Chemotherapy-Induced Hepatotoxicity in HIV Patients
Abstract
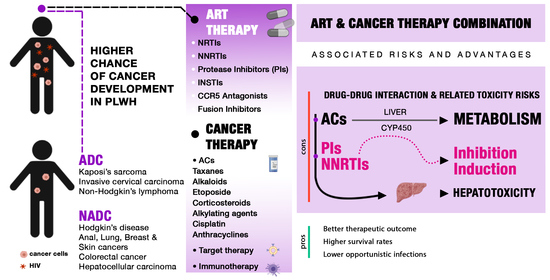
1. Introduction
2. ART and Drug Metabolism
2.1. Nucleoside or Nucleotide Reverse Transcriptase Inhibitors (NRTIs)
2.2. Non-Nucleoside Reverse Transcriptase Inhibitors
2.3. Protease Inhibitors (PIs)
2.4. Integrase Strand Transfer Inhibitors (INSTIs)
2.5. CCR5 Receptor Antagonists
2.6. Fusion Inhibitors
3. AIDS Defining and Non-Defining Cancers
3.1. Kaposi Sarcoma
3.2. Non-Hodgkin’s Lymphoma
3.3. Cervical Cancer
3.4. Hodgkin’s Lymphoma
3.5. Lung Cancer
3.6. Hepatocellular Carcinoma
4. DDIs between Antiblastic Chemotherapy and ART
4.1. Taxanes
4.2. The Vinca Alkaloids
4.3. Etoposide
4.4. Corticosteroids
4.5. Alkylating Agents (Cyclophosphamide, Ifosfamide)
4.6. Cisplatin
4.7. Anthracyclines (Doxorubicin and Daunorubicin)
5. Immunotherapy in HIV Patients
6. Hepatotoxicity
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zanet, E.; Berretta, M.; Di Benedetto, F.; Talamini, R.; Ballarin, R.; Nunnari, G.; Berretta, S.; Ridolfo, A.; Lleshi, A.; Zanghì, A.; et al. Pancreatic cancer in HIV-positive patients: A clinical case-control study. Pancreas 2012, 41, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Berretta, M.; Lleshi, A.; Cappellani, A.; Bearz, A.; Spina, M.; Talamini, R.; Cacopardo, B.; Nunnari, G.; Montesarchio, V.; Izzi, I.; et al. Oxaliplatin Based Chemotherapy and Concomitant Highly Active Antiretroviral Therapy in the Treatment of 24 Patients with Colorectal Cancer and HIV Infection. Curr. HIV Res. 2010, 8, 218–222. [Google Scholar] [CrossRef]
- Berretta, M.; Di Francia, R.; Stanzione, B.; Facchini, G.; Lleshi, A.; De Paoli, P.; Spina, M.; Tirelli, U. New treatment strategies for HIV-positive cancer patients undergoing antiblastic chemotherapy. Expert Opin. Pharmacother. 2016, 17, 2391–2403. [Google Scholar] [CrossRef]
- Corona, G.; Vaccher, E.; Sandron, S.; Sartor, I.; Tirelli, U.; Innocenti, F.; Toffoli, G. Lopinavir–Ritonavir Dramatically Affects the Pharmacokinetics of Irinotecan in HIV Patients with Kaposi’s Sarcoma. Clin. Pharmacol. Ther. 2007, 83, 601–606. [Google Scholar] [CrossRef]
- Berretta, M.; Caraglia, M.; Martellotta, F.; Zappavigna, S.; Lombardi, A.; Fierro, C.; Atripaldi, L.; Muto, T.; Valente, D.; De Paoli, P.; et al. Drug–Drug Interactions Based on Pharmacogenetic Profile between Highly Active Antiretroviral Therapy and Antiblastic Chemotherapy in Cancer Patients with HIV Infection. Front. Pharmacol. 2016, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Dubrow, R.; Silverberg, M.J.; Park, L.S.; Crothers, K.; Justice, A.C. HIV infection, aging, and immune function: Implications for cancer risk and prevention. Curr. Opin. Oncol. 2012, 24, 506–516. [Google Scholar] [CrossRef]
- Flepisi, B.T.; Bouic, P.; Sissolak, G.; Rosenkranz, B. Drug–drug interactions in HIV positive cancer patients. Biomed. Pharmacother. 2014, 68, 665–677. [Google Scholar] [CrossRef]
- Torres, H.A.; Mulanovich, V. Management of HIV Infection in Patients with Cancer Receiving Chemotherapy. Clin. Infect. Dis. 2014, 59, 106–114. [Google Scholar] [CrossRef]
- Beumer, J.H.; Venkataramanan, R.; Rudek, M.A. Pharmacotherapy in cancer patients with HIV/AIDS. Clin. Pharmacol. Ther. 2014, 95, 370–372. [Google Scholar] [CrossRef] [PubMed]
- Nunnari, G. The role of micronutrients in the diet of HIV-1-infected individuals. Front. Biosci. 2012, E4, 2442–2456. [Google Scholar] [CrossRef][Green Version]
- Wonganan, P.; Limpanasithikul, W.; Jianmongkol, S.; Kerr, S.J.; Ruxrungtham, K. Pharmacokinetics of nucleoside/nucleotide reverse transcriptase inhibitors for the treatment and prevention of HIV infection. Expert Opin. Drug Metab. Toxicol. 2020, 16, 551–564. [Google Scholar] [CrossRef]
- Cossarizza, A.; Moyle, G. Antiretroviral nucleoside and nucleotide analogues and mitochondria. AIDS 2004, 18, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Arts, E.J.; Hazuda, D.J. HIV-1 Antiretroviral Drug Therapy. Cold Spring Harb. Perspect. Med. 2012, 2, a007161. [Google Scholar] [CrossRef] [PubMed]
- Vanangamudi, M.; Kurup, S.; Namasivayam, V. Non-nucleoside reverse transcriptase inhibitors (NNRTIs): A brief overview of clinically approved drugs and combination regimens. Curr. Opin. Pharmacol. 2020, 54, 179–187. [Google Scholar] [CrossRef]
- Brik, A.; Wong, C.-H. HIV-1 protease: Mechanism and drug discovery. Org. Biomol. Chem. 2002, 1, 5–14. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Osswald, H.L.; Prato, G. Recent Progress in the Development of HIV-1 Protease Inhibitors for the Treatment of HIV/AIDS. J. Med. Chem. 2016, 59, 5172–5208. [Google Scholar] [CrossRef]
- Gong, Y.; Haque, S.; Chowdhury, P.; Cory, T.J.; Kodidela, S.; Yallapu, M.; Norwood, J.M.; Kumar, S. Pharmacokinetics and pharmacodynamics of cytochrome P450 inhibitors for HIV treatment. Expert Opin. Drug Metab. Toxicol. 2019, 15, 417–427. [Google Scholar] [CrossRef]
- Scarsi, K.K.; Havens, J.P.; Podany, A.T.; Avedissian, S.N.; Fletcher, C.V. HIV-1 Integrase Inhibitors: A Comparative Review of Efficacy and Safety. Drugs 2020, 80, 1649–1676. [Google Scholar] [CrossRef]
- Qi, B.; Fang, Q.; Liu, S.; Hou, W.; Li, J.; Huang, Y.; Shi, J. Advances of CCR5 antagonists: From small molecules to macromolecules. Eur. J. Med. Chem. 2020, 208, 112819. [Google Scholar] [CrossRef] [PubMed]
- Rullo, E.V.; Ceccarelli, M.; Condorelli, F.; Visalli, G.; D’Aleo, F.; Paolucci, I.; Cacopardo, B.; Pinzone, M.R.; Di Rosa, M.; Nunnari, G. Investigational drugs in HIV: Pros and cons of entry and fusion inhibitors (Review). Mol. Med. Rep. 2019, 19, 1987–1995. [Google Scholar] [CrossRef]
- Zanet, E.; Berretta, M.; Martellotta, F.; Cacopardo, B.; Fisichella, R.; Tavio, M.; Berretta, S.; Tirelli, U. Anal cancer: Focus on HIV-positive patients in the HAART-era. Curr. HIV Res. 2011, 9, 70–81. [Google Scholar] [CrossRef]
- Navarro, A.; Martinez-Marti, A.; Felip, E. HIV-Positive Patients with Lung Cancer: Is Immunotherapy a Safe and Active Option for Them? J. Thorac. Oncol. 2018, 13, 874–876. [Google Scholar] [CrossRef] [PubMed]
- Shmakova, A.; Germini, D.; Vassetzky, Y. HIV-1, HAART and cancer: A complex relationship. Int. J. Cancer 2019, 146, 2666–2679. [Google Scholar] [CrossRef] [PubMed]
- Shiels, M.S.; Althoff, K.N.; Pfeiffer, R.M.; Achenbach, C.J.; Abraham, A.G.; Castilho, J.; Cescon, A.; D’Souza, G.; Dubrow, R.; Eron, J.J.; et al. HIV Infection, Immunosuppression, and Age at Diagnosis of Non-AIDS-Defining Cancers. Clin. Infect. Dis. 2016, 64, 468–475. [Google Scholar] [CrossRef]
- Shiels, M.S.; Pfeiffer, R.M.; Engels, E.A. Age at Cancer Diagnosis among Persons with AIDS in the United States. Ann. Intern. Med. 2010, 153, 452. [Google Scholar] [CrossRef]
- Coghill, A.E.; Shiels, M.S.; Suneja, G.; Engels, E.A. Elevated Cancer-Specific Mortality among HIV-Infected Patients in the United States. J. Clin. Oncol. 2015, 33, 2376–2383. [Google Scholar] [CrossRef] [PubMed]
- Cesarman, E.; Damania, B.; Krown, S.E.; Martin, J.; Bower, M.; Whitby, D. Kaposi Sarcoma. Nat. Rev. Dis. Primers 2019, 5, 9. [Google Scholar] [CrossRef]
- Martellotta, F.; Berretta, M.; Vaccher, E.; Schioppa, O.; Zanet, E.; Tirelli, U. AIDS-Related Kaposis Sarcoma: State of the Art and Therapeutic Strategies. Curr. HIV Res. 2009, 7, 634–638. [Google Scholar] [CrossRef]
- La Ferla, L.; Pinzone, M.R.; Nunnari, G.; Martellotta, F.; Lleshi, A.; Tirelli, U.; De Paoli, P.; Berretta, M.; Cacopardo, B. Kaposi’ s sarcoma in HIV-positive patients: The state of art in the HAART-era. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2354–2365. [Google Scholar] [PubMed]
- Pinzone, M.R.; Berretta, M.; Cacopardo, B.; Nunnari, G. Epstein-Barr Virus– and Kaposi Sarcoma-Associated Herpesvirus–Related Malignancies in the Setting of Human Immunodeficiency Virus Infection. Semin. Oncol. 2015, 42, 258–271. [Google Scholar] [CrossRef]
- Liu, Z.; Fang, Q.; Zuo, J.; Minhas, V.; Wood, C.; Zhang, T. The world-wide incidence of Kaposi’s sarcoma in the HIV/AIDS era. HIV Med. 2018, 19, 355–364. [Google Scholar] [CrossRef]
- Ford, N.; Meintjes, G.; Vitoria, M.; Greene, G.; Chiller, T. The evolving role of CD4 cell counts in HIV care. Curr. Opin. HIV AIDS 2017, 12, 123–128. [Google Scholar] [CrossRef]
- Levine, A.; Tulpule, A. Clinical aspects and management of AIDS-related Kaposi’s sarcoma. Eur. J. Cancer 2001, 37, 1288–1295. [Google Scholar] [CrossRef]
- Di Benedetto, F.; Di Sandro, S.; De Ruvo, N.; Berretta, M.; Masetti, M.; Montalti, R.; Ballarin, R.; Cocchi, S.; Potenza, L.; Luppi, M.; et al. Kaposi’s sarcoma after liver transplantation. J. Cancer Res. Clin. Oncol. 2008, 134, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.C.; Pantanowitz, L.; Dezube, B.J. AIDS-Related Malignancies: Emerging Challenges in the Era of Highly Active Antiretroviral Therapy. Oncol. 2005, 10, 412–426. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, L.; Bellinvia, M.; Tourlaki, A.; Scoppio, B.; Gaiani, F.; Boneschi, V. Intralesional vincristine as first-line therapy for nodular lesions in classic Kaposi sarcoma: A prospective study in 151 patients. Br. J. Dermatol. 2009, 162, 854–859. [Google Scholar] [CrossRef]
- Erickson, M.K.; Choi, J.N. Successful treatment of nodular human immunodeficiency virus–associated Kaposi sarcoma of the foot utilizing combination intralesional bleomycin and cryotherapy. JAAD Case Rep. 2021, 10, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Udhrain, A.; Skubitz, K.M.; Northfelt, D.W. Pegylated liposomal doxorubicin in the treatment of AIDS-related Kaposi’s sarcoma. Int. J. Nanomed. 2007, 2, 345–352. [Google Scholar]
- Cianfrocca, M.; Lee, S.; Von Roenn, J.; Tulpule, A.; Dezube, B.J.; Aboulafia, D.M.; Ambinder, R.F.; Lee, J.Y.; Krown, S.E.; Sparano, J.A. Randomized trial of paclitaxel versus pegylated liposomal doxorubicin for advanced human immunodeficiency virus-associated Kaposi sarcoma. Cancer 2010, 116, 3969–3977. [Google Scholar] [CrossRef]
- Ezzat, H.M.; Cheung, M.C.; Hicks, L.K.; Boro, J.; Montaner, J.; Lima, V.D.; Harris, M.; Leitch, H.A. Incidence, predictors and significance of severe toxicity in patients with human immunodeficiency virus-associated Hodgkin lymphoma. Leuk. Lymphoma 2012, 53, 2390–2396. [Google Scholar] [CrossRef]
- Noy, A. Optimizing treatment of HIV-associated lymphoma. Blood 2019, 134, 1385–1394. [Google Scholar] [CrossRef]
- Simonelli, C.; Tedeschi, R.; Gloghini, A.; Talamini, R.; Bortolin, M.T.; Berretta, M.; Spina, M.; Morassut, S.; Vaccher, E.; De Paoli, P.; et al. Plasma HHV-8 viral load in HHV-8-related lymphoproliferative disorders associated with HIV infection. J. Med. Virol. 2009, 81, 888–896. [Google Scholar] [CrossRef]
- Carbone, A.; Cesarman, E.; Spina, M.; Gloghini, A.; Schulz, T.F. HIV-associated lymphomas and gamma-herpesviruses. Blood 2009, 113, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Okello, C.D.; Omoding, A.; Ddungu, H.; Mulumba, Y.; Orem, J. Outcomes of treatment with CHOP and EPOCH in patients with HIV associated NHL in a low resource setting. BMC Cancer 2020, 20, 798. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.A.; LaCasce, A.S.; Feng, Y.; Toomey, C.E.; Neuberg, D.; Michaelson, J.S.; Hochberg, E.P.; Abramson, J.S. Evaluation of the addition of rituximab to CODOX-M/IVAC for Burkitt’s lymphoma: A retrospective analysis. Ann. Oncol. 2011, 22, 1859–1864. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, F.; Pellicanò, G.F.; Venanzi Rullo, E.; d’Aleo, F.; Facciolà, A.; Micali, C.; Coco, M.; Visalli, G.; Picerno, I.; Condorelli, F.; et al. Cervical cancer in women living with HIV: A review of the literature. World Cancer Res. J. 2019, 6, e1224. [Google Scholar]
- Stelzle, D.; Tanaka, L.F.; Lee, K.K.; Khalil, A.I.; Baussano, I.; Shah, A.S.V.; McAllister, D.A.; Gottlieb, S.L.; Klug, S.J.; Winkler, A.S.; et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob. Health 2020, 9, e161–e169. [Google Scholar] [CrossRef]
- D’andrea, F.; Ceccarelli, M.; RULLO, E.V.; Facciolà, A.; Marino, A.; Cacopardo, B.; Pellicanò, G.F.; Nunnari, G. Vaccines against HPV in people living with HIV: A review. World Cancer Res. J. 2019, 6, E1348. [Google Scholar]
- D’Aleo, F.; Ceccarelli, M.; VenanziRullo, E.; Facciolà, A.; d’Andrea, F.; Micali, C.; Coco, M.; Pinzone, M.R.; Focà, E.; Condorelli, F.; et al. Anal cancer in people living with HIV: The importance of the screening and of early diagnosis. World Cancer Res. J. 2019, 6, e1319. [Google Scholar]
- Wang, C.-C.J.; Palefsky, J.M. Human Papillomavirus (HPV) Infections and the Importance of HPV Vaccination. Curr. Epidemiol. Rep. 2015, 2, 101–109. [Google Scholar] [CrossRef]
- Kelly, H.; Weiss, H.A.; Benavente, Y.; de Sanjose, S.; Mayaud, P.; Qiao, Y.-L.; Feng, R.-M.; DeVuyst, H.; Tenet, V.; Jaquet, A.; et al. Association of antiretroviral therapy with high-risk human papillomavirus, cervical intraepithelial neoplasia, and invasive cervical cancer in women living with HIV: A systematic review and meta-analysis. Lancet HIV 2017, 5, e45–e58. [Google Scholar] [CrossRef]
- Spina, M.; Carbone, A.; Gloghini, A.; Serraino, D.; Berretta, M.; Tirelli, U. Hodgkin’s Disease in Patients with HIV Infection. Adv. Hematol. 2010, 2011, 402682. [Google Scholar] [CrossRef]
- Martis, N.; Mounier, N. Hodgkin Lymphoma in Patients with HIV Infection: A Review. Curr. Hematol. Malign. Rep. 2012, 7, 228–234. [Google Scholar] [CrossRef]
- Rudek, M.A.; Flexner, C.; Ambinder, R.F. Use of antineoplastic agents in cancer patients with HIV/AIDS. Lancet Oncol. 2011, 12, 905–912. [Google Scholar] [CrossRef]
- Hooker, C.M.; Meguid, R.A.; Hulbert, A.; Taylor, J.T.; Shin, J.; Wrangle, J.; Rodgers, K.; Lee, B.; Laskshmanan, S.; Brown, T.; et al. Human Immunodeficiency Virus Infection as a Prognostic Factor in Surgical Patients with Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2012, 93, 405–412. [Google Scholar] [CrossRef]
- Sigel, K.M.; Stone, K.; Wisnivesky, J.P.; Park, L.S.; Kong, C.Y.; Silverberg, M.J.; Brown, S.; Goetz, M.; Rodriguez-Barradas, M.C.; Gibert, C.; et al. Short-term outcomes for lung cancer resection surgery in HIV infection. AIDS 2019, 33, 1353–1360. [Google Scholar] [CrossRef]
- Rowe, J.H.; Ghouri, Y.A.; Mian, I. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J. Carcinog. 2017, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, F.; Tarantino, G.; Ercolani, G.; Baccarani, U.; Montalti, R.; De Ruvo, N.; Berretta, M.; Adani, G.L.; Zanello, M.; Tavio, M.; et al. Multicenter Italian Experience in Liver Transplantation for Hepatocellular Carcinoma in HIV-Infected Patients. Oncology 2013, 18, 592. [Google Scholar] [CrossRef]
- Di Benedetto, F.; Di Sandro, S.; De Ruvo, N.; Berretta, M.; Montalti, R.; Guerrini, G.; Ballarin, R.; De Blasiis, M.; Spaggiari, M.; Smerieri, N.; et al. Human Immunodeficiency Virus and Liver Transplantation: Our Point of View. Transplant. Proc. 2008, 40, 1965–1971. [Google Scholar] [CrossRef]
- Berretta, M.; Di Benedetto, F.; Maso, L.D.; Cacopardo, B.; Nasti, G.; Facchini, G.; Bearz, A.; Spina, M.; Garlassi, E.; De Re, V.; et al. Sorafenib for the treatment of unresectable hepatocellular carcinoma in HIV-positive patients. Anti-Cancer Drugs 2013, 24, 212–218. [Google Scholar] [CrossRef]
- D’Aleo, F.; Ceccarelli, M.; Rullo, E.V.; Facciolà, A.; Di Rosa, M.; Pinzone, M.R.; Condorelli, F.; Visalli, G.; Picerno, I.; Berretta, M.; et al. Hepatitis C-related hepatocellular carcinoma: Diagnostic and therapeutic management in HIV-patients. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5859–5867. [Google Scholar] [PubMed]
- Di Benedetto, F.; De Ruvo, N.; Berretta, M.; Masetti, M.; Montalti, R.; Di Sandro, S.; Ballarin, R.; Codeluppi, M.; Guaraldi, G.; Gerunda, G. Hepatocellular carcinoma in HIV patients treated by liver transplantation. Eur. J. Surg. Oncol. 2008, 34, 422–427. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, F.; Venanzi Rullo, E.; Marino, A.; Moscatt, V.; Celesia, B.M.; Cacopardo, B.; Condorelli, F.; La Rocca, G.; Di Rosa, M.; Pellicanò, G.F. Hepatitis B virus infection and hepatocellular carcinoma in PLWH: Epidemiology, pathogenesis and treatment. World Cancer Res. J. 2020, 7, e1537. [Google Scholar]
- Krown, S.E.; Moser, C.B.; MacPhail, P.; Matining, R.M.; Godfrey, C.; Caruso, S.R.; Hosseinipour, M.C.; Samaneka, W.; Nyirenda, M.; Busakhala, N.W.; et al. Treatment of advanced AIDS-associated Kaposi sarcoma in resource-limited settings: A three-arm, open-label, randomised, non-inferiority trial. Lancet 2020, 395, 1195–1207. [Google Scholar] [CrossRef]
- Einstein, M.H.; Phaëton, R. Issues in cervical cancer incidence and treatment in HIV. Curr. Opin. Oncol. 2010, 22, 449–455. [Google Scholar] [CrossRef]
- Ostios-Garcia, L.; Faig, J.; Leonardi, G.C.; Adeni, A.E.; Subegdjo, S.J.; Lydon, C.A.; Rangachari, D.; Huberman, M.S.; Sehgal, K.; Shea, M.; et al. Safety and Efficacy of PD-1 Inhibitors among HIV-Positive Patients with Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 1037–1042. [Google Scholar] [CrossRef]
- Meier, A.; Bagchi, A.; Sidhu, H.K.; Alter, G.; Suscovich, T.J.; Kavanagh, D.G.; Streeck, H.; Brockman, M.A.; LeGall, S.; Hellman, J.; et al. Up-regulation of PD-L1 on Monocytes and Dendritic Cells by HIV-1 derived TLR Ligands. AIDS 2008, 22, 655–658. [Google Scholar] [CrossRef]
- Schadendorf, D.; van Akkooi, A.C.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef]
- Heppt, M.; Schlaak, M.; Eigentler, T.; Kähler, K.; Kiecker, F.; Loquai, C.; Meier, F.; Tomsitz, D.; Brenner, N.; Niesert, A.; et al. Checkpoint blockade for metastatic melanoma and Merkel cell carcinoma in HIV-positive patients. Ann. Oncol. 2017, 28, 3104–3106. [Google Scholar] [CrossRef]
- Ruzevick, J.; Nicholas, S.; Redmond, K.; Kleinberg, L.; Lipson, E.J.; Lim, M. A Patient with HIV Treated with Ipilimumab and Stereotactic Radiosurgery for Melanoma Metastases to the Brain. Case Rep. Oncol. Med. 2013, 2013, e946392. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.M.; Kluger, H.M.; Golden, M.; Heller, K.N.; Hoos, A.; Sznol, M. Case Report: Response to Ipilimumab in a Patient with HIV with Metastatic Melanoma. J. Clin. Oncol. 2011, 29, e792–e794. [Google Scholar] [CrossRef] [PubMed]
- Wightman, F.; Solomon, A.; Kumar, S.; Urriola, N.; Gallagher, K.; Hiener, B.; Palmer, S.; McNeil, C.; Garsia, R.; Lewin, S.R. Effect of ipilimumab on the HIV reservoir in an HIV-infected individual with metastatic melanoma. AIDS 2015, 29, 504–506. [Google Scholar] [CrossRef]
- Chang, E.; Rivero, G.; Patel, N.R.; Chiao, E.Y.; Lai, S.; Bajaj, K.; Mbue, J.E.; Yellapragada, S.V. HIV-related Refractory Hodgkin Lymphoma: A Case Report of Complete Response to Nivolumab. Clin. Lymphoma Myeloma Leuk. 2018, 18, e143–e146. [Google Scholar] [CrossRef] [PubMed]
- Le Garff, G.; Samri, A.; Lambert-Niclot, S.; Even, S.; Lavolé, A.; Cadranel, J.; Spano, J.-P.; Autran, B.; Marcelin, A.-G.; Guihot, A. Transient HIV-specific T cells increase and inflammation in an HIV-infected patient treated with nivolumab. AIDS 2017, 31, 1048–1051. [Google Scholar] [CrossRef] [PubMed]
- Makinson, A.; Pujol, J.-L.; Le Moing, V.; Peyriere, H.; Reynes, J. Interactions Between Cytotoxic Chemotherapy and Antiretroviral Treatment in Human Immunodeficiency Virus-Infected Patients with Lung Cancer. J. Thorac. Oncol. 2010, 5, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Vaccher, E.; Spina, M.; di Gennaro, G.; Talamini, R.; Nasti, G.; Schioppa, O.; Vultaggio, G.; Tirelli, U. Concomitant cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy plus highly active antiretroviral therapy in patients with human immunodeficiency virus-related, non-Hodgkin lymphoma. Cancer 2001, 91, 155–163. [Google Scholar] [CrossRef]
- Kaplan, J.E.; Benson, C.; Holmes, K.K.; Brooks, J.T.; Pau, A.; Masur, H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm. Rep. 2009, 58, 1–207. [Google Scholar] [CrossRef] [PubMed]


| Antiblastic Drug | Metabolism | Tumor | Reported Interaction |
|---|---|---|---|
| Taxanes | |||
| Paclitaxel | CYP2C8, CYP3A4, and CYP3A5 | KS | Co-administration of paclitaxel and CYP3A4 inhibitors, such as ritonavir can increased plasma concentration of the drug. |
| Docetaxel | CYP3A4 | Minor adverse event with the administration of paclitaxel in combination with CYP3A4 inducers such as efavirenz (NNRTIs) and tenofovir disoproxil fumarate (NRTIs). | |
| The vinca alkaloids | |||
| Vincristine, vinblastine and vinorelbine | CYP3A4 | NHL HL KS Lung cancer | Concomitant administration with protease inhibitors, can increase the plasma concentration of vinblastine leading to possible hematological and neurological side effects. |
| Etoposide | CYP3A4 with a minor contribution of CYP1A2A and CYP2E1 isoforms. | hematological malignancies and non-Hodgkin’s lymphoma | The inhibition of the CYP3A4 pathway may increase etoposide plasma concentration levels. Leading to an increased risk of mucositis, myelosuppression, and transaminitis. |
| Corticosteroids | CYP3A4 | HL NHL | PIs and NNRTIs are modulators of the activity of the CYP450 enzyme system and therefore may interact with corticosteroids. PIs may increase the pharmacodynamic effects of corticosteroids when used concurrently. Conversely, CYP3A4 inducers may reduce the efficacy of these drugs. |
| Alkylating Agents | |||
| Cyclophosphamide | CYP3A4 and CYP2B6 | HD and NHL | Induction of CYP3A4 may increase neurotoxicity byincreasing the substrate availability for N-dechloroethylation. |
| Ifosfamide | CYP3A4 and CYP2B6 | CYP3A4 inhibition could compromise its antitumor activity. CYP3A4 induction can increase the presence of toxic metabolites. | |
| Platin-derivates | |||
| Cisplatin | Primary renal elimination post Glutathione additions (GSTP1, GSTM1, and others) | Cervical cancer Lung cancer | It is not known if the combination with PIs could have an impact on toxicity and possible adverse events. |
| Anthracyclines | |||
| Doxorubicin | Aldoketoreductase and NADPH-dependent cytochrome reductase. | HL KS NHL | Interaction between ART via CYP system appear to be unlikely. |
| Daunorubicin | Involved in free radical generation. Substrate of P-gp which may influence Intracellular concentrations. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bressan, S.; Pierantoni, A.; Sharifi, S.; Facchini, S.; Quagliariello, V.; Berretta, M.; Montopoli, M. Chemotherapy-Induced Hepatotoxicity in HIV Patients. Cells 2021, 10, 2871. https://doi.org/10.3390/cells10112871
Bressan S, Pierantoni A, Sharifi S, Facchini S, Quagliariello V, Berretta M, Montopoli M. Chemotherapy-Induced Hepatotoxicity in HIV Patients. Cells. 2021; 10(11):2871. https://doi.org/10.3390/cells10112871
Chicago/Turabian StyleBressan, Silvia, Alessandra Pierantoni, Saman Sharifi, Sergio Facchini, Vincenzo Quagliariello, Massimiliano Berretta, and Monica Montopoli. 2021. "Chemotherapy-Induced Hepatotoxicity in HIV Patients" Cells 10, no. 11: 2871. https://doi.org/10.3390/cells10112871
APA StyleBressan, S., Pierantoni, A., Sharifi, S., Facchini, S., Quagliariello, V., Berretta, M., & Montopoli, M. (2021). Chemotherapy-Induced Hepatotoxicity in HIV Patients. Cells, 10(11), 2871. https://doi.org/10.3390/cells10112871