Modeling Hypoxic Stress In Vitro Using Human Embryonic Stem Cells Derived Cardiomyocytes Matured by FGF4 and Ascorbic Acid Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture and Cardiac Differentiation of hESCs
2.2. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.3. Immunofluorescence Staining
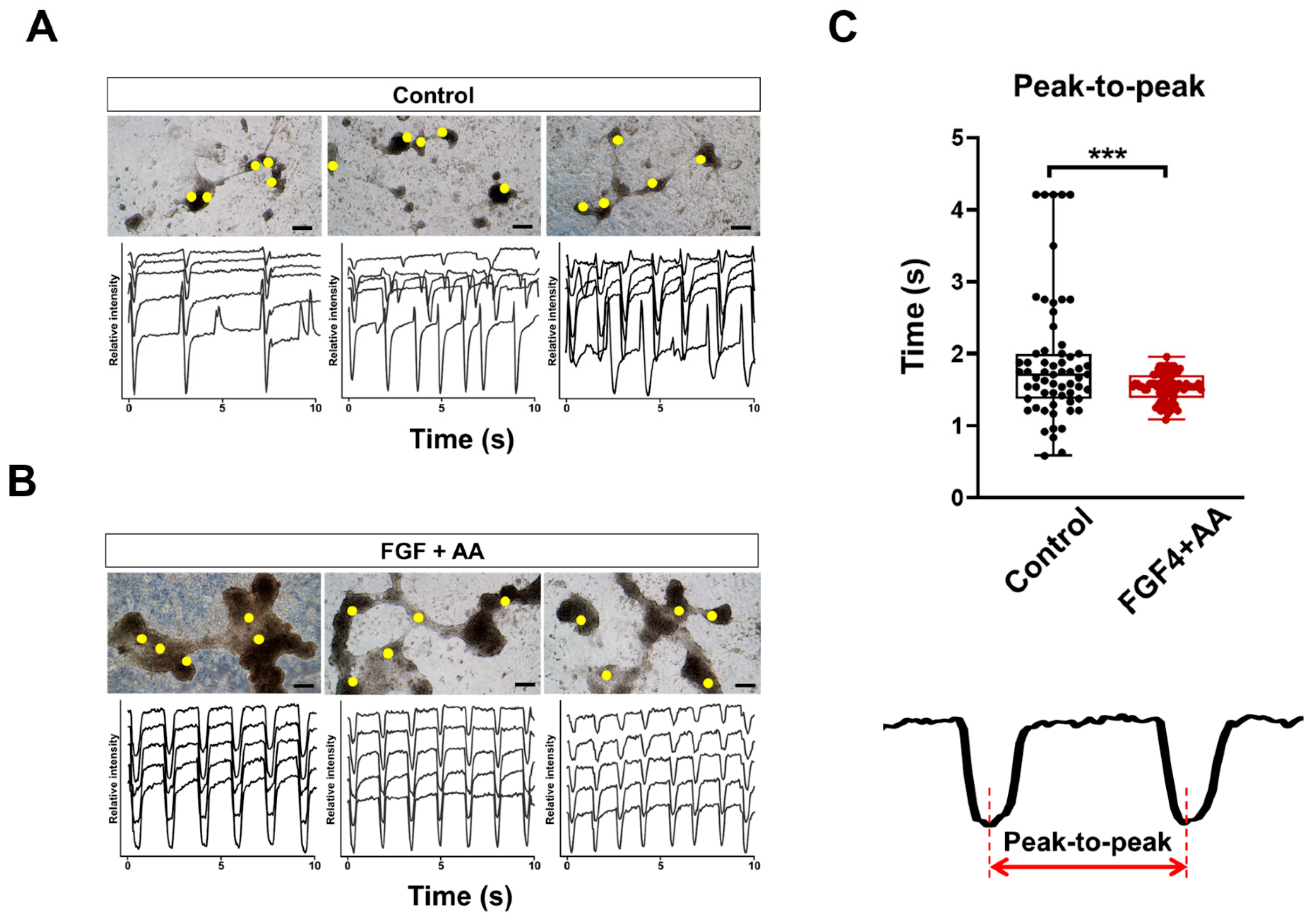
2.4. Beating Analysis Using Captured Movies
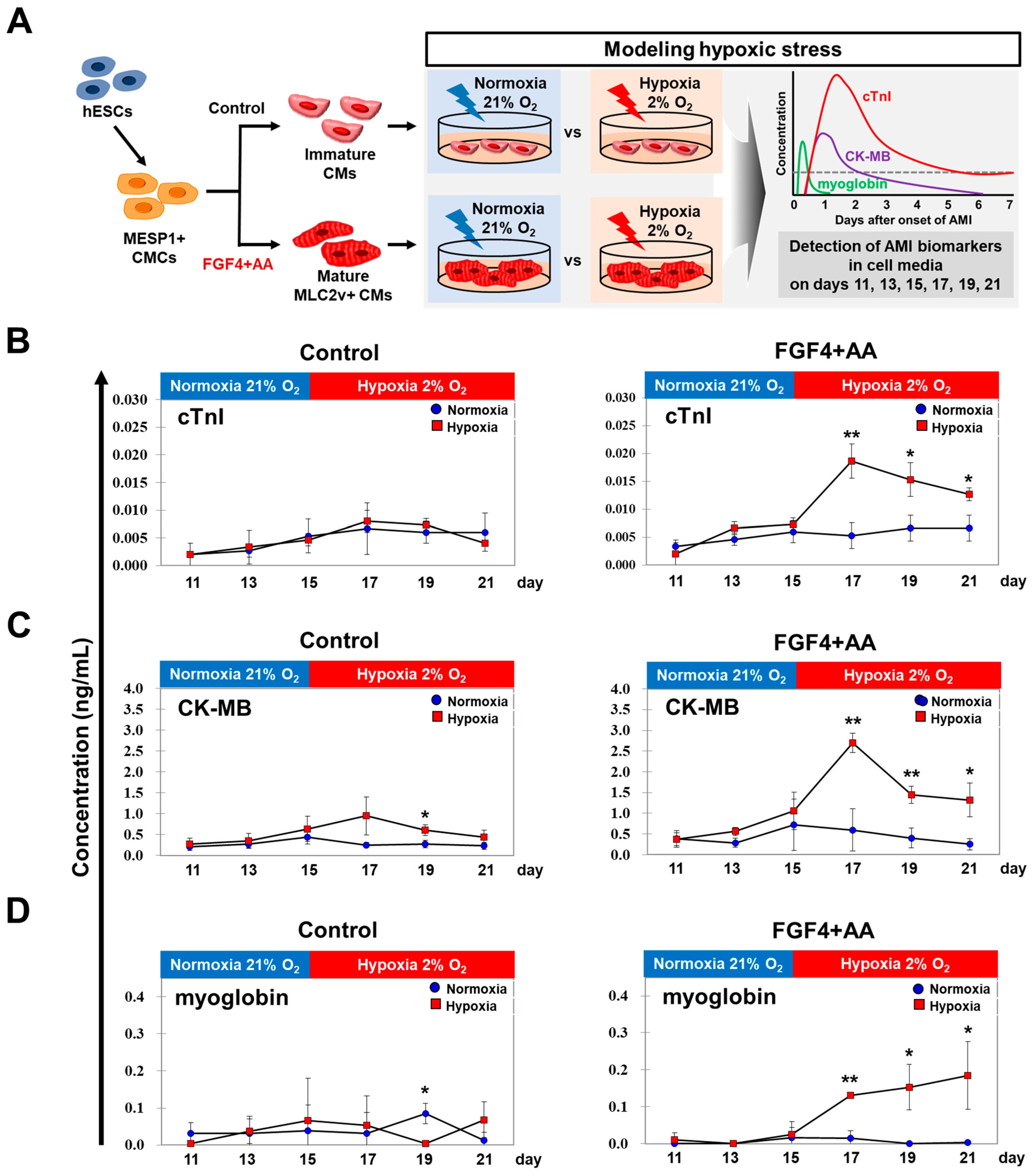
2.5. AMI Biomarker Analysis Using In Vitro Hypoxic Model
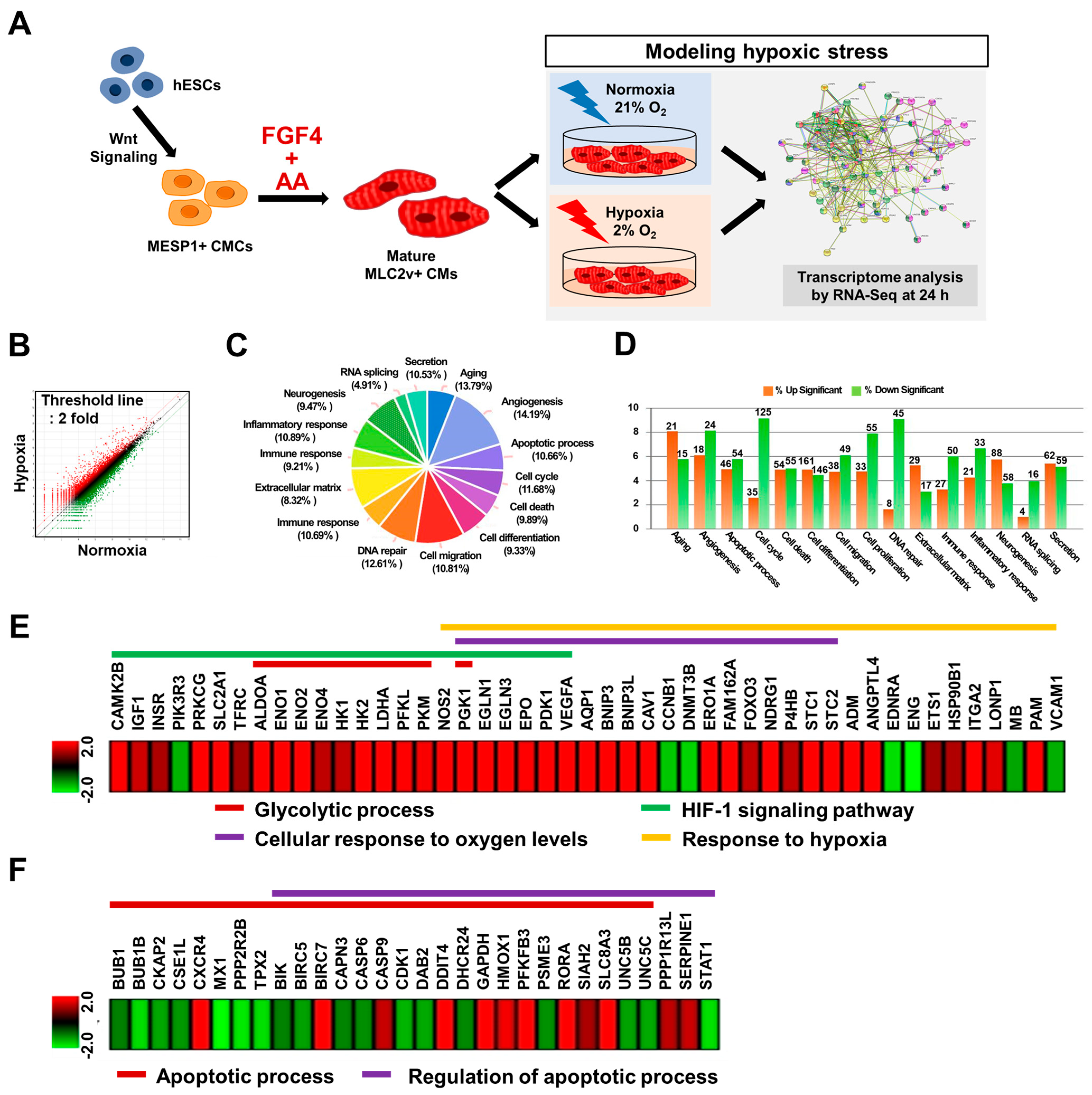
2.6. RNA Sequencing (RNA-Seq) Analyses
2.7. Statistical Analysis
3. Results
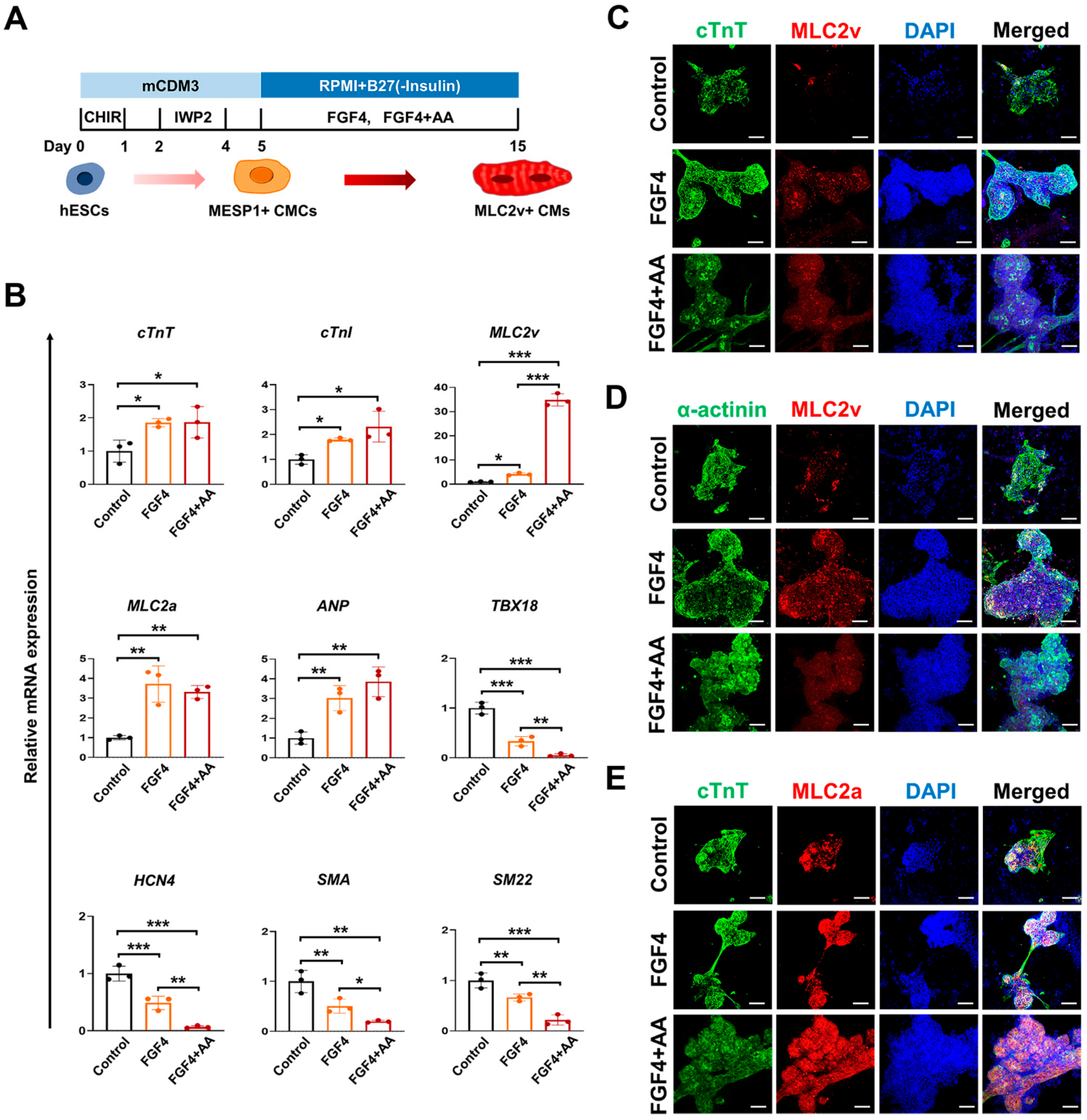
3.1. FGF4 and AA Induce Differentiation of BG01 hESC-CMCs into Mature Ventricular CMs
3.2. Co-Treatment of FGF4+AA Synergistically Induces Differentiation of Immature BG01 hESC-CMCs into Mature Ventricular CMs
3.3. AMI Biomarkers Are Released into the Culture Medium of FGF4+AA-Treated BG01 hESC-CMs in Response to Hypoxic Injury
3.4. Levels of AMI Biomarkers Released into Culture Medium Are Correlated with Sequential Changes in the Contractile Properties of Hypoxia-Exposed BG01 hESC-CMs
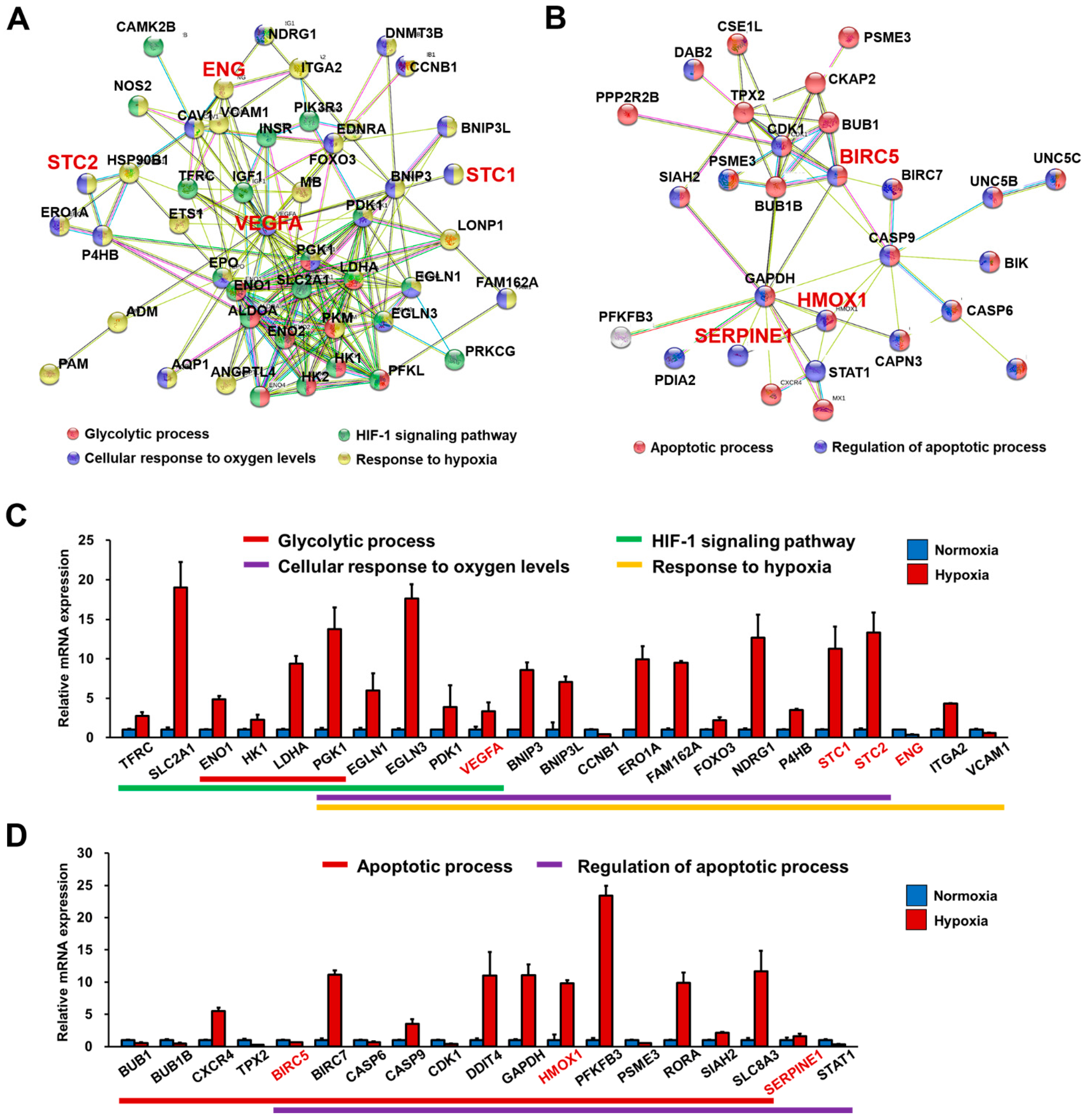
3.5. RNA-Seq Analysis Demonstrated That FGF4+AA-Treated BG01 hESC-CMs Are a Suitable In Vitro Hypoxic Stress Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, X.; Pabon, L.; Murry, C.E. Engineering adolescence: Maturation of human pluripotent stem cell-derived cardiomyocytes. Circ. Res. 2014, 114, 511–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.K.; Ng, K.M.; Chan, Y.C.; Lai, W.H.; Au, K.W.; Ho, C.Y.; Wong, L.Y.; Lau, C.P.; Tse, H.F.; Siu, C.W. Triiodothyronine promotes cardiac differentiation and maturation of embryonic stem cells via the classical genomic pathway. Mol. Endocrinol. 2010, 24, 1728–1736. [Google Scholar] [CrossRef] [Green Version]
- Chattergoon, N.N.; Giraud, G.D.; Louey, S.; Stork, P.; Fowden, A.L.; Thornburg, K.L. Thyroid hormone drives fetal cardiomyocyte maturation. FASEB J. 2012, 26, 397–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Rodriguez, M.; Pabon, L.; Fischer, K.A.; Reinecke, H.; Regnier, M.; Sniadecki, N.J.; Ruohola-Baker, H.; Murry, C.E. Tri-iodo-l-thyronine promotes the maturation of human cardiomyocytes-derived from induced pluripotent stem cells. J. Mol. Cell. Cardiol. 2014, 72, 296–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rog-Zielinska, E.A.; Craig, M.A.; Manning, J.R.; Richardson, R.V.; Gowans, G.J.; Dunbar, D.R.; Gharbi, K.; Kenyon, C.J.; Holmes, M.C.; Hardie, D.G.; et al. Glucocorticoids promote structural and functional maturation of foetal cardiomyocytes: A role for PGC-1alpha. Cell Death Differ. 2015, 22, 1106–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parikh, S.S.; Blackwell, D.J.; Gomez-Hurtado, N.; Frisk, M.; Wang, L.; Kim, K.; Dahl, C.P.; Fiane, A.; Tonnessen, T.; Kryshtal, D.O.; et al. Thyroid and Glucocorticoid Hormones Promote Functional T-Tubule Development in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Circ. Res. 2017, 121, 1323–1330. [Google Scholar] [CrossRef]
- Cao, N.; Liu, Z.; Chen, Z.; Wang, J.; Chen, T.; Zhao, X.; Ma, Y.; Qin, L.; Kang, J.; Wei, B.; et al. Ascorbic acid enhances the cardiac differentiation of induced pluripotent stem cells through promoting the proliferation of cardiac progenitor cells. Cell Res. 2012, 22, 219–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karakikes, I.; Senyei, G.D.; Hansen, J.; Kong, C.W.; Azeloglu, E.U.; Stillitano, F.; Lieu, D.K.; Wang, J.; Ren, L.; Hulot, J.S.; et al. Small molecule-mediated directed differentiation of human embryonic stem cells toward ventricular cardiomyocytes. Stem Cells Transl. Med. 2014, 3, 18–31. [Google Scholar] [CrossRef]
- Iglesias-Garcia, O.; Baumgartner, S.; Macri-Pellizzeri, L.; Rodriguez-Madoz, J.R.; Abizanda, G.; Guruceaga, E.; Albiasu, E.; Corbacho, D.; Benavides-Vallve, C.; Soriano-Navarro, M.; et al. Neuregulin-1beta induces mature ventricular cardiac differentiation from induced pluripotent stem cells contributing to cardiac tissue repair. Stem Cells Dev. 2015, 24, 484–496. [Google Scholar] [CrossRef] [Green Version]
- Itoh, N.; Ohta, H.; Nakayama, Y.; Konishi, M. Roles of FGF Signals in Heart Development, Health, and Disease. Front. Cell Dev. Biol. 2016, 4, 110. [Google Scholar] [CrossRef] [Green Version]
- Rosenblatt-Velin, N.; Lepore, M.G.; Cartoni, C.; Beermann, F.; Pedrazzini, T. FGF-2 controls the differentiation of resident cardiac precursors into functional cardiomyocytes. J. Clin. Investig. 2005, 115, 1724–1733. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Sanchez, C.; Climent, V.; Schoenwolf, G.C.; Alvarez, I.S.; Garcia-Martinez, V. Induction of cardiogenesis by Hensen’s node and fibroblast growth factors. Cell Tissue Res. 2002, 309, 237–249. [Google Scholar] [CrossRef]
- Pradhan, A.; Zeng, X.I.; Sidhwani, P.; Marques, S.R.; George, V.; Targoff, K.L.; Chi, N.C.; Yelon, D. FGF signaling enforces cardiac chamber identity in the developing ventricle. Development 2017, 144, 1328–1338. [Google Scholar] [CrossRef] [Green Version]
- Apple, F.S.; Smith, S.W.; Pearce, L.A.; Murakami, M.M. Assessment of the multiple-biomarker approach for diagnosis of myocardial infarction in patients presenting with symptoms suggestive of acute coronary syndrome. Clin. Chem. 2009, 55, 93–100. [Google Scholar] [CrossRef]
- Jaffe, A.S.; Apple, F.S. The third Universal Definition of Myocardial Infarction—Moving forward. Clin. Chem. 2012, 58, 1727–1728. [Google Scholar] [CrossRef] [Green Version]
- Christenson, R.H.; Mullins, K.; Duh, S.H. Validation of high-sensitivity performance for a United States Food and Drug Administration cleared cardiac troponin I assay. Clin. Biochem. 2018, 56, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Mythili, S.; Malathi, N. Diagnostic markers of acute myocardial infarction. Biomed. Rep. 2015, 3, 743–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, Y.; Fujiwara, H.; Takatsu, Y. Cardiac troponin and heart failure in the era of high-sensitivity assays. J. Cardiol. 2012, 60, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Lewandrowski, K.; Chen, A.; Januzzi, J. Cardiac markers for myocardial infarction. A brief review. Am. J. Clin. Pathol. 2002, 118 (Suppl.), S93–S99. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Seo, S.M.; Cho, H.M.; Hong, S.J.; Lim, D.S.; Paek, S.H. Continuous immunosensing of myoglobin in human serum as potential companion diagnostics technique. Biosens. Bioelectron. 2014, 62, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Paek, S.H.; Lim, G.S.; Jeon, J.W.; Paek, S.H. Performance characteristics of monoclonal antibodies as recyclable binders to cardiac troponin I. Anal. Biochem. 2012, 431, 11–18. [Google Scholar] [CrossRef]
- Zhao, K.; Tang, M.; Wang, H.; Zhou, Z.; Wu, Y.; Liu, S. Simultaneous detection of three biomarkers related to acute myocardial infarction based on immunosensing biochip. Biosens. Bioelectron. 2019, 126, 767–772. [Google Scholar] [CrossRef]
- Howe, E.A.; Sinha, R.; Schlauch, D.; Quackenbush, J. RNA-Seq analysis in MeV. Bioinformatics 2011, 27, 3209–3210. [Google Scholar] [CrossRef] [Green Version]
- Eiselleova, L.; Matulka, K.; Kriz, V.; Kunova, M.; Schmidtova, Z.; Neradil, J.; Tichy, B.; Dvorakova, D.; Pospisilova, S.; Hampl, A.; et al. A complex role for FGF-2 in self-renewal, survival, and adhesion of human embryonic stem cells. Stem Cells 2009, 27, 1847–1857. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Gulbranson, D.R.; Yu, P.; Hou, Z.; Thomson, J.A. Thermal stability of fibroblast growth factor protein is a determinant factor in regulating self-renewal, differentiation, and reprogramming in human pluripotent stem cells. Stem Cells 2012, 30, 623–630. [Google Scholar] [CrossRef] [Green Version]
- Peacock, W.F.; Diercks, D.; Birkhahn, R.; Singer, A.J.; Hollander, J.E.; Nowak, R.; Safdar, B.; Miller, C.D.; Peberdy, M.; Counselman, F.; et al. Can a Point-of-Care Troponin I Assay be as Good as a Central Laboratory Assay? A MIDAS Investigation. Ann. Lab. Med. 2016, 36, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, N.A.; Neumann, J.T.; Ojeda, F.; Giannitsis, E.; Spanuth, E.; Blankenberg, S.; Westermann, D.; Zeller, T. Diagnostic Evaluation of a High-Sensitivity Troponin I Point-of-Care Assay. Clin. Chem. 2019, 65, 1592–1601. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Kim, M.H.; Mok, R.S.; Jeon, J.W.; Lim, G.S.; Chai, C.Y.; Paek, S.H. Two-dimensional paper chromatography-based fluorescent immunosensor for detecting acute myocardial infarction markers. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 967, 139. [Google Scholar] [CrossRef] [PubMed]
- Markovic, D.; Jevtovic-Stoimenov, T.; Cosic, V.; Stosic, B.; Dinic, V.; Markovic-Zivkovic, B.; Jankovic, R.J. Clinical Utility of Survivin (BIRC5), Novel Cardiac Biomarker, as a Prognostic Tool Compared to High-sensitivity C-reactive Protein, Heart-type Fatty Acid Binding Protein and Revised Lee Score in Elderly Patients Scheduled for Major Non-cardiac Surgery: A Prospective Pilot Study. J. Med. Biochem. 2018, 37, 110–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markovic, D.Z.; Jevtovic-Stoimenov, T.; Stojanovic, M.; Vukovic, A.Z.; Dinic, V.; Markovic-Zivkovic, B.Z.; Jankovic, R.J. Cardiac biomarkers improve prediction performance of the combination of American Society of Anesthesiologists physical status classification and Americal College of Surgeons National Surgical Quality Improvement Program calculator for postoperative mortality in elderly patients: A pilot study. Aging Clin. Exp. Res. 2019, 31, 1207–1217. [Google Scholar] [CrossRef]
- Cruz-Gonzalez, I.; Pabon, P.; Rodriguez-Barbero, A.; Martin-Moreiras, J.; Pericacho, M.; Sanchez, P.L.; Ramirez, V.; Sanchez-Ledesma, M.; Martin-Herrero, F.; Jimenez-Candil, J.; et al. Identification of serum endoglin as a novel prognostic marker after acute myocardial infarction. J. Cell. Mol. Med. 2008, 12, 955–961. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.M.; Li, Y.G.; Wang, D.M. Study on changes of heme oxygenase-1 expression in patients with coronary heart disease. Clin. Cardiol. 2005, 28, 197–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soeki, T.; Tamura, Y.; Shinohara, H.; Tanaka, H.; Bando, K.; Fukuda, N. Serial changes in serum VEGF and HGF in patients with acute myocardial infarction. Cardiology 2000, 93, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Hwang, I.; Park, C.S.; Lee, H.; Park, D.W.; Kang, S.J.; Lee, S.W.; Kim, Y.H.; Park, S.W.; Park, S.J. Expression of stanniocalcin-1 in culprit coronary plaques of patients with acute myocardial infarction or stable angina. J. Clin. Pathol. 2013, 66, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Cediel, G.; Rueda, F.; Oxvig, C.; Oliveras, T.; Labata, C.; de Diego, O.; Ferrer, M.; Aranda-Nevado, M.C.; Serra-Gregori, J.; Nunez, J.; et al. Prognostic value of the Stanniocalcin-2/PAPP-A/IGFBP-4 axis in ST-segment elevation myocardial infarction. Cardiovasc. Diabetol. 2018, 17, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, R.G.; Simard, T.; Di Santo, P.; Labinaz, A.; Moreland, R.; Duchez, A.C.; Majeed, K.; Motazedian, P.; Rochman, R.; Jung, Y.; et al. Performance of plasminogen activator inhibitor-1 as a biomarker in patients undergoing coronary angiography: Analytical and biological considerations. Diab. Vasc. Dis. Res. 2019, 16, 478–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.-C.; Seo, H.-R.; Cui, L.-H.; Song, M.-H.; Noh, J.-M.; Kim, K.-S.; Choi, J.-H.; Kim, J.-H.; Park, C.-Y.; Joo, H.J.; et al. Modeling Hypoxic Stress In Vitro Using Human Embryonic Stem Cells Derived Cardiomyocytes Matured by FGF4 and Ascorbic Acid Treatment. Cells 2021, 10, 2741. https://doi.org/10.3390/cells10102741
Choi S-C, Seo H-R, Cui L-H, Song M-H, Noh J-M, Kim K-S, Choi J-H, Kim J-H, Park C-Y, Joo HJ, et al. Modeling Hypoxic Stress In Vitro Using Human Embryonic Stem Cells Derived Cardiomyocytes Matured by FGF4 and Ascorbic Acid Treatment. Cells. 2021; 10(10):2741. https://doi.org/10.3390/cells10102741
Chicago/Turabian StyleChoi, Seung-Cheol, Ha-Rim Seo, Long-Hui Cui, Myeong-Hwa Song, Ji-Min Noh, Kyung-Seob Kim, Ji-Hyun Choi, Jong-Ho Kim, Chi-Yeon Park, Hyung Joon Joo, and et al. 2021. "Modeling Hypoxic Stress In Vitro Using Human Embryonic Stem Cells Derived Cardiomyocytes Matured by FGF4 and Ascorbic Acid Treatment" Cells 10, no. 10: 2741. https://doi.org/10.3390/cells10102741
APA StyleChoi, S.-C., Seo, H.-R., Cui, L.-H., Song, M.-H., Noh, J.-M., Kim, K.-S., Choi, J.-H., Kim, J.-H., Park, C.-Y., Joo, H. J., Hong, S. J., Ko, T. H., Choi, J.-I., Kim, H. J., Kim, J.-H., Paek, S.-H., Park, J.-N., Kim, D.-H., Jang, Y., ... Lim, D.-S. (2021). Modeling Hypoxic Stress In Vitro Using Human Embryonic Stem Cells Derived Cardiomyocytes Matured by FGF4 and Ascorbic Acid Treatment. Cells, 10(10), 2741. https://doi.org/10.3390/cells10102741







