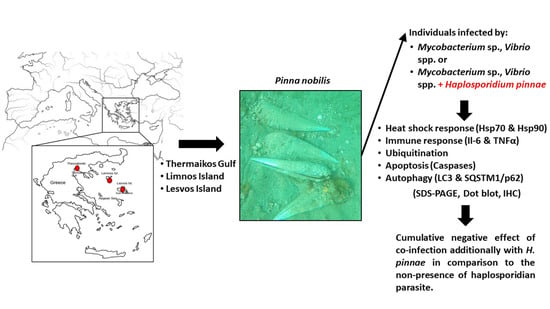
Pathophysiological Responses of Pinna nobilis Individuals Enlightens the Etiology of Mass Mortality Situation in the Mediterranean Populations
Abstract
1. Introduction
2. Materials and Methods
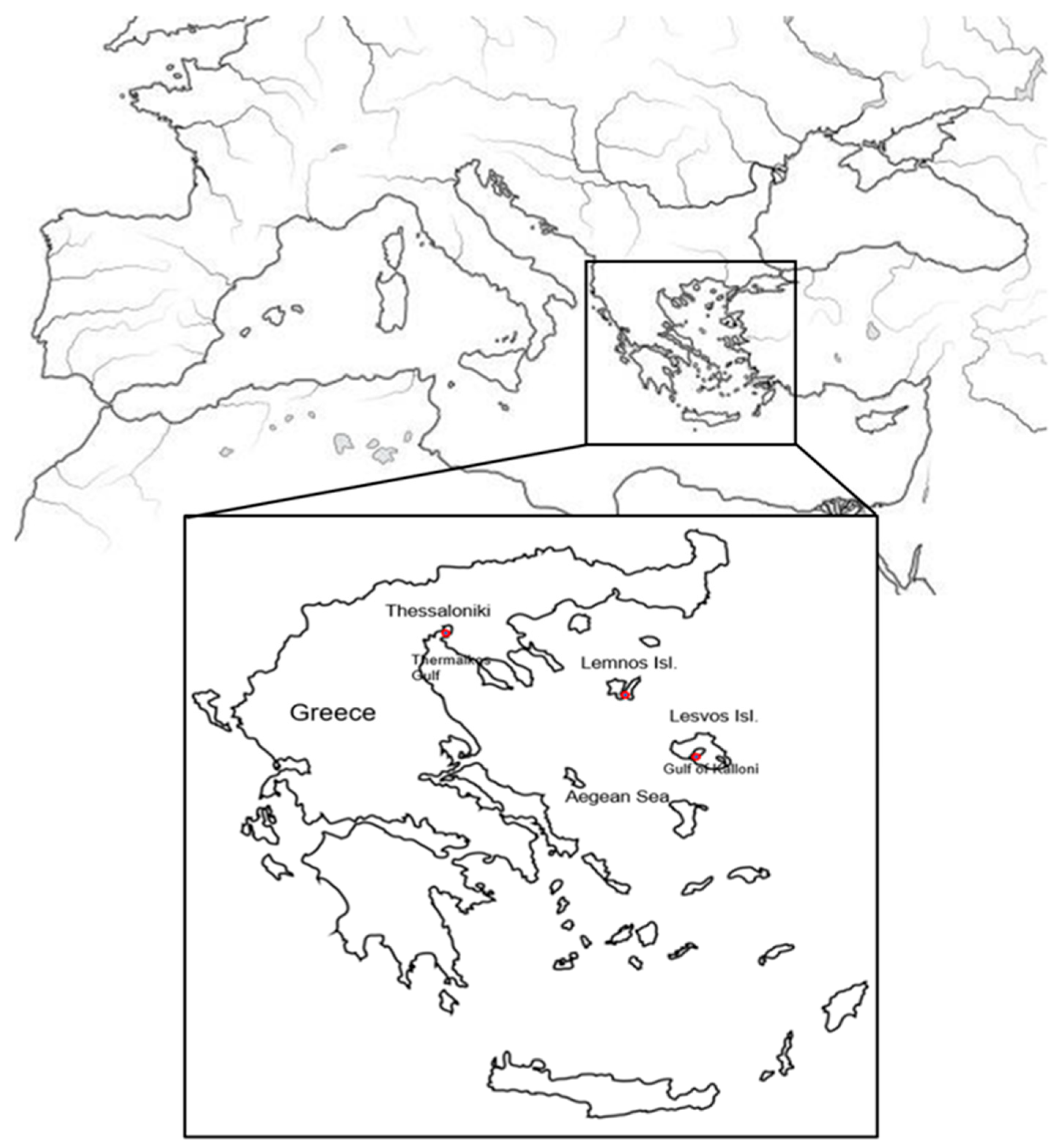
2.1. Animal and Tissue Sampling
2.2. Histopathological Procedures
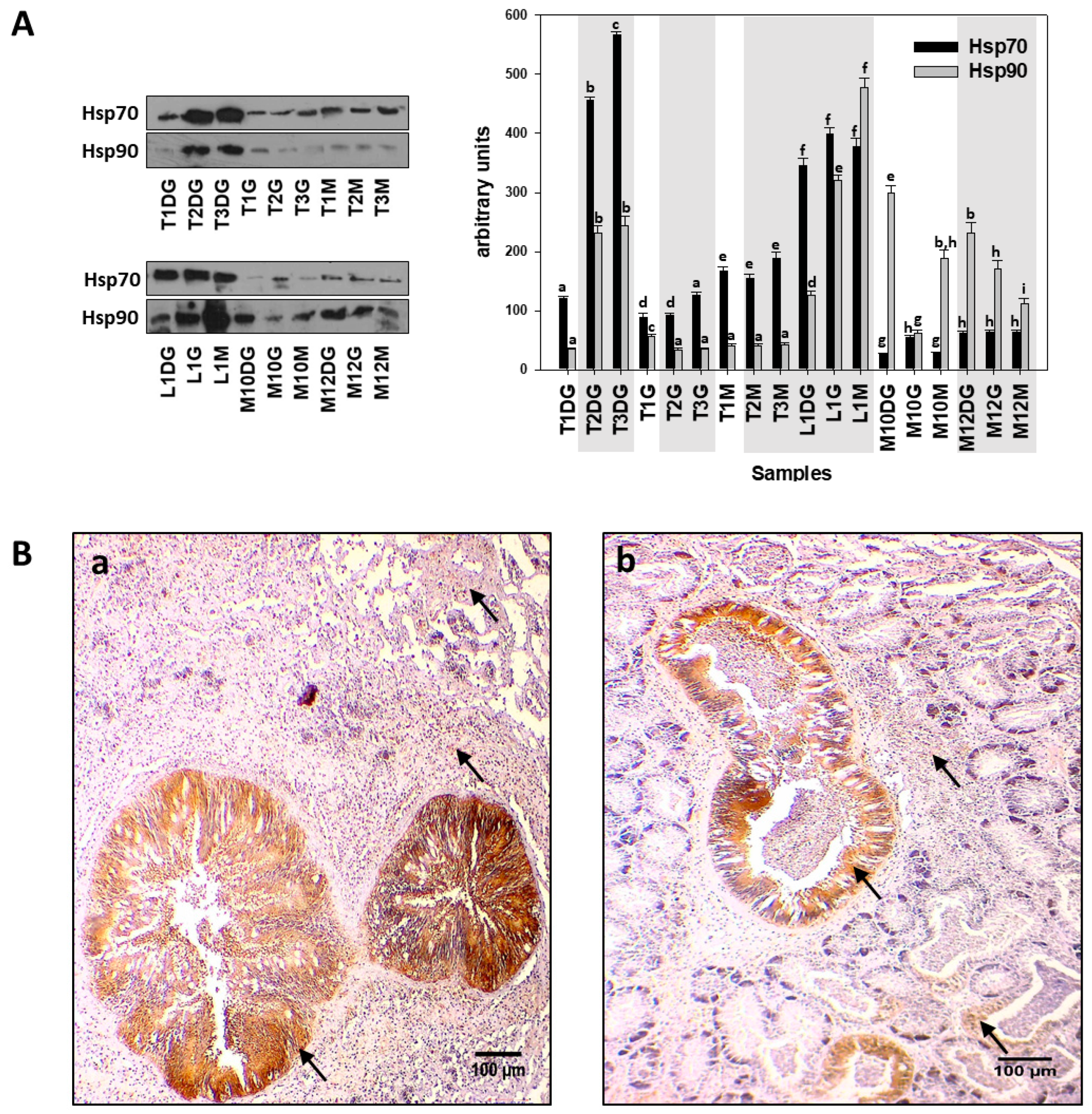
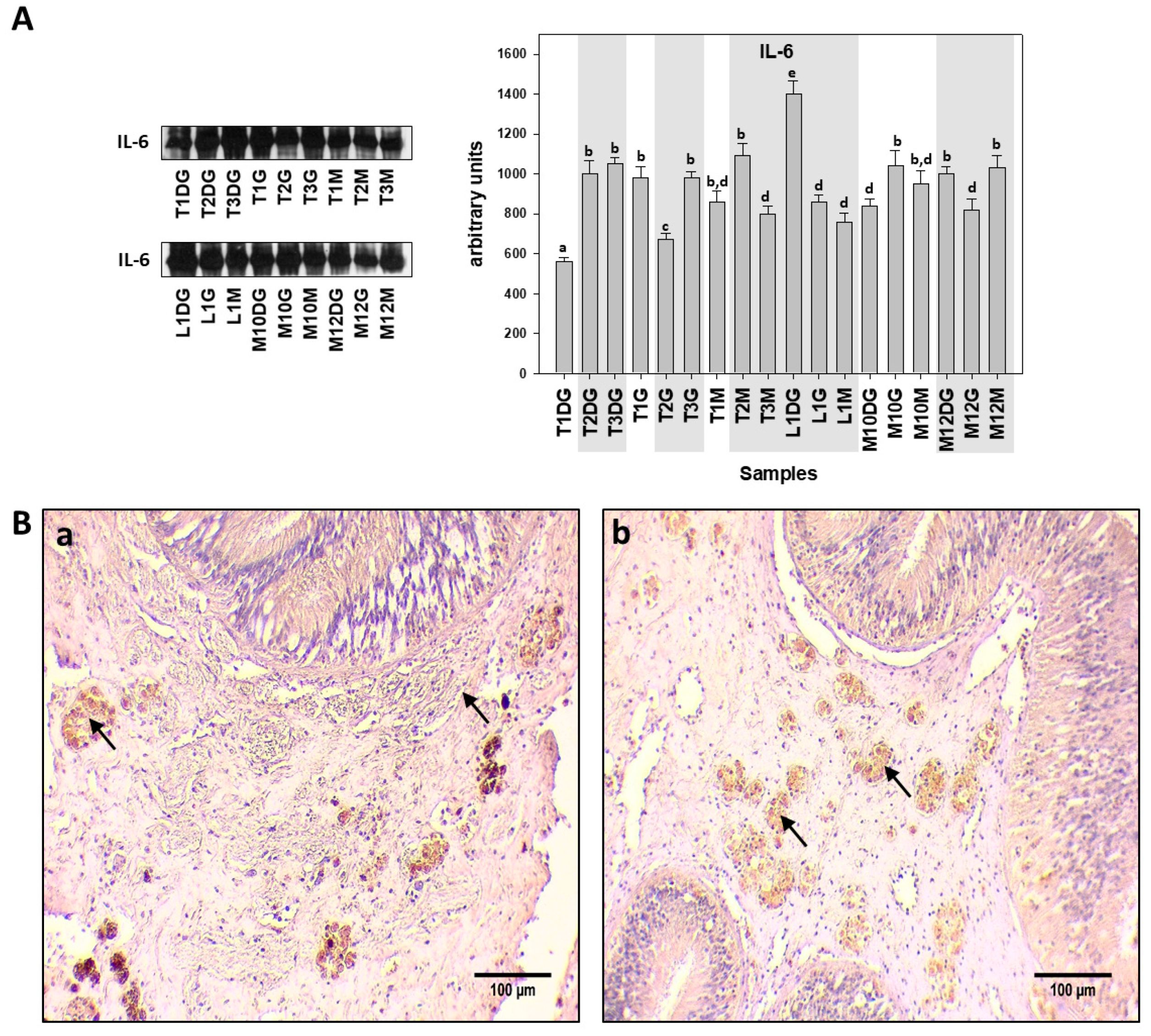
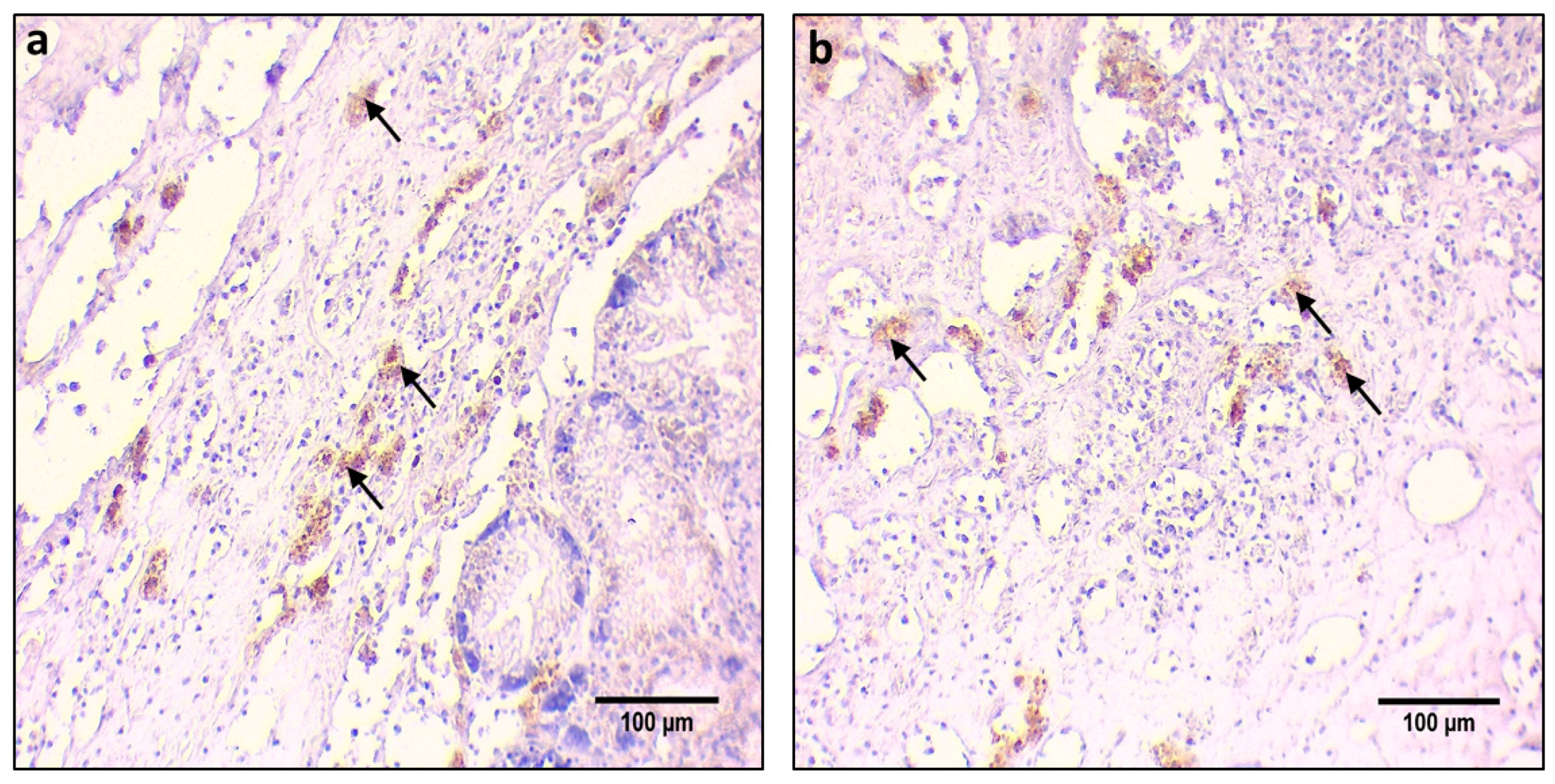
2.3. Immunohistochemistry Procedure for Protein Localization
2.4. SDS-PAGE/Immunoblot and Dot Blot Analysis
2.4.1. Preparation of Tissue Samples
2.4.2. SDS-PAGE/Immunoblot
2.4.3. Dot Blot Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richardson, C.A.; Kennedy, H.; Duarte, C.M.; Kennedy, D.P.; Proud, S.V. Age and growth of the fan mussel Pinna nobilis from south-east Spanish Mediterranean seagrass (Posidonia oceanica) meadows. Mar. Biol. 1999, 133, 205–212. [Google Scholar] [CrossRef]
- García-March, J.R.; García-Carrascosa, A.M.; Peña Cantero, A.L.; Wang, Y.G. Population structure, mortality and growth of Pinna nobilis Linnaeus, 1758 (Mollusca, Bivalvia) at different depths in Moraira bay (Alicante, Western Mediterranean). Mar. Biol. 2007, 150, 861–871. [Google Scholar] [CrossRef]
- Butler, A.; Vicente, N.; de Gaulejac, B. Ecology of the pterioid bivalves Pinna bicolor Gmelin and Pinna nobilis L. Mar. Life 1993, 3, 37–45. [Google Scholar]
- Lattos, A.; Giantsis, I.A.; Karagiannis, D.; Michaelidis, B. First detection of the invasive Haplosporidian and Mycobacteria parasites hosting the endangered bivalve Pinna nobilis in Thermaikos Gulf, North Greece. Mar. Environ. Res. 2020, 155, 104889. [Google Scholar] [CrossRef] [PubMed]
- Kersting, D.; Benabdi, M.; Čižmek, H.; Grau, A.; Jimenez, C.; Katsanevakis, S.; Öztürk, B.; Tuncer, S.; Tunesi, L.; Vázquez-Luis, M.; et al. Pinna nobilis. IUCN Red List Threat. Species 2019, 2019, 8235. [Google Scholar]
- Katsanevakis, S. Population ecology of the endangered fan mussel Pinna nobilis in a marine lake. Endanger Species Res. 2004, 1, 51–59. [Google Scholar] [CrossRef]
- Katsanevakis, S. Growth and mortality rates of the fan mussel Pinna nobilis in Lake Vouliagmeni (Korinthiakos Gulf, Greece): A generalized additive modelling approach. Mar. Biol. 2007, 152, 1319–1331. [Google Scholar] [CrossRef]
- Lattos, A.; Bitchava, K.; Giantsis, I.A.; Theodorou, J.A.; Batargias, C.; Michaelidis, B. The implication of vibrio bacteria in the winter mortalities of the critically endangered Pinna nobilis. Microorganisms 2021, 9, 922. [Google Scholar] [CrossRef]
- Darriba, S. First haplosporidan parasite reported infecting a member of the Superfamily Pinnoidea (Pinna nobilis) during a mortality event in Alicante (Spain, Western Mediterranean). J. Invertebr. Pathol. 2017, 148, 14–19. [Google Scholar] [CrossRef]
- Cabanellas-Reboredo, M.; Vázquez-Luis, M.; Mourre, B.; Alvarez, E.; Deudero, S.; Amores, A.; Addis, P.; Ballesteros, E.; Barrajon, A.; Coppa, S.; et al. Tracking a mass mortality outbreak of pen shell Pinna nobilis populations: A collaborative effort of scientists and citizens. Sci. Rep. 2019, 9, 13355. [Google Scholar] [CrossRef]
- Vázquez-Luis, M.; Álvarez, E.; Barrajón, A.; García-March, J.R.; Grau, A.; Hendriks, I.E.; Jiménez, S.; Kersting, D.; Moreno, D.; Pérez, M.; et al. S.O.S. Pinna nobilis: A mass mortality event in western Mediterranean Sea. Front. Mar. Sci. 2017, 4, 1–6. [Google Scholar] [CrossRef]
- Katsanevakis, S. The cryptogenic parasite Haplosporidium pinnae invades the Aegean Sea and causes the collapse of Pinna nobilis populations. Aquat. Invasions 2019, 14, 150–164. [Google Scholar] [CrossRef]
- Panarese, R.; Tedesco, P.; Chimienti, G.; Latrofa, M.S.; Quaglio, F.; Passantino, G.; Buonavoglia, C.; Gustinelli, A.; Tursi, A.; Otranto, D. Haplosporidium pinnae associated with mass mortality in endangered Pinna nobilis (Linnaeus 1758)fan mussels. J. Invertebr. Pathol. 2019, 164, 32–37. [Google Scholar] [CrossRef]
- Özalp, H.B.; Kersting, D.K. A pan-Mediterranean extinction? Pinna nobilis mass mortality has reached the Turkish straits system. Mar. Biodivers. 2020, 50, 81. [Google Scholar] [CrossRef]
- Öndes, F.; Alan, V.; Akçalı, B.; Güçlüsoy, H. Mass mortality of the fan mussel, Pinna nobilis in Turkey (eastern Mediterranean). Mar. Ecol. 2020, 41, 1–5. [Google Scholar] [CrossRef]
- Šarić, T.; Župan, I.; Aceto, S.; Villari, G.; Palić, D.; De Vico, G.; Carella, F. Epidemiology of Noble Pen Shell (Pinna nobilis L. 1758) Mass Mortality Events in Adriatic Sea Is Characterised with Rapid Spreading and Acute Disease Progression. Pathogens 2020, 9, 776. [Google Scholar] [CrossRef]
- Catanese, G.; Grau, A.; Valencia, J.M.; Garcia-March, J.R.; Vázquez-Luis, M.; Alvarez, E.; Deudero, S.; Darriba, S.; Carballal, M.J.; Villalba, A. Haplosporidium pinnae sp. nov., a haplosporidan parasite associated with mass mortalities of the fan mussel, Pinna nobilis, in the Western Mediterranean Sea. J. Invertebr. Pathol. 2018, 157, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Carella, F.; Aceto, S.; Pollaro, F.; Miccio, A.; Iaria, C.; Carrasco, N.; Prado, P.; De Vico, G. A mycobacterial disease is associated with the silent mass mortality of the pen shell Pinna nobilis along the Tyrrhenian coastline of Italy. Sci. Rep. 2019, 9, 2725. [Google Scholar] [CrossRef] [PubMed]
- Andree, K.B.; Carrasco, N.; Carella, F.; Furones, D.; Prado, P. Vibrio mediterranei, a potential emerging pathogen of marine fauna: Investigation of pathogenicity using a bacterial challenge in Pinna nobilis and development of a species-specific PCR. J. Appl. Microbiol. 2020, 130, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Prado, P.; Carrasco, N.; Catanese, G.; Grau, A.; Cabanes, P.; Carella, F.; García-March, J.R.; Tena, J.; Roque, A.; Bertomeu, E.; et al. Presence of Vibrio mediterranei associated to major mortality in stabled individuals of Pinna nobilis L. Aquaculture 2020, 519, 734899. [Google Scholar] [CrossRef]
- Lattos, A.; Giantsis, I.A.; Karagiannis, D.; Theodorou, J.A.; Michaelidis, B. Gut Symbiotic Microbial Communities in the IUCN Critically Endangered Pinna nobilis Suffering from Mass Mortalities, Revealed by 16S rRNA Amplicon NGS. Pathogens 2020, 9, 1002. [Google Scholar] [CrossRef] [PubMed]
- Pavlinec, Ž.; Zupičić, I.G.; Oraić, D.; Petani, B.; Mustać, B.; Mihaljević, Ž.; Beck, R.; Zrnčić, S. Assessment of predominant bacteria in noble pen shell (Pinna nobilis) collected in the Eastern Adriatic Sea. Environ. Monit. Assess 2020, 192. [Google Scholar] [CrossRef]
- Carella, F.; Antuofermo, E.; Farina, S.; Salati, F.; Mandas, D.; Prado, P.; Panarese, R.; Marino, F.; Fiocchi, E.; Pretto, T.; et al. In the Wake of the Ongoing Mass Mortality Events: Co-occurrence of Mycobacterium, Haplosporidium and Other Pathogens in Pinna nobilis Collected in Italy and Spain (Mediterranean Sea). Front. Mar. Sci. 2020, 7, 1–14. [Google Scholar] [CrossRef]
- Scarpa, F.; Sanna, D.; Azzena, I.; Mugetti, D.; Cerruti, F.; Hosseini, S.; Cossu, P.; Pinna, S.; Grech, D.; Cabana, D.; et al. Multiple non-species-specific pathogens possibly triggered the mass mortality in Pinna nobilis. Life 2020, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Xiang, Z.; Zhou, Y.; Qin, Y.; Yu, Z. Fish and Shell fi sh Immunology Tumor necrosis factor receptor-associated factor 3 from Anodonta woodiana is an important factor in bivalve immune response to pathogen infection. Fish Shellfish Immunol. 2017, 71, 151–159. [Google Scholar] [CrossRef]
- Wang, M.; Yang, J.; Zhou, Z.; Qiu, L.; Wang, L.; Zhang, H.; Gao, Y.; Wang, X.; Zhang, L.; Zhao, J.; et al. A primitive Toll-like receptor signaling pathway in mollusk Zhikong scallop Chlamys farreri. Dev. Comp. Immunol. 2011, 35, 511–520. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, F.; Li, J.; Tong, Y.; Zhang, Y.; Yu, Z. Fish & Shell fish Immunology The first invertebrate RIG-I-like receptor (RLR) homolog gene in the pacific oyster Crassostrea gigas. Fish Shellfish Immunol. 2014, 40, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.V.; Alfaro, A.C.; Merien, F.; Young, T. In vitro study of apoptosis in mussel (Perna canaliculus) haemocytes induced by lipopolysaccharide. Aquaculture 2019, 503, 8–15. [Google Scholar] [CrossRef]
- Ashida, H.; Mimuro, H.; Ogawa, M.; Kobayashi, T.; Sanada, T.; Kim, M.; Sasakawa, C. Cell death and infection: A double-edged sword for host and pathogen survival. J. Cell Biol. 2011, 195, 931–942. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Deretic, V.; Levine, B. Autophagy, Immunity, and Microbial Adaptations. Cell Host Microbe 2009, 5, 527–549. [Google Scholar] [CrossRef]
- Balbi, T.; Cortese, K.; Ciacci, C.; Bellese, G.; Vezzulli, L.; Pruzzo, C.; Canesi, L. Autophagic processes in Mytilus galloprovincialis hemocytes: Effects of Vibrio tapetis. Fish Shellfish Immunol. 2018, 73, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Picot, S.; Morga, B.; Faury, N.; Chollet, B.; Dégremont, L.; Travers, M.A.; Renault, T.; Arzul, I. A study of autophagy in hemocytes of the Pacific oyster, Crassostrea gigas. Autophagy 2019, 15, 1801–1809. [Google Scholar] [CrossRef] [PubMed]
- Choy, A.; Roy, C.R. Autophagy and bacterial infection: An evolving arms race. Trends Microbiol. 2013, 21, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Picot, S.; Faury, N.; Arzul, I.; Chollet, B.; Renault, T.; Morga, B. Identification of the autophagy pathway in a mollusk bivalve, Crassostrea gigas. Autophagy 2020, 16, 2017–2035. [Google Scholar] [CrossRef]
- Parzych, K.R.; Klionsky, D.J. An Overview of Autophagy: Morphology, Mechanism, and Regulation. Antiox. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Klionsky, D.J.A. Eaten alive: A history of macroautophagy. Nat. Cell Biol. 2013, 12, 814–822. [Google Scholar] [CrossRef]
- Pickart, C.M. Ubiquitin enters the new millennium: Meeting review. Mol. Cell 2001, 8, 499–504. [Google Scholar] [CrossRef]
- Capt, C.; Sietman, B.E.; Stewart, D.T.; Breton, S. GBE Deciphering the Link between Doubly Uniparental Inheritance of mtDNA and Sex Determination in Bivalves: Clues from Comparative Transcriptomics. Genom. Biol. Evol. 2018, 10, 577–590. [Google Scholar] [CrossRef]
- Sung, Y.Y.; MacRae, T.H. Heat Shock Proteins and Disease Control in Aquatic Organisms. J. Aquac. Res. Developent. 2011. [Google Scholar] [CrossRef]
- Sung, Y.Y.; Macrae, T.H.; Sorgeloos, P.; Bossier, P. Stress response for disease control in aquaculture. Rev. Aquaq. 2011, 120–137. [Google Scholar] [CrossRef]
- Box, A.; Capó, X.; Tejada, S.; Catanese, G.; Grau, A.; Deudero, S.; Sureda, A.; Valencia, J.M. Reduced antioxidant response of the fan mussel Pinna nobilis related to the presence of Haplosporidium pinnae. Pathogens 2020, 9, 932. [Google Scholar] [CrossRef] [PubMed]
- Karunanithi, S.; Brown, I.R. Heat shock response and homeostatic plasticity. Front. Cell. Neurosci. 2015, 9, 68. [Google Scholar] [CrossRef]
- Kanwar, R.K.; Kanwar, J.R.; Wang, D.; Ormrod, D.J.; Krissansen, G.W. Temporal expression of heat shock proteins 60 and 70 at lesion-prone sites during atherogenesis in ApoE-/- deficient mice. Arterioscler. Τhromb. Vasc. Biol. 2001, 21, 1991–1997. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qin, J.G.; Abbott, C.A.; Li, X.; Benkendorff, K. Synergistic impacts of heat shock and spawning on the physiology and immune health of Crassostrea gigas: An explanation for summer mortality in Pacific oysters. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2007, 293, 2353–2362. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.H.; He, J.Y.; Chi, C.F.; Shao, J. Differential HSP70 expression in Mytilus coruscus under various stressors. Gene 2014, 543, 166–173. [Google Scholar] [CrossRef]
- Liu, H.H.; He, J.Y.; Chi, C.F.; Lv, Z.M. Identification and analysis of HSP70 from Sepiella maindroni under stress of Vibrio harveyi and Cd2+. Gene 2015, 572, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Tsan, M.F.; Gao, B. Cytokine function of heat shock proteins. Am. J. Physiol. Cell Physiol. 2004, 286, C739–C744. [Google Scholar] [CrossRef]
- Li, W.; Xu, C.; Zhang, C.; Cao, W.; Qin, X.; Gao, J.; Zheng, H. The purification and identification of immunoregulatory peptides from oyster (Crassostrea hongkongensis) enzymatic hydrolysate. RSC Adv. 2019, 9, 32854–32863. [Google Scholar] [CrossRef]
- Ammon, H.P.T. Modulation of the immune system by Boswellia serrata extracts and boswellic acids. Phytomedicine 2010, 17, 862–867. [Google Scholar] [CrossRef]
- Ciechanover, A. The Ubiquitin-Proteasome Proteolytic Pathway. Cell 1994, 79, 13–21. [Google Scholar] [CrossRef]
- Dingkun, F.U.; Yang, Z.; Ziniu, Y.U. Cloning and expression analysis of a ubiquitin gene (Ub L40) in the haemocytes of Crassostrea hongkongensis under bacterial challenge. J. Oceanol. Limnol. 2011, 29, 80–86. [Google Scholar] [CrossRef]
- Varum, S.B.; Bento, C.B.; Sousa, P.M.B.; Gomes-Santos, C.S.S. Characterization of human sperm populations using conventional parameters, surface ubiquitination, and apoptotic markers. Fertil. Steril. 2007, 87. [Google Scholar] [CrossRef][Green Version]
- Cooper, K.F. Till Death Do Us Part: The Marriage of Autophagy and Apoptosis. Oxid. Med. Cell Longev. 2018. [Google Scholar] [CrossRef]
- Parisi, G.M.; Maisano, M.; Cappello, T.; Oliva, S. Comparative Biochemistry and Physiology, Part C Responses of marine mussel Mytilus galloprovincialis (Bivalvia: Mytilidae) after infection with the pathogen Vibrio splendidus. Comp. Biochem. Physiol. Part C 2019, 221, 1–9. [Google Scholar] [CrossRef]
- Bjorkoy, G.; Lamark, T.; Pankiv, S. Monitoring Autophagic Degradation Meth. Enzymol. 2009, 452, 181–197. [Google Scholar] [CrossRef]
- Feidantsis, K.; Georgoulis, I.; Zachariou, A.; Campaz, B.; Christoforou, M. Energetic, antioxidant, inflammatory and cell death responses in the red muscle of thermally stressed Sparus aurata. J. Comp. Physiol. B 2020, 190, 403–418. [Google Scholar] [CrossRef]
- Kusaka, M.; Ikeda, D.; Funabara, D.; Hartshorne, D.J.; Watabe, S. The occurrence of tissue-specific twitch in isoforms in the mussel Mytilus galloprovincialis. Fish. Sci. 2008, 1, 677–686. [Google Scholar] [CrossRef][Green Version]
- Feidantsis, K.; Pörtner, H.O.; Vlachonikola, E.; Antonopoulou, E.; Michaelidis, B. Seasonal Changes in Metabolism and Cellular Stress Phenomena in the Seasonal Changes in Metabolism and Cellular Stress Phenomena in the Gilthead Sea Bream (Sparus aurata). Physiol Biochem. Zool. 2018. [Google Scholar] [CrossRef]
- Anestis, A.; Pörtner, H.O.; Karagiannis, D.; Angelidis, P.; Staikou, A.; Michaelidis, B. Response of Mytilus galloprovincialis (L.) to increasing seawater temperature and to marteliosis: Metabolic and physiological parameters. Comp. Biochem. Physiol.-A Mol. Integr. Physiol. 2010, 156, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, C.L.; Lynch, S.A.; Culloty, S.C.; Malham, S.K. Future oceanic warming and acidification alter immune response and disease status in a commercial shellfish species, Mytilus edulis L. PLoS ONE 2014, 9, e99712. [Google Scholar] [CrossRef]
- Marcos-López, M.; Gale, P.; Oidtmann, B.C.; Peeler, E.J. Assessing the impact of climate change on disease emergence in freshwater fish in the United Kingdom. Transbound. Emerg. Dis. 2010, 57, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Matozzo, V.; Marin, M.G. Bivalve immune responses and climate changes: Is there a relationship? Invertebr. Surviv. J. 2011, 8, 70–77. [Google Scholar]
- Doney, S.C.; Ruckelshaus, M.; Emmett Duffy, J.; Barry, J.P.; Chan, F.; English, C.A.; Galindo, H.M.; Grebmeier, J.M.; Hollowed, A.B.; Knowlton, N.; et al. Climate change impacts on marine ecosystems. Ann. Rev. Mar. Sci. 2012, 4, 11–37. [Google Scholar] [CrossRef] [PubMed]
- Garnier, M.; Labreuche, Y.; Garcia, C.; Robert, M.; Nicolas, J.L. Evidence for the involvement of pathogenic bacteria in summer mortalities of the pacific oyster Crassostrea gigas. Microb. Ecol. 2007, 53, 187–196. [Google Scholar] [CrossRef]
- Lokmer, A.; Mathias Wegner, K. Hemolymph microbiome of Pacific oysters in response to temperature, temperature stress and infection. ISME J. 2015, 9, 670–682. [Google Scholar] [CrossRef]
- Burgos-Aceves, M.A.; Faggio, C. An approach to the study of the immunity functions of bivalve haemocytes: Physiology and molecular aspects. Fish Shellfish Immunol. 2017, 67, 513–517. [Google Scholar] [CrossRef] [PubMed]

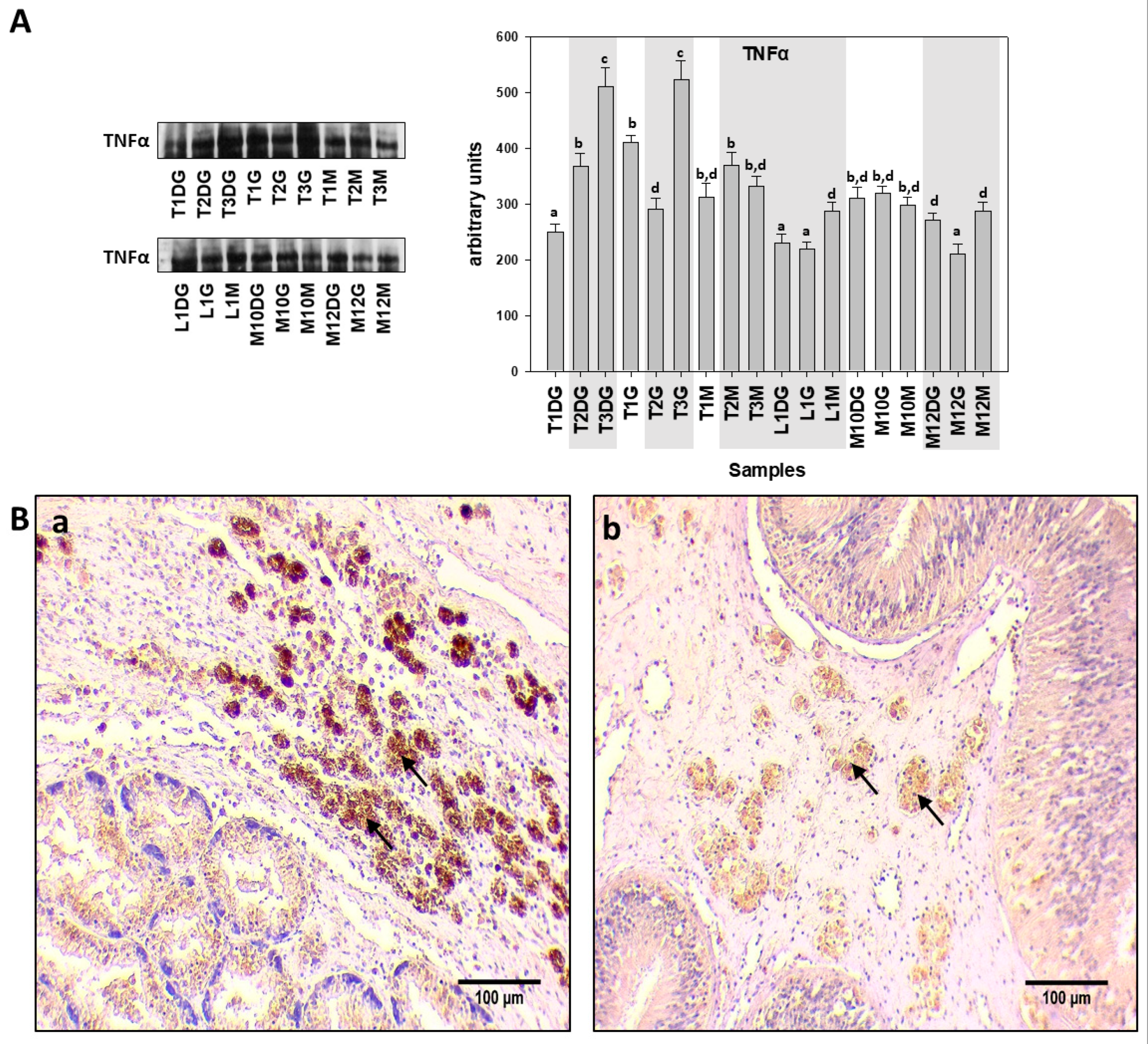
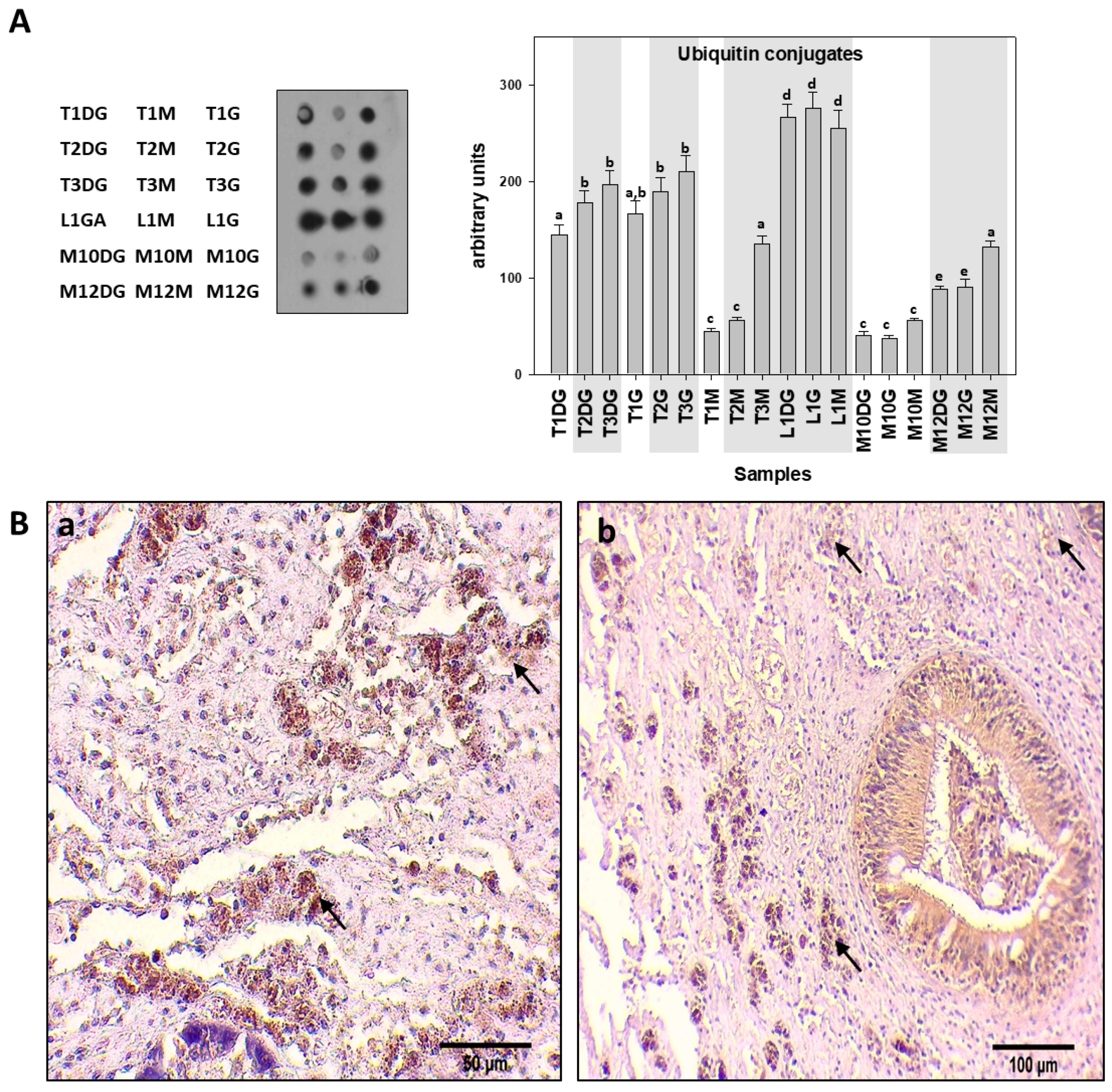
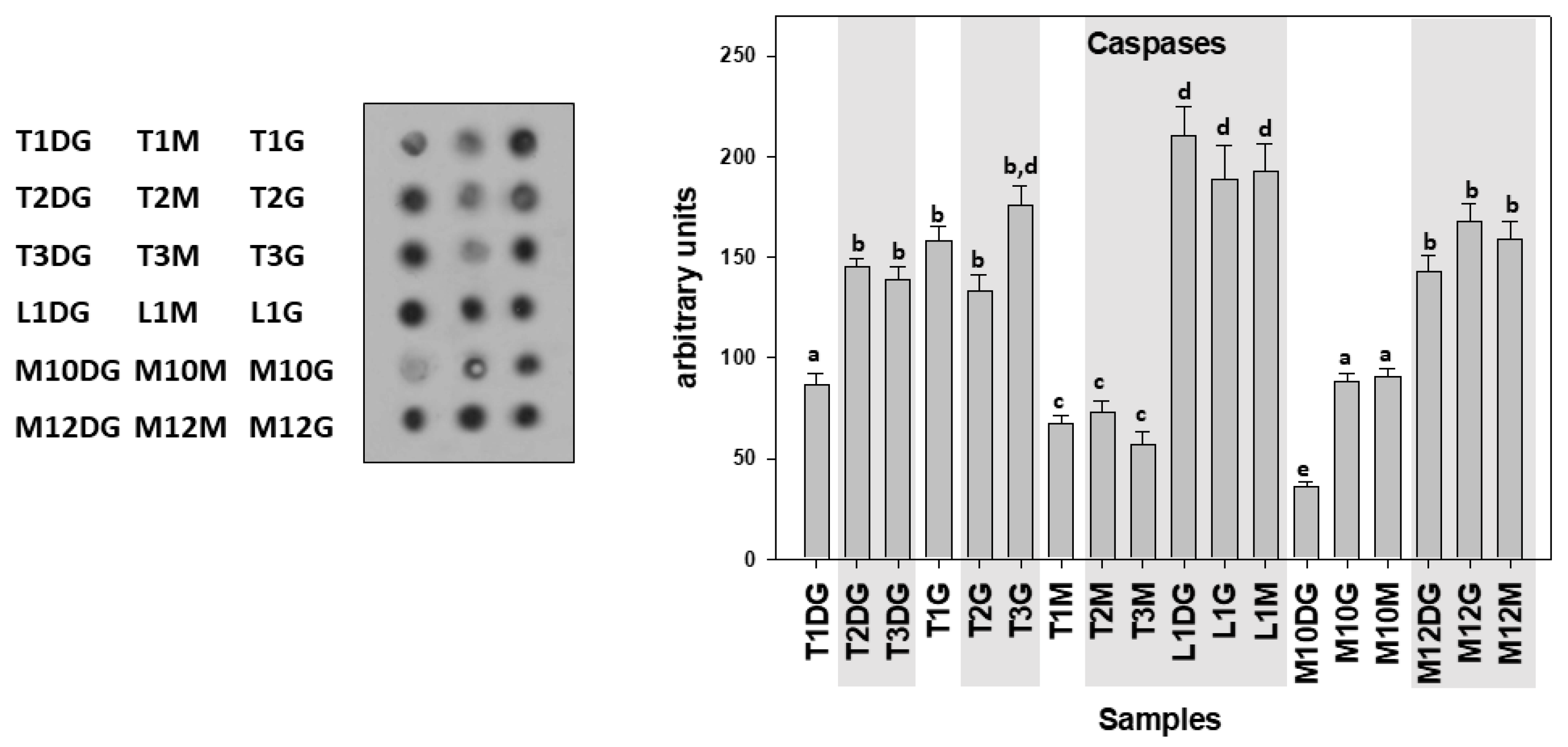
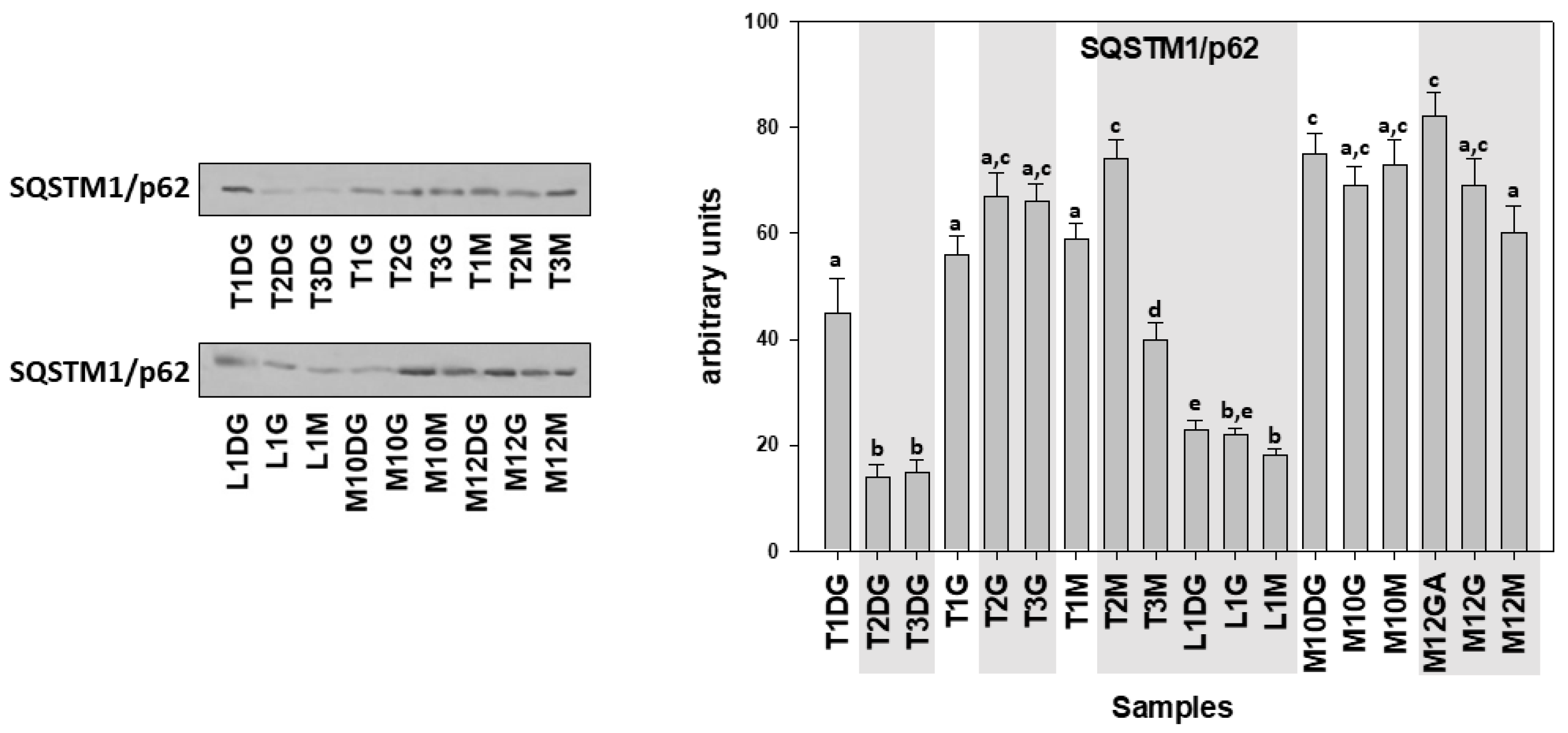
| Specimen ID | Shell Length, Height, Width (cm) | Weight (gr) | Tissue | Haplosporidiosis | Mycobacteriosis | Vibriosis |
|---|---|---|---|---|---|---|
| Ther01 | DG | + | + | |||
| 52.2, 14.2, 6.6 | 1482.26 | G | ||||
| M | ||||||
| Ther02 | DG | + | + | + | ||
| 56.5, 13.5, 6.3 | 1543.72 | G | ||||
| M | ||||||
| Ther03 | DG | + | + | + | ||
| 68.6, 18.4, 7.3 | 3080.42 | G | ||||
| M | ||||||
| Lim01 | DG | + | + | + | ||
| 22.3, 8.1, 2.5 | 160.2 | G | ||||
| M | ||||||
| Kal10 | DG | + | + | |||
| 34.2, 12.8, 4.3 | 298.26 | G | ||||
| M | ||||||
| Kal12 | DG | + | + | + | ||
| 29.3, 11.4, 3.5 | 208.75 | G | ||||
| M |
| Hsp70 | Hsp90 | Il-6 | TNF-α | Ubiquitin | Caspases | SQSTM1/p62 | |
|---|---|---|---|---|---|---|---|
| Hsp70 | 1 | ||||||
| Hsp90 | 0.463 | 1 | |||||
| Il-6 | 0.228 | −0.042 | 1 | ||||
| TNF-α | 0.228 | −0.197 | 0.211 | 1 | |||
| Ubiquitin | 0.673 | 0.318 | 0.085 | 0.049 | 1 | ||
| Caspases | 0.421 | 0.332 | 0.314 | −0.065 | 0.787 | 1 | |
| SQSTM1/62 | −0.921 | −0.425 | −0.102 | −0.063 | −0.754 | −0.447 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lattos, A.; Feidantsis, K.; Georgoulis, I.; Giantsis, I.A.; Karagiannis, D.; Theodorou, J.A.; Staikou, A.; Michaelidis, B. Pathophysiological Responses of Pinna nobilis Individuals Enlightens the Etiology of Mass Mortality Situation in the Mediterranean Populations. Cells 2021, 10, 2838. https://doi.org/10.3390/cells10112838
Lattos A, Feidantsis K, Georgoulis I, Giantsis IA, Karagiannis D, Theodorou JA, Staikou A, Michaelidis B. Pathophysiological Responses of Pinna nobilis Individuals Enlightens the Etiology of Mass Mortality Situation in the Mediterranean Populations. Cells. 2021; 10(11):2838. https://doi.org/10.3390/cells10112838
Chicago/Turabian StyleLattos, Athanasios, Konstantinos Feidantsis, Ioannis Georgoulis, Ioannis A. Giantsis, Dimitrios Karagiannis, John A. Theodorou, Alexandra Staikou, and Basile Michaelidis. 2021. "Pathophysiological Responses of Pinna nobilis Individuals Enlightens the Etiology of Mass Mortality Situation in the Mediterranean Populations" Cells 10, no. 11: 2838. https://doi.org/10.3390/cells10112838
APA StyleLattos, A., Feidantsis, K., Georgoulis, I., Giantsis, I. A., Karagiannis, D., Theodorou, J. A., Staikou, A., & Michaelidis, B. (2021). Pathophysiological Responses of Pinna nobilis Individuals Enlightens the Etiology of Mass Mortality Situation in the Mediterranean Populations. Cells, 10(11), 2838. https://doi.org/10.3390/cells10112838