The Pro-Survival Oct4/Stat1/Mcl-1 Axis Is Associated with Poor Prognosis in Lung Adenocarcinoma Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Oncomine Database Analysis
2.2. Cell Lines
2.3. Plasmids
2.4. Luciferase Reporter Assay
2.5. Quantification of mRNA Expression
2.6. Immunological Assays
2.7. Chromatin Immunoprecipitation (ChIP) Assay
2.8. TUNEL Assay
2.9. Animal Experiments
2.10. Statistical Analyses
3. Results
3.1. Oct4 and Stat1 Expression Is Elevated and Associated with Poor Prognosis of Human Lung Adenocarcinoma
3.2. Oct4 Transactivates the Stat1 Promoter
3.3. Oct4 Modulates Anti-Apoptosis via the Stat1 Downstream Gene Mcl-1 and Inhibition of Stat1 Expression Induces Apoptosis
3.4. Silencing of Stat1 Expression Ameliorates Oct4-Induced Anti-Apoptosis
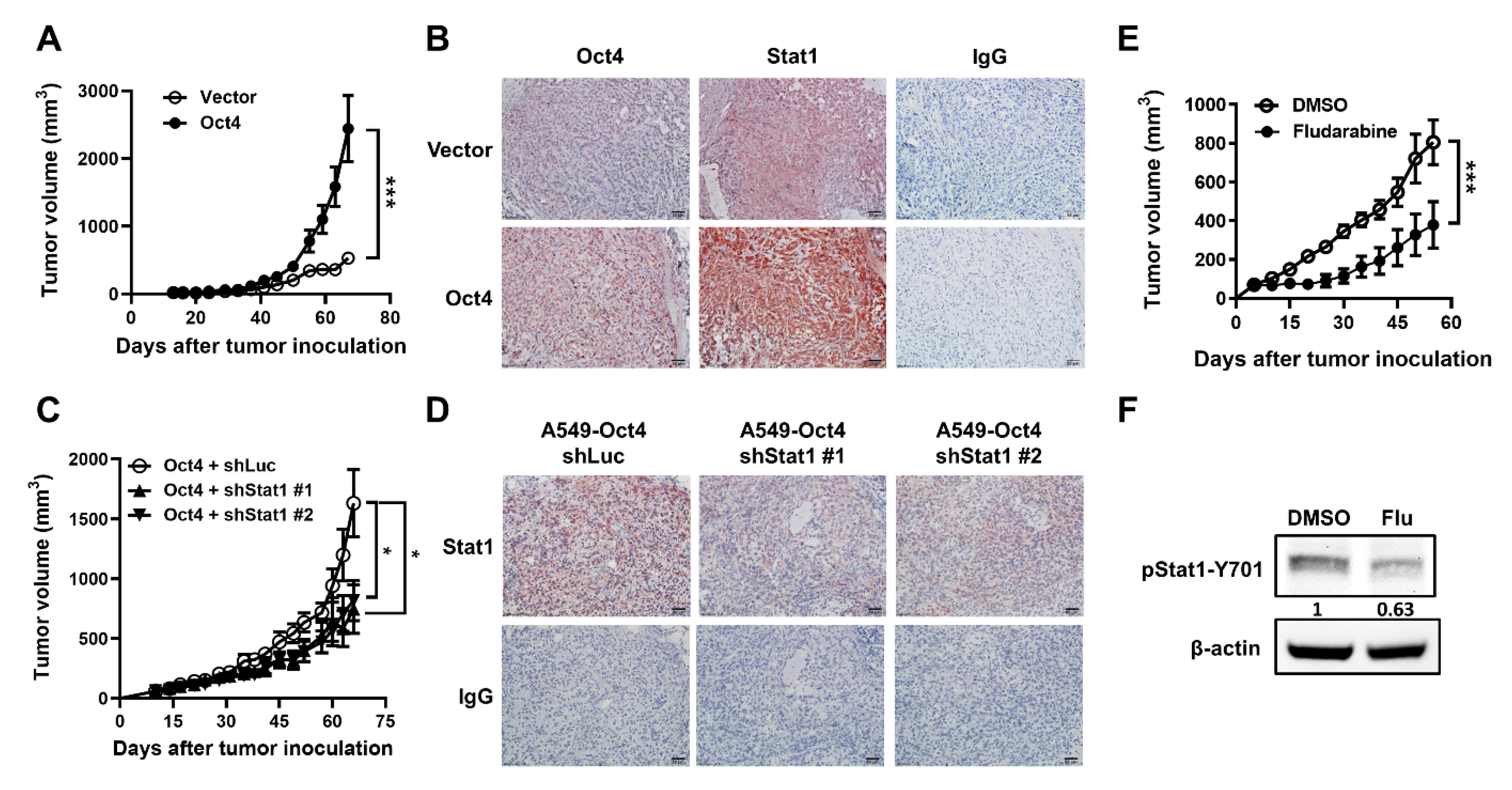
3.5. Oct4-Promoted Tumor Growth Is Attenuated by The Reduction in Stat1 Expression in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walker, S. Updates in non-small cell lung cancer. Clin. J. Oncol Nurs 2008, 12, 587–596. [Google Scholar] [CrossRef] [Green Version]
- Zarogoulidis, K.; Zarogoulidis, P.; Darwiche, K.; Boutsikou, E.; Machairiotis, N.; Tsakiridis, K.; Katsikogiannis, N.; Kougioumtzi, I.; Karapantzos, I.; Huang, H.; et al. Treatment of non-small cell lung cancer (NSCLC). J. Thorac Dis 2013, 5 (Suppl. 4), S389–S396. [Google Scholar]
- Niwa, H.; Miyazaki, J.; Smith, A.G. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat. Genet. 2000, 24, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Pesce, M.; Schöler, H.R. Oct-4: Gatekeeper in the beginnings of mammalian development. Stem Cells 2001, 19, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Schöler, H.R. Octamania: The POU factors in murine development. Trends Genet. 1991, 7, 323–329. [Google Scholar] [CrossRef]
- Chang, C.C.; Shieh, G.S.; Wu, P.; Lin, C.C.; Shiau, A.L.; Wu, C.L. Oct-3/4 expression reflects tumor progression and regulates motility of bladder cancer cells. Cancer Res. 2008, 68, 6281–6291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karoubi, G.; Gugger, M.; Schmid, R.; Dutly, A. OCT4 expression in human non-small cell lung cancer: Implications for therapeutic intervention. Interact. Cardiovasc. Thorac. Surg. 2009, 8, 393–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monk, M.; Holding, C. Human embryonic genes re-expressed in cancer cells. Oncogene 2001, 20, 8085–8091. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Branch, D.R.; Bali, M.; Schultz, G.A.; Goss, P.E.; Jin, T. The POU homeodomain protein OCT3 as a potential transcriptional activator for fibroblast growth factor-4 (FGF-4) in human breast cancer cells. Biochem. J. 2003, 375, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Han, B.; Huang, J.; Zheng, B.; Geng, Q.; Aziz, F.; Dong, Q. Prognostic significance of OCT4 expression in adenocarcinoma of the lung. Jpn. J. Clin. Oncol 2010, 40, 961–966. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.H.; Su, Y.C.; Lin, S.F.; Lin, P.R.; Wu, C.L.; Tung, C.L.; Li, C.F.; Shieh, G.S.; Shiau, A.L. Oct4 upregulates osteopontin via Egr1 and is associated with poor outcome in human lung cancer. BMC Cancer 2019, 19, 791. [Google Scholar] [CrossRef]
- Lu, C.S.; Shiau, A.L.; Su, B.H.; Hsu, T.S.; Wang, C.T.; Su, Y.C.; Tsai, M.S.; Feng, Y.H.; Tseng, Y.L.; Yen, Y.T.; et al. Oct4 promotes M2 macrophage polarization through upregulation of macrophage colony-stimulating factor in lung cancer. J. Hematol. Oncol. 2020, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Benekli, M.; Baer, M.R.; Baumann, H.; Wetzler, M. Signal transducer and activator of transcription proteins in leukemias. Blood 2003, 101, 2940–2954. [Google Scholar] [CrossRef] [Green Version]
- Dunn, G.P.; Koebel, C.M.; Schreiber, R.D. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol 2006, 6, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Schultz, J.; Koczan, D.; Schmitz, U.; Ibrahim, S.M.; Pilch, D.; Landsberg, J.; Kunz, M. Tumor-promoting role of signal transducer and activator of transcription (Stat)1 in late-stage melanoma growth. Clin. Exp. Metastasis 2010, 27, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Skrzypski, M.; Dziadziuszko, R.; Jassem, E.; Szymanowska-Narloch, A.; Gulida, G.; Rzepko, R.; Biernat, W.; Taron, M.; Jelitto-Górska, M.; Marjański, T.; et al. Main histologic types of non-small-cell lung cancer differ in expression of prognosis-related genes. Clin. Lung Cancer 2013, 14, 666–673.e662. [Google Scholar] [CrossRef]
- Timofeeva, O.A.; Plisov, S.; Evseev, A.A.; Peng, S.; Jose-Kampfner, M.; Lovvorn, H.N.; Dome, J.S.; Perantoni, A.O. Serine-phosphorylated STAT1 is a prosurvival factor in Wilms’ tumor pathogenesis. Oncogene 2006, 25, 7555–7564. [Google Scholar] [CrossRef] [Green Version]
- Khodarev, N.N.; Beckett, M.; Labay, E.; Darga, T.; Roizman, B.; Weichselbaum, R.R. STAT1 is overexpressed in tumors selected for radioresistance and confers protection from radiation in transduced sensitive cells. Proc. Natl. Acad. Sci. USA 2004, 101, 1714–1719. [Google Scholar] [CrossRef] [Green Version]
- Khodarev, N.N.; Roach, P.; Pitroda, S.P.; Golden, D.W.; Bhayani, M.; Shao, M.Y.; Darga, T.E.; Beveridge, M.G.; Sood, R.F.; Sutton, H.G.; et al. STAT1 pathway mediates amplification of metastatic potential and resistance to therapy. PLoS ONE 2009, 4, e5821. [Google Scholar] [CrossRef] [Green Version]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef]
- Lowe, S.W.; Lin, A.W. Apoptosis in cancer. Carcinogenesis 2000, 21, 485–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debatin, K.M.; Krammer, P.H. Death receptors in chemotherapy and cancer. Oncogene 2004, 23, 2950–2966. [Google Scholar] [CrossRef] [Green Version]
- Johnstone, R.W.; Ruefli, A.A.; Lowe, S.W. Apoptosis: A link between cancer genetics and chemotherapy. Cell 2002, 108, 153–164. [Google Scholar] [CrossRef] [Green Version]
- Green, D.R. Apoptotic pathways: Ten minutes to dead. Cell 2005, 121, 671–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malo, M.S.; Abedrapo, M.; Chen, A.; Mozumder, M.; Pushpakaran, P.; Alkhoury, F.; Zhang, W.; Fleming, E.; Hodin, R.A. Improved eukaryotic promoter-detection vector carrying two luciferase reporter genes. Biotechniques 2003, 35, 1150–1152, 1154. [Google Scholar] [CrossRef]
- Su, Y.C.; Ou, H.Y.; Wu, H.T.; Wu, P.; Chen, Y.C.; Su, B.H.; Shiau, A.L.; Chang, C.J.; Wu, C.L. Prothymosin-α Overexpression Contributes to the Development of Insulin Resistance. J. Clin. Endocrinol. Metab. 2015, 100, 4114–4123. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.L.; Shieh, G.S.; Chang, C.C.; Yo, Y.T.; Su, C.H.; Chang, M.Y.; Huang, Y.H.; Wu, P.; Shiau, A.L. Tumor-selective replication of an oncolytic adenovirus carrying oct-3/4 response elements in murine metastatic bladder cancer models. Clin. Cancer Res. 2008, 14, 1228–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okayama, H.; Kohno, T.; Ishii, Y.; Shimada, Y.; Shiraishi, K.; Iwakawa, R.; Furuta, K.; Tsuta, K.; Shibata, T.; Yamamoto, S.; et al. Identification of genes upregulated in ALK-positive and EGFR/KRAS/ALK-negative lung adenocarcinomas. Cancer Res. 2012, 72, 100–111. [Google Scholar] [CrossRef] [Green Version]
- Győrffy, B.; Surowiak, P.; Budczies, J.; Lánczky, A. Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PLoS ONE 2013, 8, e82241. [Google Scholar]
- Messeguer, X.; Escudero, R.; Farré, D.; Núñez, O.; Martínez, J.; Albà, M.M. PROMO: Detection of known transcription regulatory elements using species-tailored searches. Bioinformatics 2002, 18, 333–334. [Google Scholar] [CrossRef]
- Hsu, W.L.; Chiu, T.H.; Tai, D.J.; Ma, Y.L.; Lee, E.H. A novel defense mechanism that is activated on amyloid-beta insult to mediate cell survival: Role of SGK1-STAT1/STAT2 signaling. Cell Death Differ. 2009, 16, 1515–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Mantel, C.; Hromas, R.A.; Broxmeyer, H.E. Oct-4 is critical for survival/antiapoptosis of murine embryonic stem cells subjected to stress: Effects associated with Stat3/survivin. Stem Cells 2008, 26, 30–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, K.; Fu, Z.; Wu, X.; Feng, J.; Chen, W.; Qian, J. Oct-4 is required for an antiapoptotic behavior of chemoresistant colorectal cancer cells enriched for cancer stem cells: Effects associated with STAT3/Survivin. Cancer Lett. 2013, 333, 56–65. [Google Scholar] [CrossRef]
- Katze, M.G.; He, Y.; Gale, M., Jr. Viruses and interferon: A fight for supremacy. Nat. Rev. Immunol. 2002, 2, 675–687. [Google Scholar] [CrossRef]
- Xie, B.; Zhao, J.; Kitagawa, M.; Durbin, J.; Madri, J.A.; Guan, J.L.; Fu, X.Y. Focal adhesion kinase activates Stat1 in integrin-mediated cell migration and adhesion. J. Biol Chem. 2001, 276, 19512–19523. [Google Scholar] [CrossRef] [Green Version]
- Xu, N.; Papagiannakopoulos, T.; Pan, G.; Thomson, J.A.; Kosik, K.S. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell 2009, 137, 647–658. [Google Scholar] [CrossRef] [Green Version]
- Yin, R.; Zhang, S.; Wu, Y.; Fan, X.; Jiang, F.; Zhang, Z.; Feng, D.; Guo, X.; Xu, L. microRNA-145 suppresses lung adenocarcinoma-initiating cell proliferation by targeting OCT4. Oncol. Rep. 2011, 25, 1747–1754. [Google Scholar]
- Shen, H.; Shen, J.; Wang, L.; Shi, Z.; Wang, M.; Jiang, B.H.; Shu, Y. Low miR-145 expression level is associated with poor pathological differentiation and poor prognosis in non-small cell lung cancer. Biomed. Pharmacother. 2015, 69, 301–305. [Google Scholar] [CrossRef]
- Lin, L.; Hou, J.; Ma, F.; Wang, P.; Liu, X.; Li, N.; Wang, J.; Wang, Q.; Cao, X. Type I IFN inhibits innate IL-10 production in macrophages through histone deacetylase 11 by downregulating microRNA-145. J. Immunol. 2013, 191, 3896–3904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittelman, A.; Ashikari, R.; Ahmed, T.; Savona, S.; Arnold, P.; Arlin, Z. Phase II trial of fludarabine phosphate (F-Ara-AMP) in patients with advanced breast cancer. Cancer Chemother. Pharmacol. 1988, 22, 63–64. [Google Scholar] [CrossRef]
- Weiss, G.B.; Metch, B.; Von Hoff, D.D.; Taylor, S.A.; Saiers, J.H. Phase II trial of fludarabine phosphate in patients with head and neck cancer: A Southwest Oncology Group Study. Cancer Treat. Rep. 1987, 71, 1313–1314. [Google Scholar] [PubMed]
- Wang, K.; Shrestha, R.; Wyatt, A.W.; Reddy, A.; Lehár, J.; Wang, Y.; Lapuk, A.; Collins, C.C. A meta-analysis approach for characterizing pan-cancer mechanisms of drug sensitivity in cell lines. PLoS ONE 2014, 9, e103050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petta, V.; Gkiozos, I.; Strimpakos, A.; Syrigos, K. Histones and lung cancer: Are the histone deacetylases a promising therapeutic target? Cancer Chemother. Pharmacol. 2013, 72, 935–952. [Google Scholar] [CrossRef]
- Lu, C.S.; Shieh, G.S.; Wang, C.T.; Su, B.H.; Su, Y.C.; Chen, Y.C.; Su, W.C.; Wu, P.; Yang, W.H.; Shiau, A.L.; et al. Chemotherapeutics-induced Oct4 expression contributes to drug resistance and tumor recurrence in bladder cancer. Oncotarget 2017, 8, 30844–30858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedberg, J.W.; Dong, D.A.; Li, S.; Kim, H.; Stephans, K.; Noonan, K.; Neuberg, D.; Gribben, J.G.; Fisher, D.C.; Freedman, A.S.; et al. Oral fludarabine has significant activity in patients with previously untreated chronic lymphocytic leukemia, and leads to increased STAT1 levels in vivo. Leuk. Res. 2004, 28, 139–147. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.-C.; Chen, Y.-C.; Tseng, Y.-L.; Shieh, G.-S.; Wu, P.; Shiau, A.-L.; Wu, C.-L. The Pro-Survival Oct4/Stat1/Mcl-1 Axis Is Associated with Poor Prognosis in Lung Adenocarcinoma Patients. Cells 2021, 10, 2642. https://doi.org/10.3390/cells10102642
Su Y-C, Chen Y-C, Tseng Y-L, Shieh G-S, Wu P, Shiau A-L, Wu C-L. The Pro-Survival Oct4/Stat1/Mcl-1 Axis Is Associated with Poor Prognosis in Lung Adenocarcinoma Patients. Cells. 2021; 10(10):2642. https://doi.org/10.3390/cells10102642
Chicago/Turabian StyleSu, Yu-Chu, Yi-Cheng Chen, Yau-Lin Tseng, Gia-Shing Shieh, Pensee Wu, Ai-Li Shiau, and Chao-Liang Wu. 2021. "The Pro-Survival Oct4/Stat1/Mcl-1 Axis Is Associated with Poor Prognosis in Lung Adenocarcinoma Patients" Cells 10, no. 10: 2642. https://doi.org/10.3390/cells10102642





