‒ | “Rutgers,” 42 dpi | |
| 1U RNA(G16/U15) | 3/3 | ‒ | ‒ | “Rutgers,” 42 dpi | |
| 2U RNA | 3/3 + 3/3 | 3/3 + 3/3 | 0/3 + 3/3 | “Rutgers,” 42 dpi | |
| 1U cDNA-pUC9 | 1/3 + 3/3 | 1/3 + 2/3 | 0/3 + 0/3 | “Rutgers,” 42 dpi | |
| Tomato chlorotic dwarf viroid (TCDVd) | 1U RNA(G97/G96) | 5/5 | 4/5 | 3/4 | “Rutgers,” 42 dpi |
| 1U cDNA-pUC9 | 4/5 | 1/5 | 0/4 | “Rutgers,” 42 dpi | |
| Chrysanthemum stunt viroid (CSVd) | 1U RNA(G93/G92) | 5/5 | 5/5 | 3/5 | “Newskij,”2 56 dpi |
| Hop stunt viroid (HSVd) | 1U RNA(G82/G81) | 3/3 | 3/3 | 3/3 | “Suyo,”2 56 dpi |
| 1U RNA(G284/G283) | 3/3 | 3/3 | 3/3 | “Suyo,” 56 dpi | |
| 2U RNA | 3/3 | 3/3 | 3/3 | “Suyo,” 56 dpi | |
| Viroid | Inoculum Nucleic Acid 1 | Days Post-Inoculation | ||||
|---|---|---|---|---|---|---|
| 14 | 21 | 28 | 35 | 42 | ||
| Potato spindle tuber viroid (PSTVd) | 1U RNA(G96/G95) | 4/5 2 | 5/5 | |||
| 1U RNA(G265/C264) | 0/5 | 1/5 | 4/5 | 4/5 | 5/5 | |
| 1U RNA(G16/U15) | 2/5 | 4/5 | 5/5 | |||
| 2U RNA | 1/5 | 4/5 | 5/5 | |||
| Hop stunt viroid (HSVd) | 1U RNA(G82/G81) | 1/5 | 2/5 | 3/5 | 5/5 | |
| 1U RNA(G284/G283) | 0/5 | 0/5 | 2/5 | 5/5 | ||
| 2U RNA | 0/4 | 0/4 | 3/4 | 4/4 | ||
| Viroid | Inoculum Nucleic Acid 1 | Days Post-Inoculation | |||
|---|---|---|---|---|---|
| 14 | 21 | 28 | 35 | ||
| Potato spindle Tuber viroid (PSTVd) | 5′-[ppp]G96/G95[OH]-3′ 1U RNA | (6/6), 5/5 2 | |||
| 5′-[p]G96/G95[OH]-3′ 1U RNA | (6/6), 4/5 | 5/5 | |||
| 5′-[OH]G96/G95[OH]-3′ 1U RNA | (6/6), 3/5 | 5/5 | |||
| 5′-[m⁷G(5′)ppp(5′)]G96/G95[OH]-3′ (Co-cap) 3 1U RNA | (6/6), 3/5 | 5/5 | |||
| 5′-[m⁷G(5′)ppp(5′)]G96/G95[OH]-3′ (Post-cap) 3 1U RNA | (6/6), 4/5 | 5/5 | |||
| 2U RNA | (6/6), 1/5 | 5/5 | |||
| Hop stunt viroid (HSVd) | 5′-[ppp]G82/G81[OH]-3′ 1U RNA | 0/5 | 0/5 | 3/5 | 5/5 |
| 5′-[p]G82/G81[OH]-3′ 1U RNA | 0/5 | 0/5 | 1/5 | 5/5 | |
| 5′-[OH]G82/G81[OH]-3′ 1U RNA | 0/5 | 1/5 | 2/5 | 5/5 | |
| 5′-[m⁷G(5′)ppp(5′)]G82/G81[OH]-3′ (Co-cap) 1U RNA | 0/5 | 0/5 | 0/5 | 5/5 | |
| 5′-[m⁷G(5′)ppp(5′)]G82/G81[OH]-3′ (Post-cap) 1U RNA | 0/5 | 0/5 | 0/5 | 5/5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hataya, T.; Naoi, T. Precisely Monomeric Linear RNAs of Viroids Belonging to Pospiviroid and Hostuviroid Genera Are Infectious Regardless of Transcription Initiation Site and 5′-Terminal Structure. Cells 2021, 10, 2971. https://doi.org/10.3390/cells10112971
Hataya T, Naoi T. Precisely Monomeric Linear RNAs of Viroids Belonging to Pospiviroid and Hostuviroid Genera Are Infectious Regardless of Transcription Initiation Site and 5′-Terminal Structure. Cells. 2021; 10(11):2971. https://doi.org/10.3390/cells10112971
Chicago/Turabian StyleHataya, Tatsuji, and Takashi Naoi. 2021. "Precisely Monomeric Linear RNAs of Viroids Belonging to Pospiviroid and Hostuviroid Genera Are Infectious Regardless of Transcription Initiation Site and 5′-Terminal Structure" Cells 10, no. 11: 2971. https://doi.org/10.3390/cells10112971
APA StyleHataya, T., & Naoi, T. (2021). Precisely Monomeric Linear RNAs of Viroids Belonging to Pospiviroid and Hostuviroid Genera Are Infectious Regardless of Transcription Initiation Site and 5′-Terminal Structure. Cells, 10(11), 2971. https://doi.org/10.3390/cells10112971






