Hormonal Regulation of Oxidative Phosphorylation in the Brain in Health and Disease
Abstract
1. Introduction
2. Glucoregulatory Hormones Affect Energy Homeostasis and Mitochondrial Function in the Brain
3. Role of Thyroid Hormones in Brain Metabolism Regulation: Focus on Changes in Oxidative Phosphorylation
4. Insights into Glucocorticoid Effects on Brain Oxidative Phosphorylation Processes
5. Sex Hormone Regulation of Oxidative Phosphorylation in the Brain
5.1. The Influence of Ovarian Hormones on Oxidative Phosphorylation
5.2. The Influence of Androgens on Oxidative Phosphorylation
5.3. The Role of Sex Steroids in Brain Damage—Gender Differences in Brain Cell Damage Mechanisms
5.4. Mitochondrial Dynamics and Neurosteroid Synthesis
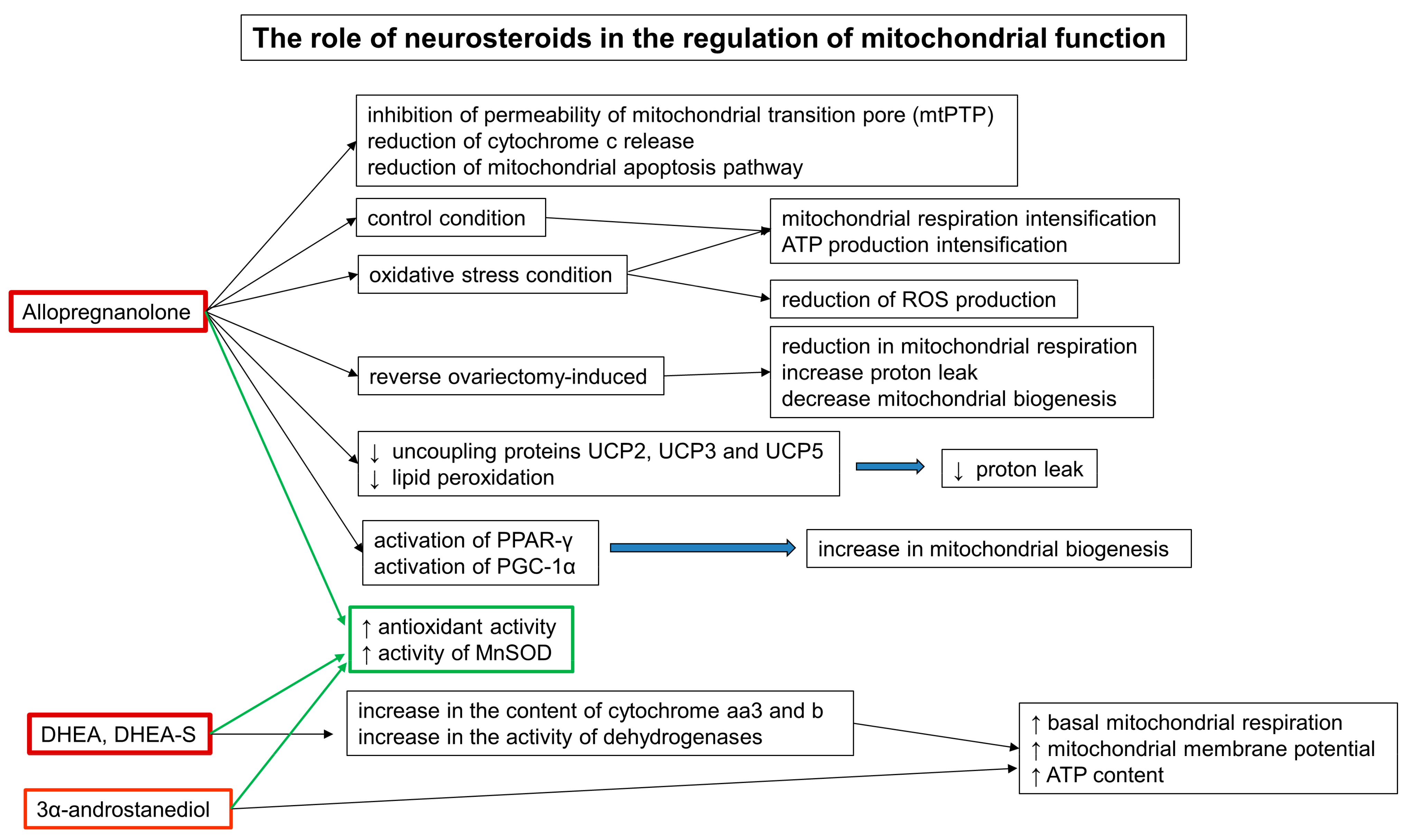
5.5. The Role of Neurosteroids in the Regulation of Mitochondrial Function
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Malcomson, R.D.G.; Nagy, A. The Endocrine System; Springer: Cham, Switzerland, 2015; pp. 671–702. [Google Scholar] [CrossRef]
- Clarke, I. Hypothalamus as an Endocrine Organ. Compr. Physiol. 2014, 5, 217–253. [Google Scholar] [CrossRef]
- McEwen, B.S. Hormones and behavior and the integration of brain-body science. Horm. Behav. 2020, 119, 104619. [Google Scholar] [CrossRef] [PubMed]
- Biessels, G.J.; Reagan, L.P. Hippocampal insulin resistance and cognitive dysfunction. Nat. Rev. Neurosci. 2015, 16, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Sheline, Y.I.; Liston, C.; McEwen, B.S. Parsing the Hippocampus in Depression: Chronic Stress, Hippocampal Volume, and Major Depressive Disorder. Biol. Psychiatry 2019, 85, 436–438. [Google Scholar] [CrossRef]
- Macdonald, R.; Barnes, K.; Hastings, C.; Mortiboys, H. Mitochondrial abnormalities in Parkinson’s disease and Alzheimer’s disease: Can mitochondria be targeted therapeutically? Biochem. Soc. Trans. 2018, 46, 891–909. [Google Scholar] [CrossRef]
- Allen, J.; Romay-Tallon, R.; Brymer, K.J.; Caruncho, H.J.; Kalynchuk, L.E. Mitochondria and Mood: Mitochondrial Dysfunction as a Key Player in the Manifestation of Depression. Front. Neurosci. 2018, 12, 386. [Google Scholar] [CrossRef]
- Roberts, R.C. Postmortem studies on mitochondria in schizophrenia. Schizophr. Res. 2017, 187, 17–25. [Google Scholar] [CrossRef]
- Pei, L.; Wallace, D.C. Mitochondrial Etiology of Neuropsychiatric Disorders. Biol. Psychiatry 2018, 83, 722–730. [Google Scholar] [CrossRef]
- Jardim, F.R.; de Rossi, F.T.; Nascimento, M.X.; Barros, R.G.D.S.; Borges, P.A.; Prescilio, I.C.; De Oliveira, M.R. Resveratrol and Brain Mitochondria: A Review. Mol. Neurobiol. 2017, 55, 2085–2101. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, J.; Levichkin, I.V.; Stasinopoulos, S.; Ryan, M.; Hoogenraad, N.J. A mitochondrial specific stress response in mammalian cells. EMBO J. 2002, 21, 4411–4419. [Google Scholar] [CrossRef] [PubMed]
- Wai, T.; Langer, T. Mitochondrial Dynamics and Metabolic Regulation. Trends Endocrinol. Metab. 2016, 27, 105–117. [Google Scholar] [CrossRef]
- El-Hattab, A.W.; Suleiman, J.; Almannai, M.; Scaglia, F. Mitochondrial dynamics: Biological roles, molecular machinery, and related diseases. Mol. Genet. Metab. 2018, 125, 315–321. [Google Scholar] [CrossRef]
- Pellerin, L.; Magistretti, P.J. Food for Thought: Challenging the Dogmas. Br. J. Pharmacol. 2003, 23, 1282–1286. [Google Scholar] [CrossRef]
- Bélanger, M.; Allaman, I.; Magistretti, P.J. Brain Energy Metabolism: Focus on Astrocyte-Neuron Metabolic Cooperation. Cell Metab. 2011, 14, 724–738. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; McEwen, B.S. Psychological Stress and Mitochondria: A Conceptual Framework. Psychosom. Med. 2018, 80, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Röder, P.V.; Wu, B.; Liu, Y.; Han, W. Pancreatic regulation of glucose homeostasis. Exp. Mol. Med. 2016, 48, e219. [Google Scholar] [CrossRef]
- Gray, S.M.; Meijer, R.I.; Barrett, E.J. Insulin Regulates Brain Function, but How Does It Get There? Diabetes 2014, 63, 3992–3997. [Google Scholar] [CrossRef] [PubMed]
- Vogt, M.C.; Brüning, J.C. CNS insulin signaling in the control of energy homeostasis and glucose metabolism—From embryo to old age. Trends Endocrinol. Metab. 2013, 24, 76–84. [Google Scholar] [CrossRef]
- Blazquez, E.; Velã¡zquez, E.; Hurtado-Carneiro, V.; Ruiz-Albusac, J.M. Insulin in the Brain: Its Pathophysiological Implications for States Related with Central Insulin Resistance, Type 2 Diabetes and Alzheimer’sDisease. Front. Endocrinol. 2014, 5, 161. [Google Scholar] [CrossRef]
- Garcia-Segura, L.; Pérez, J.; Pons, S.; Rejas, M.; Aleman, I.T. Localization of insulin-like growth factor I (IGF-I)-like immunoreactivity in the developing and adult rat brain. Brain Res. 1991, 560, 167–174. [Google Scholar] [CrossRef]
- Russo, V.C.; Gluckman, P.D.; Feldman, E.; Werther, G.A. The Insulin-Like Growth Factor System and Its Pleiotropic Functions in Brain. Endocr. Rev. 2005, 26, 916–943. [Google Scholar] [CrossRef]
- Kleinridders, A. Deciphering Brain Insulin Receptor and Insulin-Like Growth Factor 1 Receptor Signalling. J. Neuroendocr. 2016, 28, 1–13. [Google Scholar] [CrossRef]
- Leonard, B.E.; Wegener, G. Inflammation, insulin resistance and neuroprogression in depression. Acta Neuropsychiatr. 2019, 32, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shieh, J.C.-C.; Huang, P.-T.; Lin, Y.-F. Alzheimer’s Disease and Diabetes: Insulin Signaling as the Bridge Linking Two Pathologies. Mol. Neurobiol. 2020, 57, 1966–1977. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Żebrowska, E.; Chabowski, A. Insulin Resistance and Oxidative Stress in the Brain: What’s New? Int. J. Mol. Sci. 2019, 20, 874. [Google Scholar] [CrossRef]
- Mastrocola, R.; Restivo, F.; Vercellinatto, I.; Danni, O.; Brignardello, E.; Aragno, M.; Boccuzzi, G. Oxidative and nitrosative stress in brain mitochondria of diabetic rats. J. Endocrinol. 2005, 187, 37–44. [Google Scholar] [CrossRef]
- Logan, S.; Pharaoh, G.A.; Marlin, M.C.; Masser, D.R.; Matsuzaki, S.; Wronowski, B.; Yeganeh, A.; Parks, E.E.; Premkumar, P.; Farley, J.A.; et al. Insulin-like growth factor receptor signaling regulates working memory, mitochondrial metabolism, and amyloid-β uptake in astrocytes. Mol. Metab. 2018, 9, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Pharaoh, G.; Owen, D.; Yeganeh, A.; Premkumar, P.; Farley, J.; Bhaskaran, S.; Ashpole, N.; Kinter, M.; Van Remmen, H.; Logan, S. Disparate Central and Peripheral Effects of Circulating IGF-1 Deficiency on Tissue Mitochondrial Function. Mol. Neurobiol. 2019, 57, 1317–1331. [Google Scholar] [CrossRef]
- Wardelmann, K.; Blümel, S.; Rath, M.; Alfine, E.; Chudoba, C.; Schell, M.; Cai, W.; Hauffe, R.; Warnke, K.; Flore, T.; et al. Insulin action in the brain regulates mitochondrial stress responses and reduces diet-induced weight gain. Mol. Metab. 2019, 21, 68–81. [Google Scholar] [CrossRef]
- Ruegsegger, G.N.; Vanderboom, P.M.; Dasari, S.; Klaus, K.A.; Kabiraj, P.; McCarthy, C.B.; Lucchinetti, C.F.; Nair, K.S. Exercise and metformin counteract altered mitochondrial function in the insulin-resistant brain. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Schell, M.; Wardelmann, K.; Kleinridders, A. Untangling the effect of insulin action on brain mitochondria and metabolism. J. Neuroendocr. 2021, 33, e12932. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, C.-L.; Wu, Z.; Iqbal, K.; Liu, F.; Zhang, B.; Gong, C.-X. Intranasal Insulin Prevents Anesthesia-Induced Cognitive Impairment and Chronic Neurobehavioral Changes. Front. Aging Neurosci. 2017, 9, 136. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, Y.; Mao, Y.-F.; Zheng, T.; Jiang, Y.; Yan, Y.; Yin, X.; Zhang, B. Long-term treatment with intranasal insulin ameliorates cognitive impairment, tau hyperphosphorylation, and microglial activation in a streptozotocin-induced Alzheimer’s rat model. Sci. Rep. 2017, 7, 45971. [Google Scholar] [CrossRef]
- Benedict, C. Intranasal insulin improves memory in humans. Psychoneuroendocrinology 2004, 29, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Hallschmid, M.; Benedict, C.; Schultes, B.; Fehm, H.-L.; Born, J.; Kern, W. Intranasal Insulin Reduces Body Fat in Men but not in Women. Diabetes 2004, 53, 3024–3029. [Google Scholar] [CrossRef]
- Iravanpour, F.; Dargahi, L.; Rezaei, M.; Haghani, M.; Heidari, R.; Valian, N.; Ahmadiani, A. Intranasal insulin improves mitochondrial function and attenuates motor deficits in a rat 6-OHDA model of Parkinson’s disease. CNS Neurosci. Ther. 2021, 27, 308–319. [Google Scholar] [CrossRef]
- Torabi, N.; Noursadeghi, E.; Shayanfar, F.; Nazari, M.; Fahanik-Babaei, J.; Saghiri, R.; Khodagholi, F.; Eliassi, A. Intranasal insulin improves the structure–function of the brain mitochondrial ATP–sensitive Ca2+ activated potassium channel and respiratory chain activities under diabetic conditions. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2021, 1867, 166075. [Google Scholar] [CrossRef]
- Jauch-Chara, K.; Friedrich, A.; Rezmer, M.; Melchert, U.H.; Scholand-Engler, H.G.; Hallschmid, M.; Oltmanns, K.M. Intranasal Insulin Suppresses Food Intake via Enhancement of Brain Energy Levels in Humans. Diabetes 2012, 61, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.R.; Doran, E.; Halestrap, A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 2000, 348, 607–614. [Google Scholar] [CrossRef]
- Portela, L.V.; Gnoatto, J.; Brochier, A.; Haas, C.B.; de Assis, A.; De Carvalho, A.K.; Hansel, G.; Zimmer, E.R.; Oses, J.P.; Muller, A.P. Intracerebroventricular Metformin Decreases Body Weight But Has Pro-oxidant Effects and Decreases Survival. Neurochem. Res. 2014, 40, 514–523. [Google Scholar] [CrossRef]
- Skemiene, K.; Rekuviene, E.; Jekabsone, A.; Cizas, P.; Morkuniene, R.; Borutaite, V. Comparison of Effects of Metformin, Phenformin, and Inhibitors of Mitochondrial Complex I on Mitochondrial Permeability Transition and Ischemic Brain Injury. Biomolecules 2020, 10, 1400. [Google Scholar] [CrossRef]
- Pintana, H.; Apaijai, N.; Pratchayasakul, W.; Chattipakorn, N.; Chattipakorn, S.C. Effects of metformin on learning and memory behaviors and brain mitochondrial functions in high fat diet induced insulin resistant rats. Life Sci. 2012, 91, 409–414. [Google Scholar] [CrossRef]
- Pipatpiboon, N.; Pratchayasakul, W.; Chattipakorn, N.; Chattipakorn, S.C. PPARγ Agonist Improves Neuronal Insulin Receptor Function in Hippocampus and Brain Mitochondria Function in Rats with Insulin Resistance Induced by Long Term High-Fat Diets. Endocrinology 2012, 153, 329–338. [Google Scholar] [CrossRef]
- Sa-Nguanmoo, P.; Tanajak, P.; Kerdphoo, S.; Jaiwongkam, T.; Pratchayasakul, W.; Chattipakorn, N.; Chattipakorn, S.C. SGLT2-inhibitor and DPP-4 inhibitor improve brain function via attenuating mitochondrial dysfunction, insulin resistance, inflammation, and apoptosis in HFD-induced obese rats. Toxicol. Appl. Pharmacol. 2017, 333, 43–50. [Google Scholar] [CrossRef]
- Müller, T.; Finan, B.; Bloom, S.; D’Alessio, D.; Drucker, D.; Flatt, P.; Fritsche, A.; Gribble, F.; Grill, H.; Habener, J.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Kastin, A.J.; Akerstrom, V.; Pan, W. Interactions of Glucagon-Like Peptide-1 (GLP-1) with the Blood-Brain Barrier. J. Mol. Neurosci. 2002, 18, 7–13. [Google Scholar] [CrossRef]
- Yamamoto, H.; Kishi, T.; Lee, C.E.; Choi, B.J.; Fang, H.; Hollenberg, A.N.; Drucker, D.J.; Elmquist, J.K. Glucagon-Like Peptide-1-Responsive Catecholamine Neurons in the Area Postrema Link Peripheral Glucagon-Like Peptide-1 with Central Autonomic Control Sites. J. Neurosci. 2003, 23, 2939–2946. [Google Scholar] [CrossRef] [PubMed]
- Daniels, D.; Mietlicki-Baase, E.G. Glucagon-Like Peptide 1 in the Brain: Where Is It Coming From, Where Is It Going? Diabetes 2018, 68, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Turton, M.D.; O’Shea, D.; Gunn, I.; Beak, S.A.; Edwards, C.M.B.; Meeran, K.; Choi, S.J.; Taylor, G.M.; Heath, M.M.; Lambert, P.D.; et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 1996, 379, 69–72. [Google Scholar] [CrossRef]
- Kanoski, S.E.; Hayes, M.R.; Skibicka, K.P. GLP-1 and weight loss: Unraveling the diverse neural circuitry. Am. J. Physiol. Integr. Comp. Physiol. 2016, 310, R885–R895. [Google Scholar] [CrossRef]
- Brierley, D.I.; Holt, M.K.; Singh, A.; de Araujo, A.; McDougle, M.; Vergara, M.; Afaghani, M.H.; Lee, S.J.; Scott, K.; Maske, C.; et al. Central and peripheral GLP-1 systems independently suppress eating. Nat. Metab. 2021, 3, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Diz-Chaves, Y.; Herrera-Pérez, S.; González-Matías, L.C.; Lamas, J.A.; Mallo, F. Glucagon-Like Peptide-1 (GLP-1) in the Integration of Neural and Endocrine Responses to Stress. Nutrients 2020, 12, 3304. [Google Scholar] [CrossRef]
- Heppner, K.M.; Kirigiti, M.; Secher, A.; Paulsen, S.J.; Buckingham, R.; Pyke, C.; Knudsen, L.B.; Vrang, N.; Grove, K.L. Expression and distribution of glucagon-like peptide-1 receptor mRNA, protein and binding in the male nonhuman primate (Macaca mulatta) brain. Endocrinology 2015, 156, 255-267. [Google Scholar] [CrossRef]
- Spielman, L.J.; Gibson, D.L.; Klegeris, A. Incretin hormones regulate microglia oxidative stress, survival and expression of trophic factors. Eur. J. Cell Biol. 2017, 96, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Timper, K.; del Río-Martín, A.; Cremer, A.L.; Bremser, S.; Alber, J.; Giavalisco, P.; Varela, L.; Heilinger, C.; Nolte, H.; Trifunovic, A.; et al. GLP-1 Receptor Signaling in Astrocytes Regulates Fatty Acid Oxidation, Mitochondrial Integrity, and Function. Cell Metab. 2020, 31, 1189–1205.e13. [Google Scholar] [CrossRef]
- Hamilton, A.; Patterson, S.; Porter, D.; Gault, V.; Holscher, C. Novel GLP-1 mimetics developed to treat type 2 diabetes promote progenitor cell proliferation in the brain. J. Neurosci. Res. 2011, 89, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Athauda, D.; Foltynie, T. The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson’s disease: Mechanisms of action. Drug Discov. Today 2016, 21, 802–818. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Kim, O.Y.; Song, J. Alleviation of Depression by Glucagon-Like Peptide 1 through the Regulation of Neuroinflammation, Neurotransmitters, Neurogenesis, and Synaptic Function. Front. Pharmacol. 2020, 11, 1270. [Google Scholar] [CrossRef]
- Kastin, A.J.; Akerstrom, V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int. J. Obes. 2003, 27, 313–318. [Google Scholar] [CrossRef]
- Hunter, K.; Hölscher, C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci. 2012, 13, 33. [Google Scholar] [CrossRef]
- Pintana, H.; Apaijai, N.; Chattipakorn, N.; Chattipakorn, S.C. DPP-4 inhibitors improve cognition and brain mitochondrial function of insulin-resistant rats. J. Endocrinol. 2013, 218, 1–11. [Google Scholar] [CrossRef]
- He, W.; Wang, H.; Zhao, C.; Tian, X.; Li, L.; Wang, H. Role of liraglutide in brain repair promotion through Sirt1-mediated mitochondrial improvement in stroke. J. Cell. Physiol. 2019, 235, 2986–3001. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zheng, J.; Li, S.; Li, H.; Zhou, Y.; Zheng, W.; Zhang, M.; Liu, L.; Chen, Z. GLP-1 improves the neuronal supportive ability of astrocytes in Alzheimer’s disease by regulating mitochondrial dysfunction via the cAMP/PKA pathway. Biochem. Pharmacol. 2021, 188, 114578. [Google Scholar] [CrossRef]
- Zheng, J.; Xie, Y.; Ren, L.; Qi, L.; Wu, L.; Pan, X.; Zhou, J.; Chen, Z.; Liu, L. GLP-1 improves the supportive ability of astrocytes to neurons by promoting aerobic glycolysis in Alzheimer’s disease. Mol. Metab. 2021, 47, 101180. [Google Scholar] [CrossRef]
- Shupnik, M.A.; Ridgway, E.C.; Chin, W.W. Molecular Biology of Thyrotropin. Endocr. Rev. 1989, 10, 459–475. [Google Scholar] [CrossRef]
- Taylor, P.N.; Albrecht, D.; Scholz, A.; Gutierrez-Buey, G.; Lazarus, J.H.; Dayan, C.M.; Okosieme, O.E. Global epidemiology of hyperthyroidism and hypothyroidism. Nat. Rev. Endocrinol. 2018, 14, 301–316. [Google Scholar] [CrossRef]
- Emorte, B.; Bernal, J. Thyroid Hormone Action: Astrocyte–NeuronCommunication. Front. Endocrinol. 2014, 5, 82. [Google Scholar] [CrossRef]
- Weiss, R.E.; Refetoff, S. Effect of thyroid hormone on growth. Endocrinol. Metab. Clin. North Am. 1996, 25, 719–730. [Google Scholar] [CrossRef]
- Oppenheimer, J.H. Molecular Basis of Thyroid Hormone-Dependent Brain Development. Endocr. Rev. 1997, 18, 462–475. [Google Scholar] [CrossRef]
- Schroeder, A.C.; Privalsky, M.L. Thyroid Hormones, T3 and T4, in the Brain. Front. Endocrinol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Wrutniak-Cabello, C.; Casas, F.; Cabello, G. Thyroid hormone action in mitochondria. J. Mol. Endocrinol. 2001, 26, 67–77. [Google Scholar] [CrossRef]
- Chaker, L.; Bianco, A.; Jonklaas, J.; Peeters, R.P. Hypothyroidism. Lancet 2017, 390, 1550–1562. [Google Scholar] [CrossRef]
- De Leo, S.; Lee, S.Y.; Braverman, L.E.; Unit, E.; Sciences, C. Hyperthyroidism_Lancet review. Lancet 2016, 388, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Heinz, A.; Whybrow, P.C. Thyroid hormones, serotonin and mood: Of synergy and significance in the adult brain. Mol. Psychiatry 2002, 7, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Upadhyay, G.; Godbole, M.M. Hypothyroidism alters mitochondrial morphology and induces release of apoptogenic proteins during rat cerebellar development. J. Endocrinol. 2003, 176, 321–329. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fazekas, J.F.; Graves, F.B.; Alman, R.W. The influence of the thyroid on cerebral metabolism. Endocrinology 1951, 48, 169–174. [Google Scholar] [CrossRef]
- Reiss, J.M.; Reiss, M.; Wyatt, A. Action of Thyroid Hormones on Brain Metabolism of Newborn Rats. Exp. Biol. Med. 1956, 93, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.L.; Smith, D.J.; El-Bacha, T.; Stewart, M.E.; Easwaran, A.; Wooding, P.F.P.; Forhead, A.J.; Murray, A.J.; Fowden, A.L.; Camm, E.J. Development of cerebral mitochondrial respiratory function is impaired by thyroid hormone deficiency before birth in a region-specific manner. FASEB J. 2021, 35, e21591. [Google Scholar] [CrossRef]
- Martinez, B.; Del Hoyo, P.; Martin, M.A.; Arenas, J.; Perez-Castillo, A.; Santos, A. Thyroid hormone regulates oxidative phosphorylation in the cerebral cortex and striatum of neonatal rats. J. Neurochem. 2001, 78, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Rosenfeld, M.G. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000, 14, 121–141. [Google Scholar]
- Głombik, K.; Detka, J.; Kurek, A.; Budziszewska, B. Impaired Brain Energy Metabolism: Involvement in Depression and Hypothyroidism. Front. Neurosci. 2020, 14, 1239. [Google Scholar] [CrossRef]
- Zhuravliova, E.; Barbakadze, T.; Jojua, N.; Zaalishvili, E.; Shanshiashvili, L.; Natsvlishvili, N.; Kalandadze, I.; Narmania, N.; Chogovadze, I.; Mikeladze, D. Synaptic and Non-Synaptic Mitochondria in Hippocampus of Adult Rats Differ in Their Sensitivity to Hypothyroidism. Cell. Mol. Neurobiol. 2012, 32, 1311–1321. [Google Scholar] [CrossRef]
- Vega-Núñez, E.; Álvarez, A.M.; Menéndez-Hurtado, A.; Santos, A.; Pérez-Castillo, A. Neuronal Mitochondrial Morphology and Transmembrane Potential Are Severely Altered by Hypothyroidism during Rat Brain Development1. Endocrinology 1997, 138, 3771–3778. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martinez, B.; Rodrigues, T.; Gine, E.; Kaninda, J.P.; Perez-Castillo, A.; Santos, A. Hypothyroidism Decreases the Biogenesis in Free Mitochondria and Neuronal Oxygen Consumption in the Cerebral Cortex of Developing Rats. Endocrinology 2009, 150, 3953–3959. [Google Scholar] [CrossRef]
- Satav, J.G.; Katyare, S.S. Effect of experimental thyrotoxicosis on oxidative phosphorylation in rat liver, kidney and brain mitochondria. Mol. Cell. Endocrinol. 1982, 28, 173–189. [Google Scholar] [CrossRef]
- Schwartz, H.L.; Oppenheimer, J.H. Ontogenesis of 3,5,3′-Triiodothyronine Receptors in Neonatal Rat Brain: Dissociation between Receptor Concentration and Stimulation of Oxygen Consumption by 3,5,3′-Triiodothyronine. Endocrinology 1978, 103, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.E.; Matthews, P.S. Production rates and turnover of triiodothyronine in rat-developing cerebral cortex and cerebellum. Responses to hypothyroidism. J. Clin. Investig. 1984, 74, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Katyare, S.S.; Rajan, R.R. Influence of thyroid hormone treatment on the respiratory activity of cerebral mitochondria from hypothyroid rats. A critical re-assessment. Exp. Neurol. 2005, 195, 416–422. [Google Scholar] [CrossRef]
- Sheehan, T.E.; Kumar, P.A.; Hood, D.A. Tissue-specific regulation of cytochromecoxidase subunit expression by thyroid hormone. Am. J. Physiol. Metab. 2004, 286, E968–E974. [Google Scholar] [CrossRef]
- Bauer, M.; Whybrow, P.C. Role of thyroid hormone therapy in depressive disorders. J. Endocrinol. Investig. 2021, 44, 2341–2347. [Google Scholar] [CrossRef]
- Bauer, M.; London, E.D.; Rasgon, N.; Berman, S.M.; Frye, M.A.; Altshuler, L.L.; Mandelkern, M.A.; Bramen, J.; Voytek, B.; Woods, R.; et al. Supraphysiological doses of levothyroxine alter regional cerebral metabolism and improve mood in bipolar depression. Mol. Psychiatry 2005, 10, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Głombik, K.; Detka, J.; Budziszewska, B. Venlafaxine and L-Thyroxine Treatment Combination: Impact on Metabolic and Synaptic Plasticity Changes in an Animal Model of Coexisting Depression and Hypothyroidism. Cells 2021, 10, 1394. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, L.R.; Wang, Y.T.; Phillips, A.G. Evaluation of the Wistar-Kyoto rat model of depression and the role of synaptic plasticity in depression and antidepressant response. Neurosci. Biobehav. Rev. 2019, 105, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Manoli, I.; Alesci, S.; Blackman, M.R.; Su, Y.A.; Rennert, O.M.; Chrousos, G.P. Mitochondria as key components of the stress response. Trends Endocrinol. Metab. 2007, 18, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Vale, W.; Spiess, J.; Rivier, C.; Rivier, J. Characterization of a 41-Residue Ovine Hypothalamic Peptide that Stimulates Secretion of Corticotropin and β-Endorphin. Obstet. Gynecol. Surv. 1982, 37, 334. [Google Scholar] [CrossRef]
- Antoni, F.A. Hypothalamic Control of Adrenocorticotropin Secretion: Advances since the Discovery of 41-Residue Corticotropin-Releasing Factor. Endocr. Rev. 1986, 7, 351–378. [Google Scholar] [CrossRef] [PubMed]
- Gjerstad, J.K.; Lightman, S.; Spiga, F. Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress 2018, 21, 403–416. [Google Scholar] [CrossRef]
- Dallman, M.F.; Akana, S.F.; Jacobson, L.; Levin, N.; Cascio, C.S.; Shinsako, J. Characterization of Corticosterone Feedback Regulation of ACTH Secretion. Ann. N. Y. Acad. Sci. 1987, 512, 402–414. [Google Scholar] [CrossRef]
- Zalachoras, I.; Houtman, R.; Meijer, O. Understanding stress-effects in the brain via transcriptional signal transduction pathways. Neuroscience 2013, 242, 97–109. [Google Scholar] [CrossRef]
- McNally, J.G.; Muller, W.G.; Walker, D.; Wolford, R.; Hager, G.L. The Glucocorticoid Receptor: Rapid Exchange with Regulatory Sites in Living Cells. Science 2000, 287, 1262–1265. [Google Scholar] [CrossRef] [PubMed]
- Finsterwald, C.; Alberini, C.M. Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: From adaptive responses to psychopathologies. Neurobiol. Learn. Mem. 2013, 112, 17–29. [Google Scholar] [CrossRef]
- Karstens, A.J.; Korzun, I.; Avery, E.T.; Kassel, M.T.; Keelan, R.; Kales, H.; Abercrombie, H.; Eisenlohr-Moul, T.; Langenecker, S.A.; Weisenbach, S. Examining HPA-axis functioning as a mediator of the relationship between depression and cognition across the adult lifespan. Aging, Neuropsychol. Cogn. 2018, 26, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.; Zini, R.; Simon, N.; Charbonnier, P.; Tillement, J.-P.; Le Louet, H. Low glucocorticoid concentrations decrease oxidative phosphorylation of isolated rat brain mitochondria: An additional effect of dexamethasone. Fundam. Clin. Pharmacol. 2000, 14, 493–500. [Google Scholar] [CrossRef]
- Katyare, S.; Balasubramanian, S.; Parmar, D. Effect of corticosterone treatment on mitochondrial oxidative energy metabolism in developing rat brain. Exp. Neurol. 2003, 183, 241–248. [Google Scholar] [CrossRef]
- Picard, M.; Juster, R.-P.; McEwen, B.S. Mitochondrial allostatic load puts the ‘gluc’ back in glucocorticoids. Nat. Rev. Endocrinol. 2014, 10, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Adzic, M.; Lukic, I.; Mitic, M.; Djordjevic, J.; Elaković, I.; Djordjevic, A.; Krstic-Demonacos, M.; Matić, G.; Radojcic, M. Brain region- and sex-specific modulation of mitochondrial glucocorticoid receptor phosphorylation in fluoxetine treated stressed rats: Effects on energy metabolism. Psychoneuroendocrinology 2013, 38, 2914–2924. [Google Scholar] [CrossRef] [PubMed]
- Jaszczyk, A.; Juszczak, G.R. Glucocorticoids, metabolism and brain activity. Neurosci. Biobehav. Rev. 2021, 126, 113–145. [Google Scholar] [CrossRef] [PubMed]
- Scheller, K.; Sekeris, C.E.; Krohne, G.; Hock, R.; Hansen, I.A.; Scheer, U. Localization of glucocorticoid hormone receptors in mitochondria of human cells. Eur. J. Cell Biol. 2000, 79, 299–307. [Google Scholar] [CrossRef]
- Moutsatsou, P.; Psarra, A.-M.; Tsiapara, A.; Paraskevakou, H.; Davaris, P.; Sekeris, C. Localization of the Glucocorticoid Receptor in Rat Brain Mitochondria. Arch. Biochem. Biophys. 2001, 386, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Lapp, H.E.; Bartlett, A.A.; Hunter, R.G. Stress and glucocorticoid receptor regulation of mitochondrial gene expression. J. Mol. Endocrinol. 2019, 62, R121–R128. [Google Scholar] [CrossRef]
- Demonacos, C.; Karayanni, N.; Chatzoglou, E.; Tsiriyiotis, C.; Spandidos, D.; Sekeris, C.E. Mitochondrial genes as sites of primary action of steroid hormones. Steroids 1996, 61, 226–232. [Google Scholar] [CrossRef]
- Tsiriyotis, C.; Spandidos, D.; Sekeris, C.E. The Mitochondrion as a Primary Site of Action of Glucocorticoids: Mitochondrial Nucleotide Sequences, Showing Similarity to Hormone Response Elements, Confer Dexamethasone Inducibility to Chimaeric Genes Transfected in LATK−Cells. Biochem. Biophys. Res. Commun. 1997, 235, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, C.; Zecca, E.; Vento, G.; Maggio, L.; Papacci, P.; Tortorolo, G. Effect on Growth of Two Different Dexamethasone Courses for Preterm Infants at Risk of Chronic Lung Disease. Pharmacology 1999, 59, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P. Effect of Decreased Use of Postnatal Corticosteroids on Morbidity in Extremely Low Birthweight Infants. Am. J. Perinatol. 2005, 22, 77–81. [Google Scholar] [CrossRef]
- Lorscheider, M.; Tsapis, N.; Ur-Rehman, M.; Gaudin, F.; Stolfa, I.; Abreu, S.; Mura, S.; Chaminade, P.; Espéli, M.; Fattal, E. Dexamethasone palmitate nanoparticles: An efficient treatment for rheumatoid arthritis. J. Control. Release 2019, 296, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Gilstrap, L.C.; Christensen, R.; Clewell, W.H.; D’Alton, M.E.; Davidson, E.C.; Escobedo, M.B.; Gjerdingen, D.K.; Goddard-Finegold, J.; Goldenberg, R.L.; Grimes, D.A.; et al. Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA 1995, 273, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Neal, C.R.; Weidemann, G.; Kabbaj, M.; Vázquez, D.M. Effect of neonatal dexamethasone exposure on growth and neurological development in the adult rat. Am. J. Physiol. Integr. Comp. Physiol. 2004, 287, R375–R385. [Google Scholar] [CrossRef]
- Prieur, B.; Bismuth, J.; Delaval, E. Effects of adrenal steroid hormones on mitochondrial maturation during the late fetal period. JBIC J. Biol. Inorg. Chem. 1998, 252, 194–199. [Google Scholar] [CrossRef]
- Holt, P.; Oliver, I.T. Plasma corticosterone concentrations in the perinatal rat. Biochem. J. 1968, 108, 339–341. [Google Scholar] [CrossRef]
- Nakai, A.; Shibazaki, Y.; Taniuchi, Y.; Oya, A.; Asakura, H.; Koshino, T.; Araki, T. Effect of dexamethasone on mitochondrial maturation in the fetal rat brain. Am. J. Obstet. Gynecol. 2002, 186, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Nakai, A.; Taniuchi, Y.; Asakura, H.; Oya, A.; Yokota, A.; Koshino, T.; Araki, T. Developmental changes in mitochondrial activity and energy metabolism in fetal and neonatal rat brain. Dev. Brain Res. 2000, 121, 67–72. [Google Scholar] [CrossRef]
- Pandya, J.D.; Agarwal, N.A.; Katyare, S.S. Dexamethasone treatment differentially affects the oxidative energy metabolism of rat brain mitochondria in developing and adult animals. Int. J. Dev. Neurosci. 2007, 25, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M.; Meaney, M.J. Maturation of the adrenocortical stress response: Neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986, 11, 65–76. [Google Scholar] [CrossRef]
- Poyton, R.O.; McEwen, J.E. Crosstalk between nuclear and mitochondrial genomes. Annu. Rev. Biochem. 1996, 65, 563–607. [Google Scholar] [CrossRef]
- McEwen, B.S. Glucocorticoids, depression, and mood disorders: Structural remodeling in the brain. Metabolism 2005, 54, 20–23. [Google Scholar] [CrossRef]
- Yu, S.; Holsboer, F.; Almeida, O.F. Neuronal actions of glucocorticoids: Focus on depression. J. Steroid Biochem. Mol. Biol. 2008, 108, 300–309. [Google Scholar] [CrossRef]
- Cabib, S.; Campus, P.; Conversi, D.; Orsini, C.; Puglisi-Allegra, S. Functional and Dysfunctional Neuroplasticity in Learning to Cope with Stress. Brain Sci. 2020, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.E.; Han, H.J. Glucocorticoid impairs mitochondrial quality control in neurons. Neurobiol. Dis. 2021, 152, 105301. [Google Scholar] [CrossRef]
- Gregus, A.; Wintink, A.J.; Davis, A.C.; Kalynchuk, L.E. Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behav. Brain Res. 2005, 156, 105–114. [Google Scholar] [CrossRef]
- Johnson, S.A.; Fournier, N.M.; Kalynchuk, L.E. Effect of different doses of corticosterone on depression-like behavior and HPA axis responses to a novel stressor. Behav. Brain Res. 2006, 168, 280–288. [Google Scholar] [CrossRef]
- Mayer, J.L.; Klumpers, L.; Maslam, S.; De Kloet, E.R.; Joëls, M.; Lucassen, P.J. Brief Treatment With the Glucocorticoid Receptor Antagonist Mifepristone Normalises the Corticosterone-Induced Reduction of Adult Hippocampal Neurogenesis. J. Neuroendocr. 2006, 18, 629–631. [Google Scholar] [CrossRef]
- Sheline, Y.I.; Wang, P.W.; Gado, M.H.; Csernansky, J.G.; Vannier, M. Hippocampal atrophy in recurrent major depression. Proc. Natl. Acad. Sci. USA 1996, 93, 3908–3913. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Yan, X.-J.; Lei, F.; Wang, M.-L.; He, L.-L.; Luo, Y.-Y.; Gao, H.-W.; Feng, Y.-L.; Yang, S.-L.; Li, J.; et al. Proteomic profiling of the neurons in mice with depressive-like behavior induced by corticosterone and the regulation of berberine: Pivotal sites of oxidative phosphorylation. Mol. Brain 2019, 12, 1–14. [Google Scholar] [CrossRef]
- Suwanjang, W.; Wu, K.L.H.; Prachayasittikul, S.; Chetsawang, B.; Charngkaew, K. Mitochondrial Dynamics Impairment in Dexamethasone-Treated Neuronal Cells. Neurochem. Res. 2019, 44, 1567–1581. [Google Scholar] [CrossRef]
- Schumacher, M.; Weill-Engerer, S.; Liere, P.; Robert, F.; Franklin, R.; Garcia-Segura, L.; Lambert, J.; Mayo, W.; Melcangi, C.R.; Parducz, A.; et al. Steroid hormones and neurosteroids in normal and pathological aging of the nervous system. Prog. Neurobiol. 2003, 71, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Gaignard, P.; Liere, P.; Thérond, P.; Schumacher, M.; Slama, A.; Guennoun, R. Role of Sex Hormones on Brain Mitochondrial Function, with Special Reference to Aging and Neurodegenerative Diseases. Front. Aging Neurosci. 2017, 9, 406. [Google Scholar] [CrossRef]
- Ratner, M.; Kumaresan, V.; Farb, D.H. Neurosteroid Actions in Memory and Neurologic/Neuropsychiatric Disorders. Front. Endocrinol. 2019, 10, 169. [Google Scholar] [CrossRef]
- Zorumski, C.F.; Paul, S.M.; Covey, D.F.; Mennerick, S. Neurosteroids as novel antidepressants and anxiolytics: GABA-A receptors and beyond. Neurobiol. Stress 2019, 11, 100196. [Google Scholar] [CrossRef] [PubMed]
- Grimm, A.; Biliouris, E.E.; Lang, U.E.; Götz, J.; Mensah-Nyagan, A.G.; Eckert, A. Sex hormone-related neurosteroids differentially rescue bioenergetic deficits induced by amyloid-β or hyperphosphorylated tau protein. Cell. Mol. Life Sci. 2015, 73, 201–215. [Google Scholar] [CrossRef]
- Rettberg, J.R.; Yao, J.; Brinton, R.D. Estrogen: A master regulator of bioenergetic systems in the brain and body. Front. Neuroendocr. 2013, 35, 8–30. [Google Scholar] [CrossRef]
- Rasgon, N.L.; Silverman, D.; Siddarth, P.; Miller, K.; Ercoli, L.M.; Elman, S.; Lavretsky, H.; Huang, S.-C.; Phelps, M.E.; Small, G.W. Estrogen use and brain metabolic change in postmenopausal women. Neurobiol. Aging 2005, 26, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Wroolie, T.E.; Kenna, H.A.; Williams, K.E.; Rasgon, N.L. Cognitive Effects of Hormone Therapy Continuation or Discontinuation in a Sample of Women at Risk for Alzheimer Disease. Am. J. Geriatr. Psychiatry 2015, 23, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Georgakis, M.K.; Thomopoulos, T.P.; Diamantaras, A.-A.; Kalogirou, E.I.; Skalkidou, A.; Daskalopoulou, S.S.; Petridou, E. Association of Age at Menopause and Duration of Reproductive Period With Depression After Menopause. JAMA Psychiatry 2016, 73, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Georgakis, M.K.; Kalogirou, E.I.; Diamantaras, A.-A.; Daskalopoulou, S.S.; Munro, C.A.; Lyketsos, C.G.; Skalkidou, A.; Petridou, E.T. Age at menopause and duration of reproductive period in association with dementia and cognitive function: A systematic review and meta-analysis. Psychoneuroendocrinology 2016, 73, 224–243. [Google Scholar] [CrossRef]
- Gurvich, C.; Hoy, K.; Thomas, N.; Kulkarni, J. Sex Differences and the Influence of Sex Hormones on Cognition through Adulthood and the Aging Process. Brain Sci. 2018, 8, 163. [Google Scholar] [CrossRef] [PubMed]
- Zárate, S.; Astiz, M.; Magnani, N.; Imsen, M.; Merino, F.; Álvarez, S.; Reinés, A.; Seilicovich, A. Hormone deprivation alters mitochondrial function and lipid profile in the hippocampus. J. Endocrinol. 2017, 233, 1–14. [Google Scholar] [CrossRef]
- Jones, T.T.; Brewer, G.J. Critical age-related loss of cofactors of neuron cytochrome C oxidase reversed by estrogen. Exp. Neurol. 2009, 215, 212–219. [Google Scholar] [CrossRef]
- Irwin, R.W.; Yao, J.; Hamilton, R.T.; Cadenas, E.; Brinton, R.D.; Nilsen, J. Progesterone and Estrogen Regulate Oxidative Metabolism in Brain Mitochondria. Endocrinology 2008, 149, 3167–3175. [Google Scholar] [CrossRef]
- Irwin, R.W.; Yao, J.; Ahmed, S.S.; Hamilton, R.T.; Cadenas, E.; Brinton, R.D. Medroxyprogesterone Acetate Antagonizes Estrogen Up-Regulation of Brain Mitochondrial Function. Endocrinology 2010, 152, 556–567. [Google Scholar] [CrossRef]
- Zhao, L.; Morgan, T.E.; Mao, Z.; Lin, S.; Cadenas, E.; Finch, C.E.; Pike, C.; Mack, W.J.; Brinton, R.D. Continuous versus Cyclic Progesterone Exposure Differentially Regulates Hippocampal Gene Expression and Functional Profiles. PLoS ONE 2012, 7, e31267. [Google Scholar] [CrossRef]
- Uchida, M.; Palmateer, J.M.; Herson, P.S.; Devries, A.C.; Cheng, J.; Hurn, P.D. Dose-Dependent Effects of Androgens on Outcome after Focal Cerebral Ischemia in Adult Male Mice. Br. J. Pharmacol. 2009, 29, 1454–1462. [Google Scholar] [CrossRef]
- Barreto, G.; Veiga, S.; Azcoitia, I.; Garcia-Segura, L.M.; Garcia-Ovejero, D. Testosterone decreases reactive astroglia and reactive microglia after brain injury in male rats: Role of its metabolites, oestradiol and dihydrotestosterone. Eur. J. Neurosci. 2007, 25, 3039–3046. [Google Scholar] [CrossRef] [PubMed]
- Son, S.-W.; Lee, J.-S.; Kim, H.-G.; Kim, D.-W.; Ahn, Y.-C.; Son, C.-G. Testosterone depletion increases the susceptibility of brain tissue to oxidative damage in a restraint stress mouse model. J. Neurochem. 2015, 136, 106–117. [Google Scholar] [CrossRef]
- Fanaei, H.; Karimian, S.M.; Sadeghipour, H.R.; Hassanzade, G.; Kasaeian, A.; Attari, F.; Khayat, S.; Ramezani, V.; Javadimehr, M. Testosterone enhances functional recovery after stroke through promotion of antioxidant defenses, BDNF levels and neurogenesis in male rats. Brain Res. 2014, 1558, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Hioki, T.; Suzuki, S.; Morimoto, M.; Masaki, T.; Tozawa, R.; Morita, S.; Horiguchi, T. Brain Testosterone Deficiency Leads to Down-Regulation of Mitochondrial Gene Expression in Rat Hippocampus Accompanied by a Decline in Peroxisome Proliferator-Activated Receptor-γ Coactivator 1α Expression. J. Mol. Neurosci. 2013, 52, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Baez, E.; Echeverria, V.; Cabezas, R.; Ávila-Rodriguez, M.; Garcia-Segura, L.M.; Barreto, G.E. Protection by Neuroglobin Expression in Brain Pathologies. Front. Neurol. 2016, 7, 146. [Google Scholar] [CrossRef]
- Reutzel, M.; Grewal, R.; Dilberger, B.; Silaidos, C.; Joppe, A.; Eckert, G.P. Cerebral Mitochondrial Function and Cognitive Performance during Aging: A Longitudinal Study in NMRI Mice. Oxidative Med. Cell. Longev. 2020, 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- McCullough, L.D.; Zeng, Z.; Blizzard, K.K.; Debchoudhury, I.; Hurn, P.D. Ischemic Nitric Oxide and Poly (ADP-Ribose) Polymerase-1 in Cerebral Ischemia: Male Toxicity, Female Protection. Br. J. Pharmacol. 2005, 25, 502–512. [Google Scholar] [CrossRef]
- Liu, F.; Li, Z.; Li, J.; Siegel, C.; Yuan, R.; McCullough, L.D. Sex Differences in Caspase Activation After Stroke. Stroke 2009, 40, 1842–1848. [Google Scholar] [CrossRef]
- Gaignard, P.; Fréchou, M.; Schumacher, M.; Thérond, P.; Mattern, C.; Slama, A.; Guennoun, R. Progesterone reduces brain mitochondrial dysfunction after transient focal ischemia in male and female mice. Br. J. Pharmacol. 2015, 36, 562–568. [Google Scholar] [CrossRef]
- Gaignard, P.; Fréchou, M.; Liere, P.; Thérond, P.; Schumacher, M.; Slama, A.; Guennoun, R. Sex differences in brain mitochondrial metabolism: Influence of endogenous steroids and stroke. J. Neuroendocr. 2018, 30, e12497. [Google Scholar] [CrossRef] [PubMed]
- Pike, C.J.; Nguyen, T.-V.V.; Ramsden, M.; Yao, M.; Murphy, M.P.; Rosario, E.R. Androgen cell signaling pathways involved in neuroprotective actions. Horm. Behav. 2008, 53, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Witzig, M.; Grimm, A.; Schmitt, K.; Lejri, I.; Frank, S.; Brown, S.A.; Eckert, A. Clock-Controlled Mitochondrial Dynamics Correlates with Cyclic Pregnenolone Synthesis. Cells 2020, 9, 2323. [Google Scholar] [CrossRef] [PubMed]
- Lejri, I.; Grimm, A.; Hallé, F.; Abarghaz, M.; Klein, C.; Maitre, M.; Schmitt, M.; Bourguignon, J.-J.; Mensah-Nyagan, A.G.; Bihel, F.; et al. TSPO Ligands Boost Mitochondrial Function and Pregnenolone Synthesis. J. Alzheimer’s Dis. 2019, 72, 1045–1058. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.; Poderoso, C.; Cooke, M.; Soria, G.; Maciel, F.C.; Gottifredi, V.; Podesta, E.J. Mitochondrial Fusion Is Essential for Steroid Biosynthesis. PLoS ONE 2012, 7, e45829. [Google Scholar] [CrossRef] [PubMed]
- Rune, G.; Frotscher, M. Neurosteroid synthesis in the hippocampus: Role in synaptic plasticity. Neuroscience 2005, 136, 833–842. [Google Scholar] [CrossRef]
- Sayeed, I.; Parvez, S.; Wali, B.; Siemen, D.; Stein, D.G. Direct inhibition of the mitochondrial permeability transition pore: A possible mechanism for better neuroprotective effects of allopregnanolone over progesterone. Brain Res. 2009, 1263, 165–173. [Google Scholar] [CrossRef]
- Lejri, I.; Grimm, A.; Miesch, M.; Geoffroy, P.; Eckert, A.; Mensah-Nyagan, A.-G. Allopregnanolone and its analog BR 297 rescue neuronal cells from oxidative stress-induced death through bioenergetic improvement. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 631–642. [Google Scholar] [CrossRef]
- Wang, T.; Yao, J.; Chen, S.; Mao, Z.; Brinton, R.D. Allopregnanolone Reverses Bioenergetic Deficits in Female Triple Transgenic Alzheimer’s Mouse Model. Neurotherapeutics 2019, 17, 178–188. [Google Scholar] [CrossRef]
- Patel, M.A.; Katyare, S.S. Dehydroepiandrosterone (DHEA) treatment stimulates oxidative energy metabolism in the cerebral mitochondria from developing rats. Int. J. Dev. Neurosci. 2006, 24, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.A.; Katyare, S.S. Effect of dehydroepiandrosterone (DHEA) treatment on oxidative energy metabolism in rat liver and brain mitochondria. A dose–response study. Clin. Biochem. 2007, 40, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Grimm, A.; Schmitt, K.; Lang, U.E.; Mensah-Nyagan, A.G.; Eckert, A. Improvement of neuronal bioenergetics by neurosteroids: Implications for age-related neurodegenerative disorders. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2014, 1842, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-B.; Xu, C.; Zhou, M.-H.; Qiao, H.; An, S.-C. Endogenous hippocampal, not peripheral, estradiol is the key factor affecting the novel object recognition abilities of female rats. Behav. Neurosci. 2021, 135, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Segura, L.; Wozniak, A.; Azcoitia, I.; Rodriguez, J.-R.; Hutchison, R.; Hutchison, J. Aromatase expression by astrocytes after brain injury: Implications for local estrogen formation in brain repair. Neuroscience 1999, 89, 567–578. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Głombik, K.; Detka, J.; Budziszewska, B. Hormonal Regulation of Oxidative Phosphorylation in the Brain in Health and Disease. Cells 2021, 10, 2937. https://doi.org/10.3390/cells10112937
Głombik K, Detka J, Budziszewska B. Hormonal Regulation of Oxidative Phosphorylation in the Brain in Health and Disease. Cells. 2021; 10(11):2937. https://doi.org/10.3390/cells10112937
Chicago/Turabian StyleGłombik, Katarzyna, Jan Detka, and Bogusława Budziszewska. 2021. "Hormonal Regulation of Oxidative Phosphorylation in the Brain in Health and Disease" Cells 10, no. 11: 2937. https://doi.org/10.3390/cells10112937
APA StyleGłombik, K., Detka, J., & Budziszewska, B. (2021). Hormonal Regulation of Oxidative Phosphorylation in the Brain in Health and Disease. Cells, 10(11), 2937. https://doi.org/10.3390/cells10112937





