Peripartum Investigation of Red Blood Cell Properties in Women Diagnosed with Early-Onset Preeclampsia
Abstract
:1. Introduction
2. Aim
3. Materials and Methods
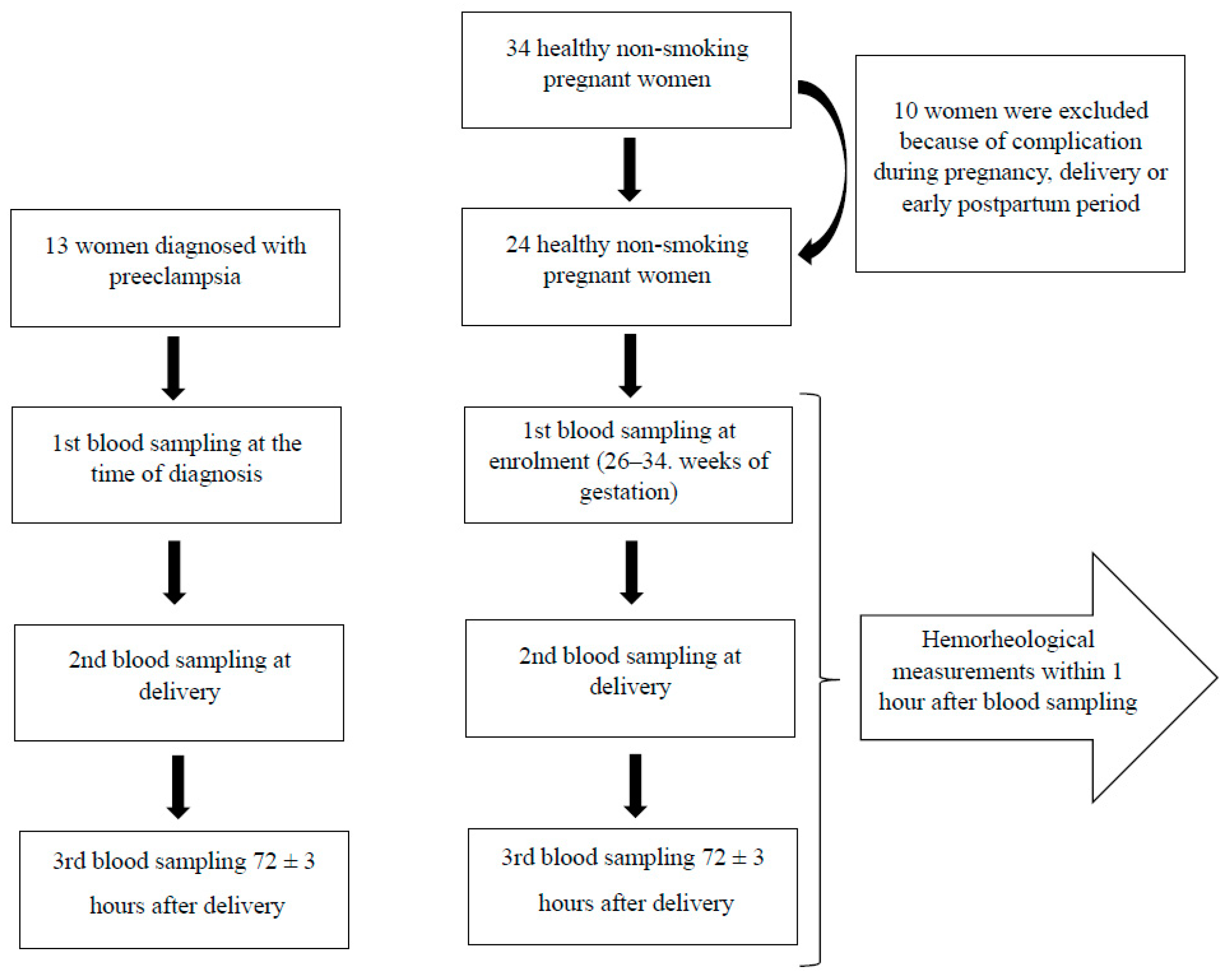
3.1. Population
3.2. Sample and Data Collection
3.3. RBC Aggregation
3.4. RBC Deformability
3.5. Statistical Analysis
4. Results
4.1. Population Characteristics
4.2. Routinely Measured RBC Laboratory Parameters
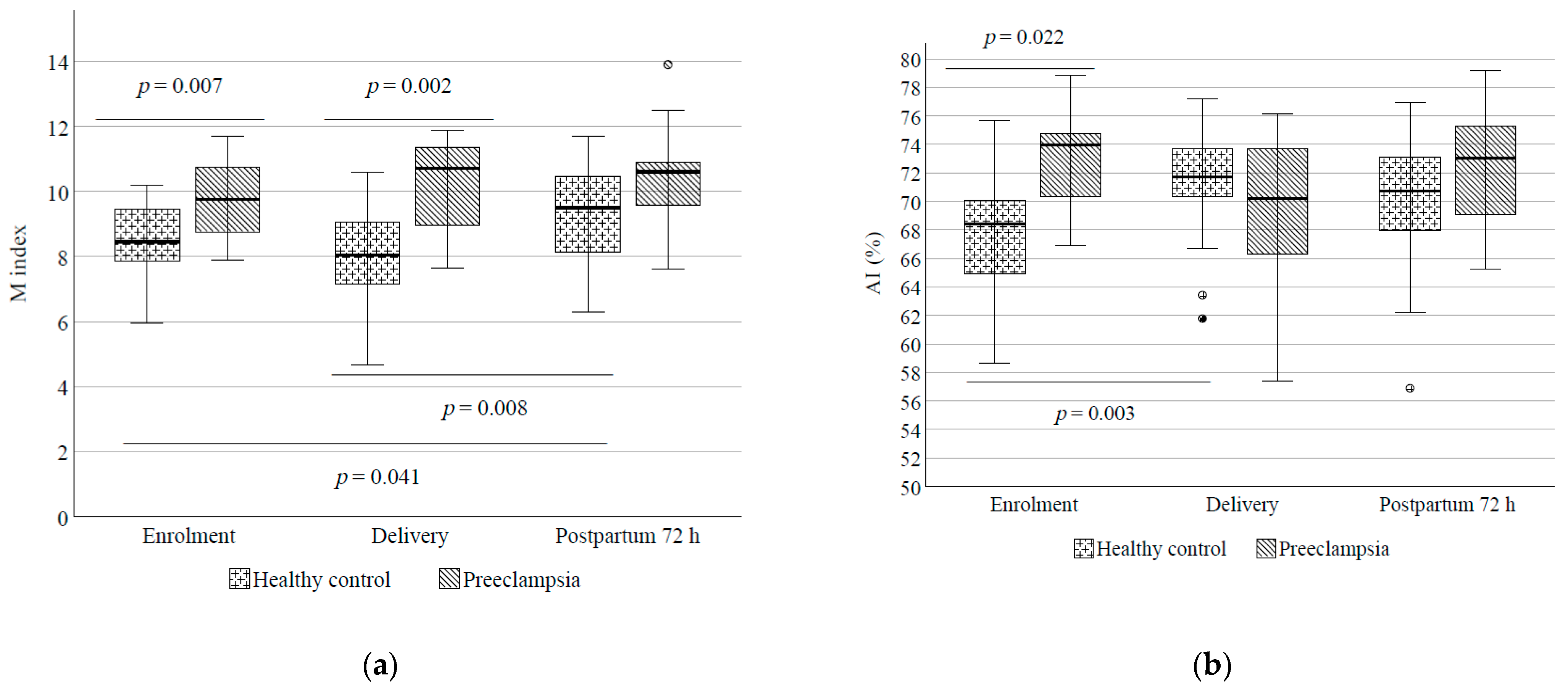
4.3. RBC Aggregation
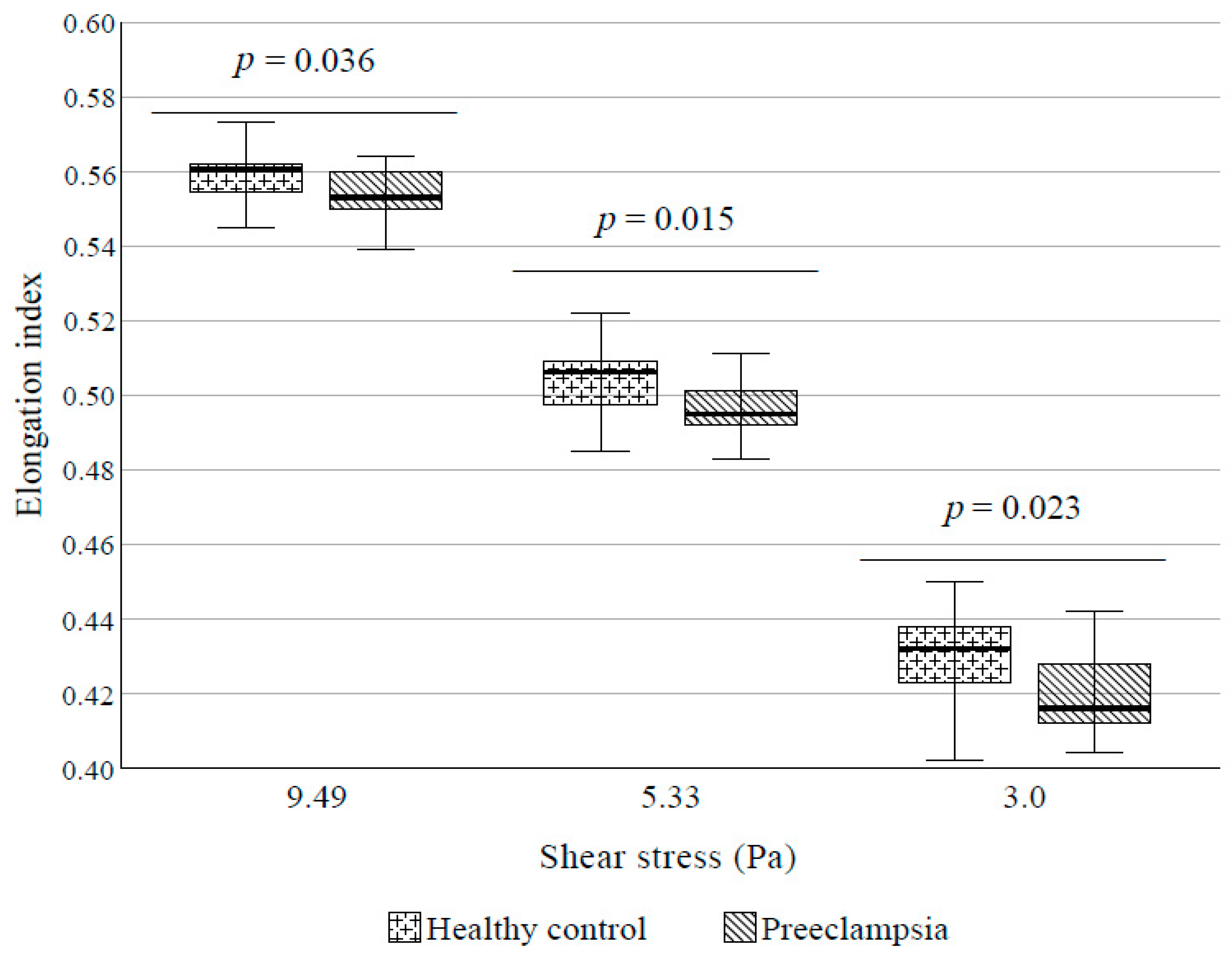
4.4. RBC Deformability
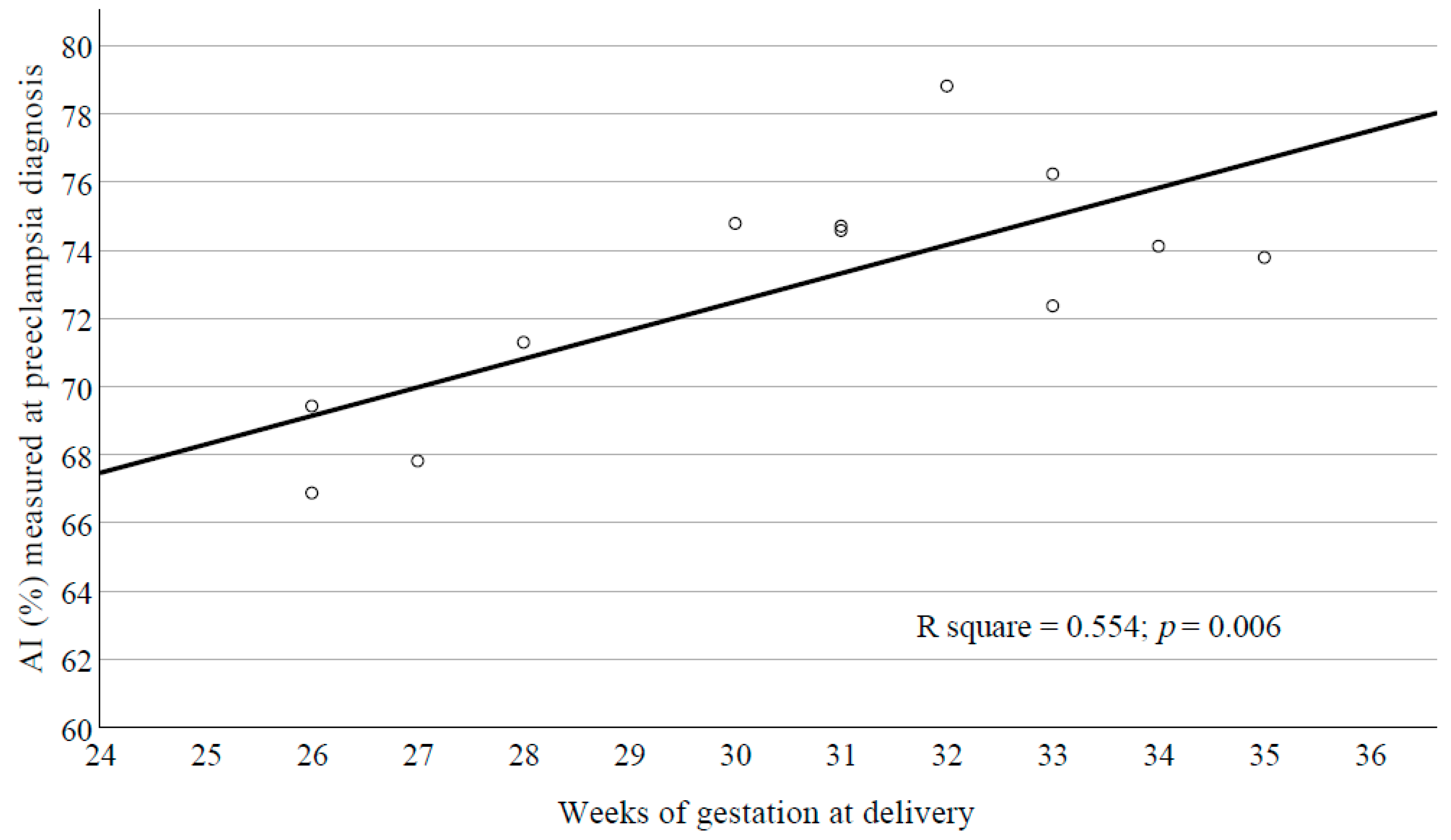
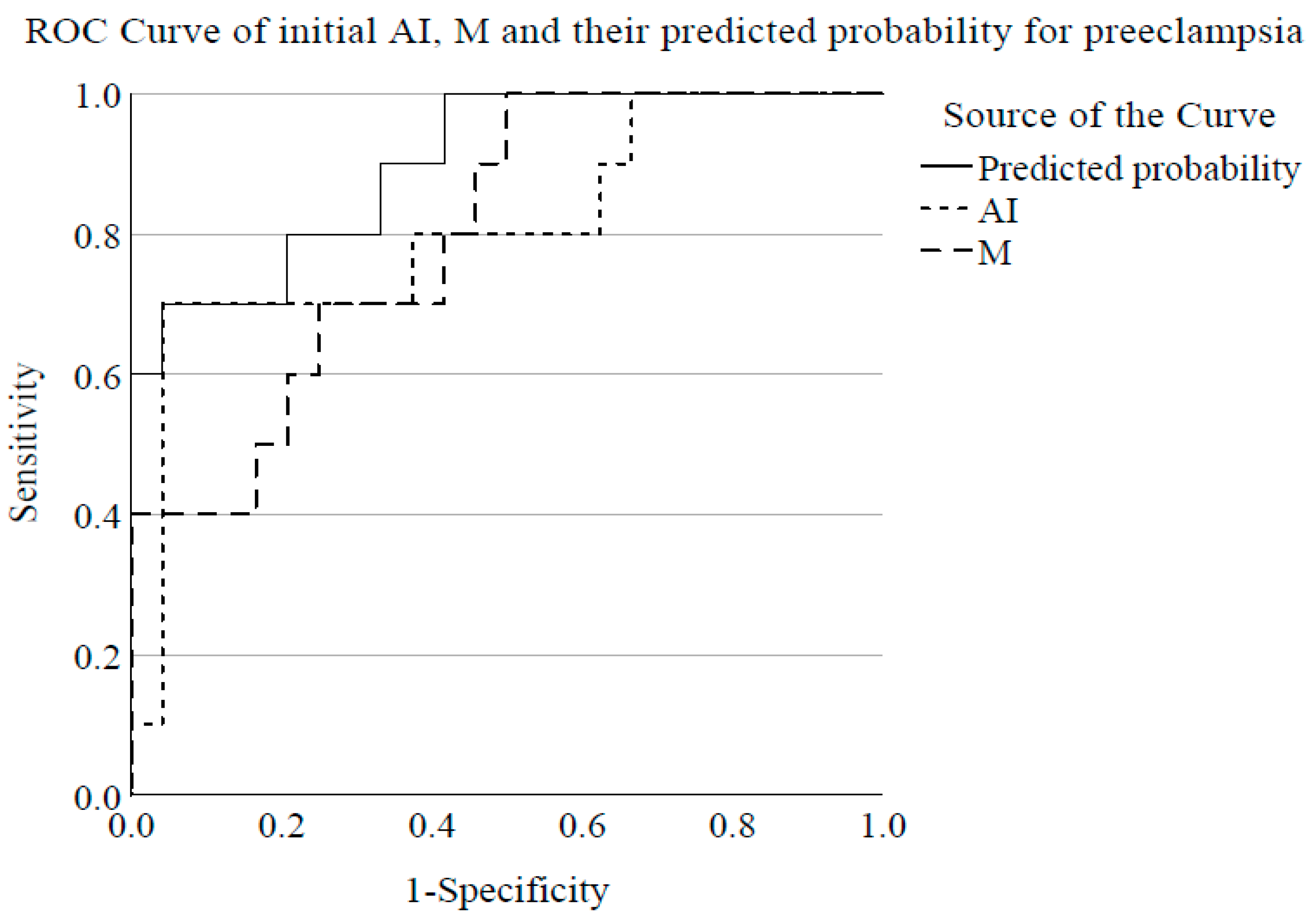
4.5. Indicators of RBC Aggregation for Preeclampsia Diagnosis
5. Discussion
5.1. Routinely Measured RBC Laboratory Parameters
5.2. RBC Aggregation
5.3. RBC Deformability
5.4. Previous Data Concerning RBC Properties
5.5. Screening
5.6. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villar, J.; Carroli, G.; Wojdyla, D.; Abalos, E.; Giordano, D.; Baaqeel, H.; Farnot, U.; Bergsjø, P.; Bakketeig, L.; Lumbiganon, P.; et al. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions? Am. J. Obstet. Gynecol. 2006, 194, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Say, L.; Chou, D.; Gemmill, A.; Tunçalp, Ö.; Moller, A.-B.; Daniels, J.; Gülmezoglu, A.M.; Temmerman, M.; Alkema, L. Global causes of maternal death: A WHO systematic analysis. Lancet Glob. Health 2014, 2, e323–e333. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018, 13, 291–310. [Google Scholar] [PubMed]
- Lisonkova, S.; Sabr, Y.; Mayer, C.; Young, C.; Skoll, A.; Joseph, K. Maternal Morbidity Associated With Early-Onset and Late-Onset Preeclampsia. Obstet. Gynecol. 2014, 124, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Vahedi, F.A.; Gholizadeh, L.; Heydari, M. Hypertensive Disorders of Pregnancy and Risk of Future Cardiovascular Disease in Women. Nurs. Women’s Health 2020, 24, 91–100. [Google Scholar] [CrossRef]
- McDonald, S.D.; Malinowski, A.; Zhou, Q.; Yusuf, S.; Devereaux, P. Cardiovascular sequelae of preeclampsia/eclampsia: A systematic review and meta-analyses. Am. Heart J. 2008, 156, 918–930. [Google Scholar] [CrossRef]
- Staff, A.C.; Fjeldstad, H.E.; Fosheim, I.K.; Moe, K.; Turowski, G.; Johnsen, G.M.; Alnaes-Katjavivi, P.; Sugulle, M. Failure of physiological transformation and spiral artery atherosis: Their roles in preeclampsia. Am. J. Obstet. Gynecol. 2020. [CrossRef] [PubMed]
- Powe, C.E.; Levine, R.J.; Karumanchi, S.A. Preeclampsia, a disease of the maternal endothelium: The role of antiangiogenic factors and implications for later cardiovascular disease. Circulation 2011, 123, 2856–2869. [Google Scholar] [CrossRef]
- Hernández Hernández, J.D.; Villaseñor, O.R.; Del Rio Alvarado, J.; Lucach, R.O.; Zárate, A.; Saucedo, R.; Hernández-Valencia, M. Morphological changes of red blood cells in peripheral blood smear of patients with pregnancy-related hypertensive disorders. Arch. Med. Res. 2015, 46, 479–483. [Google Scholar] [CrossRef]
- Gamzu, R.; Barshtein, G.; Tsipis, F.; Lessing, J.B.; Berliner, A.S.; Kupferminc, M.J.; Eldor, A.; Yedgar, S. Pregnancy-induced hypertension is associated with elevation of aggregability of red blood cells. Clin. Hemorheol. Microcirc. 2002, 27, 163–169. [Google Scholar]
- Baskurt, O.K.; Meiselman, H.J. Erythrocyte aggregation: Basic aspects and clinical importance. Clin. Hemorheol. Microcirc. 2013, 53, 23–37. [Google Scholar] [CrossRef] [Green Version]
- Soliman, A.A.; Csorba, R.; Yilmaz, A.; Tsikoaras, P.; von Tempelhoff, G.F. Rheologic results and their correlation to hemostatic changes in patients with moderate and severe preeclampsia: An observational cross-sectional study. Clin. Hemorheol. Microcirc. 2015, 59, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vayá, A.; Falcó, C.; Fernández, P.; Contreras, T.; Valls, M.; Aznar, J. Erythrocyte aggregation determined with the Myrenne aggregometer at two modes (M0, M1) and at two times (5 and 10 sec). Clin. Hemorheol. Microcirc. 2003, 29, 119–127. [Google Scholar] [PubMed]
- Hardeman, M.R.; Dobbe, J.G.; Ince, C. The Laser-assisted Optical Rotational Cell Analyzer (LORCA) as red blood cell aggregometer. Clin. Hemorheol. Microcirc. 2001, 25, 1–11. [Google Scholar] [PubMed]
- Rabai, M.; Detterich, J.A.; Wenby, R.B.; Hernandez, T.M.; Toth, K.; Meiselman, H.J.; Wood, J.C. Deformability analysis of sickle blood using ektacytometry. Biorheology 2014, 51, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Chesley, L.C. Plasma and red cell volumes during pregnancy. Am. J. Obstet. Gynecol. 1972, 112, 440–450. [Google Scholar] [CrossRef]
- Heilmann, L.; Rath, W.; Pollow, K. Hemorheological changes in women with severe preeclampsia. Clin. Hemorheol. Microcirc. 2004, 31, 49–58. [Google Scholar] [PubMed]
- Elgari, M.M.; Khabour, O.F.; Alhag, S.M. Correlations between changes in hematological indices of mothers with preeclampsia and umbilical cord blood of newborns. Clin. Exp. Hypertens. 2019, 41, 58–61. [Google Scholar] [CrossRef]
- de Freitas, M.A.R.; da Costa, A.V.; Medeiros, L.A.; Cunha, L.M.; Coutinho Filho, U.; Garrote Filho, M.D.S.; Diniz, A.L.D.; Penha-Silva, N. The role of the erythrocyte in the outcome of pregnancy with preeclampsia. PLoS ONE 2019, 14, e0212763. [Google Scholar] [CrossRef]
- Krüger-Genge, A.; Sternitzky, R.; Pindur, G.; Rampling, M.; Franke, R.P.; Jung, F. Erythrocyte aggregation in relation to plasma proteins and lipids. J. Cell. Biotechnol. 2019, 5, 65–70. [Google Scholar] [CrossRef]
- Tranquilli, A.L.; Curzi, C.M.; Moretti, N.; Nanetti, L.; Mazzanti, L. 362 Reduction of sialic acid and membrane fluidity in erythrocyte from preeclamptic patients as the ground for increased aggregation. Am. J. Obstetr. Gynecol. 2001, 185, S180. [Google Scholar] [CrossRef]
- Tranquilli, A.L.; Gioele Garzetti, G.; De Tommaso, G.; Boemi, M.; Lucino, E.; Fumelli, P.; Romanini, C. Nifedipine treatment in preeclampsia reverts the increased erythrocyte aggregation to normal. Am. J. Obstetr. Gynecol. 1992, 167, 942–945. [Google Scholar] [CrossRef]
- Sen, P.; Ghosh, D.; Sarkar, C. Erythrocytic membrane anionic charge, sialic acid content, and their correlations with urinary glycosaminoglycans in preeclampsia and eclampsia. Scand. J. Clin. Lab. Investig. 2020, 80, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Huisman, A.; Aarnoudse, J.G.; Krans, M.; Huisjes, H.J.; Fidler, V.; Zijlstra, W.G. Red cell aggregation during normal pregnancy. Br. J. Haematol. 1988, 68, 121–124. [Google Scholar] [CrossRef]
- Xu, C.; Li, Y.; Zhang, W.; Wang, Q. Analysis of perinatal coagulation function in preeclampsia. Medicine 2021, 100, e26482. [Google Scholar] [CrossRef] [PubMed]
- Friederichs, E.; Meiselman, H.J. Effects of calcium permeabilization on RBC rheologic behavior. Biorheology 1994, 31, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Schauf, B.; Aydeniz, B.; Pijnenborg, R.; Wallwiener, D. Connection between the deformability of the erythrocytes and intraerythrocyte ATP in pregnancy. Geburtshilfe Und Frauenheilkunde 2002, 62, 477–480. [Google Scholar] [CrossRef]
- Mohandas, N.; Clark, M.R.; Jacobs, M.S.; Shohet, S.B. Analysis of factors regulating erythrocyte deformability. J. Clin. Investig. 1980, 66, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Schauf, B.; Lang, U.; Stute, P.; Schneider, S.; Dietz, K.; Aydeniz, B.; Wallwiener, D. Reduced red blood cell deformability, an indicator for high fetal or maternal risk, is found in preeclampsia and IUGR. Hypertens Pregnancy 2002, 21, 147–160. [Google Scholar] [CrossRef]
- Ray, J.; Vasishta, K.; Kaur, S.; Majumdar, S.; Sawhney, H. Calcium metabolism in pre-eclampsia. Int. J. Gynaecol. Obstet. 1999, 66, 245–250. [Google Scholar] [CrossRef]
- Oviedo, N.J.; Benaim, G.; Cervino, V.; Proverbio, T.; Proverbio, F.; Marín, R. The plasma membrane Ca2+-ATPase protein from red blood cells is not modified in preeclampsia. Biochim. Biophys. Acta 2006, 1762, 381–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srour, M.A.; Bilto, Y.Y.; Juma, M.; Irhimeh, M.R. Exposure of human erythrocytes to oxygen radicals causes loss of deformability, increased osmotic fragility, lipid peroxidation and protein degradation. Clin. Hemorheol. Microcirc. 2000, 23, 13–21. [Google Scholar] [PubMed]
- Gresele, P.; Guerciolini, R.; Nenci, G.G. Erythrocyte deformability changes in normal pregnancy and pre-eclampsia. Br. J. Haematol. 1982, 52, 340–342. [Google Scholar] [CrossRef]
- Inglis, T.C.; Stuart, J.; George, A.J.; Davies, A.J. Haemostatic and rheological changes in normal pregnancy and pre-eclampsia. Br. J. Haematol. 1982, 50, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Lang, G.D.; Lowe, G.D.; Walker, J.J.; Forbes, C.D.; Prentice, C.R.; Calder, A.A. Blood rheology in pre-eclampsia and intrauterine growth retardation: Effects of blood pressure reduction with labetalol. Br. J. Obstet. Gynaecol. 1984, 91, 438–443. [Google Scholar] [CrossRef]
- Pepple, D.J.; Hardeman, M.R.; Mullings, A.M.; Reid, H.L. Erythrocyte deformability and erythrocyte aggregation in preeclampsia. Clin. Hemorheol. Microcirc. 2001, 24, 43–48. [Google Scholar] [PubMed]
- Ozanne, P.; Miller, F.C.; Meiselman, H.J. RBC aggregation in diabetic and pre-eclamptic pregnancy. Clin. Hemorheol. Microcirc. 1983, 3, 145–154. [Google Scholar] [CrossRef]
- Burton, G.J.; Redman, C.W.; Roberts, J.M.; Moffett, A. Pre-eclampsia: Pathophysiology and clinical implications. BMJ 2019, 366, l2381. [Google Scholar] [CrossRef] [Green Version]
- Jakobsen, C.; Larsen, J.B.; Fuglsang, J.; Hvas, A.M. Platelet function in preeclampsia—A systematic review and meta-analysis. Platelets 2019, 30, 549–562. [Google Scholar] [CrossRef]
- Rolnik, D.L.; Wright, D.; Poon, L.C.; O’Gorman, N.; Syngelaki, A.; de Paco Matallana, C.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N. Engl. J. Med. 2017, 377, 613–622. [Google Scholar] [CrossRef]
- Poon, L.C.; Shennan, A.; Hyett, J.A.; Kapur, A.; Hadar, E.; Divakar, H.; McAuliffe, F.; da Silva Costa, F.; von Dadelszen, P.; McIntyre, H.D.; et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int. J. Gynaecol. Obstet. 2019, 145 (Suppl. 1), 1–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Maternal | Baseline Characteristics | Preeclampsia | Control | p-Value |
| Age (years) | 29.08 ± 2.13 | 30.42 ± 1.39 | 0.589 | |
| Gestation age at first sampling (weeks) | 29.69 ± 0.67 | 28.71 ± 0.48 | 0.240 | |
| Systolic blood pressure at admission (mmHg) | 160 (146–175) | 120 (119–130) | <0.001 | |
| Diastolic blood pressure at admission (mmHg) | 100 (90–110) | 80 (70–80) | <0.001 | |
| Heart rate at admission (/min) | 82 (80–95) | 82 (77–88) | 0.320 | |
| Body height (m) | 1.63 ± 0.06 | 1.67 ± 0.07 | 0.094 | |
| Body weight (kg) | 78 (70–87) | 80 (70–86) | 0.824 | |
| BMI (kg/m2) | 30.1 (26.8–33.5) | 28.2 (25.7–30.2) | 0.227 | |
| Change in body weight (kg) | 10 ± 7 | 13 ± 4 | 0.132 | |
| Mode of delivery: cesarean section (n; %) | 13; 100% | 9; 37.5% | <0.001 | |
| Length of hospital stay (day) | 8 (6–15) | 4 (4–5) | <0.001 | |
| Neonatal | Gestational age at birth (weeks) | 30.23 ± 0.86 | 39.06 ± 0.28 | <0.001 |
| Birth weight (gram) | 1355.83 ± 157.69 | 3420.00 ± 89.04 | <0.001 | |
| Birth length (cm) | 36.67 ± 1.64 | 49.76 ± 0.80 | <0.001 | |
| Head circumference (cm) | 27.81 ± 1.01 | 34.24 ± 0.35 | <0.001 | |
| Shoulder width (cm) | 28.09 ± 1.30 | 36.88 ± 0.57 cm | <0.001 | |
| Apgar 1 | 7.0 (6.0–8.0) | 9.0 (9.0–9.0) | <0.001 | |
| Apgar 5 | 9.0 (8.0–9.0) | 10.0 (10.0–10.0) | <0.001 | |
| IUGR (n; %) | 7; 53.8% | 0 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csiszar, B.; Galos, G.; Funke, S.; Kevey, D.K.; Meggyes, M.; Szereday, L.; Kenyeres, P.; Toth, K.; Sandor, B. Peripartum Investigation of Red Blood Cell Properties in Women Diagnosed with Early-Onset Preeclampsia. Cells 2021, 10, 2714. https://doi.org/10.3390/cells10102714
Csiszar B, Galos G, Funke S, Kevey DK, Meggyes M, Szereday L, Kenyeres P, Toth K, Sandor B. Peripartum Investigation of Red Blood Cell Properties in Women Diagnosed with Early-Onset Preeclampsia. Cells. 2021; 10(10):2714. https://doi.org/10.3390/cells10102714
Chicago/Turabian StyleCsiszar, Beata, Gergely Galos, Simone Funke, Dora Kinga Kevey, Matyas Meggyes, Laszlo Szereday, Peter Kenyeres, Kalman Toth, and Barbara Sandor. 2021. "Peripartum Investigation of Red Blood Cell Properties in Women Diagnosed with Early-Onset Preeclampsia" Cells 10, no. 10: 2714. https://doi.org/10.3390/cells10102714
APA StyleCsiszar, B., Galos, G., Funke, S., Kevey, D. K., Meggyes, M., Szereday, L., Kenyeres, P., Toth, K., & Sandor, B. (2021). Peripartum Investigation of Red Blood Cell Properties in Women Diagnosed with Early-Onset Preeclampsia. Cells, 10(10), 2714. https://doi.org/10.3390/cells10102714






