Sirtuins as Interesting Players in the Course of HIV Infection and Comorbidities
Abstract
:1. Introduction
2. Sirtuins Family—The General Role in Organisms
3. Role of Sirtuins during HIV and Other Viral Infections
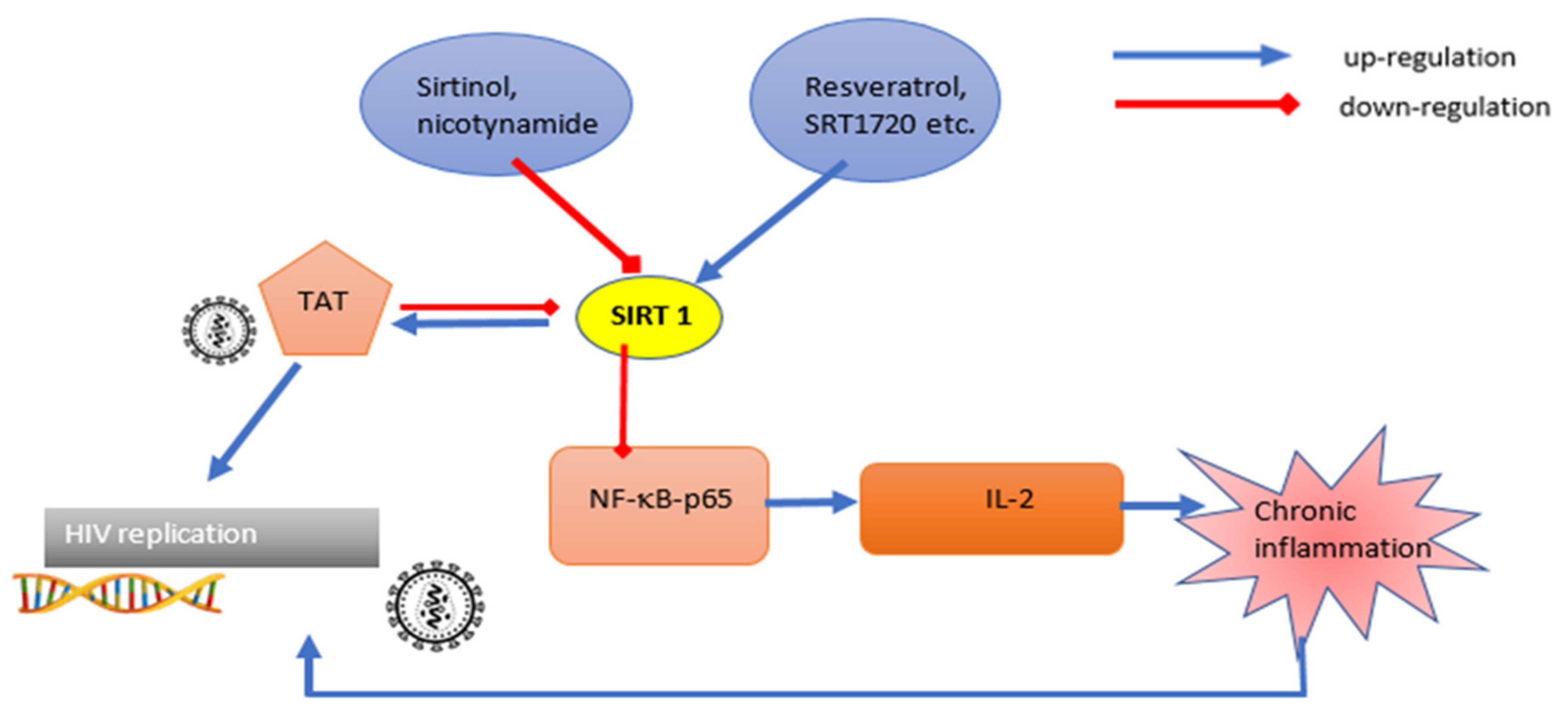
3.1. Viral Targets and Sirtuin Action
3.2. Host Targets and Sirtuins Action in Viral Infections
4. Liver Disturbances in HIV and Sirtuin’s Role
5. Cardiovascular Risk in HIV-Infected Patients and Sirtuin Participation
6. Insulin Resistance and Diabetes in HIV-Infected Patients and Sirtuins Participation
7. Bone Metabolism Disturbances in HIV-Infected Patients and the Role of Sirtuins
8. Kidney Diseases in HIV-Infected Patients and the Role of Sirtuins
9. NeuroAIDS in Aging HIV Population and Sirtuin Participation
10. Other Disturbances
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fauci, A.S.; Lane, H.C. Four Decades of HIV/AIDS—Much Accomplished, Much to Do. N. Engl. J. Med. 2020, 383, 1–4. [Google Scholar] [CrossRef]
- Zhan, J.; Qin, S.; Lu, L.; Hu, X.; Zhou, J.; Sun, Y.; Yang, J.; Liu, Y.; Wang, Z.; Tan, N.; et al. miR-34a is a common link in both HIV- and antiretroviral therapy-induced vascular aging. Aging 2016, 8, 3298–3310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrão, R.; Piñero, C.; Velez, J.; Coutinho, D.; Maltez, F.; Lino, S.; Sarmento e Castro, R.; Tavares, A.P.; Pacheco, P.; Lopes, M.J.; et al. Non-AIDS-related comorbidities in people living with HIV-1 aged 50 years and older: The AGING POSITIVE study. Int. J. Infect. Dis. 2019, 79, 94–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houtkooper, R.; Pirinen, E.; Auwerx, J. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 2012, 13, 225–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiedel, M.; Robaa, D.; Rumpf, T.; Sippl, W.; Jung, M. The Current State of NAD+-Dependent Histone Deacetylases (Sirtuins) as Novel Therapeutic Targets. Med. Res. Rev. 2017, 38, 147–200. [Google Scholar] [CrossRef]
- Chang, H.-C.; Guarente, L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 2013, 25, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Kupis, W.; Pałyga, J.; Tomal, E.; Niewiadomska, E. The role of sirtuins in cellular homeostasis. J. Physiol. Biochem. 2016, 72, 371–380. [Google Scholar] [CrossRef] [Green Version]
- O’Callaghan, C.; Vassilopoulos, A. Sirtuins at the crossroads of stemness, aging, and cancer. Aging Cell 2017, 16, 1208–1218. [Google Scholar] [CrossRef]
- Kratz, E.M.; Sołkiewicz, K.; Kubis-Kubiak, A.; Piwowar, A. Sirtuins as Important Factors in Pathological States and the Role of Their Molecular Activity Modulators. Int. J. Mol. Sci. 2021, 22, 630. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Zhang, X.; Yi, J.; Huang, J.; He, J.; Tao, Y. Sirtuins in metabolism, DNA repair and cancer. J. Exp. Clin. Cancer Res. 2016, 35, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Alqarni, M.; Foudah, A.; Muharram, M.; Labrou, N. The Pleiotropic Function of Human Sirtuins as Modulators of Metabolic Pathways and Viral Infections. Cells 2021, 10, 460. [Google Scholar] [CrossRef]
- Pinzone, M.R.; Cacopardo, B.; Condorelli, F.; Di Rosa, M.; Nunnari, G. Sirtuin-1 and HIV-1: An Overview. Curr. Drug Targets 2013, 14, 648–652. [Google Scholar] [CrossRef]
- Budayeva, H.G.; Rowland, E.A.; Cristea, I.M. Intricate Roles of Mammalian Sirtuins in Defense against Viral Pathogens. J. Virol. 2016, 90, 5–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogoi, R.N.; De Pablo, A.; Valencia, E.; Martín-Carbonero, L.; Moreno, V.; Vilchez-Rueda, H.H.; Asensi, V.; Rodriguez, R.; Toledano, V.; Rodés, B. Expression profiling of chromatin-modifying enzymes and global DNA methylation in CD4+ T cells from patients with chronic HIV infection at different HIV control and progression states. Clin. Epigenet. 2018, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.-S.; Brent, M.M.; Getachew, R.; Jayakumar, P.; Chen, L.-F.; Schnolzer, M.; McBurney, M.W.; Marmorstein, R.; Greene, W.C.; Ott, M. Human Immunodeficiency Virus Type 1 Tat Protein Inhibits the SIRT1 Deacetylase and Induces T Cell Hyperactivation. Cell Host Microbe 2008, 3, 158–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, B.; Kong, Q.; Kemp, K.; Zhao, Y.-S.; Fang, D. Analysis of sirtuin 1 expression reveals a molecular explanation of IL-2-mediated reversal of T-cell tolerance. Proc. Natl. Acad. Sci. USA 2012, 109, 899–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samer, S.; Arif, M.S.; Giron, L.B.; Zukurov, J.P.L.; Hunter, J.; Santillo, B.T.; Namiyama, G.; Galinskas, J.; Komninakis, S.V.; Oshiro, T.M.; et al. Nicotinamide activates latent HIV-1 ex vivo in ART suppressed individuals, revealing higher potency than the association of two methyltransferase inhibitors, chaetocin and BIX01294. Braz. J. Infect. Dis. 2020, 24, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Tuyama, A.C.; Hong, F.; Saiman, Y.; Wang, C.; Ozkok, D.; Mosoian, A.; Chen, P.; Chen, B.K.; Klotman, M.E.; Bansal, M.B. Human immunodeficiency virus (HIV)-1 infects human hepatic stellate cells and promotes collagen I and monocyte chemoattractant protein-1 expression: Implications for the pathogenesis of HIV/hepatitis C virus-induced liver fibrosis. Hepatology 2010, 52, 612–622. [Google Scholar] [CrossRef] [Green Version]
- Kovari, H.; Ledergerber, B.; Battegay, M.; Rauch, A.; Hirschel, B.; Foguena, A.K.; Vernazza, P.; Bernasconi, E.; Mueller, N.J.; Weber, R. Incidence and Risk Factors for Chronic Elevation of Alanine Aminotransferase Levels in HIV-Infected Persons without Hepatitis B or C Virus Co-Infection. Clin. Infect. Dis. 2010, 50, 502–511. [Google Scholar] [CrossRef]
- Zhang, H.S.; Sang, W.W.; Wang, Y.O.; Liu, W. Nicotinamide phosphoribosyltransferase/sirtuin 1 pathway is involved in human immunodeficiency virus type 1 Tat-mediated long terminal repeat transactivation. J. Cell. Biochem. 2010, 110, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Chamroonkul, N.; Bansal, M.B. HIV and the liver. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.; Galastri, S.; Sacchi, P.; Cima, S.; Caligiuri, A.; DeFranco, R.; Milani, S.; Gessani, S.; Fantuzzi, L.; Liotta, F.; et al. gp120 modulates the biology of human hepatic stellate cells: A link between HIV infection and liver fibrogenesis. Gut 2009, 59, 513–520. [Google Scholar] [CrossRef]
- Platt, L.; Easterbrook, P.; Gower, E.; McDonald, B.; Sabin, K.; McGowan, C.; Yanny, I.; Razavi, H.; Vickerman, P. Prevalence and burden of HCV co-infection in people living with HIV: A global systematic review and meta-analysis. Lancet Infect. Dis. 2016, 16, 797–808. [Google Scholar] [CrossRef]
- Hernandez, M.D.; Sherman, K.E. HIV/hepatitis C coinfection natural history and disease progression. Curr. Opin. HIV AIDS 2011, 6, 478–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Zhang, W.; Xu, K.; Lu, J. miR-34a promotes liver fibrosis in patients with chronic hepatitis via mediating Sirt1/p53 signaling pathway. Pathol. Res. Pract. 2020, 216, 152876. [Google Scholar] [CrossRef]
- Gupta, D.; Rani, M.; Khan, N.; Jameel, S. HIV-1 Infected Peripheral Blood Mononuclear Cells Modulate the Fibrogenic Activity of Hepatic Stellate Cells through Secreted TGF-β and JNK Signaling. PLoS ONE 2014, 9, e91569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Liu, Q.; Huang, Y.; Li, R.; Wu, T.; Zhang, Z.; Zhou, J.; Huang, H.; Tang, Q.; et al. Sirt6 Alleviated Liver Fibrosis by Deacetylating Conserved Lysine 54 on Smad2 in Hepatic Stellate Cells. Hepatology 2020, 73, 1140–1157. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Gu, J.; Chen, C.; Duanmu, J.; Miao, J.; Yao, W.; Tao, J.; Tu, M.; Xiong, B.; et al. Celastrol exerts anti-inflammatory effect in liver fibrosis via activation of AMPK-SIRT3 signalling. J. Cell. Mol. Med. 2019, 24, 941–953. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Xin, T.; Li, D.; Wang, C.; Zhu, H.; Zhou, H. Therapeutic effect of Sirtuin 3 on ameliorating nonalcoholic fatty liver disease: The role of the ERK-CREB pathway and Bnip3-mediated mitophagy. Redox Biol. 2018, 18, 229–243. [Google Scholar] [CrossRef]
- Gu, J.; Chen, C.; Wang, J.; Chen, T.; Yao, W.; Yan, T.; Liu, Z. Withaferin A Exerts Preventive Effect on Liver Fibrosis through Oxidative Stress Inhibition in a Sirtuin 3-Dependent Manner. Oxid. Med. Cell. Longev. 2020, 2020, 1–17. [Google Scholar] [CrossRef]
- Ye, X.; Li, M.; Hou, T.; Gao, T.; Zhu, W.-G.; Yang, Y. Sirtuins in glucose and lipid metabolism. Oncotarget 2016, 8, 1845–1859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Sun, Y.; Liu, W.; Dong, J.; Chen, J. SIRT1 mediates the role of RNA-binding protein QKI 5 in the synthesis of triglycerides in non-alcoholic fatty liver disease mice via the PPARα/FoxO1 signaling pathway. Int. J. Mol. Med. 2019, 43, 1271–1280. [Google Scholar] [CrossRef]
- Tobita, T.; Guzman-Lepe, J.; Takeishi, K.; Nakao, T.; Wang, Y.; Meng, F.; Deng, C.-X.; De L’Hortet, A.C.; Soto-Gutierrez, A. SIRT1 Disruption in Human Fetal Hepatocytes Leads to Increased Accumulation of Glucose and Lipids. PLoS ONE 2016, 11, e0149344. [Google Scholar] [CrossRef] [Green Version]
- Vecchi, V.L.; Soresi, M.; Giannitrapani, L.; Di Carlo, P.; Mazzola, G.; Colletti, P.; Terranova, A.; Vizzini, G.; Montalto, G. Prospective evaluation of hepatic steatosis in HIV-infected patients with or without hepatitis C virus co-infection. Int. J. Infect. Dis. 2012, 16, e397–e402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuille-Lessard, É.; Lebouché, B.; Lennox, L.; Routy, J.-P.; Costiniuk, C.T.; Pexos, C.; Giannakis, A.; Szabo, J.; Klein, M.B.; Sebastiani, G. Nonalcoholic fatty liver disease diagnosed by transient elastography with controlled attenuation parameter in unselected HIV monoinfected patients. AIDS 2016, 30, 2635–2643. [Google Scholar] [CrossRef]
- Agarwal, N.; Iyer, D.; Gabbi, C.; Saha, P.; Patel, S.G.; Mo, Q.; Chang, B.; Goswami, B.; Schubert, U.; Kopp, J.B.; et al. HIV-1 viral protein R (Vpr) induces fatty liver in mice via LXRα and PPARα dysregulation: Implications for HIV-specific pathogenesis of NAFLD. Sci. Rep. 2017, 7, 13362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponugoti, B.; Kim, D.-H.; Xiao, Z.; Smith, Z.; Miao, J.; Zang, M.; Wu, S.-Y.; Chiang, C.-M.; Veenstra, T.D.; Kemper, J.K. SIRT1 Deacetylates and Inhibits SREBP-1C Activity in Regulation of Hepatic Lipid Metabolism. J. Biol. Chem. 2010, 285, 33959–33970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raposo, M.A.; Armiliato, G.N.D.A.; Guimarães, N.S.; Caram, C.A.; Silveira, R.D.D.S.; Tupinambás, U. Metabolic disorders and cardiovascular risk in people living with HIV/AIDS without the use of antiretroviral therapy. Rev. Soc. Bras. Med. Trop. 2017, 50, 598–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, C.; Ryom, L.; Weber, R.; Morlat, P.; Pradier, C.; Reiss, P.; Kowalska, J.D.; de Wit, S.; Law, M.; el Sadr, W.; et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): A multicohort collaboration. Lancet 2014, 384, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Eyawo, O.; Brockman, G.; Goldsmith, C.H.; Hull, M.W.; Lear, S.A.; Bennett, M.; Guillemi, S.; Franco-Villalobos, C.; Adam, A.; Mills, E.J.; et al. Risk of myocardial infarction among people living with HIV: An updated systematic review and meta-analysis. BMJ Open 2019, 9, e025874. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.R.; Rachel, G.; Parthasarathy, D. HIV Proteins and Endothelial Dysfunction: Implications in Cardiovascular Disease. Front. Cardiovasc. Med. 2018, 5, 185. [Google Scholar] [CrossRef]
- D’Ascenzo, F.; Cerrato, E.; Calcagno, A.; Grossomarra, W.; Ballocca, F.; Omedè, P.; Montefusco, A.; Veglia, S.; Barbero, U.; Gili, S.; et al. High prevalence at computed coronary tomography of non-calcified plaques in asymptomatic HIV patients treated with HAART: A meta-analysis. Atherosclerosis 2015, 240, 197–204. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Servillo, L.; Balestrieri, M.L. SIRT1 and SIRT6 Signaling Pathways in Cardiovascular Disease Protection. Antioxid. Redox Signal. 2018, 28, 711–732. [Google Scholar] [CrossRef] [PubMed]
- Charles, S.; Raj, V.; Arokiaraj, J.; Mala, K. Caveolin1/protein arginine methyltransferase1/sirtuin1 axis as a potential target against endothelial dysfunction. Pharmacol. Res. 2017, 119, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mattagajasingh, I.; Kim, C.-S.; Naqvi, A.; Yamamori, T.; Hoffman, T.A.; Jung, S.-B.; DeRicco, J.; Kasuno, K.; Irani, K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 2007, 104, 14855–14860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, S.-B.; Kim, C.-S.; Kim, Y.-R.; Naqvi, A.; Yamamori, T.; Kumar, S.; Kumar, A.; Irani, K. Redox Factor-1 Activates Endothelial SIRTUIN1 through Reduction of Conserved Cysteine Sulfhydryls in Its Deacetylase Domain. PLoS ONE 2013, 8, e65415. [Google Scholar] [CrossRef] [Green Version]
- Volonte, D.; Zou, H.; Bartholomew, J.N.; Liu, Z.; Morel, P.A.; Galbiati, F. Oxidative Stress-induced Inhibition of Sirt1 by Caveolin-1 Promotes p53-dependent Premature Senescence and Stimulates the Secretion of Interleukin 6 (IL-6). J. Biol. Chem. 2015, 290, 4202–4214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlomosti, F.; D’Agostino, M.; Beji, S.; Torcinaro, A.; Rizzi, R.; Zaccagnini, G.; Maimone, B.; Di Stefano, V.; De Santa, F.; Cordisco, S.; et al. Oxidative Stress-Induced miR-200c Disrupts the Regulatory Loop Among SIRT1, FOXO1, and eNOS. Antioxid. Redox Signal. 2017, 27, 328–344. [Google Scholar] [CrossRef]
- Ren, Z.; Yao, Q.; Chen, C. HIV-1 Envelope Glycoprotein 120 Increases Intercellular Adhesion Molecule-1 Expression by Human Endothelial Cells. Lab. Investig. 2002, 82, 245–255. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Fu, W.; Wang, X.; Lin, P.H.; Yao, Q.; Chen, C. HIV gp120 induces endothelial dysfunction in tumour necrosis factor-α-activated porcine and human endothelial cells. Cardiovasc. Res. 2010, 87, 366–374. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-W.; Sung, H.-C.; Lin, S.-R.; Wu, C.-W.; Lee, C.-W.; Lee, I.-T.; Yang, Y.-F.; Yu, I.-S.; Chiang, M.-H.; Liang, C.-J.; et al. Resveratrol attenuates ICAM-1 expression and monocyte adhesiveness to TNF-α-treated endothelial cells: Evidence for an anti-inflammatory cascade mediated by the miR-221/222/AMPK/p38/NF-κB pathway. Sci. Rep. 2017, 7, srep44689. [Google Scholar] [CrossRef]
- Pillai, V.B.; Sundaresan, N.R.; Jeevanandam, V.; Gupta, M.P. Mitochondrial SIRT3 and heart disease. Cardiovasc. Res. 2010, 88, 250–256. [Google Scholar] [CrossRef] [Green Version]
- Pan, W.; Yu, H.; Huang, S.; Zhu, P. Resveratrol Protects against TNF-α-Induced Injury in Human Umbilical Endothelial Cells through Promoting Sirtuin-1-Induced Repression of NF-KB and p38 MAPK. PLoS ONE 2016, 11, e0147034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, M.; Yao, H.; Hu, G.; Chen, X.; Lund, A.K.; Buch, S. HIV Tat Induces Expression of ICAM-1 in HUVECs: Implications for miR-221/-222 in HIV-Associated Cardiomyopathy. PLoS ONE 2013, 8, e60170. [Google Scholar] [CrossRef]
- Wang, T.; Green, L.A.; Gupta, S.K.; Kim, C.; Wang, L.; Almodovar, S.; Flores, S.C.; Prudovsky, I.A.; Jolicoeur, P.; Liu, Z.; et al. Transfer of Intracellular HIV Nef to Endothelium Causes Endothelial Dysfunction. PLoS ONE 2014, 9, e91063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundaresan, N.R.; Vasudevan, P.; Zhong, L.; Kim, G.; Samant, S.; Parekh, V.; Pillai, V.B.; Ravindra, P.V.; Gupta, M.; Jeevanandam, V.; et al. The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nat. Med. 2012, 18, 1643–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winnik, S.; Auwerx, J.; Sinclair, D.; Matter, C.M. Protective effects of sirtuins in cardiovascular diseases: From bench to bedside. Eur. Heart J. 2015, 36, 3404–3412. [Google Scholar] [CrossRef] [PubMed]
- Tseng, A.; Szadkowski, L.; Walmsley, S.; Salit, I.; Raboud, J. Association of Age with Polypharmacy and Risk of Drug Interactions with Antiretroviral Medications in HIV-Positive Patients. Ann. Pharmacother. 2013, 47, 1429–1439. [Google Scholar] [CrossRef]
- Zeng, H.; Chen, J.X. Sirtuin 3, Endothelial Metabolic Reprogramming, and Heart Failure with Preserved Ejection Fraction. J. Cardiovasc. Pharmacol. 2019, 74, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Bugger, H.; Witt, C.N.; Bode, C. Mitochondrial sirtuins in the heart. Heart Fail. Rev. 2016, 215, 519–528. [Google Scholar] [CrossRef]
- Luo, Y.; Tang, X.; An, X.-Z.; Xie, X.-M.; Chen, X.-F.; Zhao, X.; Hao, D.-L.; Liu, D.-P. Sirt4 accelerates Ang II-induced pathological cardiac hypertrophy by inhibiting manganese superoxide dismutase activity. Eur. Heart J. 2016. [Google Scholar] [CrossRef] [Green Version]
- Nishida, Y.; Rardin, M.J.; Carrico, C.; He, W.; Sahu, A.K.; Gut, P.; Najjar, R.; Fitch, M.; Hellerstein, M.; Gibson, B.W.; et al. SIRT5 Regulates both Cytosolic and Mitochondrial Protein Malonylation with Glycolysis as a Major Target. Mol. Cell 2015, 59, 321–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hershberger, K.; Abraham, D.M.; Martin, A.S.; Mao, L.; Liu, J.; Gu, H.; Locasale, J.W.; Hirschey, M.D. Sirtuin 5 is required for mouse survival in response to cardiac pressure overload. J. Biol. Chem. 2017, 292, 19767–19781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberale, L.; Akhmedov, A.; Vlachogiannis, N.I.; Bonetti, N.R.; Nageswaran, V.; Miranda, M.X.; Puspitasari, Y.M.; Schwarz, L.; Costantino, S.; Paneni, F.; et al. Sirtuin 5 promotes arterial thrombosis by blunting the fibrinolytic system. Cardiovasc. Res. 2020, 117, 2275–2288. [Google Scholar] [CrossRef] [PubMed]
- Brar, I.; Shuter, J.; Thomas, A.; Daniels, E.; Absalon, J. A Comparison of Factors Associated With Prevalent Diabetes Mellitus Among HIV-Infected Antiretroviral-Naive Individuals Versus Individuals in the National Health and Nutritional Examination Survey Cohort. JAIDS J. Acquir. Immune Defic. Syndr. 2007, 45, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Monroe, A.K.; Glesby, M.J.; Brown, T.T. Diagnosing and Managing Diabetes in HIV-Infected Patients: Current Concepts. Clin. Infect. Dis. 2014, 60, 453–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez-Romieu, A.C.; Garg, S.; Rosenberg, E.S.; Thompson-Paul, A.M.; Skarbinski, J. Is diabetes prevalence higher among HIV-infected individuals compared with the general population? Evidence from MMP and NHANES 2009–2010. BMJ Open Diabetes Res. Care 2017, 5, e000304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, K.A.; Peer, N.; Mills, E.J.; Kengne, A.P. A Meta-Analysis of the Metabolic Syndrome Prevalence in the Global HIV-Infected Population. PLoS ONE 2016, 11, e0150970. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.D.; Schwarzer, M.; Schrepper, A.; Amorim, P.A.; Blum, D.; Hain, C.; Faerber, G.; Haendeler, J.; Altschmied, J.; Doenst, T. Increased Protein Tyrosine Phosphatase 1B (PTP1B) Activity and Cardiac Insulin Resistance Precede Mitochondrial and Contractile Dysfunction in Pressure-Overloaded Hearts. J. Am. Heart Assoc. 2018, 7, e008865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigouroux, C.; Maachi, M.; Nguyên, T.-H.; Coussieu, C.; Gharakhanian, S.; Funahashi, T.; Matsuzawa, Y.; Shimomura, I.; Rozenbaum, W.; Capeau, J.; et al. Serum adipocytokines are related to lipodystrophy and metabolic disorders in HIV-infected men under antiretroviral therapy. AIDS 2003, 17, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Ogura, Y.; Monno, I.; Koya, D. Sirtuins and Type 2 Diabetes: Role in Inflammation, Oxidative Stress, and Mitochondrial Function. Front. Endocrinol. 2019, 10, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, L.; Mostoslavsky, R. SIRT6. Transcription 2010, 1, 17–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, W.; Wang, S.; Xiao, M.; Lin, Y.; Zhou, L.; Lei, Q.-Y.; Xiong, Y.; Guan, K.-L.; Zhao, S. Acetylation Regulates Gluconeogenesis by Promoting PEPCK1 Degradation via Recruiting the UBR5 Ubiquitin Ligase. Mol. Cell 2011, 43, 33–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Dentin, R.; Chen, D.; Hedrick, S.; Ravnskjaer, K.; Schenk, S.; Milne, J.; Meyers, D.J.; Cole, P.; Iii, J.Y.; et al. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature 2008, 456, 269–273. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, F.; Ge, X.; Yan, T.; Chen, X.; Shi, X.; Zhai, Q. SIRT1 Improves Insulin Sensitivity under Insulin-Resistant Conditions by Repressing PTP1B. Cell Metab. 2007, 6, 307–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandra, S.; Mondal, D.; Agrawal, K.C. HIV-1 Protease Inhibitor Induced Oxidative Stress Suppresses Glucose Stimulated Insulin Release: Protection with Thymoquinone. Exp. Biol. Med. 2009, 234, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Nzuza, S.; Zondi, S.; Owira, P.M.O. Naringin prevents HIV-1 protease inhibitors-induced metabolic complications in vivo. PLoS ONE 2017, 12, e0183355. [Google Scholar] [CrossRef] [Green Version]
- Hallows, W.C.; Yu, W.; Denu, J.M. Regulation of Glycolytic Enzyme Phosphoglycerate Mutase-1 by Sirt1 Protein-mediated Deacetylation. J. Biol. Chem. 2012, 287, 3850–3858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemos, V.; de Oliveira, R.M.; Naia, L.; Szegö, É.; Ramos, E.; Pinho, S.; Magro, F.; Cavadas, C.; Rego, A.C.; Costa, V.; et al. The NAD+-dependent deacetylase SIRT2 attenuates oxidative stress and mitochondrial dysfunction and improves insulin sensitivity in hepatocytes. Hum. Mol. Genet. 2017, 26, 4105–4117. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, J.; Danzer, C.; Simka, T.; Ukropec, J.; Walter, K.M.; Kumpf, S.; Mirtschink, P.; Ukropcova, B.; Gasperikova, D.; Pedrazzini, T.; et al. Dietary obesity-associated Hif1 activation in adipocytes restricts fatty acid oxidation and energy expenditure via suppression of the Sirt2-NAD+ system. Genes Dev. 2012, 26, 259–270. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Tong, Q. SIRT2 Suppresses Adipocyte Differentiation by Deacetylating FOXO1 and Enhancing FOXO1’s Repressive Interaction with PPARγ. Mol. Biol. Cell 2009, 20, 801–808. [Google Scholar] [CrossRef] [Green Version]
- Palacios, O.M.; Carmona, J.J.; Michan, S.; Chen, K.Y.; Manabe, Y.; Iii, J.L.W.; Goodyear, L.J.; Tong, Q. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1α in skeletal muscle. Aging 2009, 1, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Gudiksen, A.; Pilegaard, H. PGC-1αand fasting-induced PDH regulation in mouse skeletal muscle. Physiol. Rep. 2017, 5, e13222. [Google Scholar] [CrossRef]
- Jing, E.; O’Neill, B.T.; Rardin, M.J.; Kleinridders, A.; Ilkeyeva, O.R.; Ussar, S.; Bain, J.R.; Lee, K.Y.; Verdin, E.M.; Newgard, C.B.; et al. Sirt3 Regulates Metabolic Flexibility of Skeletal Muscle Through Reversible Enzymatic Deacetylation. Diabetes 2013, 62, 3404–3417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirschey, M.; Shimazu, T.; Goetzman, E.; Jing, E.; Schwer, B.; Lombard, D.; Grueter, C.; Harris, C.; Biddinger, S.; Ilkayeva, O.R.; et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 2010, 464, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Xiong, X.; Wang, G.; Tao, R.; Wu, P.; Kono, T.; Li, K.; Ding, W.-X.; Tong, X.; Tersey, S.A.; Harris, R.A.; et al. Sirtuin 6 regulates glucose-stimulated insulin secretion in mouse pancreatic beta cells. Diabetologia 2015, 59, 151–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.G.; Ramadori, G.; Ioris, R.M.; Galiè, M.; Berglund, E.D.; Coate, K.; Fujikawa, T.; Pucciarelli, S.; Moreschini, B.; Amici, A.; et al. Enhanced insulin sensitivity in skeletal muscle and liver by physiological overexpression of SIRT6. Mol. Metab. 2015, 4, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Hruz, P.W. Molecular mechanisms for insulin resistance in treated HIV-infection. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 459–468. [Google Scholar] [CrossRef] [Green Version]
- Bresciani, E.; Saletti, C.; Squillace, N.; Rizzi, L.; Molteni, L.; Meanti, R.; Omeljaniuk, R.J.; Biagini, G.; Gori, A.; Locatelli, V.; et al. miRNA-218 Targets Lipin-1 and Glucose Transporter Type 4 Genes in 3T3-L1 Cells Treated with Lopinavir/Ritonavir. Front. Pharmacol. 2019, 10, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sociali, G.; Magnone, M.; Ravera, S.; Damonte, P.; Vigliarolo, T.; Von Holtey, M.; Vellone, V.G.; Millo, E.; Caffa, I.; Cea, M.; et al. Pharmacological Sirt6 inhibition improves glucose tolerance in a type 2 diabetes mouse model. FASEB J. 2017, 31, 3138–3149. [Google Scholar] [CrossRef] [Green Version]
- Young, B.; Dao, C.N.; Buchacz, K.; Baker, R.; Brooks, J.T.; The HIV Outpatient Study (HOPS) Investigators. Increased Rates of Bone Fracture Among HIV-Infected Persons in the HIV Outpatient Study (HOPS) Compared with the US General Population, 2000–2006. Clin. Infect. Dis. 2011, 52, 1061–1068. [Google Scholar] [CrossRef] [Green Version]
- Gibellini, D.; Borderi, M.; De Crignis, E.; Cicola, R.; Vescini, F.; Caudarella, R.; Chiodo, F.; Re, M.C. RANKL/OPG/TRAIL plasma levels and bone mass loss evaluation in antiretroviral naive HIV-1-positive men. J. Med. Virol. 2007, 79, 1446–1454. [Google Scholar] [CrossRef]
- Womack, J.A.; Goulet, J.; Gibert, C.; Brandt, C.; Chang, C.C.; Gulanski, B.; Fraenkel, L.; Mattocks, K.; Rimland, D.; Rodriguez-Barradas, M.C.; et al. Increased Risk of Fragility Fractures among HIV Infected Compared to Uninfected Male Veterans. PLoS ONE 2011, 6, e17217. [Google Scholar] [CrossRef] [Green Version]
- Titanji, K.; Vunnava, A.; Foster, A.; Sheth, A.N.; Lennox, J.L.; Knezevic, A.; Shenvi, N.; Easley, K.; Ofotokun, I.; Weitzmann, M.N. T-cell receptor activator of nuclear factor-κB ligand/osteoprotegerin imbalance is associated with HIV-induced bone loss in patients with higher CD4+ T-cell counts. AIDS 2018, 32, 885–894. [Google Scholar] [CrossRef]
- Titanji, K.; Vunnava, A.; Sheth, A.N.; Delille, C.; Lennox, J.L.; Sanford, S.E.; Foster, A.; Knezevic, A.; Easley, K.; Weitzmann, M.N.; et al. Dysregulated B Cell Expression of RANKL and OPG Correlates with Loss of Bone Mineral Density in HIV Infection. PLoS Pathog. 2014, 10, e1004497. [Google Scholar] [CrossRef]
- Duvivier, C.; Kolta, S.; Assoumou, L.; Ghosn, J.; Rozenberg, S.; Murphy, R.; Katlama, C.; Costagliola, D. Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. AIDS 2009, 23, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Malizia, A.P.; Cotter, E.; Chew, N.; Powderly, W.; Doran, P. HIV Protease Inhibitors Selectively Induce Gene Expression Alterations Associated with Reduced Calcium Deposition in Primary Human Osteoblasts. AIDS Res. Hum. Retrovir. 2007, 23, 243–250. [Google Scholar] [CrossRef]
- Cozzolino, M.; Vidal, M.; Arcidiacono, M.V.; Tebas, P.; Yarasheski, K.E.; Dusso, A.S. HIV-protease inhibitors impair vitamin D bioactivation to 1,25-dihydroxyvitamin D. AIDS 2003, 17, 513–520. [Google Scholar] [CrossRef]
- Grigsby, I.F.; Pham, L.; Mansky, L.M.; Gopalakrishnan, R.; Mansky, K. Tenofovir-associated bone density loss. Ther. Clin. Risk Manag. 2009, 6, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Negredo, E.; Diez-Pérez, A.; Bonjoch, A.; Domingo, P.; Pérez-Álvarez, N.; Gutierrez, M.; Mateo, G.; Puig, J.; Echeverría, P.; Escrig, R.; et al. Switching from tenofovir to abacavir in HIV-1-infected patients with low bone mineral density: Changes in bone turnover markers and circulating sclerostin levels. J. Antimicrob. Chemother. 2015, 70, 2104–2107. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.; Yoon, D.S.; Lee, K.-M.; Choi, S.M.; Lee, M.-H.; Park, K.H.; Han, S.H.; Lee, J.W. Enhancement of Mesenchymal Stem Cell-Driven Bone Regeneration by Resveratrol-Mediated SOX2 Regulation. Aging Dis. 2019, 10, 818–833. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Liu, S.; Ma, S.; Zhao, J.; Zhang, W.; Qi, W.; Cao, P.; Wang, Z.; Lei, W. Protective effects of resveratrol on postmenopausal osteoporosis: Regulation of SIRT1-NF-κB signaling pathway. Acta Biochim. Biophys. Sin. 2014, 46, 1024–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zainabadi, K.; Liu, C.J.; Caldwell, A.L.M.; Guarente, L. SIRT1 is a positive regulator of in vivo bone mass and a therapeutic target for osteoporosis. PLoS ONE 2017, 12, e0185236. [Google Scholar] [CrossRef]
- Cohen-Kfir, E.; Artsi, H.; Levin, A.; Abramowitz, E.; Bajayo, A.; Gurt, I.; Zhong, L.; D’Urso, A.; Toiber, D.; Mostoslavsky, R.; et al. Sirt1 Is a Regulator of Bone Mass and a Repressor of Sost Encoding for Sclerostin, a Bone Formation Inhibitor. Endocrinology 2011, 152, 4514–4524. [Google Scholar] [CrossRef]
- Abed, É.; Couchourel, D.; Delalandre, A.; Duval, N.; Pelletier, J.-P.; Martel-Pelletier, J.; Lajeunesse, D. Low sirtuin 1 levels in human osteoarthritis subchondral osteoblasts lead to abnormal sclerostin expression which decreases Wnt/β-catenin activity. Bone 2014, 59, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Mora, S.; Puzzovio, M.; Giacomet, V.; Fabiano, V.; Maruca, K.; Capelli, S.; Nannini, P.; Lombardi, G.; Zuccotti, G.V. Sclerostin and DKK-1: Two important regulators of bone metabolism in HIV-infected youths. Endocrine 2015, 49, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Erlandson, K.M.; O’Riordan, M.; Hileman, C.O.; Rapaport, E.; Labbato, D.; Campbell, T.B.; Mccomsey, G.A. Plasma Sclerostin in HIV-Infected Adults on Effective Antiretroviral Therapy. AIDS Res. Hum. Retroviruses 2015, 31, 731–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Liu, Y.; Wang, Y.; Zhang, M.; Lv, L.; Zhang, X.; Zhou, Y. SIRT6 promotes osteogenic differentiation of mesenchymal stem cells through BMP signaling. Sci. Rep. 2017, 7, 10229. [Google Scholar] [CrossRef] [Green Version]
- Hou, K.-L.; Lin, S.-K.; Chao, L.-H.; Lai, E.H.-H.; Chang, C.-C.; Shun, C.-T.; Lu, W.-Y.; Wang, J.H.; Hsiao, M.; Hong, C.-Y.; et al. Sirtuin 6 suppresses hypoxia-induced inflammatory response in human osteoblasts via inhibition of reactive oxygen species production and glycolysis-A therapeutic implication in inflammatory bone resorption. BioFactors 2016, 43, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.I.; Li, Y.; Parikh, C.; Volberding, P.A.; Shlipak, M.G. Long-term clinical consequences of acute kidney injury in the HIV-infected. Kidney Int. 2010, 78, 478–485. [Google Scholar] [CrossRef] [Green Version]
- Wyatt, C.M. Kidney Disease and HIV Infection. Top. Antivir. Med. 2017, 25, 13–16. [Google Scholar] [PubMed]
- Calza, L.; Vanino, E.; Magistrelli, E.; Salvadori, C.; Cascavilla, A.; Colangeli, V.; Di Bari, M.A.; Manfredi, R.; Viale, P. Prevalence of renal disease within an urban HIV-infected cohort in northern Italy. Clin. Exp. Nephrol. 2013, 18, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, J.; Fonseca, J.A.; Jorge, S.; Lopes, J. Acute kidney injury in HIV-infected patients: A critical review. HIV Med. 2018, 20, 77–87. [Google Scholar] [CrossRef]
- Kooij, K.W.; Vogt, L.; Wit, F.W.N.M.; Van Der Valk, M.; Van Zoest, R.A.; Goorhuis, A.; Prins, M.; Post, F.A.; Reiss, P. AGEhIV Cohort Study Higher Prevalence and Faster Progression of Chronic Kidney Disease in Human Immunodeficiency Virus-Infected Middle-Aged Individuals Compared with Human Immunodeficiency Virus-Uninfected Controls. J. Infect. Dis. 2017, 216, 622–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calza, L.; Sachs, M.; Colangeli, V.; Borderi, M.; Granozzi, B.; Malosso, P.; Comai, G.; Corradetti, V.; La Manna, G.; Viale, P. Prevalence of chronic kidney disease among HIV-1-infected patients receiving a combination antiretroviral therapy. Clin. Exp. Nephrol. 2019, 23, 1272–1279. [Google Scholar] [CrossRef]
- Casado, J.L.; del Rey, J.M.; Bañón, S.; Santiuste, C.; Rodriguez, M.; Moreno, A.; Elias, M.J.P.; Liaño, F.; Moreno, S. Changes in Kidney Function and in the Rate of Tubular Dysfunction After Tenofovir Withdrawal or Continuation in HIV-Infected Patients. J. Acquir. Immune Defic. Syndr. 2016, 72, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Mocroft, A.; Lundgren, J.; Ross, M.; Fux, A.C.; Reiss, P.; Moranne, O.; Morlat, P.; Monforte, A.D.; Kirk, O.; Ryom, L. Cumulative and current exposure to potentially nephrotoxic antiretrovirals and development of chronic kidney disease in HIV-positive individuals with a normal baseline estimated glomerular filtration rate: A prospective international cohort study. Lancet HIV 2015, 3, e23–e32. [Google Scholar] [CrossRef]
- Mikulak, J.; Singhals, P.C. HIV-1 and kidney cells: Better understanding of viral interaction. Nephron Exp. Nephrol. 2010, 115, e15–e21. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.L.; Guda, P.R.; Asemu, G.; Subedi, R.; Ray, S.; Khalid, O.S.; Shukla, V.; Patel, D.; Davis, H.; Nimmagadda, V.K.C.; et al. Glomerular mitochondrial changes in HIV associated renal injury. Exp. Mol. Pathol. 2018, 104, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Lempiäinen, J.; Finckenberg, P.; Mervaala, E.E.; Sankari, S.; Levijoki, J. Caloric restriction ameliorates kidney ischaemia/reperfusion injury through PGC-1α-eNOS pathway and enhanced autophagy. Acta Physiol. 2013, 208, 410–421. [Google Scholar] [CrossRef]
- Khader, A.; Yang, W.-L.; Kuncewitch, M.; Jacob, A.; Prince, J.M.; Asirvatham, J.R.; Nicastro, J.; Coppa, G.F.; Wang, P. Sirtuin 1 Activation Stimulates Mitochondrial Biogenesis and Attenuates Renal Injury After Ischemia-Reperfusion. Transplantation 2014, 98, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Morigi, M.; Perico, L.; Rota, C.; Longaretti, L.; Conti, S.; Rottoli, D.; Novelli, R.; Remuzzi, G.; Benigni, A. Sirtuin 3–dependent mitochondrial dynamic improvements protect against acute kidney injury. J. Clin. Investig. 2015, 125, 715–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, N.E.; Belyaev, N.D.; Lambert, D.W.; Turner, A.J. Epigenetic regulation of angiotensin-converting enzyme 2 (ACE2) by SIRT1 under conditions of cell energy stress. Clin. Sci. 2013, 126, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Modulation of renin angiotensin system predominantly alters sclerotic phenotype of glomeruli in HIVAN. Histol. Histopathol. 2014, 29, 1575–1581. [CrossRef]
- Chuang, P.Y.; Dai, Y.; Liu, R.; He, H.; Kretzler, M.; Jim, B.; Cohen, C.D.; He, J.C. Alteration of Forkhead Box O (Foxo4) Acetylation Mediates Apoptosis of Podocytes in Diabetes Mellitus. PLoS ONE 2011, 6, e23566. [Google Scholar] [CrossRef] [PubMed]
- Husain, M.; Meggs, L.G.; Vashistha, H.; Simoes, S.; Griffiths, K.O.; Kumar, D.; Mikulak, J.; Mathieson, P.W.; Saleem, M.A.; Del Valle, L.; et al. Inhibition of p66ShcA Longevity Gene Rescues Podocytes from HIV-1-induced Oxidative Stress and Apoptosis. J. Biol. Chem. 2009, 284, 16648–16658. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Zhao, L.; Han, Y.; Liu, Y.; Chen, C.; Zhan, M.; Xiong, X.; Zhu, X.; Xiao, L.; Hu, C.; et al. Probucol ameliorates renal injury in diabetic nephropathy by inhibiting the expression of the redox enzyme p66Shc. Redox Biol. 2017, 13, 482–497. [Google Scholar] [CrossRef]
- Kumar, S.; Kim, Y.-R.; Vikram, A.; Naqvi, A.; Li, Q.; Kassan, M.; Kumar, V.; Bachschmid, M.M.; Jacobs, J.S.; Kumar, A.; et al. Sirtuin1-regulated lysine acetylation of p66Shc governs diabetes-induced vascular oxidative stress and endothelial dysfunction. Proc. Natl. Acad. Sci. USA 2017, 114, 1714–1719. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Meng, L.; Zhao, L.; Wang, Z.; Liu, H.; Liu, G.; Guan, G. Resveratrol ameliorates hyperglycemia-induced renal tubular oxidative stress damage via modulating the SIRT1/FOXO3a pathway. Diabetes Res. Clin. Pract. 2017, 126, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Motonishi, S.; Nangaku, M.; Wada, T.; Ishimoto, Y.; Ohse, T.; Matsusaka, T.; Kubota, N.; Shimizu, A.; Kadowaki, T.; Tobe, K.; et al. Sirtuin1 Maintains Actin Cytoskeleton by Deacetylation of Cortactin in Injured Podocytes. J. Am. Soc. Nephrol. 2014, 26, 1939–1959. [Google Scholar] [CrossRef]
- Wang, X.; Liu, R.; Zhang, W.; Hyink, D.P.; Das, G.C.; Das, B.; Li, Z.; Wang, A.; Yuan, W.; Klotman, P.E.; et al. Role of SIRT1 in HIV-associated kidney disease. Am. J. Physiol. Physiol. 2020, 319, F335–F344. [Google Scholar] [CrossRef] [PubMed]
- High, K.P.; Brennan-Ing, M.; Clifford, D.B.; Cohen, M.H.; Currier, J.; Deeks, S.G.; Deren, S.; Effros, R.B.; Gebo, K.; Goronzy, J.J.; et al. HIV and Aging. JAIDS J. Acquir. Immune Defic. Syndr. 2012, 60, S1–S18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guaraldi, G.; Milic, J.; Mussini, C. Aging with HIV. Curr. HIV/AIDS Rep. 2019, 16, 475–481. [Google Scholar] [CrossRef]
- Liu, J.C.Y.; Leung, J.M.; Ngan, D.A.; Nashta, N.F.; Guillemi, S.; Harris, M.; Lima, V.D.; Um, S.-J.; Li, Y.; Tam, S.; et al. Absolute Leukocyte Telomere Length in HIV-Infected and Uninfected Individuals: Evidence of Accelerated Cell Senescence in HIV-Associated Chronic Obstructive Pulmonary Disease. PLoS ONE 2015, 10, e0124426. [Google Scholar] [CrossRef] [Green Version]
- Wątroba, M.; Dudek, I.; Skoda, M.; Stangret, A.; Rzodkiewicz, P.; Szukiewicz, D. Sirtuins, epigenetics and longevity. Ageing Res. Rev. 2017, 40, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Amano, H.; Sahin, E. Telomeres and sirtuins: At the end we meet again. Mol. Cell. Oncol. 2019, 6, e1632613. [Google Scholar] [CrossRef] [Green Version]
- Tasselli, L.; Zheng, W.; Chua, K.F. SIRT6: Novel Mechanisms and Links to Aging and Disease. Trends Endocrinol. Metab. 2016, 28, 168–185. [Google Scholar] [CrossRef] [Green Version]
- Farhadian, S.; Patel, P.; Spudich, S. Neurological Complications of HIV Infection. Curr. Infect. Dis. Rep. 2017, 19, 1–7. [Google Scholar] [CrossRef]
- Eggers, C.; Arendt, G.; Hahn, K.; Husstedt, I.W.; Maschke, M.; Neuen-Jacob, E.; Obermann, M.; Rosenkranz, T.; Schielke, E.; et al.; The German Association of Neuro-AIDS und Neuro-Infectiology (DGNANI) HIV-1-associated neurocognitive disorder: Epidemiology, pathogenesis, diagnosis, and treatment. J. Neurol. 2017, 264, 1715–1727. [Google Scholar] [CrossRef]
- Kakad, S.P. Neuro-AIDS: Current Status and Challenges to Antiretroviral Drug Therapy (ART) for Its Treatment. Curr. Drug Ther. 2021, 15, 469–481. [Google Scholar] [CrossRef]
- Dahl, V.; Peterson, J.; Fuchs, D.; Gisslen, M.; Palmer, S.; Price, R.W. Low levels of HIV-1 RNA detected in the cerebrospinal fluid after up to 10 years of suppressive therapy are associated with local immune activation. AIDS 2014, 28, 2251–2258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yilmaz, A.; Yiannoutsos, C.T.; Fuchs, D.; Price, R.W.; Crozier, K.; Hagberg, L.; Spudich, S.; Gisslen, M. Cerebrospinal fluid neopterin decay characteristics after initiation of antiretroviral therapy. J. Neuroinflamm. 2013, 10, 828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nookala, A.R.; Kumar, A. Molecular mechanisms involved in HIV-1 Tat-mediated induction of IL-6 and IL-8 in astrocytes. J. Neuroinflamm. 2014, 11, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandramowlishwaran, P.; Vijay, A.; Abraham, D.; Li, G.; Mwangi, S.M.; Srinivasan, S. Role of Sirtuins in Modulating Neurodegeneration of the Enteric Nervous System and Central Nervous System. Front. Neurosci. 2020, 14, 1368. [Google Scholar] [CrossRef] [PubMed]
- Dobbin, M.M.; Madabhushi, R.; Pan, L.; Chen, Y.; Kim, D.; Gao, J.; Ahanonu, B.; Pao, P.-C.; Qiu, Y.; Zhao, Y.; et al. SIRT1 collaborates with ATM and HDAC1 to maintain genomic stability in neurons. Nat. Neurosci. 2013, 16, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Swinton, M.K.; Carson, A.; Telese, F.; Sanchez, A.B.; Soontornniyomkij, B.; Rad, L.; Batki, I.; Quintanilla, B.; Pérez-Santiago, J.; Achim, C.L.; et al. Mitochondrial biogenesis is altered in HIV+ brains exposed to ART: Implications for therapeutic targeting of astroglia. Neurobiol. Dis. 2019, 130, 104502. [Google Scholar] [CrossRef]
- Wareski, P.; Vaarmann, A.; Choubey, V.; Safiulina, D.; Liiv, J.; Kuum, M.; Kaasik, A. PGC-1α and PGC-1Β Regulate Mitochondrial Density in Neurons. J. Biol. Chem. 2009, 284, 21379–21385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozzi, S.J.; Avdoshina, V.; Fields, J.A.; Trejo, M.; Ton, H.T.; Ahern, G.P.; Mocchetti, I. Human Immunodeficiency Virus Promotes Mitochondrial Toxicity. Neurotox. Res. 2017, 32, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, A.; Chivero, E.T.; Tripathi, A.; Singh, S.; Niu, F.; Guo, M.-L.; Pillai, P.; Periyasamy, P.; Buch, S. HIV TAT-mediated microglial senescence: Role of SIRT3-dependent mitochondrial oxidative stress. Redox Biol. 2020, 40, 101843. [Google Scholar] [CrossRef]
- Fields, J.A.; Serger, E.; Campos, S.; Divakaruni, A.S.; Kim, C.; Smith, K.; Trejo, M.; Adame, A.; Spencer, B.; Rockenstein, E.; et al. HIV alters neuronal mitochondrial fission/fusion in the brain during HIV-associated neurocognitive disorders. Neurobiol. Dis. 2015, 86, 154–169. [Google Scholar] [CrossRef] [Green Version]
- Meng, H.; Yan, W.-Y.; Lei, Y.-H.; Wan, Z.; Hou, Y.-Y.; Sun, L.-K.; Zhou, J.-P. SIRT3 Regulation of Mitochondrial Quality Control in Neurodegenerative Diseases. Front. Aging Neurosci. 2019, 11, 313. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, M.F.; Genebra, T.; Rego, A.C.; Rodrigues, C.M.P.; Solá, S. Amyloid β Peptide Compromises Neural Stem Cell Fate by Irreversibly Disturbing Mitochondrial Oxidative State and Blocking Mitochondrial Biogenesis and Dynamics. Mol. Neurobiol. 2018, 56, 3922–3936. [Google Scholar] [CrossRef]
- Thangaraj, A.; Periyasamy, P.; Liao, K.; Bendi, V.S.; Callen, S.; Pendyala, G.; Buch, S. HIV-1 TAT-mediated microglial activation: Role of mitochondrial dysfunction and defective mitophagy. Autophagy 2018, 14, 1596–1619. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.; Liao, K.; Yang, L.; Pendyala, G.; Kook, Y.; Fox, H.S.; Buch, S. Tat-Mediated Induction of miRs-34a & -138 Promotes Astrocytic Activation via Downregulation of SIRT1: Implications for Aging in HAND. J. Neuroimmune Pharmacol. 2017, 12, 420–432. [Google Scholar] [CrossRef]
- Castro, V.; Bertrand, L.; Luethen, M.; Dabrowski, S.; Lombardi, J.; Morgan, L.; Sharova, N.; Stevenson, M.; Blasig, I.E.; Toborek, M. Occludin controls HIV transcription in brain pericytes via regulation of SIRT-1 activation. FASEB J. 2015, 30, 1234–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhuri, A.D.; Yelamanchili, S.V.; Fox, H.S. MicroRNA-142 Reduces Monoamine Oxidase a Expression and Activity in Neuronal Cells by Downregulating SIRT1. PLoS ONE 2013, 8, e79579. [Google Scholar] [CrossRef] [Green Version]
- Gaskill, P.J.; Miller, D.R.; Gamble-George, J.; Yano, H.; Khoshbouei, H. HIV, Tat and dopamine transmission. Neurobiol. Dis. 2017, 105, 51–73. [Google Scholar] [CrossRef] [PubMed]
- Khanlou, N.; Moore, D.J.; Chana, G.; Cherner, M.; Lazzaretto, D.; Dawes, S.; Grant, I.; Masliah, E.; Everall, I.P.; The HNRC Group. Increased frequency of α-synuclein in the substantia nigra in human immunodeficiency virus infection. J. NeuroVirol. 2009, 15, 131–138. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, R.M.; Miranda, H.V.; Francelle, L.; Pinho, R.; Szegö, M.; Martinho, R.; Munari, F.; Lázaro, D.F.; Moniot, S.; Guerreiro, P.; et al. The mechanism of sirtuin 2–mediated exacerbation of alpha-synuclein toxicity in models of Parkinson disease. PLoS Biol. 2017, 15, e2000374. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, J.; Hong, T.-T.; Sun, Y.; Huang, H.; Chen, F.; Chen, X.; Chen, H.; Dong, S.; Cui, L.; et al. RTN4B-mediated suppression of Sirtuin 2 activity ameliorates β-amyloid pathology and cognitive impairment in Alzheimer’s disease mouse model. Aging Cell 2020, 19, e13194. [Google Scholar] [CrossRef] [PubMed]
- Reznik, A.D. Oral manifestations of HIV disease. Top. HIV Med. A Publ. Int. AIDS Soc. USA 2005, 13, 143–148. [Google Scholar]
- Aškinytė, D.; Matulionytė, R.; Rimkevičius, A. Oral manifestations of HIV disease: A review. Stomatologija 2015, 17, 21–28. [Google Scholar]
- Nokta, M. Oral manifestations associated with HIV infection. Curr. HIV/AIDS Rep. 2008, 5, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Amador, V.; Ponce-De-León, S.; Anaya-Saavedra, G.; Ramírez, B.C.; Sierra-Madero, J. Oral Lesions as Clinical Markers of Highly Active Antiretroviral Therapy Failure: A Nested Case-Control Study in Mexico City. Clin. Infect. Dis. 2007, 45, 925–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez-Amador, V.; Esquivel-Pedraza, L.; Sierra-Madero, J.; Anaya-Saavedra, G.; González-Ramírez, I.; Ponce-De-León, S. The Changing Clinical Spectrum of Human Immunodeficiency Virus (HIV)-Related Oral Lesions in 1000 Consecutive Patients. Medicine 2003, 82, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, D.; Canchola, A.J.; MacPhail, L.A.; Cheikh, B.; Greenspan, J.S. Effect of highly active antiretroviral therapy on frequency of oral warts. Lancet 2001, 357, 1411–1412. [Google Scholar] [CrossRef]
- Cameron, J.; Mercante, D.; O’Brien, M.; Gaffga, A.M.; Leigh, J.E.; Fidel, P.L.; Hagensee, M.E. The Impact of Highly Active Antiretroviral Therapy and Immunodeficiency on Human Papillomavirus Infection of the Oral Cavity of Human Immunodeficiency Virus–Seropositive Adults. Sex. Transm. Dis. 2005, 32, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Navazesh, M.; Mulligan, R.; Karim, R.B.; Mack, W.J.; Ram, S.J.; Seirawan, H.; Greenspan, D.S.; Phelan, J.; Alves, M.C.G.P.; The Oral Substudy of the WIHS Collaborative Study Group. Effect of HAART on salivary gland function in the Women’s Interagency HIV Study (WIHS). Oral Dis. 2008, 15, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Chapple, I.L.C. The significance of oral health in HIV disease. Sex. Transm. Infect. 2000, 76, 236–243. [Google Scholar] [CrossRef] [Green Version]
- Leão, J.C.; Ribeiro, C.M.B.; Carvalho, A.A.T.; Frezzini, C.; Porter, S. Oral complications of HIV disease. Clinics 2009, 64, 459–470. [Google Scholar] [CrossRef] [Green Version]
- Jang, Y.-E.; Go, S.-H.; Lee, B.-N.; Chang, H.-S.; Hwang, I.-N.; Oh, W.-M.; Hwang, Y.-C. Changes in SIRT gene expression during odontoblastic differentiation of human dental pulp cells. Restor. Dent. Endod. 2015, 40, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-J.; Kim, S.-Y.; Kim, Y.-S.; Park, S.-H.; Kim, E.-C. The Role of SIRT1 on Angiogenic and Odontogenic Potential in Human Dental Pulp Cells. J. Endod. 2012, 38, 899–906. [Google Scholar] [CrossRef]
- Islam, S.; Abiko, Y.; Uehara, O.; Chiba, I. Sirtuin 1 and oral cancer (Review). Oncol. Lett. 2018, 17, 729–738. [Google Scholar] [CrossRef] [Green Version]
- Schemies, J.; Uciechowska, U.; Sippl, W.; Jung, M. NAD+ -dependent histone deacetylases (sirtuins) as novel therapeutic targets. Med. Res. Rev. 2009, 30, 861–889. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-Y.; Sun, F.-L.; Zhang, Y.; Wang, Z. SIRT1 acts as a potential tumor suppressor in oral squamous cell carcinoma. J. Chin. Med Assoc. 2018, 81, 416–422. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurkowska, K.; Szymańska, B.; Knysz, B.; Kuźniarski, A.; Piwowar, A. Sirtuins as Interesting Players in the Course of HIV Infection and Comorbidities. Cells 2021, 10, 2739. https://doi.org/10.3390/cells10102739
Jurkowska K, Szymańska B, Knysz B, Kuźniarski A, Piwowar A. Sirtuins as Interesting Players in the Course of HIV Infection and Comorbidities. Cells. 2021; 10(10):2739. https://doi.org/10.3390/cells10102739
Chicago/Turabian StyleJurkowska, Karolina, Beata Szymańska, Brygida Knysz, Amadeusz Kuźniarski, and Agnieszka Piwowar. 2021. "Sirtuins as Interesting Players in the Course of HIV Infection and Comorbidities" Cells 10, no. 10: 2739. https://doi.org/10.3390/cells10102739
APA StyleJurkowska, K., Szymańska, B., Knysz, B., Kuźniarski, A., & Piwowar, A. (2021). Sirtuins as Interesting Players in the Course of HIV Infection and Comorbidities. Cells, 10(10), 2739. https://doi.org/10.3390/cells10102739





