Relevance of Neutrophil Neprilysin in Heart Failure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sampling and Routine Laboratory Analysis
2.3. Determination of Neutrophil mNEP Expression by Flow Cytometry
2.4. Determination of NEP Activity in Plasma and the Cellular Compartment Liquid Chromatography-Tandem Mass Spectrometry
2.5. Echocardiographic Assessment
2.6. Follow-Up and Study Endpoints
2.7. Statistical Analysis
3. Results
3.1. Study Population Characteristics
3.2. Heart Failure Is Characterized by Low Neutrophil mNEP Expression
3.3. Neutrophil mNEP Is Robust over Time
3.4. Blood Cell NEP Activity Is Determined by Neutrophil mNEP Expression
3.5. Worsening of HFrEF Is Associated with a Continuous Decrease in Neutrophil mNEP
3.6. Lower Neutrophil mNEP Is Related to Adverse Cardiac Remodeling
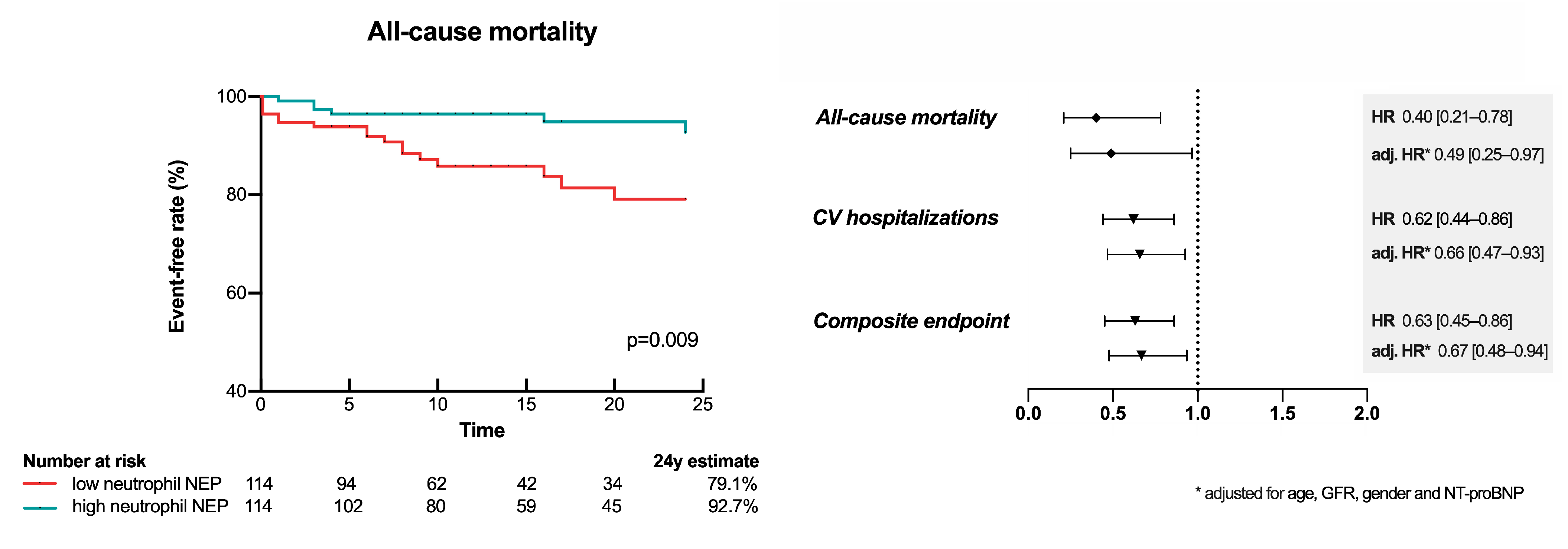
3.7. Low Neutrophil mNEP Is a Risk Factor for Future CV Hospitalization and Mortality
4. Discussion
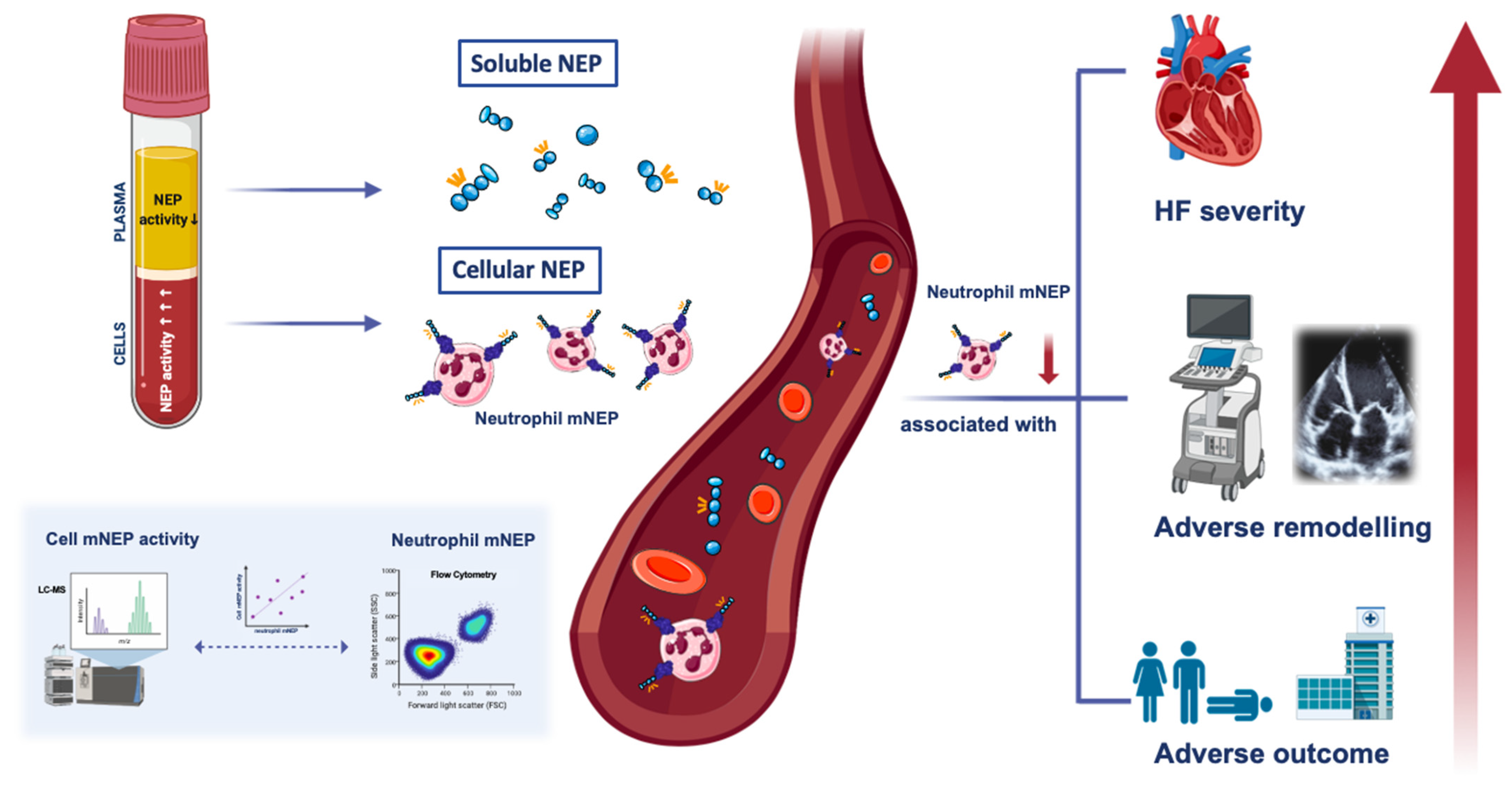
4.1. Characteristics and Significance of Neutrophil mNEP within the Circulation
4.2. Neutrophil mNEP in HFrEF: A Marker for Disease Severity
4.3. Possible Links between Neutrophil mNEP and Heart Failure: Vasoactivity and Inflammation
4.4. Implications for Clinical Practice
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [Green Version]
- Solomon, S.D.; McMurray, J.J.V.; Anand, I.S.; Ge, J.; Lam, C.S.P.; Maggioni, A.P.; Martinez, F.; Packer, M.; Pfeffer, M.A.; Pieske, B.; et al. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2019, 381, 1609–1620. [Google Scholar] [CrossRef] [Green Version]
- Senni, M.; Wachter, R.; Witte, K.K.; Straburzynska-Migaj, E.; Belohlavek, J.; Fonseca, C.; Mueller, C.; Lonn, E.; Chakrabarti, A.; Bao, W.; et al. Initiation of sacubitril/valsartan shortly after hospitalisation for acutely decompensated heart failure in patients with newly diagnosed (de novo) heart failure: A subgroup analysis of the TRANSITION study. Eur. J. Heart Fail. 2020, 22, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Erdös, E.G.; Skidgel, R.A. Neutral endopeptidase 24.11 (enkephalinase) and related regulators of peptide hormones. FASEB J. 1989, 3, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Arfsten, H.; Goliasch, G.; Bartko, P.E.; Prausmüller, S.; Spinka, G.; Cho, A.; Novak, J.; Haslacher, H.; Strunk, G.; Struck, J.; et al. Increased concentrations of bioactive adrenomedullin subsequently to angiotensin-receptor/neprilysin-inhibitor treatment in chronic systolic heart failure. Br. J. Clin. Pharmacol. 2021, 87, 916–924. [Google Scholar] [CrossRef]
- Nougue, H.; Pezel, T.; Picard, F.; Sadoune, M.; Arrigo, M.; Beauvais, F.; Launay, J.-M.; Cohen-Solal, A.; Vodovar, N.; Logeart, D. Effects of sacubitril/valsartan on neprilysin targets and the metabolism of natriuretic peptides in chronic heart failure: A mechanistic clinical study. Eur. J. Heart Fail. 2019, 21, 598–605. [Google Scholar] [CrossRef]
- Iwamoto, I.; Kimura, A.; Ochiai, K.; Tomioka, H.; Yoshida, S. Distribution of neutral endopeptidase activity in human blood leukocytes. J. Leukoc. Biol. 1991, 49, 116–125. [Google Scholar] [CrossRef]
- Katsuda, T.; Tsuchiya, R.; Kosaka, N.; Yoshioka, Y.; Takagaki, K.; Oki, K.; Takeshita, F.; Sakai, Y.; Kuroda, M.; Ochiya, T. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci. Rep. 2013, 3, 1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuruppu, S.; Rajapakse, N.W.; Minond, D.; Smith, A.I. Production of soluble Neprilysin by endothelial cells. Biochem. Biophys. Res. Commun. 2014, 446, 423–427. [Google Scholar] [CrossRef]
- Bayes-Genis, A.; Barallat, J.; Galan, A.; de Antonio, M.; Domingo, M.; Zamora, E.; Urrutia, A.; Lupon, J. Soluble neprilysin is predictive of cardiovascular death and heart failure hospitalization in heart failure patients. J. Am. Coll. Cardiol. 2015, 65, 657–665. [Google Scholar] [CrossRef]
- Prausmüller, S.; Arfsten, H.; Spinka, G.; Freitag, C.; Bartko, P.E.; Goliasch, G.; Strunk, G.; Pavo, N.; Hülsmann, M. Plasma Neprilysin Displays No Relevant Association with Neurohumoral Activation in Chronic HFrEF. J. Am. Heart Assoc. 2020, 9, e015071. [Google Scholar] [CrossRef] [PubMed]
- Pavo, N.; Arfsten, H.; Cho, A.; Goliasch, G.; Bartko, P.E.; Wurm, R.; Freitag, C.; Gisslinger, H.; Kornek, G.; Strunk, G.; et al. The circulating form of neprilysin is not a general biomarker for overall survival in treatment-naive cancer patients. Sci. Rep. 2019, 9, 2554. [Google Scholar] [CrossRef] [Green Version]
- Reddy, Y.N.; Iyer, S.R.; Scott, C.G.; Rodeheffer, R.J.; Bailey, K.; Jenkins, G.; Batzler, A.; Redfield, M.M.; Burnett, J.C.J.; Pereira, N.L. Soluble Neprilysin in the General Population: Clinical Determinants and Its Relationship to Cardiovascular Disease. J. Am. Heart Assoc. 2019, 8, e012943. [Google Scholar] [CrossRef] [Green Version]
- Goliasch, G.; Pavo, N.; Zotter-Tufaro, C.; Kammerlander, A.; Duca, F.; Mascherbauer, J.; Bonderman, D. Soluble neprilysin does not correlate with outcome in heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2016, 18, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Núñez, J.; Núñez, E.; Barallat, J.; Bodí, V.; Miñana, G.; Cruz Pastor, M.; Sanchis, J.; Lupón, J.; Bayes-Genis, A. Serum neprilysin and recurrent admissions in patients with heart failure. J. Am. Heart Assoc. 2017, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bayes-Genis, A.; Barallat, J.; Galan, A.; de Antonio, M.; Domingo, M.; Zamora, E.; Gastelurrutia, P.; Vila, J.; Penafiel, J.; Galvez-Monton, C.; et al. Multimarker Strategy for Heart Failure Prognostication. Value of Neurohormonal Biomarkers: Neprilysin vs. NT-proBNP. Rev. Esp. Cardiol. 2015, 68, 1075–1084. [Google Scholar] [CrossRef]
- Emrich, I.E.; Vodovar, N.; Feuer, L.; Untersteller, K.; Nougue, H.; Seiler-Mussler, S.; Fliser, D.; Launay, J.-M.; Heine, G.H. Do plasma neprilysin activity and plasma neprilysin concentration predict cardiac events in chronic kidney disease patients? Nephrol. Dial. Transplant 2019, 34, 100–108. [Google Scholar] [CrossRef]
- Lyle, M.A.; Iyer, S.R.; Redfield, M.M.; Reddy, Y.N.V.; Felker, G.M.; Cappola, T.P.; Hernandez, A.F.; Scott, C.G.; Burnett, J.C.J.; Pereira, N.L. Circulating Neprilysin in Patients with Heart Failure and Preserved Ejection Fraction. JACC. Heart Fail. 2020, 8, 70–80. [Google Scholar] [CrossRef]
- Shipp, M.A.; Stefano, G.B.; Switzer, S.N.; Griffin, J.D.; Reinherz, E.L. CD10 (CALLA)/neutral endopeptidase 24.11 modulates inflammatory peptide-induced changes in neutrophil morphology, migration, and adhesion proteins and is itself regulated by neutrophil activation. Blood 1991, 78, 1834–1841. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.; Gerard, N.P.; Kolakowski, L.F.J.; Bozza, M.; Zurakowski, D.; Finco, O.; Carroll, M.C.; Gerard, C. Neutral endopeptidase modulation of septic shock. J. Exp. Med. 1995, 181, 2271–2275. [Google Scholar] [CrossRef] [Green Version]
- Martens, A.; Eppink, G.J.; Woittiez, A.J.; Eidhof, H.; de Leij, L.F. Neutrophil function capacity to express CD10 is decreased in patients with septic shock. Crit. Care Med. 1999, 27, 549–553. [Google Scholar] [CrossRef]
- Pavo, N.; Gugerell, A.; Goliasch, G.; Bartko, P.E.; Arfsten, H.; Novak, J.F.; Gyöngyösi, M.; Hülsmann, M. Increased granulocyte membrane neprilysin (CD10) expression is associated with better prognosis in heart failure. Eur. J. Heart Fail. 2019, 21, 537–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Kaltenecker, C.C.; Domenig, O.; Kopecky, C.; Antlanger, M.; Poglitsch, M.; Berlakovich, G.; Kain, R.; Stegbauer, J.; Rahman, M.; Hellinger, R.; et al. Critical Role of Neprilysin in Kidney Angiotensin Metabolism. Circ. Res. 2020, 127, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- Erdos, E.G.; Wagner, B.; Harbury, C.B.; Painter, R.G.; Skidgel, R.A.; Fa, X.G. Down-regulation and inactivation of neutral endopeptidase 24.11 (enkephalinase) in human neutrophils. J. Biol. Chem. 1989, 264, 14519–14523. [Google Scholar] [CrossRef]
- Nortier, J.; Pauwels, S.; De Prez, E.; Deschodt-Lanckman, M. Human neutrophil and plasma endopeptidase 24.11: Quantification and respective roles in atrial natriuretic peptide hydrolysis. Eur. J. Clin. Investig. 1995, 25, 206–212. [Google Scholar] [CrossRef]
- Vodovar, N.; Seronde, M.-F.; Laribi, S.; Gayat, E.; Lassus, J.; Januzzi, J.L.J.; Boukef, R.; Nouira, S.; Manivet, P.; Samuel, J.-L.; et al. Elevated Plasma B-Type Natriuretic Peptide Concentrations Directly Inhibit Circulating Neprilysin Activity in Heart Failure. JACC. Heart Fail. 2015, 3, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Pavo, I.J.; Pavo, N.; Kastner, N.; Traxler, D.; Lukovic, D.; Zlabinger, K.; Spannbauer, A.; Riesenhuber, M.; Lorant, D.; Bartko, P.E.; et al. Heart Failure with Reduced Ejection Fraction Is Characterized by Systemic NEP Downregulation. JACC. Basic Transl. Sci. 2020, 5, 715–726. [Google Scholar] [CrossRef]
- Januzzi, J.L.J.; Prescott, M.F.; Butler, J.; Felker, G.M.; Maisel, A.S.; McCague, K.; Camacho, A.; Piña, I.L.; Rocha, R.A.; Shah, A.M.; et al. Association of Change in N-Terminal Pro-B-Type Natriuretic Peptide Following Initiation of Sacubitril-Valsartan Treatment with Cardiac Structure and Function in Patients with Heart Failure with Reduced Ejection Fraction. JAMA 2019, 322, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-H.; Park, S.-J.; Shin, S.-H.; Hong, G.-R.; Lee, S.; Kim, M.-S.; Yun, S.-C.; Song, J.-M.; Park, S.-W.; Kim, J.-J. Angiotensin Receptor Neprilysin Inhibitor for Functional Mitral Regurgitation. Circulation 2019, 139, 1354–1365. [Google Scholar] [CrossRef]
- Sharifi Kia, D.; Benza, E.; Bachman, T.N.; Tushak, C.; Kim, K.; Simon, M.A. Angiotensin Receptor-Neprilysin Inhibition Attenuates Right Ventricular Remodeling in Pulmonary Hypertension. J. Am. Heart Assoc. 2020, 9, e015708. [Google Scholar] [CrossRef] [PubMed]
- Yoshihisa, A.; Yokokawa, T.; Ichijo, Y.; Kimishima, Y.; Kanno, Y.; Misaka, T.; Sato, T.; Oikawa, M.; Kobayashi, A.; Yamaki, T.; et al. Soluble Neprilysin―Cardiac Function and Outcome in Hypertrophic Cardiomyopathy. Circ. Rep. 2019, 1, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Torre-Amione, G.; Kapadia, S.; Lee, J.; Durand, J.B.; Bies, R.D.; Young, J.B.; Mann, D.L. Tumor necrosis factor-alpha and tumor necrosis factor receptors in the failing human heart. Circulation 1996, 93, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Vasan, R.S.; Sullivan, L.M.; Roubenoff, R.; Dinarello, C.A.; Harris, T.; Benjamin, E.J.; Sawyer, D.B.; Levy, D.; Wilson, P.W.F.; D’Agostino, R.B. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: The Framingham Heart Study. Circulation 2003, 107, 1486–1491. [Google Scholar] [CrossRef]
- Azzam, Z.S.; Kinaneh, S.; Bahouth, F.; Ismael-Badarneh, R.; Khoury, E.; Abassi, Z. Involvement of Cytokines in the Pathogenesis of Salt and Water Imbalance in Congestive Heart Failure. Front. Immunol. 2017, 8, 716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassetta, L.; Baekkevold, E.S.; Brandau, S.; Bujko, A.; Cassatella, M.A.; Dorhoi, A.; Krieg, C.; Lin, A.; Loré, K.; Marini, O.; et al. Deciphering myeloid-derived suppressor cells: Isolation and markers in humans, mice and non-human primates. Cancer Immunol. Immunother. 2019, 68, 687–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marini, O.; Costa, S.; Bevilacqua, D.; Calzetti, F.; Tamassia, N.; Spina, C.; De Sabata, D.; Tinazzi, E.; Lunardi, C.; Scupoli, M.T.; et al. Mature CD10(+) and immature CD10(-) neutrophils present in G-CSF-treated donors display opposite effects on T cells. Blood 2017, 129, 1343–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakschevitz, F.S.; Hassanpour, S.; Rubin, A.; Fine, N.; Sun, C.; Glogauer, M. Identification of neutrophil surface marker changes in health and inflammation using high-throughput screening flow cytometry. Exp. Cell Res. 2016, 342, 200–209. [Google Scholar] [CrossRef]
- Fujimoto, H.; Sakata, T.; Hamaguchi, Y.; Shiga, S.; Tohyama, K.; Ichiyama, S.; Wang, F.S.; Houwen, B. Flow cytometric method for enumeration and classification of reactive immature granulocyte populations. Cytometry 2000, 42, 371–378. [Google Scholar] [CrossRef]
- Mackey, J.B.G.; Coffelt, S.B.; Carlin, L.M. Neutrophil Maturity in Cancer. Front. Immunol. 2019, 10, 1912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blazkova, J.; Gupta, S.; Liu, Y.; Gaudilliere, B.; Ganio, E.A.; Bolen, C.R.; Saar-Dover, R.; Fragiadakis, G.K.; Angst, M.S.; Hasni, S.; et al. Multicenter Systems Analysis of Human Blood Reveals Immature Neutrophils in Males and During Pregnancy. J. Immunol. 2017, 198, 2479–2488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, B.E.; Tabariès, S.; Johnson, R.M.; Andrzejewski, S.; Senecal, J.; Lehuédé, C.; Annis, M.G.; Ma, E.H.; Völs, S.; Ramsay, L.; et al. Immature Low-Density Neutrophils Exhibit Metabolic Flexibility that Facilitates Breast Cancer Liver Metastasis. Cell Rep. 2019, 27, 3902–3915. [Google Scholar] [CrossRef] [Green Version]
- Manz, M.G.; Boettcher, S. Emergency granulopoiesis. Nat. Rev. Immunol. 2014, 14, 302–314. [Google Scholar] [CrossRef]
- Kong, T.; Kim, T.H.; Park, Y.S.; Chung, S.P.; Lee, H.S.; Hong, J.H.; Lee, J.W.; You, J.S.; Park, I. Usefulness of the delta neutrophil index to predict 30-day mortality in patients with ST segment elevation myocardial infarction. Sci. Rep. 2017, 7, 15718. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, P.K.; Claggett, B.L.; Solomon, S.D.; McMurray, J.J.; Packer, M.; Zile, M.R.; Desai, A.S.; Rouleau, J.L.; Swedberg, K.; Fonarow, G.C. Estimated 5-Year Number Needed to Treat to Prevent Cardiovascular Death or Heart Failure Hospitalization with Angiotensin Receptor-Neprilysin Inhibition vs. Standard Therapy for Patients with Heart Failure with Reduced Ejection Fraction: An Analysis of Dat. JAMA Cardiol. 2018, 3, 1226–1231. [Google Scholar] [CrossRef] [Green Version]
- Bryan, P.M.; Xu, X.; Dickey, D.M.; Chen, Y.; Potter, L.R. Renal hyporesponsiveness to atrial natriuretic peptide in congestive heart failure results from reduced atrial natriuretic peptide receptor concentrations. Am. J. Physiol. Ren. Physiol. 2007, 292, F1636–F1644. [Google Scholar] [CrossRef]
- Charloux, A.; Piquard, F.; Doutreleau, S.; Brandenberger, G.; Geny, B. Mechanisms of renal hyporesponsiveness to ANP in heart failure. Eur. J. Clin. Investig. 2003, 33, 769–778. [Google Scholar] [CrossRef]
- Matsumura, T.; Kugiyama, K.; Sugiyama, S.; Ohgushi, M.; Amanaka, K.; Suzuki, M.; Yasue, H. Neutral endopeptidase 24.11 in neutrophils modulates protective effects of natriuretic peptides against neutrophils-induced endothelial cytotoxity. J. Clin. Investig. 1996, 97, 2192–2203. [Google Scholar] [CrossRef] [Green Version]


| Baseline Characteristics | Total Study Population (n = 228) |
|---|---|
| Age, median years (IQR) | 64 (55 to 72) |
| Male gender, n (%) | 169 (74) |
| BMI, kg/m2 (IQR) | 28 (24 to 32) |
| Systolic blood pressure, mmHg (IQR) | 122 (110 to 140) |
| Diastolic blood pressure, mmHg (IQR) | 75 (70 to 85) |
| Heart rate, min−1 (IQR) | 69 (62 to 80) |
| NYHA functional class | |
| NYHA I, n (%) | 36 (16) |
| NYHA II, n (%) | 97 (43) |
| NYHA III, n (%) | 91 (40) |
| NYHA IV, n (%) | 4 (2) |
| Comorbidities | |
| Ischemic etiology of HF, n (%) | 101 (44) |
| Non-ischemic etiology of HF, n (%) | 127 (56) |
| Hypertension, n (%) | 130 (57) |
| Type II diabetes mellitus, n (%) | 76 (33) |
| Atrial fibrillation, n (%) | 81 (36) |
| Laboratory parameters | |
| Hemoglobin, g/dl (IQR) | 13.5 (12.2 to 14.5) |
| WBC, G/l (IQR) | 7.14 (6.04 to 8.75) |
| Neutrophil count, G/l (IQR) | 4.5 (3.7 to 5.7) |
| Serum creatinine, mg/dl (IQR) | 1.19 (0.91 to 1.66) |
| Blood urea nitrogen, mg/dl (IQR) | 22.8 (16.7 to 33.7) |
| Total cholesterol, mg/dl (IQR) | 168 (134 to 192) |
| C-reactive protein, mg/dl (IQR) | 0.29 (0.14 to 0.85) |
| Total bilirubin, mg/dl (IQR) | 0.60 (0.43 to 8.40) |
| BChE, kU/I (IQR) | 7.07 (5.52 to 8.81) |
| NT-proBNP, pg/mL (IQR) | 1819 (746 to 4264) |
| Medication | |
| Beta-blocker, n (%) | 217 (95) |
| Diuretics, n (%) | 102 (45) |
| Mineralocorticoidantagonist, n (%) | 180 (79) |
| If Inhibitor (%) | 21 (9) |
| ACE-I/ARB/ARNI, n (%) Dose equivalent, ≥50% | 101/50/62 (44/22/27) (92/86/79) |
| Echocardiographic characteristics | |
| Left ventricular end-diastolic diameter, mm | 58 (52 to 65) |
| Left ventricular function (≥ moderately reduced), n (%) | 195 (86) |
| Mitral regurgitation (≥ moderate), n (%) | 124 (54) |
| Right ventricular end-diastolic diameters, mm | 37 (32 to 42) |
| Right ventricular function (≥ moderately reduced), n (%) | 82 (36) |
| Tricuspid regurgitation (≥ moderate), n (%) | 106 (46) |
| Systolic pulmonary artery pressure, mmHg | 48 (37 to 59) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prausmüller, S.; Spinka, G.; Arfsten, H.; Stasek, S.; Rettl, R.; Bartko, P.E.; Goliasch, G.; Strunk, G.; Riebandt, J.; Mascherbauer, J.; et al. Relevance of Neutrophil Neprilysin in Heart Failure. Cells 2021, 10, 2922. https://doi.org/10.3390/cells10112922
Prausmüller S, Spinka G, Arfsten H, Stasek S, Rettl R, Bartko PE, Goliasch G, Strunk G, Riebandt J, Mascherbauer J, et al. Relevance of Neutrophil Neprilysin in Heart Failure. Cells. 2021; 10(11):2922. https://doi.org/10.3390/cells10112922
Chicago/Turabian StylePrausmüller, Suriya, Georg Spinka, Henrike Arfsten, Stefanie Stasek, Rene Rettl, Philipp Emanuel Bartko, Georg Goliasch, Guido Strunk, Julia Riebandt, Julia Mascherbauer, and et al. 2021. "Relevance of Neutrophil Neprilysin in Heart Failure" Cells 10, no. 11: 2922. https://doi.org/10.3390/cells10112922
APA StylePrausmüller, S., Spinka, G., Arfsten, H., Stasek, S., Rettl, R., Bartko, P. E., Goliasch, G., Strunk, G., Riebandt, J., Mascherbauer, J., Bonderman, D., Hengstenberg, C., Hülsmann, M., & Pavo, N. (2021). Relevance of Neutrophil Neprilysin in Heart Failure. Cells, 10(11), 2922. https://doi.org/10.3390/cells10112922






