Antioxidant and Antiinflammatory Effects of Epilobium parviflorum, Melilotus officinalis and Cardiospermum halicacabum Plant Extracts in Macrophage and Microglial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Extracts
2.3. Total Phenolic Content
2.4. Flavonoid Content
2.5. Total Condensed Tannins
2.6. Cell Cultures
2.7. Cellular Treatments
2.8. DPPH Test
2.9. MTS Assay
2.10. H2DCFDA Assay
2.11. Nitric Oxide Assay
2.12. Membrane Preparation
2.13. Radioligand Binding Experiments
2.14. Statistical Analysis
3. Results
3.1. Phenols Contents of Plant Extracts
3.2. Antioxidant Properties of Plant Extracts
3.3. Cells Viability Following Treatment with Epilobium parviflorum, Melilotus officinalis and Cardiospermum halicacabum on RAW 264.7 Macrophage and N9 Microglial Cells
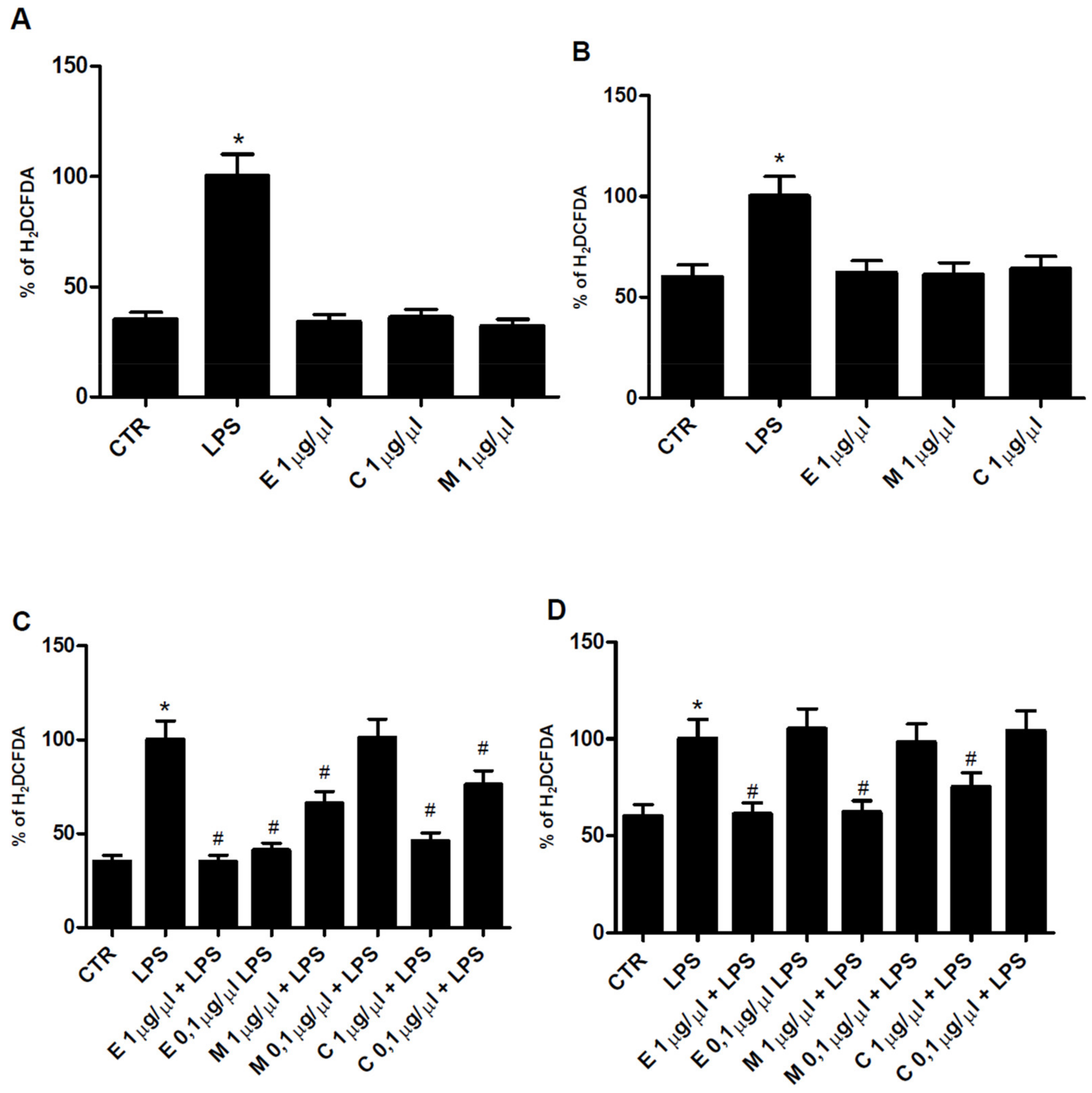
3.4. Antioxidant Properties of Epilobium parviflorum, Melilotus officinalis, and Cardiospermum halicacabum on RAW 264.7 Macrophage Cells
3.5. Antiinflammatory Properties of Epilobium parviflorum, Melilotus officinalis and Cardiospermum halicacabum on RAW 264.7 and N9 Cells
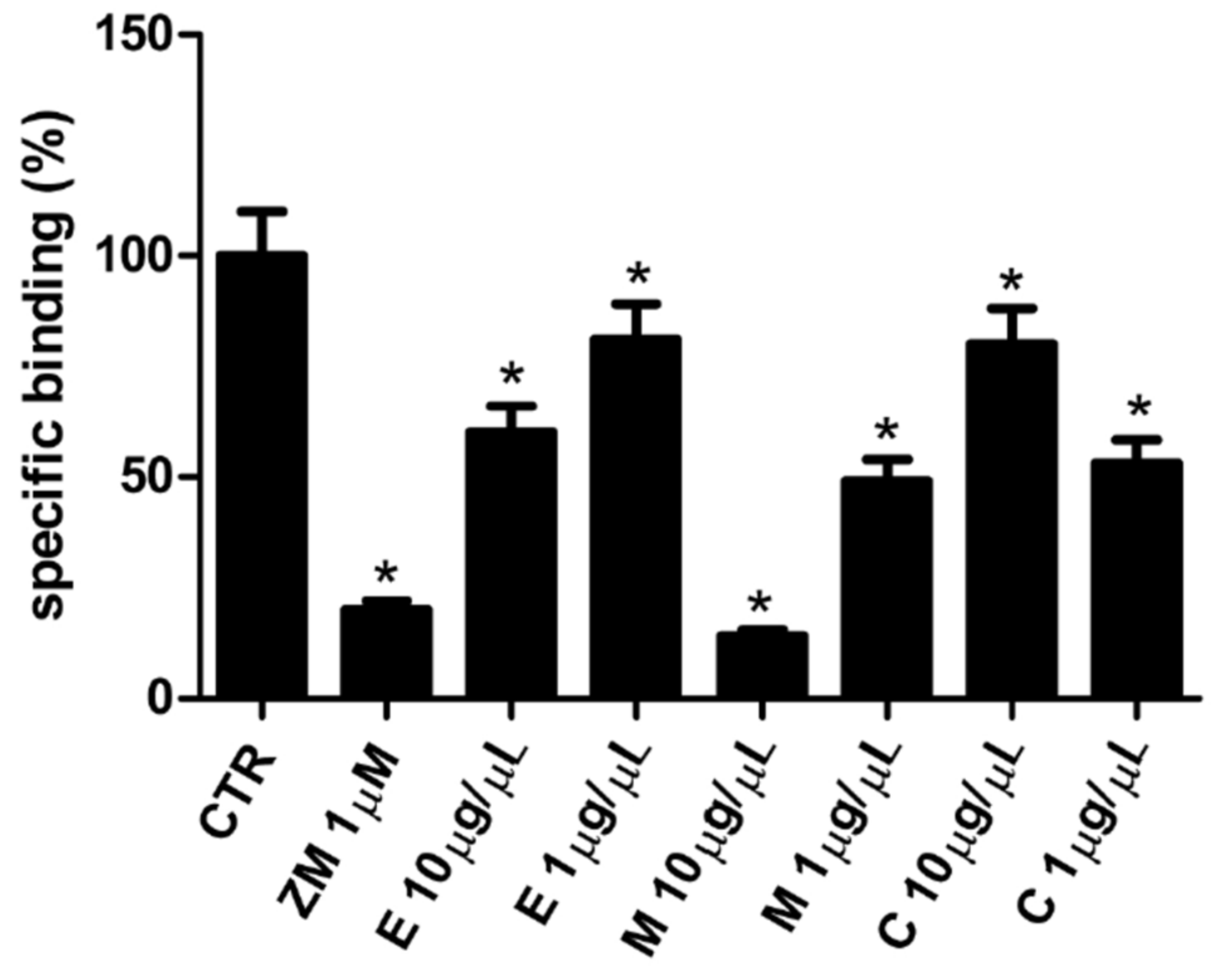
3.6. Affinity of Epilobium parviflorum, Melilotus officinalis, and Cardiospermum halicacabum for A2A Adenosine Receptors
3.7. Expression of A2A Adenosine Receptors in RAW 264.7 and N9 Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Enioutina, E.Y.; Salis, E.R.; Job, K.M.; Gubarev, M.I.; Krepkova, L.V.; Sherwin, C.M. Herbal Medicines: Challenges in the modern world. Part 5. status and current directions of complementary and alternative herbal medicine worldwide. Expert Rev. Clin. Pharmacol. 2017, 10, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Koparde, A.A.; Doijad, R.C.; Magdum, C.S. Natural Products in Drug Discovery. In Pharmacognosy; Perveen, S., Al-Taweel, A., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Thelingwani, R.; Masimirembwa, C. Evaluation of Herbal Medicines: Value Addition to Traditional Medicines Through Metabolism, Pharmacokinetic and Safety Studies. Curr. Drug Metab. 2014, 15, 942–952. [Google Scholar] [CrossRef]
- Barnes, J.; McLachlan, A.J.; Sherwin, C.M.T.; Enioutina, E.Y. Herbal Medicines: Challenges in the Modern World. Part 1 (Australia and New Zealand). Expert Rev. Clin. Pharmacol. 2016, 9, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Sammons, H.M.; Gubarev, M.I.; Krepkova, L.V.; Bortnikova, V.V.; Corrick, F.; Job, K.M.; Sherwin, C.M.; Enioutina, E.Y. Herbal medicines: Challenges in the modern world. Part 2. European Union and Russia. Expert Rev. Clin. Pharmacol. 2016, 9, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Zu, Q.; Li, G.; Yu, T.; Job, K.M.; Yang, X.; Di, L.; Sherwin, C.M.; Enioutina, E.Y. Herbal medicines: Challenges in the modern world. Part 3. China and Japan. Expert Rev. Clin. Pharmacol. 2016, 9, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Gunawardena, D.; Shanmugam, K.; Low, M.; Bennett, L.; Govindaraghavan, S.; Head, R.; Ooi, L.; Münch, G. Determination of anti-inflammatory activities of standardised preparations of plant- and mushroom-based foods. Eur. J. Nutr. 2014, 53, 335–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasneem, S.; Liu, B.; Li, B.; Choudhary, M.I.; Wang, W. Molecular pharmacology of inflammation: Medicinal plants as anti-inflammatory agents. Pharmacol. Res. 2019, 139, 126–140. [Google Scholar] [CrossRef]
- Di Sotto, A.; Vitalone, A.; Di Giacomo, S. Plant-Derived Nutraceuticals and Immune System Modulation: An Evidence-Based Overview. Vaccines 2020, 8, 468. [Google Scholar] [CrossRef]
- Zhang, G.; Ghosh, S. Molecular mechanisms of NF-kappaB activation induced by bacterial lipopolysaccharide through Toll-like receptors. J. Endotoxin Res. 2000, 6, 453–457. [Google Scholar] [CrossRef]
- Vane, J.R.; Mitchell, J.A.; Appleton, I.; Tomlinson, A.; Bishop-Bailey, D.; Croxtall, J.; Willoughby, D.A. Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proc. Natl. Acad. Sci. USA 1994, 91, 2046–2050. [Google Scholar] [CrossRef] [Green Version]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef]
- Borea, P.A.; Gessi, S.; Merighi, S.; Varani, K. Adenosine as a Multi-Signalling Guardian Angel in Human Diseases: When, Where and How Does it Exert its Protective Effects? Trends Pharmacol. Sci. 2016, 37, 419–434. [Google Scholar] [CrossRef]
- Blum, D.; Chern, Y.; Domenici, M.R.; Buée, L.; Lin, C.Y.; Rea, W.; Ferré, S.; Popoli, P. The Role of Adenosine Tone and Adenosine Receptors in Huntington’s Disease. J. Caffeine Adenosine Res. 2018, 8, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Domenici, M.R.; Chiodi, V.; Averna, M.; Armida, M.; Pèzzola, A.; Pepponi, R.; Ferrante, A.; Bader, M.; Fuxe, K.; Popoli, P. Neuronal adenosine A(2A) receptor overexpression is neuroprotective towards 3-nitropropionic acid-induced striatal toxicity: A rat model of Huntington’s disease. Purinergic Signal. 2018, 14, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Temido-Ferreira, M.; Ferreira, D.G.; Batalha, V.L.; Marques-Morgado, I.; Coelho, J.E.; Pereira, P.; Gomes, R.; Pinto, A.; Carvalho, S.; Canas, P.M.; et al. Age-related shift in LTD is dependent on neuronal adenosine A2A receptors interplay with mGluR5 and NMDA receptors. Mol. Psychiatry 2020, 25, 1876–1900. [Google Scholar] [CrossRef] [PubMed]
- Merighi, S.; Battistello, E.; Casetta, I.; Gragnaniello, D.; Poloni, T.E.; Medici, V.; Cirrincione, A.; Varani, K.; Vincenzi, F.; Borea, P.A.; et al. Upregulation of Cortical A2A Adenosine Receptors is Reflected in Platelets of Patients with Alzheimer’s Disease. J. Alzheimers Dis. 2021, 80, 1105–1117. [Google Scholar] [CrossRef]
- Jenner, P.; Mori, A.; Aradi, S.D.; Hauser, R.A. Istradefylline—A first generation adenosine A(2A) antagonist for the treatment of Parkinson’s disease. Expert Rev. Neurother. 2021, 22, 1–17. [Google Scholar]
- Sitkovsky, M.V.; Ohta, A. The ‘danger’ sensors that STOP the immune response: The A2 adenosine receptors. Trends Immunol. 2005, 26, 299–304. [Google Scholar] [CrossRef]
- Antonioli, L.; Fornai, M.; Blandizzi, C.; Pacher, P.; Haskó, G. Adenosine signaling and the immune system: When a lot could be too much. Immunol. Lett. 2019, 205, 9–15. [Google Scholar] [CrossRef]
- Vitalone, A.; Allkanjari, O. Epilobium spp: Pharmacology and Phytochemistry. Phytother Res. 2018, 32, 1229–1240. [Google Scholar] [CrossRef]
- Liu, Y.T.; Gong, P.H.; Xiao, F.Q.; Shao, S.; Zhao, D.Q.; Yan, M.M.; Yang, X.W. Chemical Constituents and Antioxidant, Anti-Inflammatory and Anti-Tumor Activities of Melilotus officinalis (Linn.) Pall. Molecules 2018, 23, 271. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.H.; Huang, S.S.; Wang, B.S.; Wu, C.H.; Sheu, M.J.; Hou, W.C.; Lin, S.S.; Huang, G.J. Antioxidant and anti-inflammatory properties of Cardiospermum halicacabum and its reference compounds ex vivo and in vivo. J. Ethnopharmacol. 2011, 133, 743–750. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Ramstead, A.G.; Kirpotina, L.N.; Voyich, J.M.; Jutila, M.A.; Quinn, M.T. Therapeutic Potential of Polyphenols from Epilobium Angustifolium (Fireweed). Phytother. Res. 2016, 30, 1287–1297. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Zhao, W.; Wang, X.; Sun, Y.; Chen, X. A pharmacological review of dicoumarol: An old natural anticoagulant agent. Pharmacol Res. 2020, 160, 105193. [Google Scholar] [CrossRef] [PubMed]
- Granica, S.; Piwowarski, J.P.; Czerwińska, M.E.; Kiss, A.K. Phytochemistry, pharmacology and traditional uses of different Epilobium species (Onagraceae): A review. J. Ethnopharmacol. 2014, 156, 316–346. [Google Scholar] [CrossRef] [PubMed]
- Fai, D.; Fai, C.; Di Vito, M.; Martini, C.; Zilio, G.; De Togni, H. Cardiospermum halicacabum in atopic dermatitis: Clinical evidence based on phytotherapic approach. Dermatol Ther. 2020, 33, e14519. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdicphosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Broadhurst, W.T.; Jones, R.B. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agr. 1978, 29, 788–794. [Google Scholar] [CrossRef]
- Gessi, S.; Bencivenni, S.; Battistello, E.; Vincenzi, F.; Colotta, V.; Catarzi, D.; Varano, F.; Merighi, S.; Borea, P.A.; Varani, K. Inhibition of A 2A Adenosine Receptor Signaling in Cancer Cells Proliferation by the Novel Antagonist TP455. Front. Pharmacol. 2017, 8, 888. [Google Scholar] [CrossRef] [Green Version]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M. International Natural Product Sciences Taskforce, Supuran CT. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell Longev. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popa-Wagner, A.; Mitran, S.; Sivanesan, S.; Chang, E.; Buga, A.M. ROS and brain diseases: The good, the bad, and the ugly. Oxidative Med. Cell. Longev. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Giuffrida Stella, A.M. Nitric oxide in the central nervous system: Neuroprotection vs. neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766–775. [Google Scholar] [CrossRef]
- Picón-Pagès, P.; Garcia-Buendia, J.; Muñoz, F.J. Functions and dysfunctions of nitric oxide in brain. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1949–1967. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Gaire, B.P.; Parveen, A.; Kim, S.Y. Nitric Oxide as a Target for Phytochemicals in Anti-Neuroinflammatory Prevention Therapy. Int. J. Mol. Sci. 2021, 22, 4771. [Google Scholar] [CrossRef] [PubMed]
- Jahan, A.A.; Anis, M.; Aref, I.M. Relative examination of antioxidative enzymatic activities in plantlets of Cardiospermum halicacabum L. differentiated from hypocotyls in in vivo and ex vitro environment. Biotechnol. Rep. 2014, 4, 66–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, H.L.; Zhang, L.J.; Liang, Y.H.; Hsu, Y.W.; Lee, I.J.; Liaw, C.C.; Hwang, S.Y.; Kuo, Y.H. Antiinflammatory and Antioxidant Flavonoids and Phenols from Cardiospermum halicacabum (Dào Dì Líng). J. Tradit. Complement. Med. 2013, 3, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pathological overproduction: The bad side of adenosine. Br. J. Pharmacol. 2017, 174, 1945–1960. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhang, Z.X.; Zheng, L.P.; Wang, L.; Liu, Y.F.; Yin, W.Y.; Chen, Y.Y.; Wang, X.S.; Hou, S.T.; Chen, J.F.; et al. The adenosine A(2A) receptor antagonist SCH58261 reduces macrophage/microglia activation and protects against experimental autoimmune encephalomyelitis in mice. Neurochem. Int. 2019, 129, 104490. [Google Scholar] [CrossRef] [PubMed]
- Eudy, B.J.; da Silva, R.P. Systematic deletion of adenosine receptors reveals novel roles in inflammation and pyroptosis in THP-1 macrophages. Mol. Immunol. 2021, 132, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ohta, A.; Sitkovsky, M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 2001, 414, 916–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinsel, J.F.; Sitkovsky, M.V. Possible targeting of G protein coupled receptors to manipulate inflammation in vivo using synthetic and natural ligands. Ann. Rheum. Dis. 2003, 62, 22–24. [Google Scholar] [CrossRef] [Green Version]
- Fredholm, B.B.; Bättig, K.; Holmén, J.; Nehlig, A.; Zvartau, E.E. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 1999, 51, 83–133. [Google Scholar]
- Sánchez-Melgar, A.; Albasanz, J.L.; Guixà-González, R.; Saleh, N.; Selent, J.; Martín, M. The antioxidant resveratrol acts as a non-selective adenosine receptor agonist. Free Radic. Biol. Med. 2019, 135, 261–273. [Google Scholar] [CrossRef]

| Plant Extracts | Solvent | Polyphenols (µg/µL Gallic Acid Eq.) | Flavonoids (µg/µL +(-) Catechin Eq.) | Tannins (µg/µL +(-) Catechin Eq.) |
|---|---|---|---|---|
| Epilobium parviflorum | hot glycerate | 14.16 ± 0.04 | 4.78 ± 0.11 | 1.04 ± 0.01 |
| cold glycerate | 14.85 ± 0.14 | 3.71 ± 0.01 | 1.43 ± 0.01 | |
| Melilotus officinalis | hot glycerate | 4.12 ± 0.06 | 1.57 ± 0.01 | 0.76 ± 0.07 |
| cold glycerate | 5.53 ± 0.07 | 2.66 ± 0.01 | 0.88 ± 0.09 | |
| Cardiospermum halicacabum | hot glycerate | 2.82 ± 0.02 | 2.76 ± 0.06 | 1.48 ± 0.02 |
| cold glycerate | 2.78 ± 0.03 | 2.08 ± 0.02 | 1.12 ± 0.01 | |
| Epilobium parviflorum | 40% ethanol | 16.79 ± 0.16 | 4.45 ± 0.04 | 0.56 ± 0.06 |
| Melilotus officinalis | 40% ethanol | 3.18 ± 0.03 | 2.74 ± 0.02 | 0.16 ± 0.02 |
| Cardiospermum halicacabum | 40% ethanol | 7.82 ± 0.07 | 5.28 ± 0.05 | 1.02 ± 0.01 |
| Plant Extracts | Solvent | 40 µg/µL | 4 µg/µL | 0.4 µg/µL | 0.04 µg/µL | IC50 (µg/µL) |
|---|---|---|---|---|---|---|
| Epilobium parviflorum | hot glycerate | 69 ± 7 * | 63 ± 4 * | 51 ± 3 * | 13 ± 1 | 0.195 ± 0.022 |
| cold glycerate | 72 ± 2 * | 71 ± 1 * | 61 ± 3 * | 21 ± 3 | 0.117 ± 0.021 | |
| Melilotus officinalis | hot glycerate | 70 ± 5 * | 67 ± 6 * | 24 ± 4 * | 5 ± 1 | 0.141 ± 0.013 |
| cold glycerate | 89 ± 4 * | 74 ± 7 * | 41 ± 5 * | 7 ± 3 | 0.510 ± 0.053 | |
| Cardiospermum halicacabum | hot glycerate | 65 ± 5 * | 60 ± 3 * | 24 ± 7 * | 15 ± 2 | 0.892 ± 0.080 |
| cold glycerate | 84 ± 9 * | 61 ± 4 * | 39 ± 3 * | 8 ± 1 | 0.587 ± 0.075 | |
| 10 µg/µL | 1 µg/µL | 0.1 µg/µL | 0.01 µg/µL | |||
| Epilobium parviflorum | 40% ethanol | 92 ± 6 * | 90 ± 5 * | 81 ± 6 * | 40 ± 5 * | 0.014 ± 0.013 |
| Melilotus officinalis | 40% ethanol | 90 ± 1 * | 86 ± 2 * | 30 ± 3 * | 9 ± 1 | 0.227 ± 0.025 |
| Cardiospermum halicacabum | 40% ethanol | 89 ± 4 * | 82 ± 3 * | 26 ± 2 * | 16 ± 1 | 0.290 ± 0.027 |
| Plant Extracts | N9 | RAW 264.7 | ||||
|---|---|---|---|---|---|---|
| 2.5 µg/µL | 1 µg/µL | 0.1 µg/µL | 2.5 µg/µL | 1 µg/µL | 0.1 µg/µL | |
| Epilobium parviflorum | 95 ± 6 | 98 ± 7 | 102 ± 8 | 33 ± 4 * | 80 ± 9 | 99 ± 8 |
| Melilotus officinalis | 97 ± 9 | 98 ± 8 | 101 ± 11 | 96 ± 8 | 98 ± 9 | 105 ± 9 |
| Cardiospermum halicacabum | 69 ± 9 * | 95 ± 3 | 97 ± 8 | 31 ± 4 * | 98 ± 8 | 101 ± 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merighi, S.; Travagli, A.; Tedeschi, P.; Marchetti, N.; Gessi, S. Antioxidant and Antiinflammatory Effects of Epilobium parviflorum, Melilotus officinalis and Cardiospermum halicacabum Plant Extracts in Macrophage and Microglial Cells. Cells 2021, 10, 2691. https://doi.org/10.3390/cells10102691
Merighi S, Travagli A, Tedeschi P, Marchetti N, Gessi S. Antioxidant and Antiinflammatory Effects of Epilobium parviflorum, Melilotus officinalis and Cardiospermum halicacabum Plant Extracts in Macrophage and Microglial Cells. Cells. 2021; 10(10):2691. https://doi.org/10.3390/cells10102691
Chicago/Turabian StyleMerighi, Stefania, Alessia Travagli, Paola Tedeschi, Nicola Marchetti, and Stefania Gessi. 2021. "Antioxidant and Antiinflammatory Effects of Epilobium parviflorum, Melilotus officinalis and Cardiospermum halicacabum Plant Extracts in Macrophage and Microglial Cells" Cells 10, no. 10: 2691. https://doi.org/10.3390/cells10102691
APA StyleMerighi, S., Travagli, A., Tedeschi, P., Marchetti, N., & Gessi, S. (2021). Antioxidant and Antiinflammatory Effects of Epilobium parviflorum, Melilotus officinalis and Cardiospermum halicacabum Plant Extracts in Macrophage and Microglial Cells. Cells, 10(10), 2691. https://doi.org/10.3390/cells10102691











