BMP-7 Attenuates Inflammation-Induced Pyroptosis and Improves Cardiac Repair in Diabetic Cardiomyopathy
Abstract
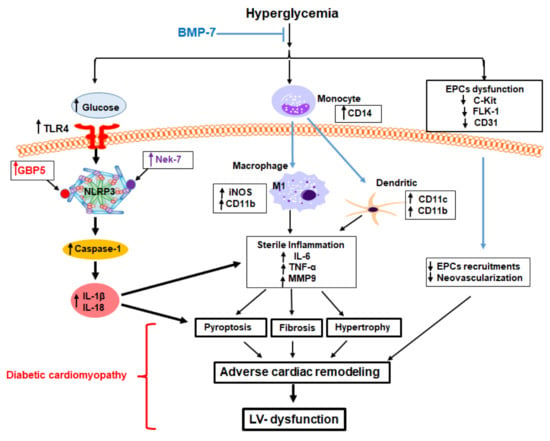
1. Introduction
2. Materials and Methods
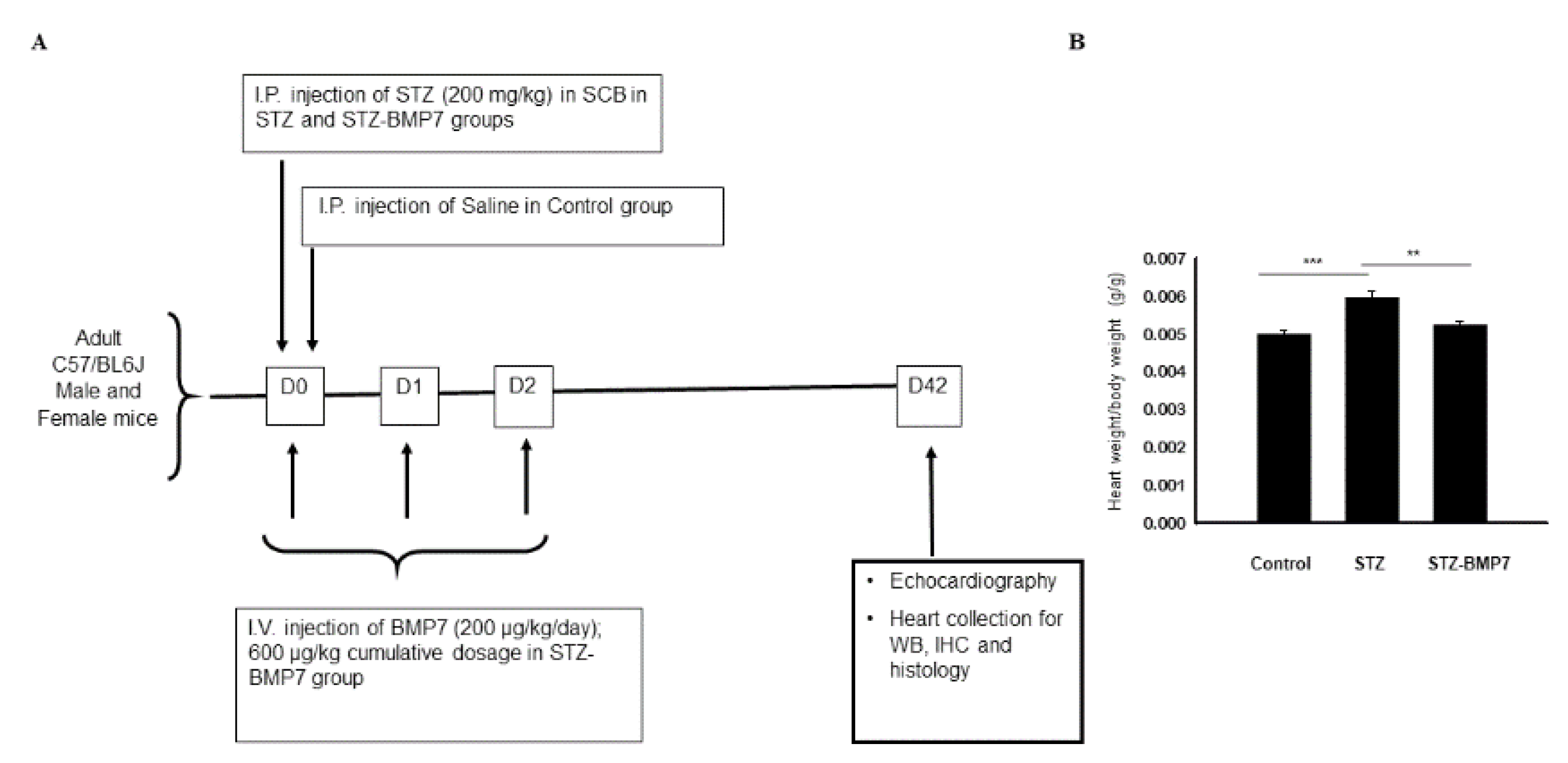
2.1. STZ-Induced Diabetes Mellitus in Mice
2.2. Immunohistochemistry (IHC) Staining
2.3. Western Blot Assay
2.4. Hematoxylin and Eosin (H&E) Staining
2.5. Masson’s Trichrome Staining
2.6. Determination of Heart Function
2.7. Statistical Analysis
3. Results
3.1. Effect of BMP-7 on Heart Weight in Diabetic Cardiomyopathy
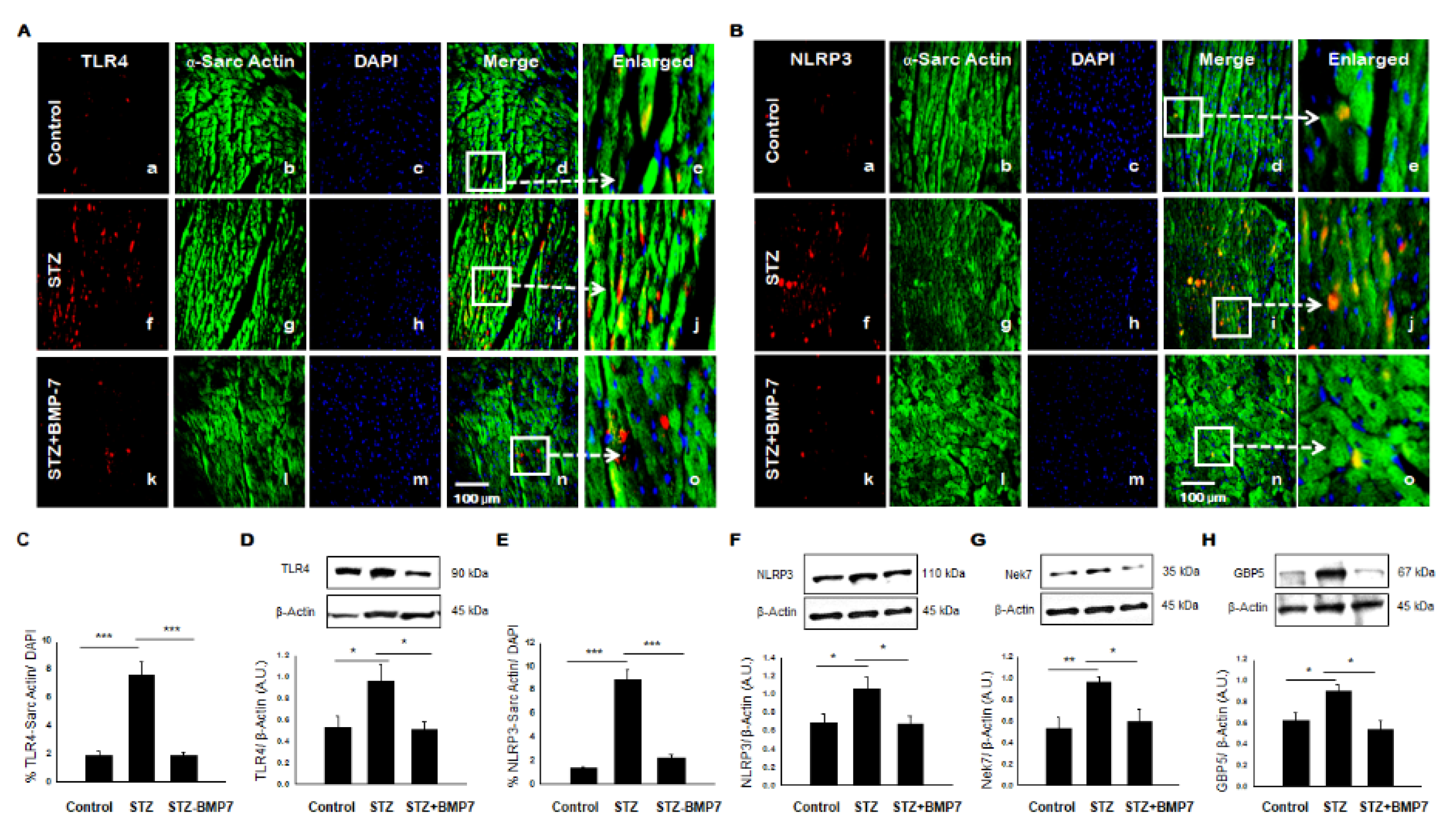
3.2. BMP-7 Inhibits TLR4-NLRP3 Inflammasome Formation in Diabetic Hearts
3.3. BMP-7 Inhibits Inflammasome Formation Protein Activators in Diabetic Hearts
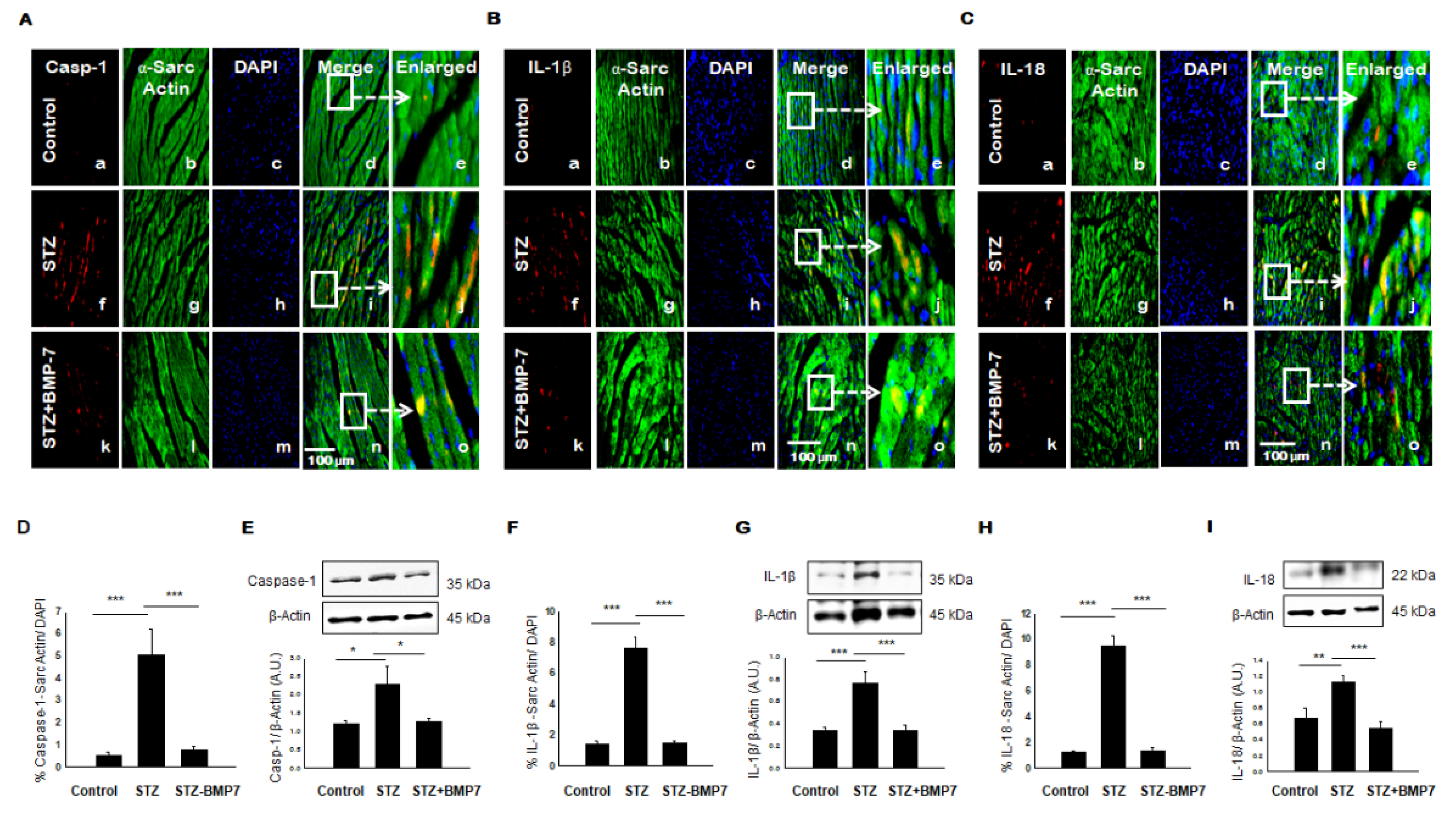
3.4. BMP-7 Inhibits Pyroptotic Protein Caspase-1
3.5. BMP-7 Inhibits Pyroptotic Proteins IL-1β and IL-18
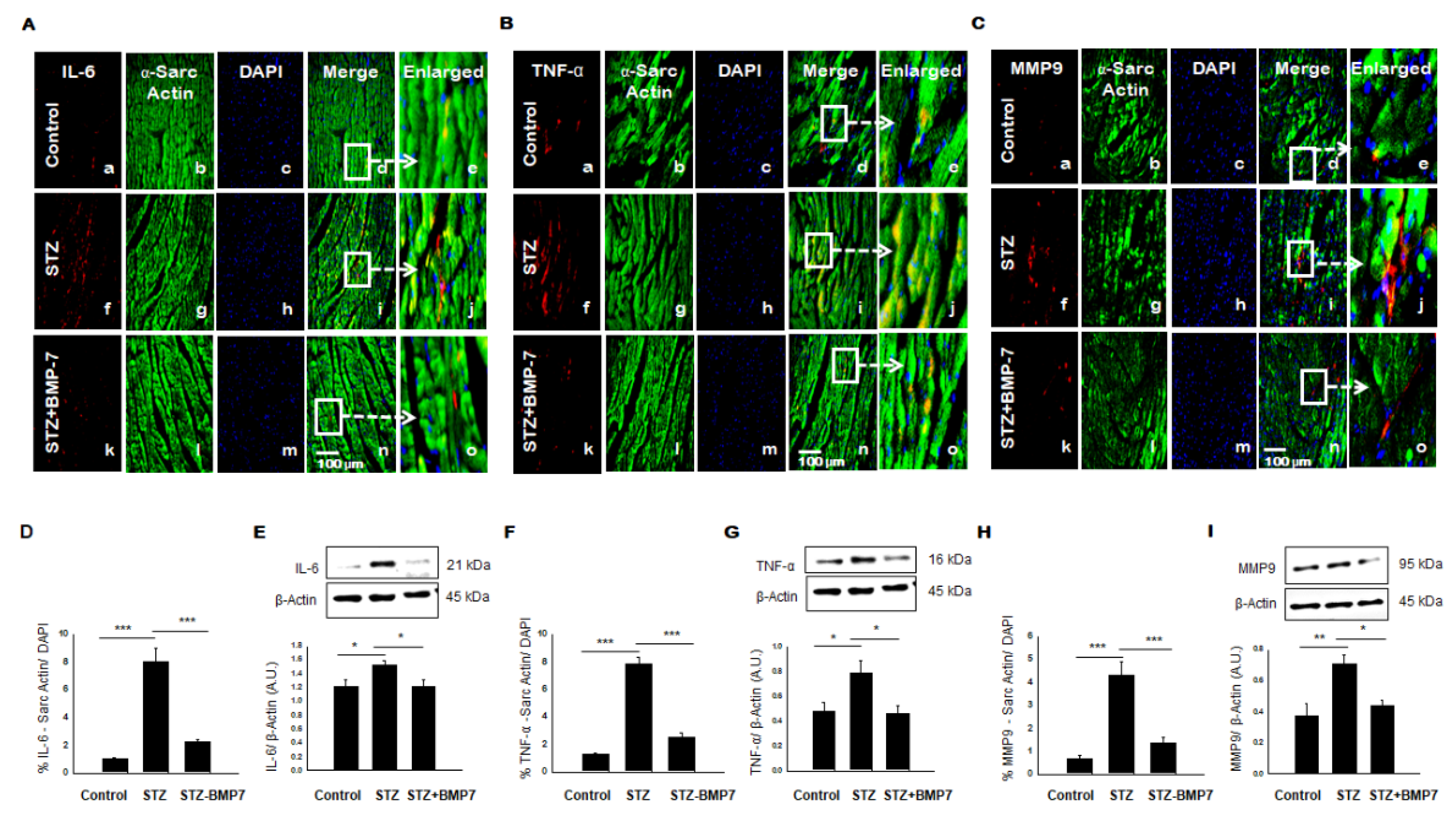
3.6. BMP-7 Inhibits Pro-Inflammatory Cytokine IL-6 and TNF-α level in Diabetic Hearts
3.7. BMP-7 Inhibits MMP9 Level in Diabetic Hearts
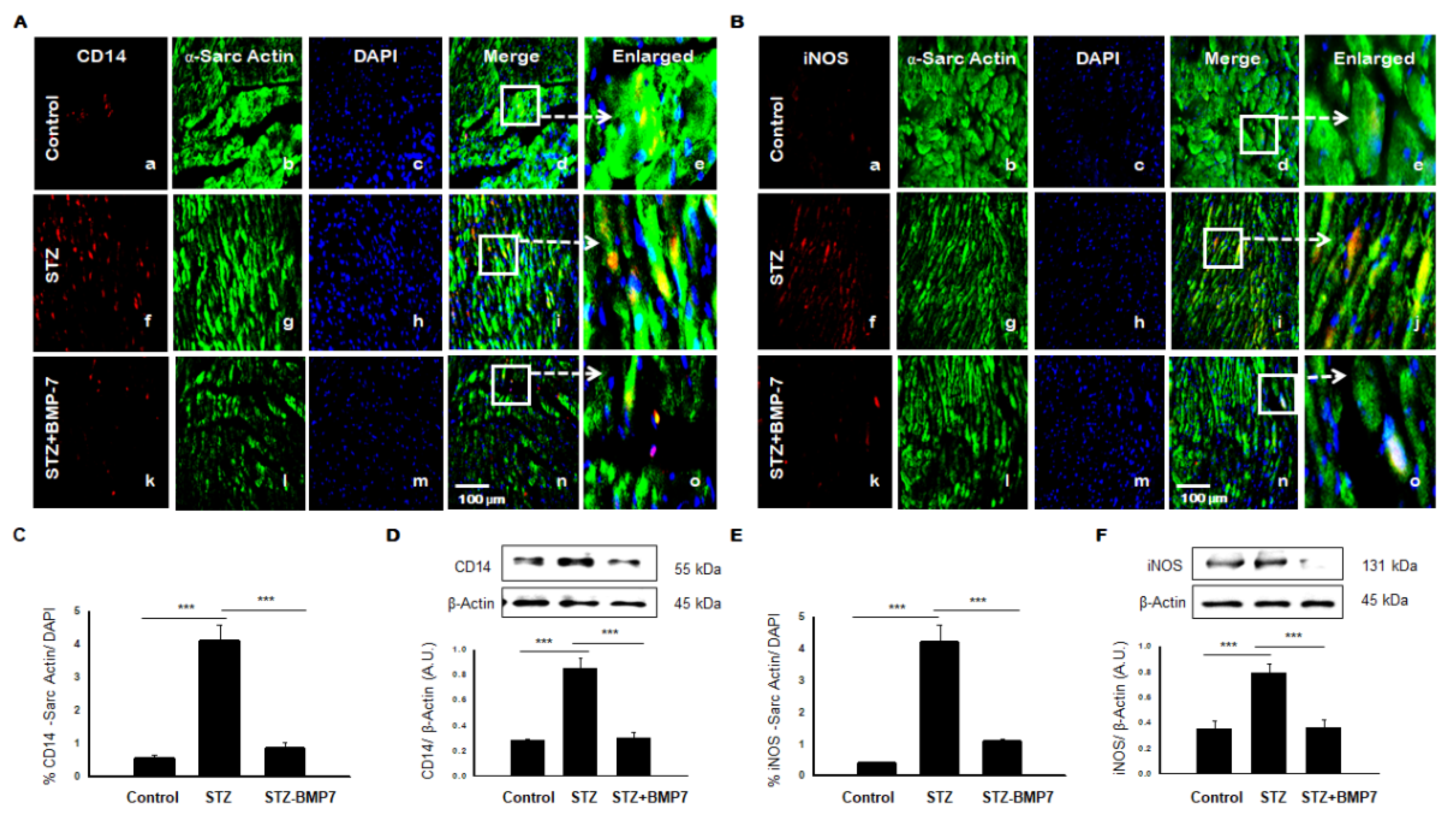
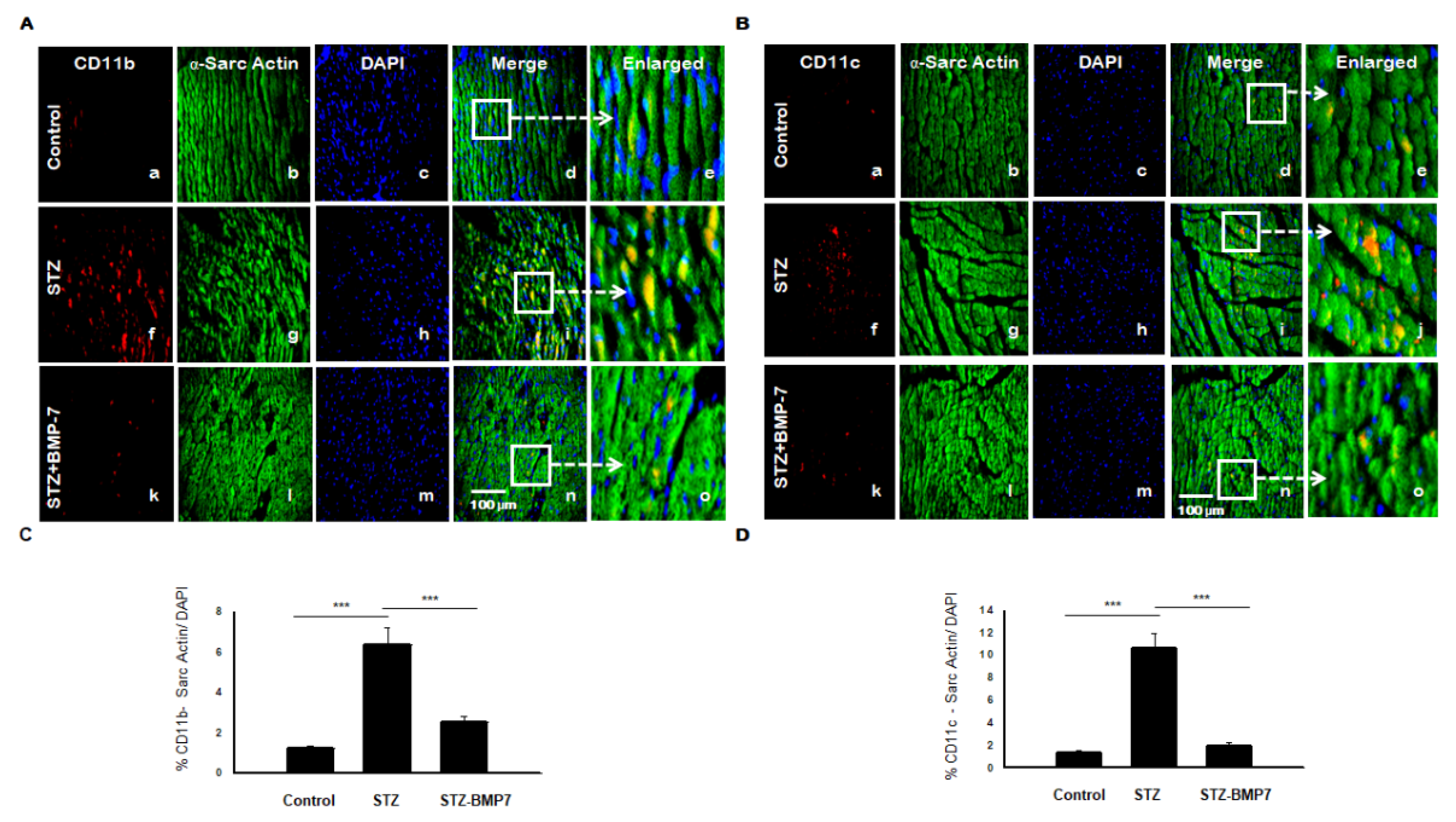
3.8. BMP-7 Reduces Monocytes/Macrophages +ve Markers in Diabetic Hearts
3.9. BMP-7 Reduces Dendritic Cells (DCs) +ve Markers in Diabetic Hearts
3.10. BMP-7 Enhances the Recruitment of EPCs and Induces Neovascularization in Diabetic Hearts
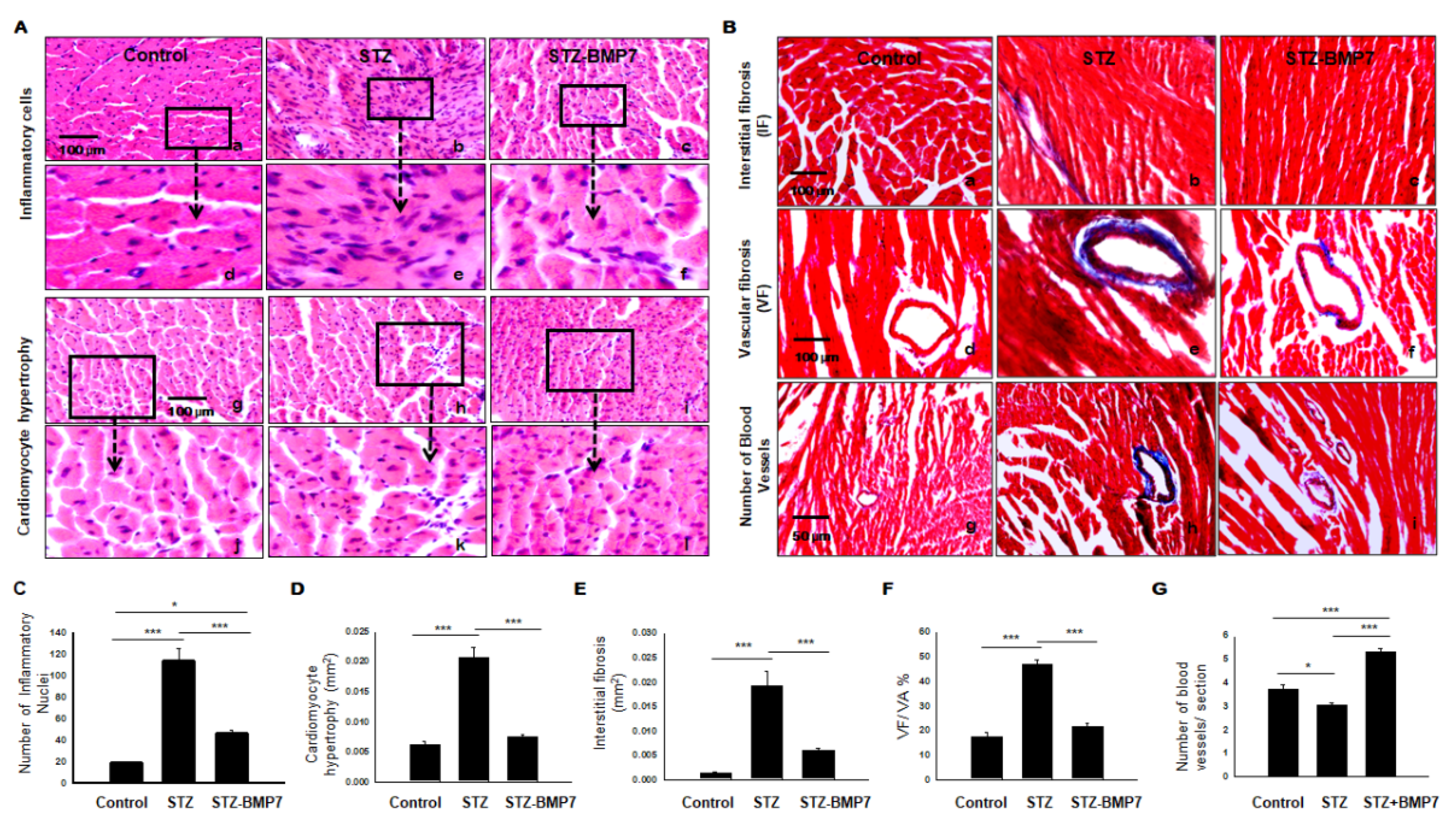
3.11. BMP-7 Decreases Inflammatory Cells and Hypertrophy in Diabetic Hearts
3.12. BMP-7 Decreases Interstitial and Vascular Fibrosis in Diabetic Hearts
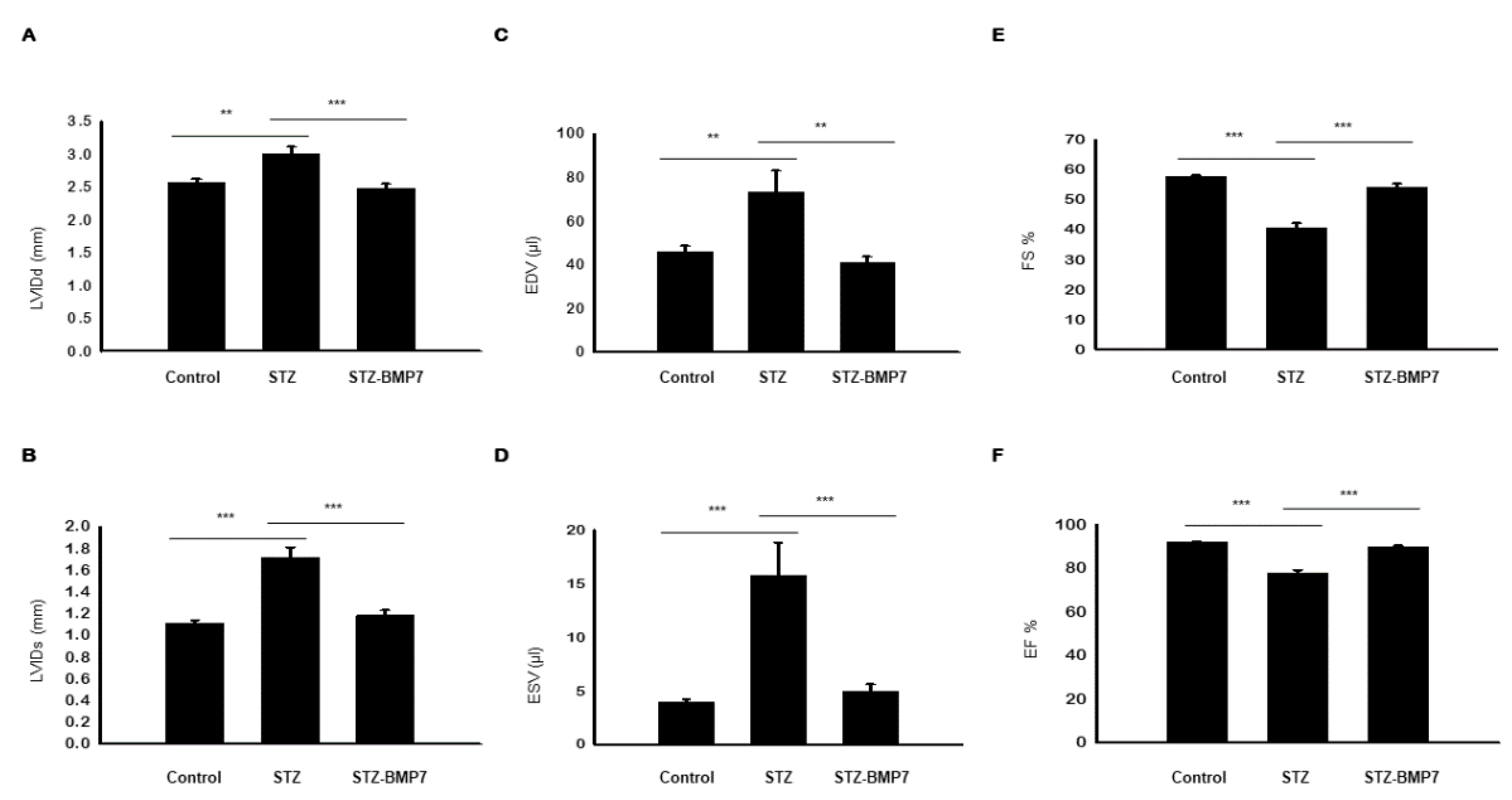
3.13. BMP-7 Improves LV Heart Function in Diabetic Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gulsin, G.S.; Athithan, L.; McCann, G.P. Diabetic cardiomyopathy: Prevalence, determinants and potential treatments. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819834869. [Google Scholar] [CrossRef]
- Filardi, T.; Ghinassi, B.; Di Baldassarre, A.; Tanzilli, G.; Morano, S.; Lenzi, A.; Basili, S.; Crescioli, C. Cardiomyopathy Associated with Diabetes: The Central Role of the Cardiomyocyte. Int. J. Mol. Sci. 2019, 20, 3299. [Google Scholar] [CrossRef]
- Huynh, K.; Bernardo, B.C.; McMullen, J.R.; Ritchie, R.H. Diabetic cardiomyopathy: Mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacol. Ther. 2014, 142, 375–415. [Google Scholar] [CrossRef]
- Urbina, P.; Singla, D.K. BMP-7 attenuates adverse cardiac remodeling mediated through M2 macrophages in prediabetic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H762–H772. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Qin, Y.; Lv, J.; Wang, Y.; Che, H.; Chen, X.; Jiang, Y.; Li, A.; Sun, X.; Yue, E.; et al. Silencing long non-coding RNA Kcnq1ot1 alleviates pyroptosis and fibrosis in diabetic cardiomyopathy. Cell Death Dis. 2018, 9, 1000. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Qin, Y.; Wang, Y.; Li, A.; Lv, J.; Sun, X.; Che, H.; Han, T.; Meng, S.; Bai, Y.; et al. LncRNA KCNQ1OT1 Mediates Pyroptosis in Diabetic Cardiomyopathy. Cell Physiol. Biochem. 2018, 50, 1230–1244. [Google Scholar] [CrossRef] [PubMed]
- Aluganti Narasimhulu, C.; Singla, D.K. Amelioration of diabetes-induced inflammation mediated pyroptosis, sarcopenia, and adverse muscle remodelling by bone morphogenetic protein-7. J. Cachexia Sarcopenia Muscle 2021, 12, 403–420. [Google Scholar] [CrossRef]
- Dessouki, F.B.A.; Kukreja, R.C.; Singla, D.K. Stem Cell-Derived Exosomes Ameliorate Doxorubicin-Induced Muscle Toxicity through Counteracting Pyroptosis. Pharmaceuticals 2020, 13, 450. [Google Scholar] [CrossRef] [PubMed]
- Singla, D.K.; Johnson, T.A.; Tavakoli Dargani, Z. Exosome Treatment Enhances Anti-Inflammatory M2 Macrophages and Reduces Inflammation-Induced Pyroptosis in Doxorubicin-Induced Cardiomyopathy. Cells 2019, 8, 1224. [Google Scholar] [CrossRef]
- Murao, A.; Aziz, M.; Wang, H.; Brenner, M.; Wang, P. Release mechanisms of major DAMPs. Apoptosis 2021, 26, 152–162. [Google Scholar] [CrossRef]
- Luo, B.; Huang, F.; Liu, Y.; Liang, Y.; Wei, Z.; Ke, H.; Zeng, Z.; Huang, W.; He, Y. NLRP3 Inflammasome as a Molecular Marker in Diabetic Cardiomyopathy. Front. Physiol. 2017, 8, 519. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lu, L.; Li, L. NEK7: A novel promising therapy target for NLRP3-related inflammatory diseases. Acta Biochim. Biophys. Sin. 2016, 48, 966–968. [Google Scholar] [CrossRef] [PubMed]
- Zhaolin, Z.; Guohua, L.; Shiyuan, W.; Zuo, W. Role of pyroptosis in cardiovascular disease. Cell Prolif. 2019, 52, e12563. [Google Scholar] [CrossRef]
- Shenoy, A.R.; Wellington, D.A.; Kumar, P.; Kassa, H.; Booth, C.J.; Cresswell, P.; MacMicking, J.D. GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science 2012, 336, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Higgins, D.F.; Ewart, L.M.; Masterson, E.; Tennant, S.; Grebnev, G.; Prunotto, M.; Pomposiello, S.; Conde-Knape, K.; Martin, F.M.; Godson, C. BMP7-induced-Pten inhibits Akt and prevents renal fibrosis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 3095–3104. [Google Scholar] [CrossRef] [PubMed]
- Singla, D.K.; Singla, R.; Wang, J. BMP-7 Treatment Increases M2 Macrophage Differentiation and Reduces Inflammation and Plaque Formation in Apo E-/- Mice. PLoS ONE 2016, 11, e0147897. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, S.; Bennet, S.J.; Arora, M. Bone morphogenetic protein-7: Review of signalling and efficacy in fracture healing. J. Orthop. Transl. 2016, 4, 28–34. [Google Scholar] [CrossRef]
- Singla, D.K. Akt-mTOR Pathway Inhibits Apoptosis and Fibrosis in Doxorubicin-Induced Cardiotoxicity Following Embryonic Stem Cell Transplantation. Cell Transplant. 2015, 24, 1031–1042. [Google Scholar] [CrossRef]
- Singla, D.K.; Lyons, G.E.; Kamp, T.J. Transplanted embryonic stem cells following mouse myocardial infarction inhibit apoptosis and cardiac remodeling. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1308–H1314. [Google Scholar] [CrossRef]
- Singla, D.K.; Hacker, T.A.; Ma, L.; Douglas, P.S.; Sullivan, R.; Lyons, G.E.; Kamp, T.J. Transplantation of embryonic stem cells into the infarcted mouse heart: Formation of multiple cell types. J. Mol. Cell Cardiol. 2006, 40, 195–200. [Google Scholar] [CrossRef]
- Schmid-Burgk, J.L.; Chauhan, D.; Schmidt, T.; Ebert, T.S.; Reinhardt, J.; Endl, E.; Hornung, V. A Genome-wide CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) Screen Identifies NEK7 as an Essential Component of NLRP3 Inflammasome Activation. J. Biol. Chem. 2016, 291, 103–109. [Google Scholar] [CrossRef]
- Li, G.; Xing, W.; Zhang, M.; Geng, F.; Yang, H.; Zhang, H.; Zhang, X.; Li, J.; Dong, L.; Gao, F. Antifibrotic cardioprotection of berberine via downregulating myocardial IGF-1 receptor-regulated MMP-2/MMP-9 expression in diabetic rats. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H802–H813. [Google Scholar] [CrossRef]
- Peet, C.; Ivetic, A.; Bromage, D.I.; Shah, A.M. Cardiac monocytes and macrophages after myocardial infarction. Cardiovasc. Res. 2020, 116, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.M.; Dong, Y.; Beskid, N.M.; Garcia, A.J.; Adams, A.B.; Babensee, J.E. Brief exposure to hyperglycemia activates dendritic cells in vitro and in vivo. J. Cell Physiol. 2019, 235, 5120–5129. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, A.; Tilley, D.G. The Role of Leukocytes in Diabetic Cardiomyopathy. Front. Physiol. 2018, 9, 1547. [Google Scholar] [CrossRef]
- Wang, H.; Kwak, D.; Fassett, J.; Liu, X.; Yao, W.; Weng, X.; Xu, X.; Xu, Y.; Bache, R.J.; Mueller, D.L.; et al. Role of bone marrow-derived CD11c(+) dendritic cells in systolic overload-induced left ventricular inflammation, fibrosis and hypertrophy. Basic Res. Cardiol. 2017, 112, 25. [Google Scholar] [CrossRef]
- Junod, A.; Lambert, A.E.; Stauffacher, W.; Renold, A.E. Diabetogenic action of streptozotocin: Relationship of dose to metabolic response. J. Clin. Investig. 1969, 48, 2129–2139. [Google Scholar] [CrossRef]
- Lu, W.T.; Juang, J.H.; Hsu, B.R.; Huang, H.S. Effects of high or low dose of streptozocin on pancreatic islets in C57BL/6 and C.B17-SCID mice. Transplant. Proc. 1998, 30, 609–610. [Google Scholar] [CrossRef]
- Jia, C.; Chen, H.; Zhang, J.; Zhou, K.; Zhuge, Y.; Niu, C.; Qiu, J.; Rong, X.; Shi, Z.; Xiao, J.; et al. Role of pyroptosis in cardiovascular diseases. Int. Immunopharmacol. 2019, 67, 311–318. [Google Scholar] [CrossRef]
- Leuschner, F.; Nahrendorf, M. Novel functions of macrophages in the heart: Insights into electrical conduction, stress, and diastolic dysfunction. Eur. Heart J. 2020, 41, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Segura, E.; Touzot, M.; Bohineust, A.; Cappuccio, A.; Chiocchia, G.; Hosmalin, A.; Dalod, M.; Soumelis, V.; Amigorena, S. Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity 2013, 38, 336–348. [Google Scholar] [CrossRef]
- Anzai, A.; Anzai, T.; Nagai, S.; Maekawa, Y.; Naito, K.; Kaneko, H.; Sugano, Y.; Takahashi, T.; Abe, H.; Mochizuki, S.; et al. Regulatory role of dendritic cells in postinfarction healing and left ventricular remodeling. Circulation 2012, 125, 1234–1245. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, E.; Monteagudo, P.T.; Marrocos, M.S.; Campa, A. Neutrophils and monocytes as potentially important sources of proinflammatory cytokines in diabetes. Clin. Exp. Immunol. 2006, 146, 443–447. [Google Scholar] [CrossRef]
- Antonov, I.B.; Kozlov, K.L.; Pal’tseva, E.M.; Polyakova, O.V.; Lin’kova, N.S. Matrix Metalloproteinases MMP-1 and MMP-9 and Their Inhibitor TIMP-1 as Markers of Dilated Cardiomyopathy in Patients of Different Age. Bull. Exp. Biol. Med. 2018, 164, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Cheng, X.; Lu, J.; Li, X. Exogenous BMP-7 Facilitates the Recovery of Cardiac Function after Acute Myocardial Infarction through Counteracting TGF-beta1 Signaling Pathway. Tohoku J. Exp. Med. 2018, 244, 1–6. [Google Scholar] [CrossRef]
- Chen, X.; Xu, J.; Jiang, B.; Liu, D. Bone Morphogenetic Protein-7 Antagonizes Myocardial Fibrosis Induced by Atrial Fibrillation by Restraining Transforming Growth Factor-beta (TGF-beta)/Smads Signaling. Med. Sci. Monit. 2016, 22, 3457–3468. [Google Scholar] [CrossRef]
- Merino, D.; Villar, A.V.; Garcia, R.; Tramullas, M.; Ruiz, L.; Ribas, C.; Cabezudo, S.; Nistal, J.F.; Hurle, M.A. BMP-7 attenuates left ventricular remodelling under pressure overload and facilitates reverse remodelling and functional recovery. Cardiovasc. Res. 2016, 110, 331–345. [Google Scholar] [CrossRef]
- Adamiak, M.; Abdel-Latif, A.; Bujko, K.; Thapa, A.; Anusz, K.; Tracz, M.; Brzezniakiewicz-Janus, K.; Ratajczak, J.; Kucia, M.; Ratajczak, M.Z. Nlrp3 Inflammasome Signaling Regulates the Homing and Engraftment of Hematopoietic Stem Cells (HSPCs) by Enhancing Incorporation of CXCR4 Receptor into Membrane Lipid Rafts. Stem Cell Rev. Rep. 2020, 16, 954–967. [Google Scholar] [CrossRef]
- Berezin, A. The endothelial progenitor cell dysfunction in type 2 diabetes mellitus: The link with heart failure developing. Biol. Markers Guided Ther. 2018, 5, 47–52. [Google Scholar] [CrossRef]
- Uthman, L.; Baartscheer, A.; Schumacher, C.A.; Fiolet, J.W.T.; Kuschma, M.C.; Hollmann, M.W.; Coronel, R.; Weber, N.C.; Zuurbier, C.J. Direct Cardiac Actions of Sodium Glucose Cotransporter 2 Inhibitors Target Pathogenic Mechanisms Underlying Heart Failure in Diabetic Patients. Front. Physiol. 2018, 9, 1575. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.F.; Green, J.B.; Janmohamed, S.; D’Agostino, R.B., Sr.; Granger, C.B.; Jones, N.P.; Leiter, L.A.; Rosenberg, A.E.; Sigmon, K.N.; Somerville, M.C.; et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): A double-blind, randomised placebo-controlled trial. Lancet 2018, 392, 1519–1529. [Google Scholar] [CrossRef]
- Larsen, C.M.; Faulenbach, M.; Vaag, A.; Volund, A.; Ehses, J.A.; Seifert, B.; Mandrup-Poulsen, T.; Donath, M.Y. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 2007, 356, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Hensen, J.; Howard, C.P.; Walter, V.; Thuren, T. Impact of interleukin-1beta antibody (canakinumab) on glycaemic indicators in patients with type 2 diabetes mellitus: Results of secondary endpoints from a randomized, placebo-controlled trial. Diabetes Metab. 2013, 39, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Zahid, A.; Li, B.; Kombe, A.J.K.; Jin, T.; Tao, J. Pharmacological Inhibitors of the NLRP3 Inflammasome. Front. Immunol. 2019, 10, 2538. [Google Scholar] [CrossRef] [PubMed]
- Bacchiega, B.C.; Bacchiega, A.B.; Usnayo, M.J.; Bedirian, R.; Singh, G.; Pinheiro, G.D. Interleukin 6 Inhibition and Coronary Artery Disease in a High-Risk Population: A Prospective Community-Based Clinical Study. J. Am. Heart Assoc. 2017, 6, e005038. [Google Scholar] [CrossRef]
- Zhang, N.; Liang, H.; Farese, R.V.; Li, J.; Musi, N.; Hussey, S.E. Pharmacological TLR4 Inhibition Protects against Acute and Chronic Fat-Induced Insulin Resistance in Rats. PLoS ONE 2015, 10, e0132575. [Google Scholar] [CrossRef]
- He, H.; Jiang, H.; Chen, Y.; Ye, J.; Wang, A.; Wang, C.; Liu, Q.; Liang, G.; Deng, X.; Jiang, W.; et al. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat. Commun. 2018, 9, 2550. [Google Scholar] [CrossRef]
- Pomerantz, B.J.; Reznikov, L.L.; Harken, A.H.; Dinarello, C.A. Inhibition of caspase 1 reduces human myocardial ischemic dysfunction via inhibition of IL-18 and IL-1beta. Proc. Natl. Acad. Sci. USA 2001, 98, 2871–2876. [Google Scholar] [CrossRef]
- Westermann, D.; Van Linthout, S.; Dhayat, S.; Dhayat, N.; Schmidt, A.; Noutsias, M.; Song, X.Y.; Spillmann, F.; Riad, A.; Schultheiss, H.P.; et al. Tumor necrosis factor-alpha antagonism protects from myocardial inflammation and fibrosis in experimental diabetic cardiomyopathy. Basic Res. Cardiol. 2007, 102, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Capucha, T.; Koren, N.; Nassar, M.; Heyman, O.; Nir, T.; Levy, M.; Zilberman-Schapira, G.; Zelentova, K.; Eli-Berchoer, L.; Zenke, M.; et al. Sequential BMP7/TGF-beta1 signaling and microbiota instruct mucosal Langerhans cell differentiation. J. Exp. Med. 2018, 215, 481–500. [Google Scholar] [CrossRef]
- Kim, Y.; Kang, B.J.; Kim, W.H.; Yun, H.S.; Kweon, O.K. Evaluation of Mesenchymal Stem Cell Sheets Overexpressing BMP-7 in Canine Critical-Sized Bone Defects. Int. J. Mol. Sci. 2018, 19, 2073. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Xing, Z.; Yu, Y.Y.; Colnot, C.; Miclau, T.; Marcucio, R.S. Recombinant human bone morphogenetic protein-7 enhances fracture healing in an ischemic environment. J. Orthop. Res. 2010, 28, 687–696. [Google Scholar] [CrossRef][Green Version]
- Kim, J.; Kim, M.; Jeong, Y.; Lee, W.B.; Park, H.; Kwon, J.Y.; Kim, Y.M.; Hwang, D.; Kwon, Y.G. BMP9 Induces Cord Blood-Derived Endothelial Progenitor Cell Differentiation and Ischemic Neovascularization via ALK1. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; An, G.; Zhu, Z.; Wang, Y.; Yang, G.; Li, X.; Niu, P.; Chen, L.; Tian, L. The protective effects of bone morphogenetic protein-7 against epithelial injury and matrix metalloproteases upregulation induced by silica in vitro. Hum. Exp. Toxicol. 2017, 36, 892–900. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmadbouh, I.; Singla, D.K. BMP-7 Attenuates Inflammation-Induced Pyroptosis and Improves Cardiac Repair in Diabetic Cardiomyopathy. Cells 2021, 10, 2640. https://doi.org/10.3390/cells10102640
Elmadbouh I, Singla DK. BMP-7 Attenuates Inflammation-Induced Pyroptosis and Improves Cardiac Repair in Diabetic Cardiomyopathy. Cells. 2021; 10(10):2640. https://doi.org/10.3390/cells10102640
Chicago/Turabian StyleElmadbouh, Ibrahim, and Dinender K. Singla. 2021. "BMP-7 Attenuates Inflammation-Induced Pyroptosis and Improves Cardiac Repair in Diabetic Cardiomyopathy" Cells 10, no. 10: 2640. https://doi.org/10.3390/cells10102640
APA StyleElmadbouh, I., & Singla, D. K. (2021). BMP-7 Attenuates Inflammation-Induced Pyroptosis and Improves Cardiac Repair in Diabetic Cardiomyopathy. Cells, 10(10), 2640. https://doi.org/10.3390/cells10102640