GM101 in Combination with Histone Deacetylase Inhibitor Enhances Anti-Tumor Effects in Desmoplastic Microenvironment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Preparation of Ad
2.2. Preparation of HDACi
2.3. Transduction Efficiency Assay
2.4. Quantification of CAR Expression Level
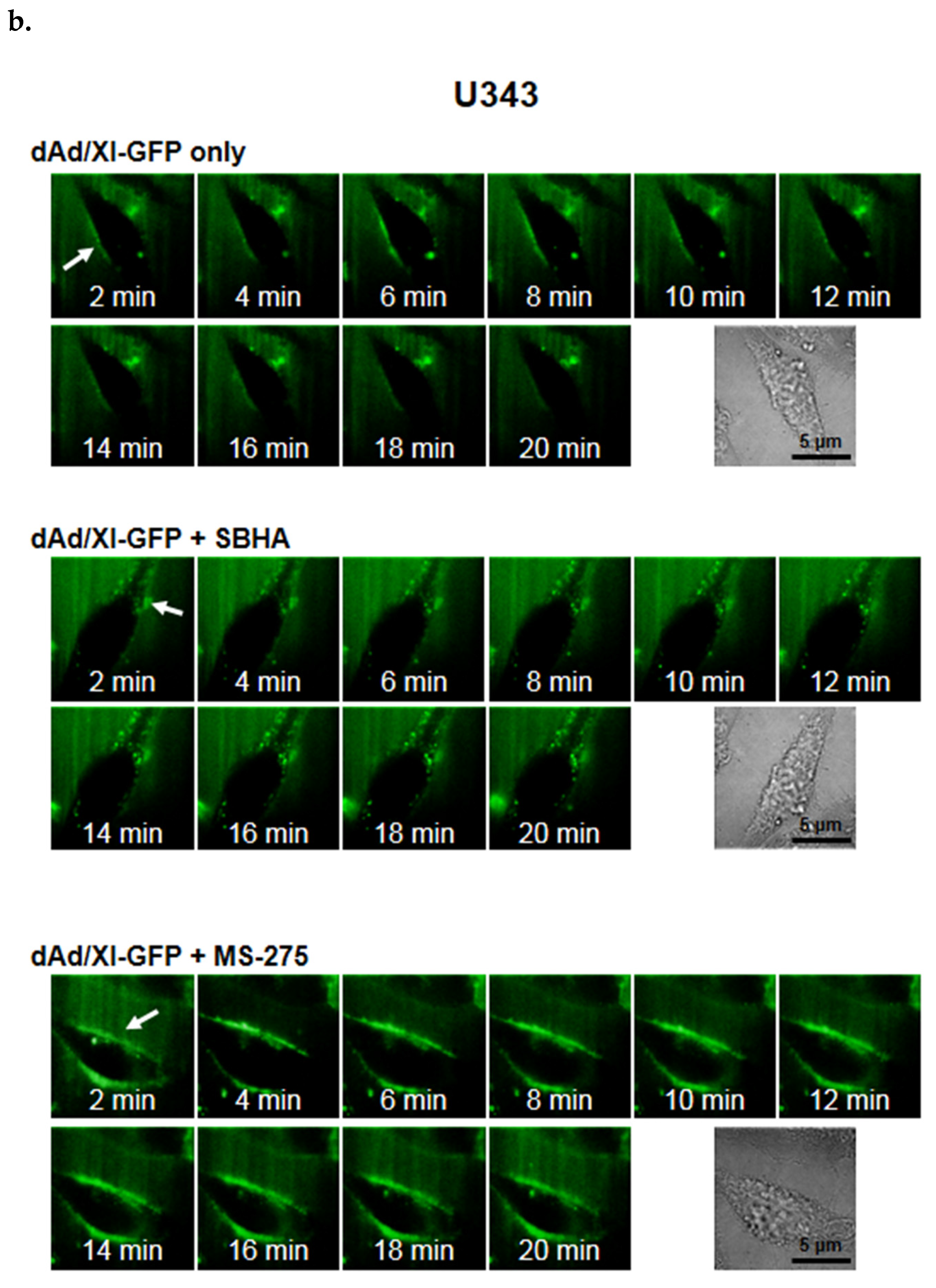
2.5. Total Internal Reflection Fluorescence (TIRF) Microscope Imaging
2.6. Analysis of Virus Attachment to Cell Membrane Following HDACi Treatment
2.7. Analysis of Clathrin- and Dynamin-Dependent Endocytosis of Ad Following HDACi Treatment
2.8. Western Blot Analysis
2.9. Cell Viability Assessment
2.10. Assessment of Antitumor Efficacy
2.11. Histological and Immunohistochemical Analysis of Tumor Tissues
2.12. Serum Toxicity Profile of Combination Therapy
2.13. Statistical Analysis
3. Results
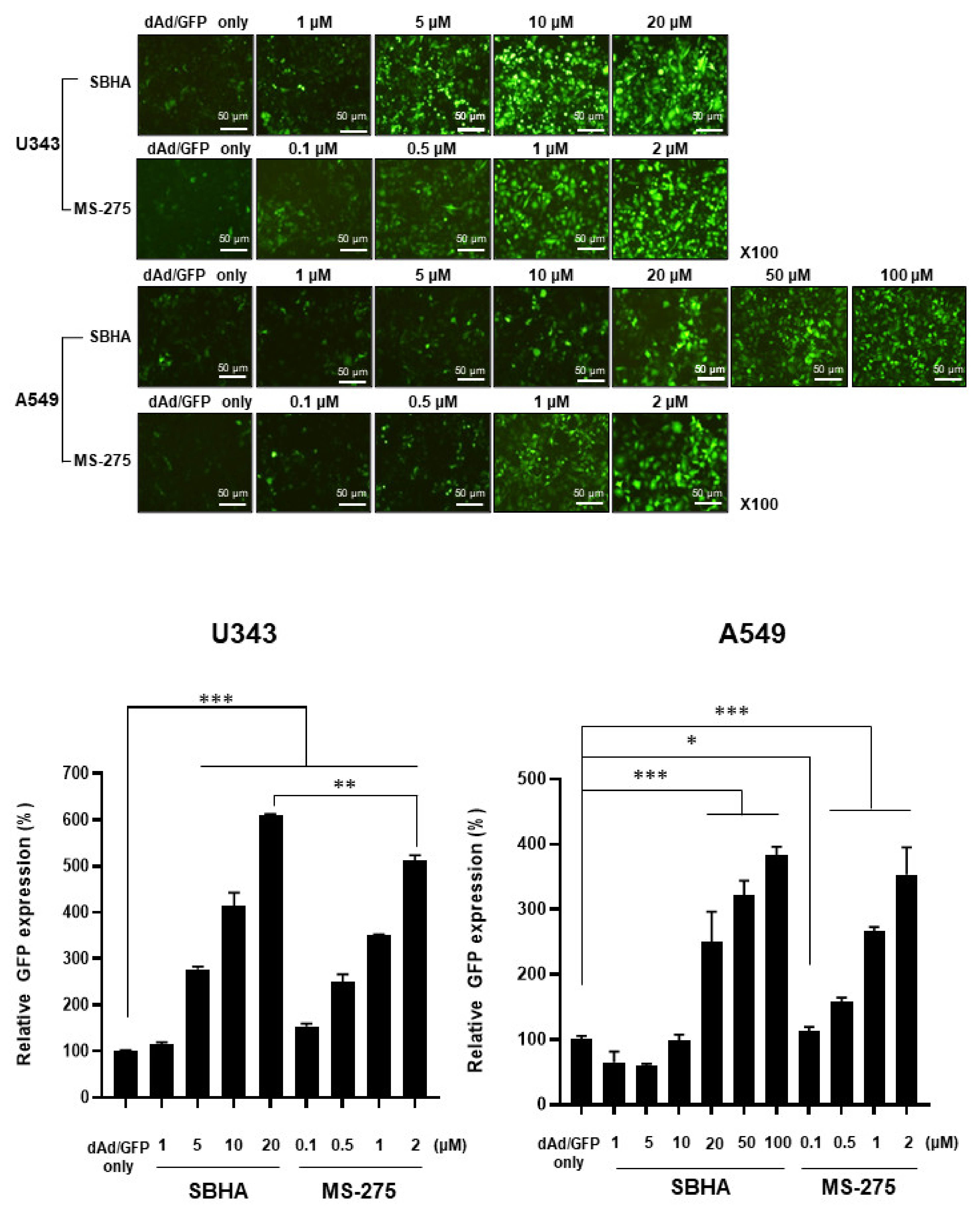
3.1. HDACi Enhances Ad-Mediated Transgene Expression
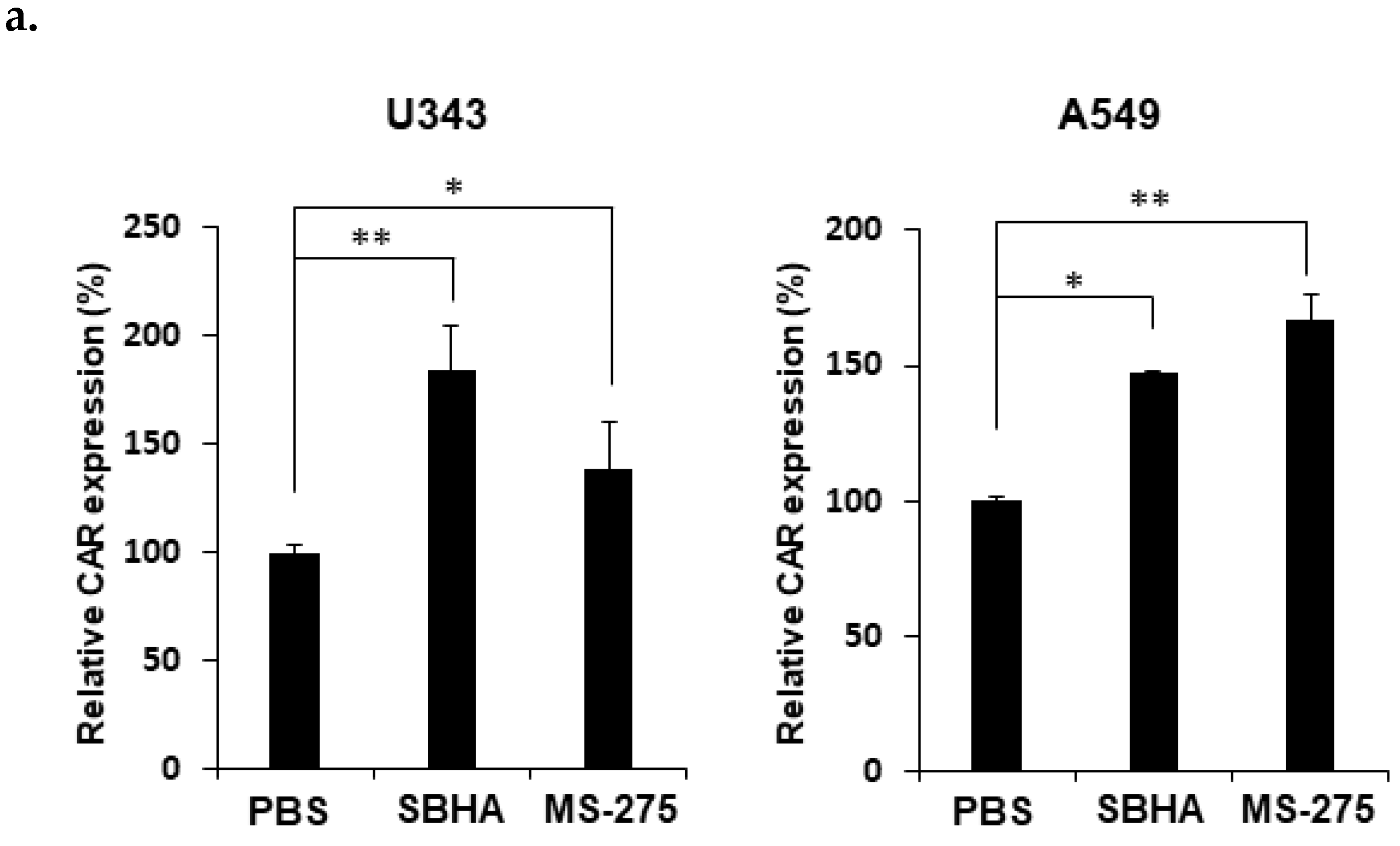
3.2. HDACi Upregulates CAR Expression and Facilitates Membrane Attachment of Ad
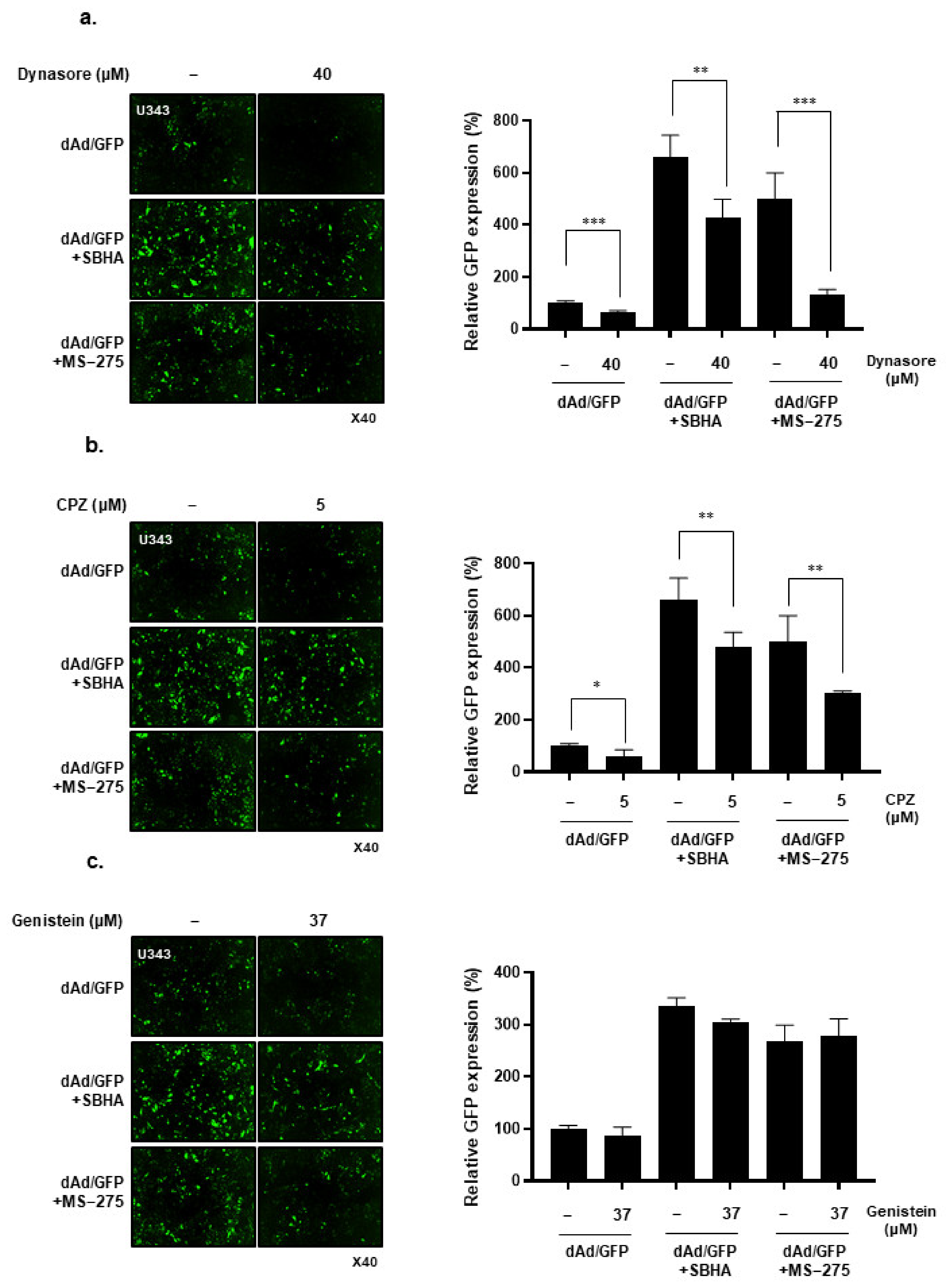
3.3. HDACi Induces Cell Uptake of Ad through Dynamin 2- and Clathrin-Mediated Endocytic Pathway
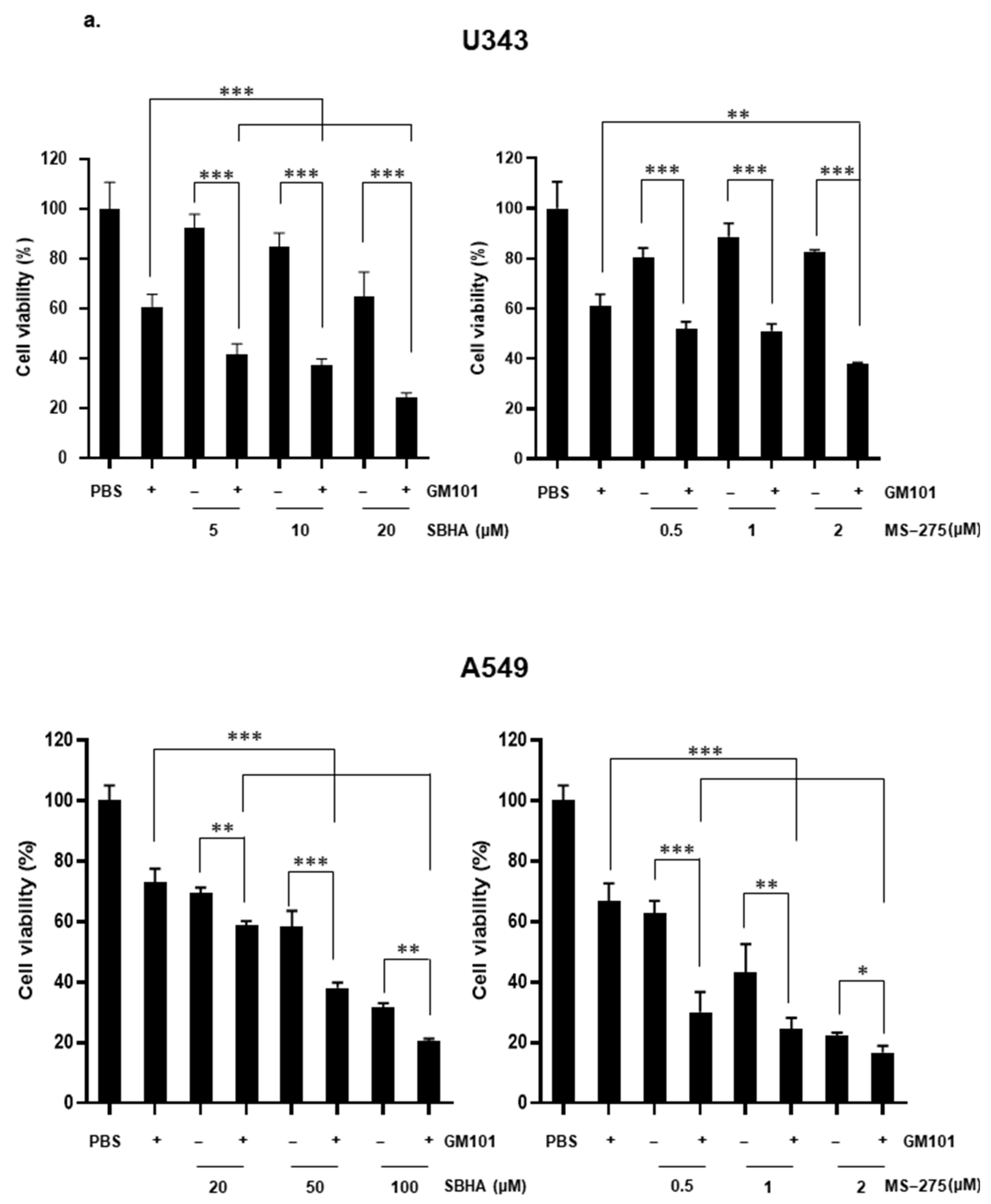
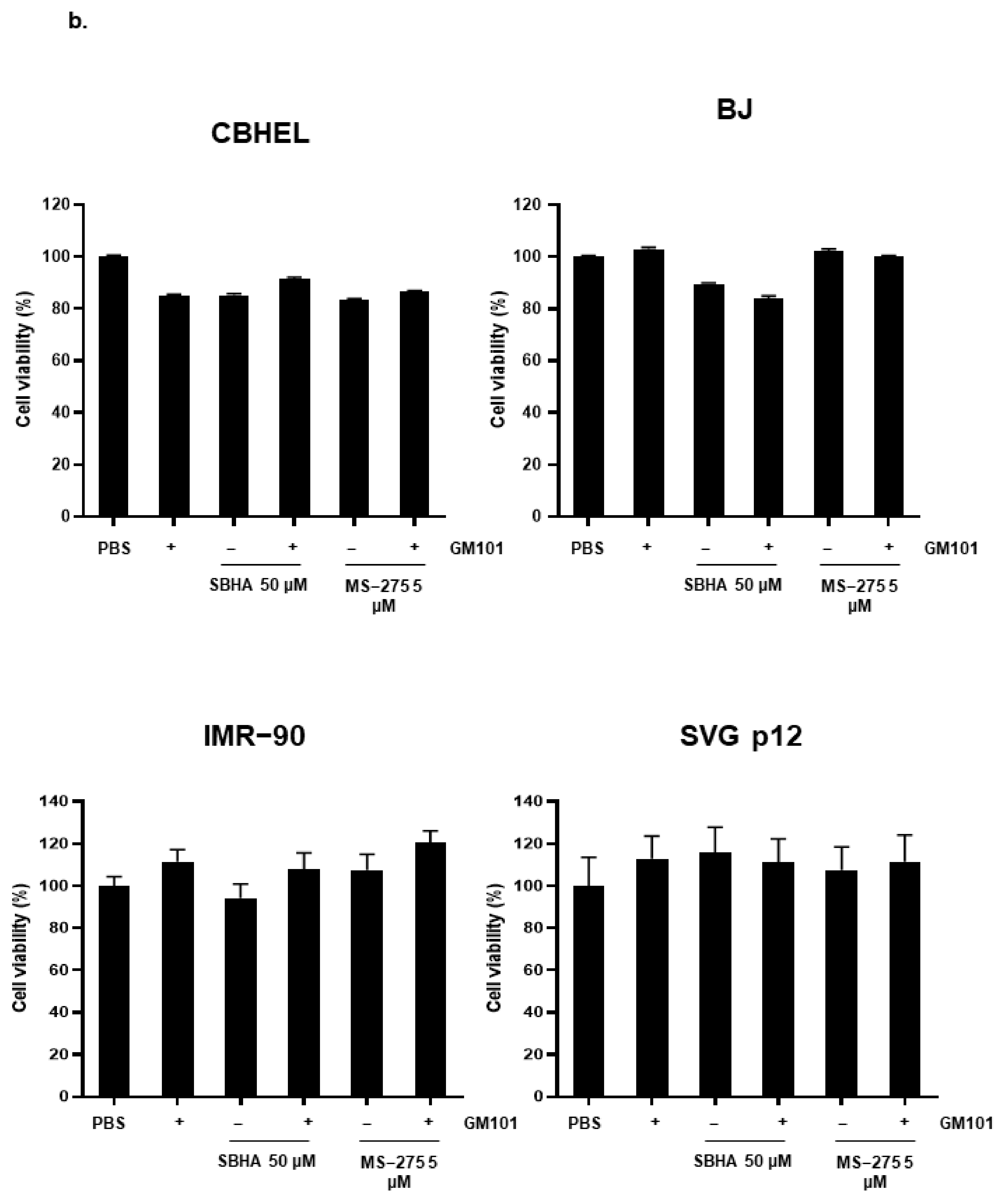
3.4. HDACi Enhances Cytotoxicity of GM101 in a Cancer-Specific Manner
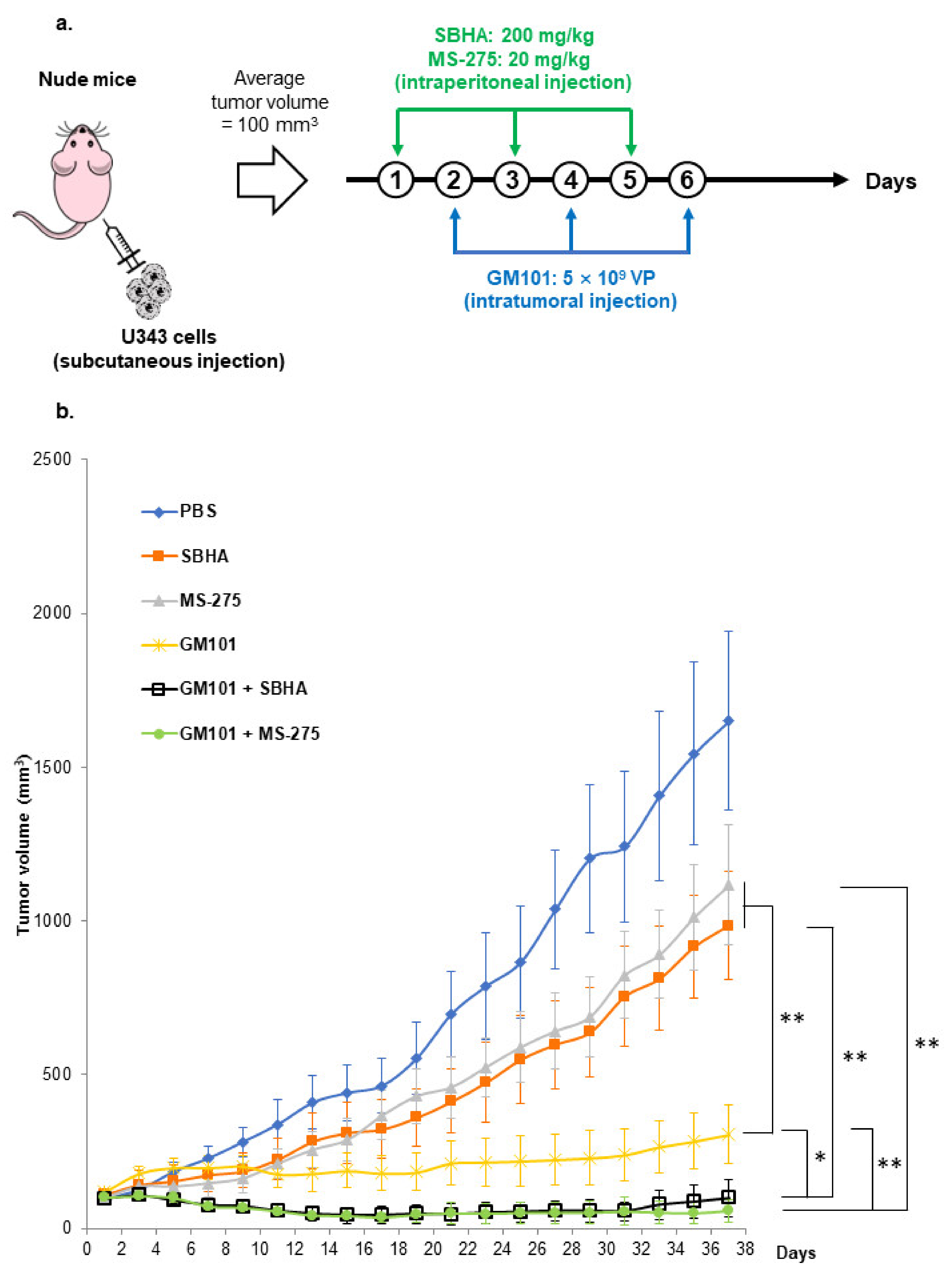
3.5. HDACi Potentiate the Antitumor Effect of GM101
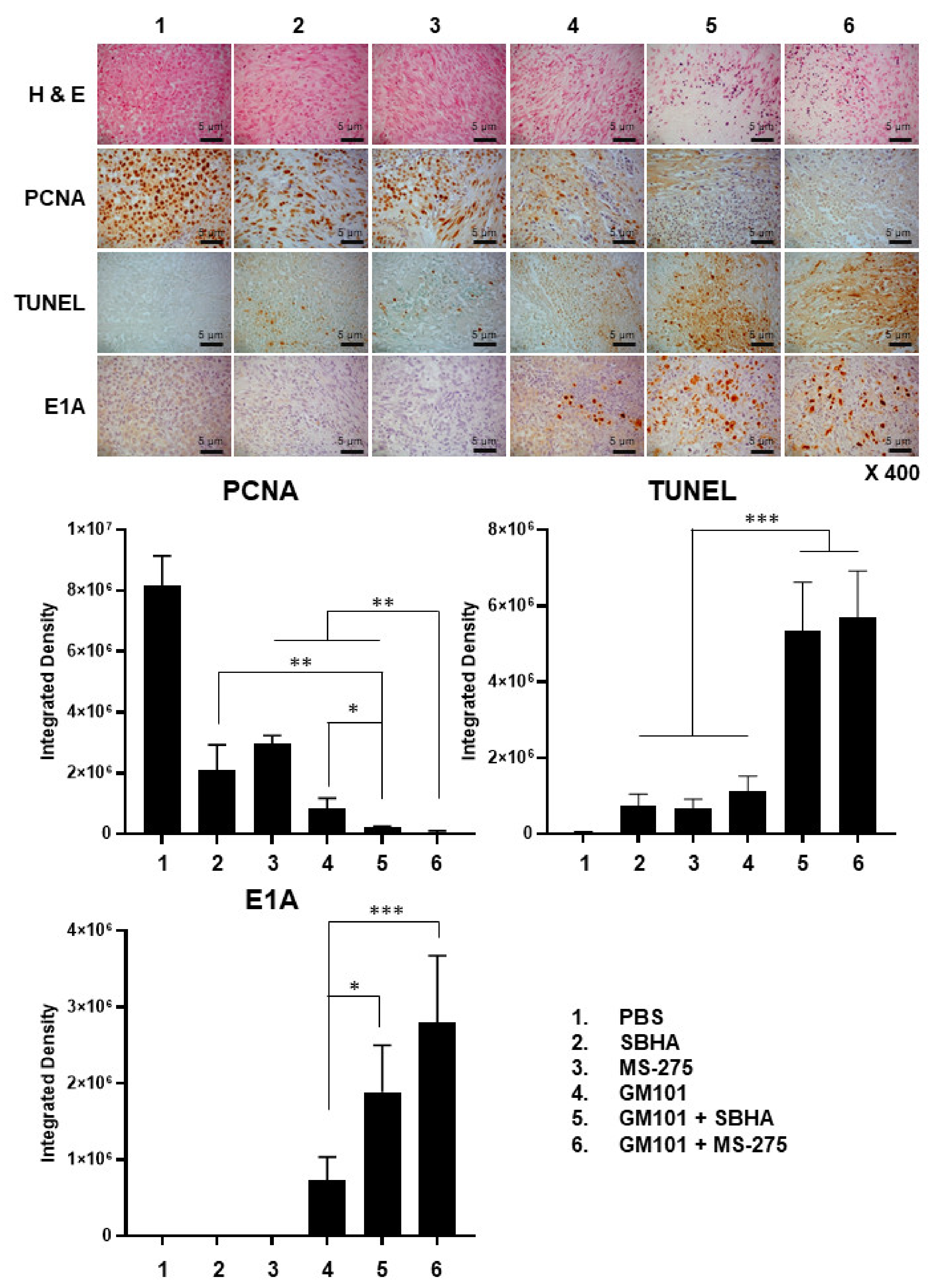
3.6. Combination of GM101 with HDACi Increases Viral Accumulation, Apoptotic Cell Death, and Degradation of Extracellular Matrix in Tumor Tissues
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hassig, C.A.; Schreiber, S.L. Nuclear histone acetylases and deacetylases and transcriptional regulation: Hats off to hdacs. Curr. Opin. Chem. Biol. 1997, 1, 300–308. [Google Scholar] [CrossRef]
- Richon, V.M.; O’Brien, J.P. Histone deacetylase inhibitors: A new class of potential therapeutic agents for cancer treatment. Clin. Cancer Res. 2002, 8, 662–664. [Google Scholar] [PubMed]
- Al-Janadi, A.; Chandana, S.R.; Conley, B.A. Histone deacetylation: An attractive target for cancer therapy? Drugs R D 2008, 9, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Mann, B.S.; Johnson, J.R.; Cohen, M.H.; Justice, R.; Pazdur, R. FDA approval summary: Vorinostat for treatment of advanced primary cutaneous t-cell lymphoma. Oncologist 2007, 12, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Raedler, L.A. Farydak (panobinostat): First hdac inhibitor approved for patients with relapsed multiple myeloma. Am. Health Drug Benefits 2016, 9, 84–87. [Google Scholar]
- Delcuve, G.P.; Khan, D.H.; Davie, J.R. Roles of histone deacetylases in epigenetic regulation: Emerging paradigms from studies with inhibitors. Clin. Epigenetics 2012, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Fouladi, M. Histone deacetylase inhibitors in cancer therapy. Cancer Investig. 2006, 24, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Khuri, F.R.; Nemunaitis, J.; Ganly, I.; Arseneau, J.; Tannock, I.F.; Romel, L.; Gore, M.; Ironside, J.; MacDougall, R.H.; Heise, C.; et al. A controlled trial of intratumoral onyx-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat. Med. 2000, 6, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Kuryk, L.; Vassilev, L.; Ranki, T.; Hemminki, A.; Karioja-Kallio, A.; Levalampi, O.; Vuolanto, A.; Cerullo, V.; Pesonen, S. Toxicological and bio-distribution profile of a gm-csf-expressing, double-targeted, chimeric oncolytic adenovirus oncos-102—Support for clinical studies on advanced cancer treatment. PLoS ONE 2017, 12, e0182715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Z.-J.; Chang, J.-H.; Zhang, L.; Jiang, W.-Q.; Guan, Z.-Z.; Liu, J.-W.; Zhang, Y.; Hu, X.-H.; Wu, G.-H.; Wang, H.-Q.; et al. [Phase iii randomized clinical trial of intratumoral injection of e1b gene-deleted adenovirus (h101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus]. Ai Zheng 2004, 23, 1666–1670. [Google Scholar]
- Moon, C.Y.; Choi, J.W.; Kasala, D.; Jung, S.J.; Kim, S.W.; Yun, C.O. Dual tumor targeting with ph-sensitive and bioreducible polymer-complexed oncolytic adenovirus. Biomaterials 2015, 41, 53–68. [Google Scholar] [CrossRef]
- Jiang, H.; Gomez-Manzano, C.; Lang, F.F.; Alemany, R.; Fueyo, J. Oncolytic adenovirus: Preclinical and clinical studies in patients with human malignant gliomas. Curr. Gene Ther. 2009, 9, 422–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergelson, J.M.; Cunningham, J.A.; Droguett, G.; Kurt-Jones, E.A.; Krithivas, A.; Hong, J.S.; Horwitz, M.S.; Crowell, R.L.; Finberg, R.W. Isolation of a common receptor for coxsackie b viruses and adenoviruses 2 and 5. Science 1997, 275, 1320–1323. [Google Scholar] [CrossRef]
- Cohen, C.J.; Shieh, J.T.; Pickles, R.J.; Okegawa, T.; Hsieh, J.T.; Bergelson, J.M. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc. Natl. Acad. Sci. USA 2001, 98, 15191–15196. [Google Scholar] [CrossRef] [Green Version]
- Korn, W.M.; Macal, M.; Christian, C.; Lacher, M.D.; McMillan, A.; Rauen, K.A.; Warren, R.S.; Ferrell, L. Expression of the coxsackievirus- and adenovirus receptor in gastrointestinal cancer correlates with tumor differentiation. Cancer Gene Ther. 2006, 13, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Wunder, T.; Schumacher, U.D.O.; Friedrich, R.E. Coxsackie adenovirus receptor expression in carcinomas of the head and neck. Anticancer Res. 2012, 32, 1057–1062. [Google Scholar] [PubMed]
- Sachs, M.D.; Ramamurthy, M.; Poel, H.; Wickham, T.J.; Lamfers, M.; Gerritsen, W.; Chowdhury, W.; Li, Y.; Schoenberg, M.P.; Rodriguez, R. Histone deacetylase inhibitors upregulate expression of the coxsackie adenovirus receptor (car) preferentially in bladder cancer cells. Cancer Gene Ther. 2004, 11, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Dion, L.D.; Goldsmith, K.T.; Tang, D.C.; Engler, J.A.; Yoshida, M.; Garver, R.I., Jr. Amplification of recombinant adenoviral transgene products occurs by inhibition of histone deacetylase. Virology 1997, 231, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Rosato, R.R.; Grant, S. Histone deacetylase inhibitors in cancer therapy. Cancer Biol. Ther. 2003, 2, 30–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.D.; Gillespie, S.K.; Borrow, J.M.; Hersey, P. The histone deacetylase inhibitor suberic bishydroxamate: A potential sensitizer of melanoma to tnf-related apoptosis-inducing ligand (trail) induced apoptosis. Biochem. Pharmacol. 2003, 66, 1537–1545. [Google Scholar] [CrossRef]
- Neuzil, J.; Swettenham, E.; Gellert, N. Sensitization of mesothelioma to trail apoptosis by inhibition of histone deacetylase: Role of bcl-xl down-regulation. Biochem. Biophys. Res. Commun. 2004, 314, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Nakata, S.; Yoshida, T.; Horinaka, M.; Shiraishi, T.; Wakada, M.; Sakai, T. Histone deacetylase inhibitors upregulate death receptor 5/trail-r2 and sensitize apoptosis induced by trail/apo2-l in human malignant tumor cells. Oncogene 2004, 23, 6261–6271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berghauser Pont, L.M.; Kleijn, A.; Kloezeman, J.J.; van den Bossche, W.; Kaufmann, J.K.; de Vrij, J.; Leenstra, S.; Dirven, C.M.; Lamfers, M.L. The hdac inhibitors scriptaid and lbh589 combined with the oncolytic virus delta24-rgd exert enhanced anti-tumor efficacy in patient-derived glioblastoma cells. PLoS ONE 2015, 10, e0127058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bressy, C.; Majhen, D.; Raddi, N.; Jdey, W.; Cornilleau, G.; Zig, L.; Guirouilh-Barbat, J.; Lopez, B.S.; Bawa, O.; Opolon, P.; et al. Combined therapy of colon carcinomas with an oncolytic adenovirus and valproic acid. Oncotarget 2017, 8, 97344–97360. [Google Scholar] [CrossRef]
- Choi, I.K.; Lee, Y.S.; Yoo, J.Y.; Yoon, A.R.; Kim, H.; Kim, D.S.; Seidler, D.G.; Kim, J.H.; Yun, C.O. Effect of decorin on overcoming the extracellular matrix barrier for oncolytic virotherapy. Gene Ther. 2010, 17, 190–201. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.; Kim, J.-H.; Shin, H.-Y.; Lee, H.; Yang, J.M.; Kim, J.; Sohn, J.-H.; Kim, H.; Yun, C.-O. Ad-mtert-delta19, a conditional replication-competent adenovirus driven by the human telomerase promoter, selectively replicates in and elicits cytopathic effect in a cancer cell-specific manner. Hum. Gene Ther. 2003, 14, 1415–1428. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Lee, Y.-S.; Kim, H.; Huang, J.-H.; Yoon, A.-R.; Yun, C.-O. Relaxin expression from tumor-targeting adenoviruses and its intratumoral spread, apoptosis induction, and efficacy. J. Natl. Cancer Inst. 2006, 98, 1482–1493. [Google Scholar] [CrossRef]
- Blundell, T.L.; Humbel, R.E. Hormone families: Pancreatic hormones and homologous growth factors. Nature 1980, 287, 781–787. [Google Scholar] [CrossRef]
- Unemori, E.N.; Pickford, L.B.; Salles, A.L.; Piercy, C.E.; Grove, B.H.; Erikson, M.E.; Amento, E.P. Relaxin induces an extracellular matrix-degrading phenotype in human lung fibroblasts in vitro and inhibits lung fibrosis in a murine model in vivo. J. Clin. Investig. 1996, 98, 2739–2745. [Google Scholar] [CrossRef]
- Kim, P.-H.; Kim, J.; Kim, T.-i.; Nam, H.Y.; Yockman, J.W.; Kim, M.; Kim, S.W.; Yun, C.-O. Bioreducible polymer-conjugated oncolytic adenovirus for hepatoma-specific therapy via systemic administration. Biomaterials 2011, 32, 9328–9342. [Google Scholar] [CrossRef]
- Kim, P.-H.; Sohn, J.-H.; Choi, J.-W.; Jung, Y.; Kim, S.W.; Haam, S.; Yun, C.-O. Active targeting and safety profile of peg-modified adenovirus conjugated with herceptin. Biomaterials 2011, 32, 2314–2326. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-W.; Kang, E.; Kwon, O.-J.; Yun, T.J.; Park, H.-K.; Kim, P.-H.; Kim, S.W.; Kim, J.H.; Yun, C.-O. Local sustained delivery of oncolytic adenovirus with injectable alginate gel for cancer virotherapy. Gene Ther. 2013, 20, 880–892. [Google Scholar] [CrossRef] [Green Version]
- Meier, O.; Greber, U.F. Adenovirus endocytosis. J. Gene Med. 2004, 6, S152–S163. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kim, J.I.; Frilot, N.; Daaka, Y. Dynamin2 s-nitrosylation regulates adenovirus type 5 infection of epithelial cells. J. Gen. Virol. 2012, 93, 2109–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Huang, S.; Kapoor-Munshi, A.; Nemerow, G. Adenovirus internalization and infection require dynamin. J. Virol. 1998, 72, 3455–3458. [Google Scholar] [CrossRef] [Green Version]
- Jung, K.H.; Choi, I.-K.; Lee, H.-S.; Yan, H.H.; Son, M.K.; Ahn, H.M.; Hong, J.; Yun, C.-O.; Hong, S.-S. Oncolytic adenovirus expressing relaxin (ydc002) enhances therapeutic efficacy of gemcitabine against pancreatic cancer. Cancer Lett. 2017, 396, 155–166. [Google Scholar] [CrossRef]
- Jung, B.-K.; Ko, H.Y.; Kang, H.; Hong, J.; Ahn, H.M.; Na, Y.; Kim, H.; Kim, J.S.; Yun, C.-O. Relaxin-expressing oncolytic adenovirus induces remodeling of physical and immunological aspects of cold tumor to potentiate PD-1 blockade. J. Immunother. Cancer 2020, 8, e000763. [Google Scholar] [CrossRef]
- Höti, N.; Chowdhury, W.; Hsieh, J.-T.; Sachs, M.D.; Lupold, S.E.; Rodriguez, R. Valproic acid, a histone deacetylase inhibitor, is an antagonist for oncolytic adenoviral gene therapy. Mol. Ther. 2006, 14, 768–778. [Google Scholar] [CrossRef]
- Kim, D.R.; Park, M.-Y.; Lim, H.-J.; Park, J.S.; Cho, Y.J.; Lee, S.-W.; Yoon, H.-I.; Lee, J.-H.; Kim, Y.-S.; Lee, C.-T. Combination therapy of conditionally replicating adenovirus and histone deacetylase inhibitors. Int. J. Mol. Med. 2012, 29, 218–224. [Google Scholar]
- Hulin-Curtis, S.L.; Davies, J.A.; Jones, R.; Hudson, E.; Hanna, L.; Chester, J.D.; Parker, A.L. Histone deacetylase inhibitor trichostatin a sensitises cisplatin-resistant ovarian cancer cells to oncolytic adenovirus. Oncotarget 2018, 9, 26328–26341. [Google Scholar] [CrossRef]
- Liu, N.; He, S.; Ma, L.; Ponnusamy, M.; Tang, J.; Tolbert, E.; Bayliss, G.; Zhao, T.C.; Yan, H.; Zhuang, S. Blocking the class i histone deacetylase ameliorates renal fibrosis and inhibits renal fibroblast activation via modulating tgf-beta and egfr signaling. PLoS ONE 2013, 8, e54001. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Zhang, R.; Sohrabi, A.; Ehsanipour, A.; Sun, S.; Liang, J.; Walthers, C.M.; Ta, L.; Nathanson, D.A.; Seidlits, S.K. Brain-mimetic 3D culture platforms allow investigation of cooperative effects of extracellular matrix features on therapeutic resistance in glioblastoma. Cancer Res. 2018, 78, 1358–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyai, M.; Tomita, H.; Soeda, A.; Yano, H.; Iwama, T.; Hara, A. Current trends in mouse models of glioblastoma. J. Neuro-Oncol. 2017, 135, 423–432. [Google Scholar] [CrossRef]
- Shu, Q.; Wong, K.-K.; Su, J.M.; Adesina, A.M.; Yu, L.T.; Tsang, Y.T.M.; Antalffy, B.C.; Baxter, P.; Perlaky, L.; Yang, J.; et al. Direct orthotopic transplantation of fresh surgical specimen preserves cd133 + tumor cells in clinically relevant mouse models of medulloblastoma and glioma. Stem Cells 2008, 26, 1414–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bürgi, S.; Seuwen, A.; Keist, R.; vom Berg, J.; Grandjean, J.; Rudin, M. In vivo imaging of hypoxia-inducible factor regulation in a subcutaneous and orthotopic GL261 glioma tumor model using a reporter gene assay. Mol. Imaging 2014, 13, 7290.2014.00029. [Google Scholar] [CrossRef] [PubMed]
- Feldman, L.A.; Fabre, M.-S.; Grasso, C.; Reid, D.; Broaddus, W.C.; Lanza, G.M.; Spiess, B.D.; Garbow, J.R.; McConnell, M.J.; Herst, P.M. Perfluorocarbon emulsions radiosensitise brain tumors in carbogen breathing mice with orthotopic gl261 gliomas. PLoS ONE 2017, 12, e0184250. [Google Scholar] [CrossRef] [Green Version]
- Pluen, A.; Boucher, Y.; Ramanujan, S.; McKee, T.D.; Gohongi, T.; di Tomaso, E.; Brown, E.B.; Izumi, Y.; Campbell, R.B.; Berk, D.A.; et al. Role of tumor–host interactions in interstitial diffusion of macromolecules: Cranial vs. Subcutaneous tumors. Proc. Natl. Acad. Sci. USA 2001, 98, 4628–4633. [Google Scholar] [CrossRef] [Green Version]
- Claes, A.; Schuuring, J.; Boots-Sprenger, S.; Hendriks-Cornelissen, S.; Dekkers, M.; van der Kogel, A.J.; Leenders, W.P.; Wesseling, P.; Jeuken, J.W. Phenotypic and genotypic characterization of orthotopic human glioma models and its relevance for the study of anti-glioma therapy. Brain Pathol. 2008, 18, 423–433. [Google Scholar] [CrossRef]
- Dong, Y.; Zheng, Q.; Wang, Z.; Lin, X.; You, Y.; Wu, S.; Wang, Y.; Hu, C.; Xie, X.; Chen, J.; et al. Higher matrix stiffness as an independent initiator triggers epithelial-mesenchymal transition and facilitates hcc metastasis. J. Hematol. Oncol. 2019, 12, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, G.J. Applications of patient-derived tumor xenograft models and tumor organoids. J. Hematol. Oncol. 2020, 13, 1–16. [Google Scholar] [CrossRef]
- Xiao, W.; Sohrabi, A.; Seidlits, S.K. Integrating the glioblastoma microenvironment into engineered experimental models. Future Sci. OA 2017, 3, FSO189. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Oliva, R.; Domínguez-García, S.; Carrascal, L.; Abalos-Martínez, J.; Pardillo-Díaz, R.; Verástegui, C.; Castro, C.; Nunez-Abades, P.; Geribaldi-Doldán, N. Evolution of experimental models in the study of glioblastoma: Toward finding efficient treatments. Front. Oncol. 2021, 10, 3245. [Google Scholar] [CrossRef]
- Heldin, C.-H.; Rubin, K.; Pietras, K.; Östman, A. High interstitial fluid pressure—An obstacle in cancer therapy. Nat. Rev. Cancer 2004, 4, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Grantab, R.H.; Tannock, I.F. Penetration of anticancer drugs through tumour tissue as a function of cellular packing density and interstitial fluid pressure and its modification by bortezomib. BMC Cancer 2012, 12, 214. [Google Scholar] [CrossRef] [Green Version]
- Oh, E.; Hong, J.; Kwon, O.-J.; Yun, C.-O. A hypoxia-and telomerase-responsive oncolytic adenovirus expressing secretable trimeric trail triggers tumour-specific apoptosis and promotes viral dispersion in trail-resistant glioblastoma. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, P.-H.; Yoo, J.Y.; Yoon, A.-R.; Choi, H.J.; Seong, J.; Kim, I.-W.; Kim, J.H.; Yun, C.-O. Double E1b 19 kDa- and E1b 55 kDa-deleted oncolytic adenovirus in combination with radiotherapy elicits an enhanced anti-tumor effect. Gene Ther. 2009, 16, 1111–1121. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, H.-G.; Choi, Y.-H.; Hong, J.; Choi, J.-W.; Yoon, A.-R.; Yun, C.-O. GM101 in Combination with Histone Deacetylase Inhibitor Enhances Anti-Tumor Effects in Desmoplastic Microenvironment. Cells 2021, 10, 2811. https://doi.org/10.3390/cells10112811
Chang H-G, Choi Y-H, Hong J, Choi J-W, Yoon A-R, Yun C-O. GM101 in Combination with Histone Deacetylase Inhibitor Enhances Anti-Tumor Effects in Desmoplastic Microenvironment. Cells. 2021; 10(11):2811. https://doi.org/10.3390/cells10112811
Chicago/Turabian StyleChang, Han-Gyu, Yong-Hyeon Choi, JinWoo Hong, Joung-Woo Choi, A-Rum Yoon, and Chae-Ok Yun. 2021. "GM101 in Combination with Histone Deacetylase Inhibitor Enhances Anti-Tumor Effects in Desmoplastic Microenvironment" Cells 10, no. 11: 2811. https://doi.org/10.3390/cells10112811
APA StyleChang, H.-G., Choi, Y.-H., Hong, J., Choi, J.-W., Yoon, A.-R., & Yun, C.-O. (2021). GM101 in Combination with Histone Deacetylase Inhibitor Enhances Anti-Tumor Effects in Desmoplastic Microenvironment. Cells, 10(11), 2811. https://doi.org/10.3390/cells10112811







