How Viruses Hijack and Modify the Secretory Transport Pathway
Abstract
:1. Introduction
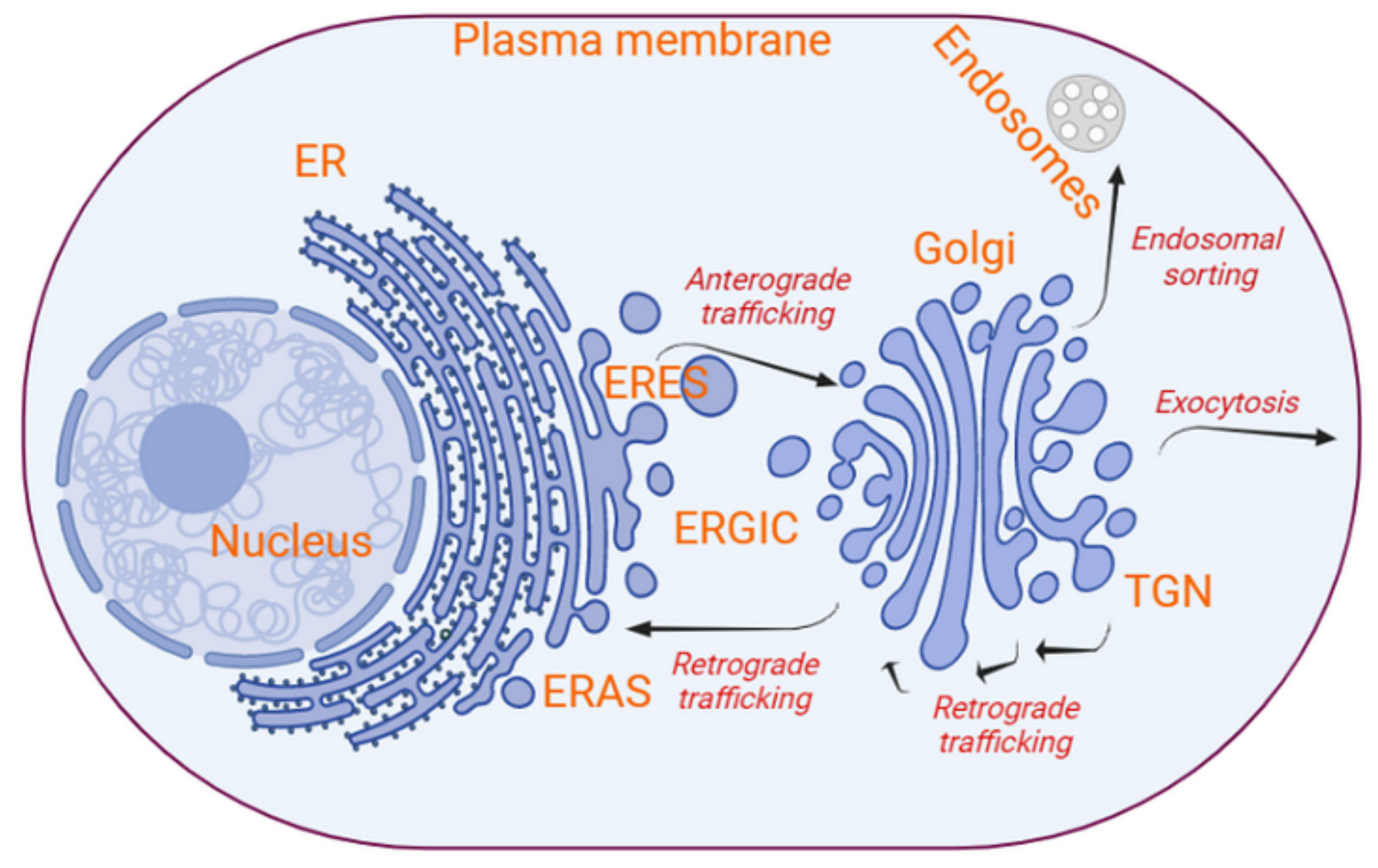
2. The Organization and Dynamics of the Secretory Pathway
2.1. Vesicle Formation and Budding at the ER, and Fusion with the ERGIC/Golgi
2.2. Vesicle Formation and Budding at the Golgi, and Fusion with the ER and within the Golgi
2.3. Vesicle Fusion at the Plasma Membrane and Exocytosis
3. Formation and Functions of Viral-Induced Membrane Rearrangements
3.1. RNA Viruses
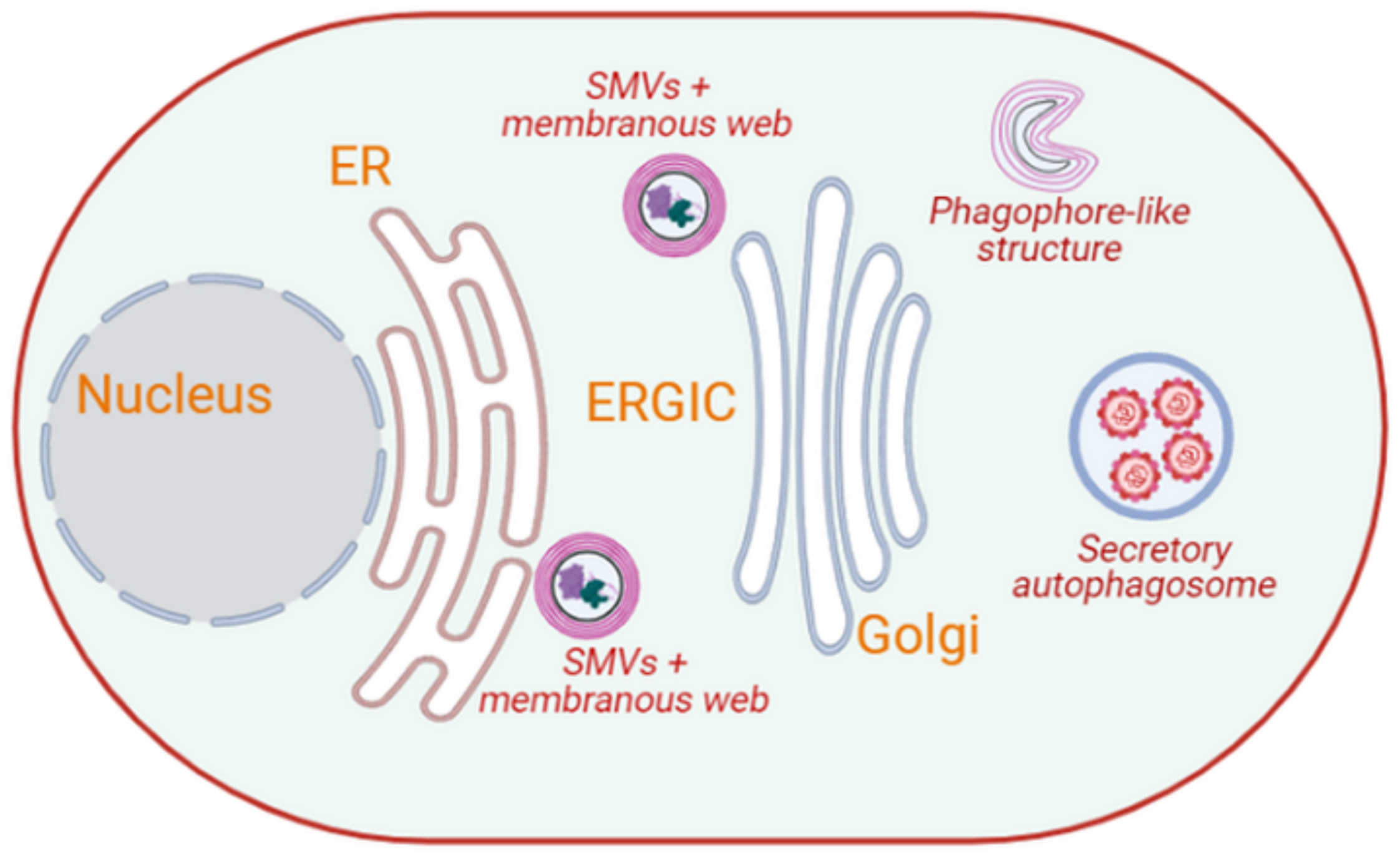
3.1.1. Picornaviruses
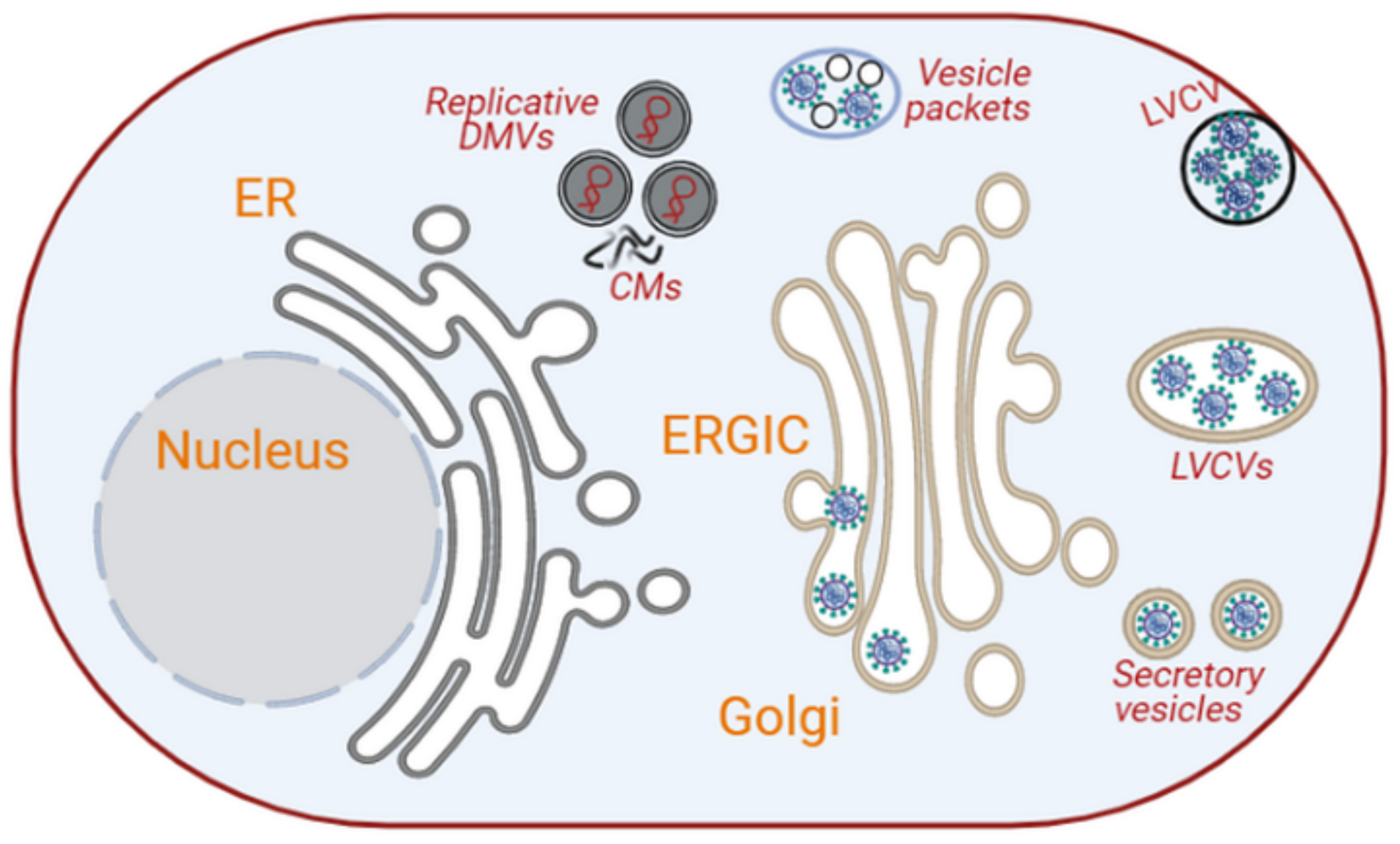
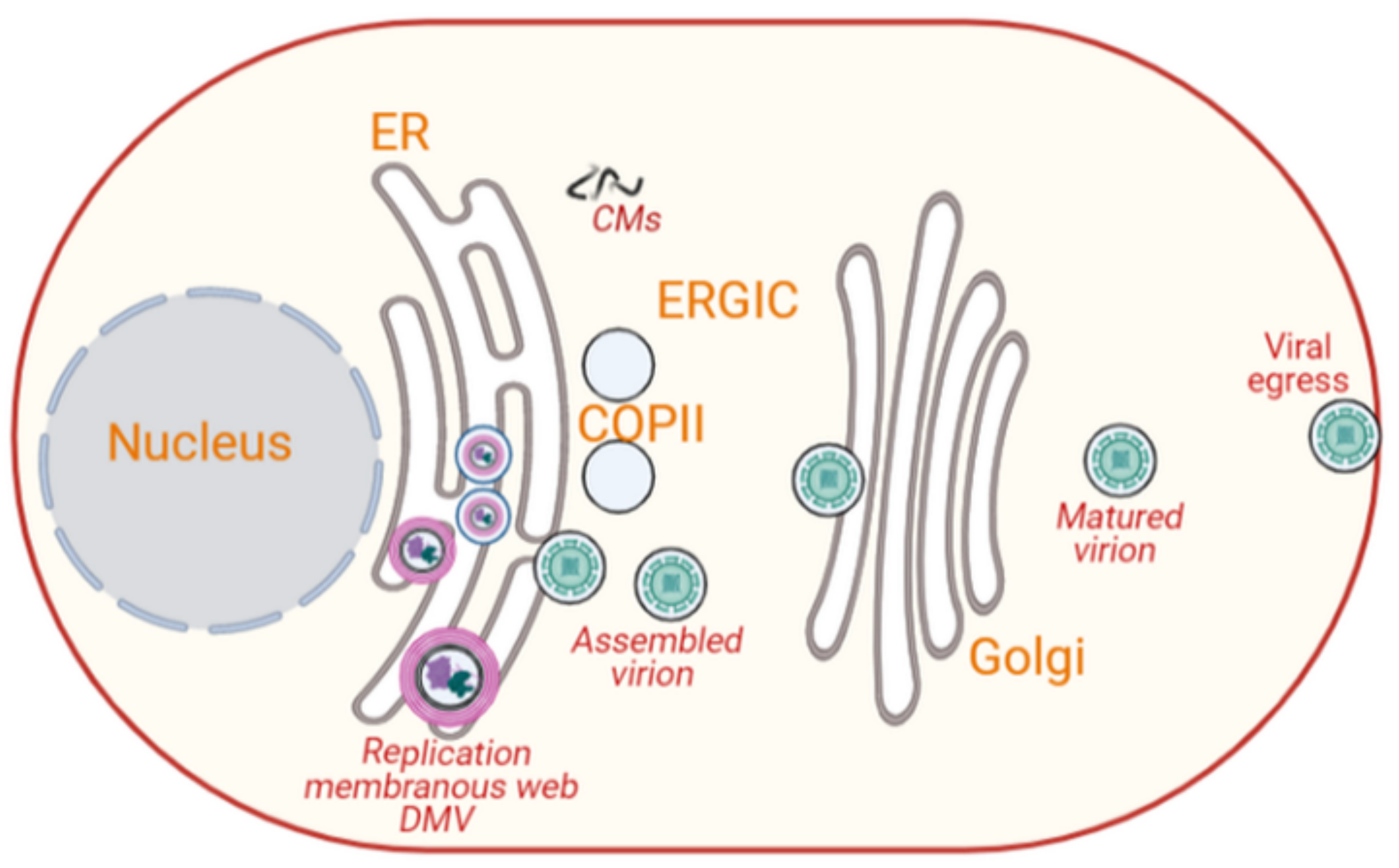
3.1.2. Coronaviruses
3.1.3. Flaviviruses
3.2. DNA Viruses
3.2.1. Poxviruses
3.2.2. Parvovirus
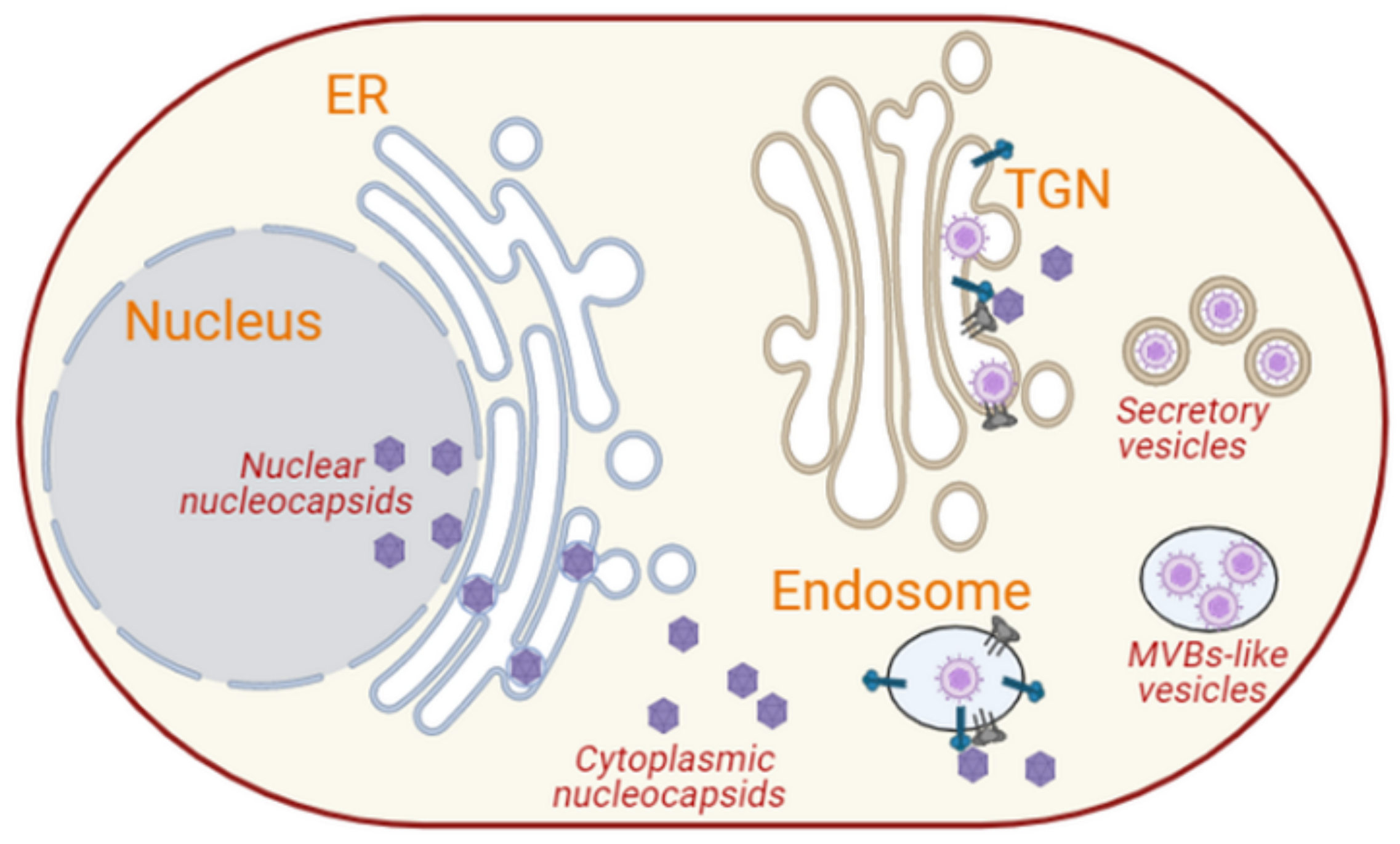
3.2.3. Herpesviruses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tokarev, A.A.; Alfonso, A.; Segev, N. Overview of Intracellular Compartments and Trafficking pathways. In Madame Curie Bioscience Database; Landes Bioscience: Austin, TX, USA, 2000; p. 8. [Google Scholar]
- Costaguta, G.; Payne, G. Overview of protein trafficking mechanisms. In Trafficking Inside Cells: Pathways, Mechanisms and Regulation; Segev, N., Ed.; Landes Bioscience: Austin, TX, USA; Springer Science+Business Media: New York, NY, USA, 2009; pp. 105–114. [Google Scholar]
- Orlando, K.; Guo, W. Membrane Organization and Dynamics in Cell Polarity. Cold Spring Harb. Perspect. Biol. 2009, 1, a001321. [Google Scholar] [CrossRef]
- Winner, M.B.; Bodt, S.M.L.; McNutt, P.M. Special Delivery: Potential Mechanisms of Botulinum Neurotoxin Uptake and Trafficking within Motor Nerve Terminals. Int. J. Mol. Sci. 2020, 21, 8715. [Google Scholar] [CrossRef]
- Morris, S.; Geoghegan, N.D.; Sadler, J.B.A.; Koester, A.M.; Black, H.L.; Laub, M.; Miller, L.; Heffernan, L.; Simpson, J.C.; Mastick, C.C.; et al. Characterisation of GLUT4 trafficking in HeLa cells: Comparable kinetics and orthologous trafficking mechanisms to 3T3-L1 adipocytes. Peer J. 2020, 8, e8751. [Google Scholar] [CrossRef]
- Marvin, J.F.; Chan, E.K.L. Golgi complex and endosome antibodies. In Autoantibodies, 2nd ed.; Shoenfeld, Y., Gershwin, M.E., Meroni, P.L., Eds.; Elsevier: Burlington, WI, USA, 2007; pp. 263–270. [Google Scholar] [CrossRef]
- Risco, C.; Fernández de Castro, I. Virus Morphogenesis in the Cell: Methods and Observations. Struct. Phys. Viruses 2013, 68, 417–440. [Google Scholar] [CrossRef]
- De Armas-Rillo, L.; Valera, M.; Marrero-Hernández, S.; Valenzuela-Fernández, A. Membrane dynamics associated with viral infection. Rev. Med. Virol. 2016, 26, 146–160. [Google Scholar] [CrossRef]
- Heath, C.M.; Windsor, M.; Wileman, T. Aggresomes Resemble Sites Specialized for Virus Assembly. J. Cell Biol. 2001, 153, 449–456. [Google Scholar] [CrossRef] [Green Version]
- Randow, F.; MacMicking, J.D.; James, L.C. Cellular Self-Defense: How Cell-Autonomous Immunity Protects Against Pathogens. Science 2013, 340, 6133. [Google Scholar] [CrossRef] [PubMed]
- Randow, F.; Münz, C. Autophagy in the regulation of pathogen replication and adaptive immunity. Trends Immunol. 2012, 33, 475–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wileman, T. Aggresomes and Pericentriolar Sites of Virus Assembly: Cellular Defense or Viral Design? Annu. Rev. Microbiol. 2007, 61, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Chen, Z.J. Intrinsic Antiviral Immunity. Nat. Immunol. 2012, 13, 214–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutagalung, A.H.; Novick, P.J. Role of Rab GTPases in Membrane Traffic and Cell Physiology. Physiol. Rev. 2011, 91, 119–149. [Google Scholar] [CrossRef] [Green Version]
- Wozniak, A.L.; Long, A.; Jones-Jamtgaard, K.N.; Weinman, S.A. Hepatitis C virus promotes virion secretion through cleavage of the Rab7 adaptor protein RILP. Proc. Natl. Acad. Sci. USA 2016, 113, 12484–12489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantalupo, G.; Alifano, P.; Roberti, V.; Bruni, C.B.; Bucci, C. Rab-interacting lysosomal protein (RILP): The Rab7 effector required for transport to lysosomes. EMBO J. 2001, 20, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Indran, S.V.; Britt, W.J. A Role for the Small GTPase Rab6 in Assembly of Human Cytomegalovirus. J. Virol. 2011, 85, 5213–5219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, M.; Schor, S.; Barouch-Bentov, R.; Einav, S. Viral journeys on the intracellular highways. Cell Mol. Life Sci. 2018, 75, 3693–3714. [Google Scholar] [CrossRef]
- Altan-Bonnet, N.; Balla, T. Phosphatidylinositol 4-kinases: Hostages harnessed to build panviral replication platforms. Trends Biochem. Sci. 2012, 37, 293–302. [Google Scholar] [CrossRef]
- Stalder, D.; Gershlick, D.C. Direct trafficking pathways from the Golgi apparatus to the plasma membrane. Semin. Cell Dev. Biol. 2020, 107, 112–125. [Google Scholar] [CrossRef]
- Miller, S.; Krijnse-Locker, J. Modification of intracellular membrane structures for virus replication. Nat. Rev. Microbiol. 2008, 6, 363–374. [Google Scholar] [CrossRef]
- Gomez-Navarro, N.; Miller, E. Protein sorting at the ER–Golgi interface. J. Cell Biol. 2016, 215, 769–778. [Google Scholar] [CrossRef] [Green Version]
- Villeneuve, J.; Bassaganyas, L.; Lepreux, S.; Chiritoiu, M.; Costet, P.; Ripoche, J.; Malhotra, V.; Schekman, R. Unconventional secretion of FABP4 by endosomes and secretory lysosomes. J. Cell Biol. 2017, 217, 649–665. [Google Scholar] [CrossRef]
- Gee, H.Y.; Kim, J.; Lee, M.G. Unconventional secretion of transmembrane proteins. Semin. Cell Dev. Biol. 2018, 83, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Okeke, E.; Dingsdale, H.; Parker, T.; Voronina, S.; Tepikin, A.V. Endoplasmic reticulum-plasma membrane junctions: Structure, function and dynamics. J. Physiol. 2016, 594, 2837–2847. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-L.; Chen, Y.-J.; Liou, J. ER-plasma membrane junctions: Why and how do we study them? Biochim. Biophys. Acta BBA-Bioenerg. 2017, 1864, 1494–1506. [Google Scholar] [CrossRef]
- Bär, S.; Rommelaere, J.; Nüesch, J.P.F. Vesicular Transport of Progeny Parvovirus Particles through ER and Golgi Regulates Maturation and Cytolysis. PLoS Pathog. 2013, 9, e1003605. [Google Scholar] [CrossRef] [Green Version]
- Pfeffer, S.; Aivazian, D. Targeting Rab GTPases to distinct membrane compartments. Nat. Rev. Mol. Cell Biol. 2004, 5, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.W.; Hughson, F.M. Chaperoning SNARE assembly and disassembly. Nat. Rev. Mol. Cell Biol. 2016, 17, 465–479. [Google Scholar] [CrossRef] [Green Version]
- Walter, A.M.; Müller, R.; Tawfik, B.; Wierda, K.D.; Pinheiro, P.S.; Nadler, A.; McCarthy, A.W.; Ziomkiewicz, I.; Kruse, M.; Reither, G.; et al. Phosphatidylinositol 4,5-bisphosphate optical uncaging potentiates exocytosis. eLife 2017, 6, 30203. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.-Y.; Yu, C.; Zhang, Y.; Ling, L.; Wang, L.; Gao, J.-L. Key proteins involved in insulin vesicle exocytosis and secretion. Biomed. Rep. 2017, 6, 134–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Dun, A.R.; Martin, K.J.; Qiu, Z.; Dunn, A.; Lord, G.J.; Lu, W.; Duncan, R.R.; Rickman, C. Secretory Vesicles Are Preferentially Targeted to Areas of Low Molecular SNARE Density. PLoS ONE 2012, 7, e49514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutton, R.B.; Fasshauer, D.; Jahn, R.; Brunger, A. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nat. Cell Biol. 1998, 395, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Zemelman, B.; McNew, J.; Westermann, B.; Gmachl, M.; Parlati, F.; Söllner, T.H.; Rothman, J.E. SNAREpins: Minimal Machinery for Membrane Fusion. Cell 1998, 92, 759–772. [Google Scholar] [CrossRef] [Green Version]
- Mostafavi, H.; Thiyagarajan, S.; Stratton, B.S.; Karatekin, E.; Warner, J.M.; Rothman, J.E.; O′Shaughnessy, B. Entropic forces drive self-organization and membrane fusion by SNARE proteins. Proc. Natl. Acad. Sci. USA 2017, 114, 5455–5460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; Wang, C.-T.; Bai, J.; Chapman, E.R.; Jackson, M.B. Transmembrane Segments of Syntaxin Line the Fusion Pore of Ca2+-Triggered Exocytosis. Science 2004, 304, 289–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popoff, V.; Adolf, F.; Brügger, B.; Wieland, F. COPI Budding within the Golgi Stack. Cold Spring Harb. Perspect. Biol. 2011, 3, a005231. [Google Scholar] [CrossRef] [Green Version]
- Aridor, M.; Bannykh, S.I.; Rowe, T.; Balch, W.E. Sequential coupling between COPII and COPI vesicle coats in endoplasmic reticulum to Golgi transport. J. Cell Biol. 1995, 131, 875–893. [Google Scholar] [CrossRef]
- Nakano, A.; Brada, D.; Schekman, R. A membrane glycoprotein, Sec12p, required for protein transport from the endoplasmic reticulum to the Golgi apparatus in yeast. J. Cell Biol. 1988, 107, 851–863. [Google Scholar] [CrossRef] [Green Version]
- Nakano, A.; Muramatsu, M. A novel GTP-binding protein, Sar1p, is involved in transport from the endoplasmic reticulum to the Golgi apparatus. J. Cell Biol. 1989, 109, 2677–2691. [Google Scholar] [CrossRef] [Green Version]
- Wendeler, M.W.; Paccaud, J.; Hauri, H. Role of Sec24 isoforms in selective export of membrane proteins from the endoplasmic reticulum. EMBO Rep. 2007, 8, 258–264. [Google Scholar] [CrossRef] [Green Version]
- Brandizzi, F.; Barlowe, C. Organization of the ER–Golgi interface for membrane traffic control. Nat. Rev. Mol. Cell Biol. 2013, 14, 382–392. [Google Scholar] [CrossRef] [Green Version]
- Lorente-Rodríguez, A.; Barlowe, C. Entry and Exit Mechanisms at the cis-Face of the Golgi Complex. Cold Spring Harb. Perspect. Biol. 2011, 3, a005207. [Google Scholar] [CrossRef] [Green Version]
- Arakel, E.C.; Schwappach, B. Formation of COPI-coated vesicles at a glance. J. Cell Sci. 2018, 131, jcs209890. [Google Scholar] [CrossRef] [Green Version]
- Faini, M.; Beck, R.; Wieland, F.T.; Briggs, J.A. Vesicle coats: Structure, function, and general principles of assembly. Trends Cell Biol. 2013, 23, 279–288. [Google Scholar] [CrossRef]
- Deng, Y.; Golinelli-Cohen, M.-P.; Smirnova, E.; Jackson, C.L. A COPI coat subunit interacts directly with an early-Golgi localized Arf exchange factor. EMBO Rep. 2009, 10, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Faini, M.; Prinz, S.; Beck, R.; Schorb, M.; Riches, J.D.; Bacia, K.; Brügger, B.; Wieland, F.T.; Briggs, J.A.G. The Structures of COPI-Coated Vesicles Reveal Alternate Coatomer Conformations and Interactions. Science 2012, 336, 1451–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, V.W.; Yang, J.-S. Mechanisms of COPI vesicle formation. FEBS Lett. 2009, 583, 3758–3763. [Google Scholar] [CrossRef] [Green Version]
- Amor, J.C.; Harrison, D.H.; Kahn, R.A.; Ringe, D. Structure of the human ADP-ribosylation factor 1 complexed with GDP. Nat. Cell Biol. 1994, 372, 704–708. [Google Scholar] [CrossRef]
- Ferguson, S.M.; De Camilli, P. Dynamin, a membrane-remodelling GTPase. Nat. Rev. Mol. Cell Biol. 2012, 13, 75–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schröter, S.; Beckmann, S.; Schmitt, H.D. ER arrival sites for COPI vesicles localize to hotspots of membrane trafficking. EMBO J. 2016, 35, 1935–1955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Bot, N.; Antony, C.; White, J.; Karsenti, E.; Vernos, I. Role of Xklp3, a Subunit of the Xenopus Kinesin II Heterotrimeric Complex, in Membrane Transport between the Endoplasmic Reticulum and the Golgi Apparatus. J. Cell Biol. 1998, 143, 1559–1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stauber, T.; Simpson, J.C.; Pepperkok, R.; Vernos, I. A Role for Kinesin-2 in COPI-Dependent Recycling between the ER and the Golgi Complex. Curr. Biol. 2006, 16, 2245–2251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zink, S.; Wenzel, D.; Wurm, C.A.; Schmitt, H.D. A Link between ER Tethering and COP-I Vesicle Uncoating. Dev. Cell 2009, 17, 403–416. [Google Scholar] [CrossRef] [Green Version]
- He, S.; O′Connell, D.; Zhang, X.; Yang, Y.; Liang, C. The intersection of Golgi-ER retrograde and autophagic trafficking. Autophagy 2013, 10, 180–181. [Google Scholar] [CrossRef] [Green Version]
- Kelly, R. Pathways of protein secretion in eukaryotes. Science 1985, 230, 25–32. [Google Scholar] [CrossRef]
- Deng, Y.; Rivera-Molina, F.E.; Toomre, D.K.; Burd, C.G. Sphingomyelin is sorted at the trans Golgi network into a distinct class of secretory vesicle. Proc. Natl. Acad. Sci. USA 2016, 113, 6677–6682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; Pakdel, M.; Blank, B.; Sundberg, E.L.; Burd, C.G.; von Blume, J. Activity of the SPCA1 Calcium Pump Couples Sphingomyelin Synthesis to Sorting of Secretory Proteins in the Trans-Golgi Network. Dev. Cell 2018, 47, 464–478.e8. [Google Scholar] [CrossRef] [Green Version]
- Von Blume, J.; Alleaume, A.-M.; Cantero-Recasens, G.; Curwin, A.; Carreras-Sureda, A.; Zimmermann, T.; van Galen, J.; Wakana, Y.; Valverde, M.A.; Malhotra, V. ADF/Cofilin Regulates Secretory Cargo Sorting at the TGN via the Ca2+ ATPase SPCA1. Dev. Cell 2011, 20, 652–662. [Google Scholar] [CrossRef] [Green Version]
- Kienzle, C.; Basnet, N.; Crevenna, A.; Beck, G.; Habermann, B.; Mizuno, N.; Von Blume, J. Cofilin recruits F-actin to SPCA1 and promotes Ca2+-mediated secretory cargo sorting. J. Cell Biol. 2014, 206, 635–654. [Google Scholar] [CrossRef] [Green Version]
- Crevenna, A.H.; Blank, B.; Maiser, A.; Emin, D.; Prescher, J.; Beck, G.; Kienzle, C.; Bartnik, K.; Habermann, B.; Pakdel, M.; et al. Secretory cargo sorting by Ca2+-dependent Cab45 oligomerization at the trans-Golgi network. J. Cell Biol. 2016, 213, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Grote, E.; Novick, P.J. Promiscuity in Rab–SNARE Interactions. Mol. Biol. Cell 1999, 10, 4149–4161. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Roth, D.; Walch-Solimena, C.; Novick, P. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 1999, 18, 1071–1080. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.; Guo, W. The Exocyst at a Glance. J. Cell Sci. 2015, 128, 2957–2964. [Google Scholar] [CrossRef] [Green Version]
- Man, K.N.M.; Imig, C.; Walter, A.M.; Pinheiro, P.S.; Stevens, D.R.; Rettig, J.; Sørensen, J.B.; Cooper, B.H.; Brose, N.; Wojcik, S.M. Identification of a Munc13-sensitive step in chromaffin cell large dense-core vesicle exocytosis. eLife 2015, 4, 10635. [Google Scholar] [CrossRef] [Green Version]
- Sheu, L.; Pasyk, E.A.; Ji, J.; Huang, X.; Gao, X.; Varoqueaux, F.; Brose, N.; Gaisano, H.Y. Regulation of Insulin Exocytosis by Munc13-1. J. Biol. Chem. 2003, 278, 27556–27563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapman, E.R.; An, S.; Barton, N.; Jahn, R. SNAP-25, a t-SNARE which binds to both syntaxin and synap-tobrevin via domains that may form coiled coils. J. Biol. Chem. 1994, 269, 27427–27432. [Google Scholar] [CrossRef]
- Yang, S.-N.; Berggren, P.-O. β-Cell CaVchannel regulation in physiology and pathophysiology. Am. J. Physiol. Metab. 2005, 288, E16–E28. [Google Scholar] [CrossRef]
- Wolff, G.; Melia, C.E.; Snijder, E.J.; Bárcena, M. Double-Membrane Vesicles as Platforms for Viral Replication. Trends Microbiol. 2020, 28, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Netherton, C.L.; Wileman, T. Virus factories, double membrane vesicles and viroplasm generated in animal cells. Curr. Opin. Virol. 2011, 1, 381–387. [Google Scholar] [CrossRef]
- Schlegel, A.; Giddings, T.H.; Ladinsky, M.S.; Kirkegaard, K. Cellular origin and ultrastructure of membranes induced during poliovirus infection. J. Virol. 1996, 70, 6576–6588. [Google Scholar] [CrossRef] [Green Version]
- Mettenleiter, T.C. Herpesvirus Assembly and Egress. J. Virol. 2002, 76, 1537–1547. [Google Scholar] [CrossRef] [Green Version]
- Novoa, R.R.; Calderita, G.; Arranz, R.; Fontana, J.; Granzow, H.; Risco, C. Virus factories: Associations of cell organelles for viral replication and morphogenesis. Biol. Cell 2005, 97, 147–172. [Google Scholar] [CrossRef]
- Tolonen, N.; Doglio, L.; Schleich, S.; Locker, J.K. Vaccinia Virus DNA Replication Occurs in Endoplasmic Reticulum-enclosed Cytoplasmic Mini-Nuclei. Mol. Biol. Cell 2001, 12, 2031–2046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granzow, H.; Klupp, B.G.; Fuchs, W.; Veits, J.; Osterrieder, N.; Mettenleiter, T.C. Egress of Alphaherpesviruses: Comparative Ultrastructural Study. J. Virol. 2001, 75, 3675–3684. [Google Scholar] [CrossRef] [Green Version]
- Nanbo, A.; Noda, T.; Ohba, Y. Epstein–Barr Virus Acquires Its Final Envelope on Intracellular Compartments With Golgi Markers. Front. Microbiol. 2018, 9, 454. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Koike, M.; Moriishi, E.; Kawabata, A.; Tang, H.; Oyaizu, H.; Uchiyama, Y.; Yamanishi, K. Human Herpesvirus-6 Induces MVB Formation, and Virus Egress Occurs by an Exosomal Release Pathway. Traffic 2008, 9, 1728–1742. [Google Scholar] [CrossRef] [Green Version]
- Cortese, M.; Goellner, S.; Acosta, E.G.; Neufeldt, C.; Oleksiuk, O.; Lampe, M.; Haselmann, U.; Funaya, C.; Schieber, N.; Ronchi, P.; et al. Ultrastructural Characterization of Zika Virus Replication Factories. Cell Rep. 2017, 18, 2113–2123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorobantu, C.M.; Van Der Schaar, H.M.; Ford, L.A.; Strating, J.R.P.M.; Ulferts, R.; Fang, Y.; Belov, G.; Van Kuppeveld, F.J.M.; Sandri-Goldin, R.M. Recruitment of PI4KIII to Coxsackievirus B3 Replication Organelles Is Independent of ACBD3, GBF1, and Arf1. J. Virol. 2013, 88, 2725–2736. [Google Scholar] [CrossRef] [Green Version]
- Brey, I.R.; Merz, A.; Chiramel, A.; Lee, J.-Y.; Chlanda, P.; Haselman, U.; Santarella-Mellwig, R.; Habermann, A.; Hoppe, S.; Kallis, S.; et al. Three-Dimensional Architecture and Biogenesis of Membrane Structures Associated with Hepatitis C Virus Replication. PLoS Pathog. 2012, 8, e1003056. [Google Scholar] [CrossRef] [Green Version]
- Harak, C.; Lohmann, V. Ultrastructure of the replication sites of positive-strand RNA viruses. Virology 2015, 479, 418–433. [Google Scholar] [CrossRef] [Green Version]
- Neuman, B.W.; Angelini, M.M.; Buchmeier, M.J. Does form meet function in the coronavirus replicative organelle? Trends Microbiol. 2014, 22, 642–647. [Google Scholar] [CrossRef] [Green Version]
- Romero-Brey, I.; Bartenschlager, R. Membranous Replication Factories Induced by Plus-Strand RNA Viruses. Viruses 2014, 6, 2826–2857. [Google Scholar] [CrossRef]
- Doyle, N.; Hawes, P.C.; Simpson, J.; Adams, L.H.; Maier, H.J. The Porcine Deltacoronavirus Replication Organelle Comprises Double-Membrane Vesicles and Zippered Endoplasmic Reticulum with Double-Membrane Spherules. Viruses 2019, 11, 1030. [Google Scholar] [CrossRef] [Green Version]
- Snijder, E.J.; Limpens, R.W.A.L.; de Wilde, A.H.; de Jong, A.W.M.; Zevenhoven-Dobbe, J.C.; Maier, H.J.; Faas, F.F.G.A.; Koster, A.J.; Bárcena, M. A unifying structural and functional model of the coronavirus replication organelle: Tracking down RNA synthesis. PLoS Biol. 2020, 18, e3000715. [Google Scholar] [CrossRef]
- Knoops, K.; Kikkert, M.; Worm, S.H.E.V.D.; Zevenhoven-Dobbe, J.C.; Van Der Meer, Y.; Koster, A.J.; Mommaas, A.M.; Snijder, E.J. SARS-Coronavirus Replication Is Supported by a Reticulovesicular Network of Modified Endoplasmic Reticulum. PLoS Biol. 2008, 6, e226. [Google Scholar] [CrossRef]
- Lachmann, S.; Bär, S.; Rommelaere, J.; Nüesch, J.P.F. Parvovirus interference with intracellular signalling: Mechanism of PKCη activation in MVM-infected A9 fibroblasts. Cell. Microbiol. 2008, 10, 755–769. [Google Scholar] [CrossRef]
- Laliberte, J.P.; Moss, B. Lipid Membranes in Poxvirus Replication. Viruses 2010, 2, 972–986. [Google Scholar] [CrossRef] [Green Version]
- Melia, C.E.; Peddie, C.J.; de Jong, A.; Snijder, E.J.; Collinson, L.M.; Koster, A.J.; van der Schaar, H.M.; van Kuppeveld, F.J.M.; Bárcena, M. Origins of Enterovirus Replication Organelles Established by Whole-Cell Electron Microscopy. mBio 2019, 10, e00951-19. [Google Scholar] [CrossRef] [Green Version]
- Tuthill, T.J.; Groppelli, E.; Hogle, J.M.; Rowlands, D.J. Picornaviruses. Foam. Viruses 2010, 343, 43–89. [Google Scholar] [CrossRef]
- Cifuente, J.O.; Moratorio, G. Evolutionary and Structural Overview of Human Picornavirus Capsid Antibody Evasion. Front. Cell Infect. Microbiol. 2019, 9, 283. [Google Scholar] [CrossRef]
- Belov, G.A.; Nair, V.; Hansen, B.T.; Hoyt, F.H.; Fischer, E.R.; Ehrenfeld, E. Complex Dynamic Development of Poliovirus Membranous Replication Complexes. J. Virol. 2011, 86, 302–312. [Google Scholar] [CrossRef] [Green Version]
- Gosert, R.; Egger, D.; Lohmann, V.; Bartenschlager, R.; Blum, H.E.; Bienz, K.; Moradpour, D. Identification of the Hepatitis C Virus RNA Replication Complex in Huh-7 Cells Harboring Subgenomic Replicons. J. Virol. 2003, 77, 5487–5492. [Google Scholar] [CrossRef] [Green Version]
- Ferraris, P.; Blanchard, E.; Roingeard, P. Ultrastructural and biochemical analyses of hepatitis C virus-associated host cell membranes. J. Gen. Virol. 2010, 91, 2230–2237. [Google Scholar] [CrossRef]
- Rust, R.C.; Landmann, L.; Gosert, R.; Tang, B.L.; Hong, W.; Hauri, H.-P.; Egger, D.; Bienz, K. Cellular COPII Proteins Are Involved in Production of the Vesicles That Form the Poliovirus Replication Complex. J. Virol. 2001, 75, 9808–9818. [Google Scholar] [CrossRef] [Green Version]
- Richards, A.L.; Jackson, W.T. Behind Closed Membranes: The Secret Lives of Picornaviruses? PLOS Pathog. 2013, 9, e1003262. [Google Scholar] [CrossRef] [Green Version]
- Belov, G.; Feng, Q.; Nikovics, K.; Jackson, C.; Ehrenfeld, E. A Critical Role of a Cellular Membrane Traffic Protein in Poliovirus RNA Replication. PLoS Pathog. 2008, 4, e1000216. [Google Scholar] [CrossRef] [Green Version]
- Hsu, N.-Y.; Ilnytska, O.; Belov, G.; Santiana, M.; Chen, Y.-H.; Takvorian, P.M.; Pau, C.; Van Der Schaar, H.; Kaushik-Basu, N.; Balla, T.; et al. Viral Reorganization of the Secretory Pathway Generates Distinct Organelles for RNA Replication. Cell 2010, 141, 799–811. [Google Scholar] [CrossRef] [Green Version]
- Belov, G.A.; Altan-Bonnet, N.; Kovtunovych, G.; Jackson, C.L.; Lippincott-Schwartz, J.; Ehrenfeld, E. Hijacking Components of the Cellular Secretory Pathway for Replication of Poliovirus RNA. J. Virol. 2006, 81, 558–567. [Google Scholar] [CrossRef] [Green Version]
- Berger, K.L.; Kelly, S.M.; Jordan, T.; Tartell, M.A.; Randall, G. Hepatitis C Virus Stimulates the Phosphatidylinositol 4-Kinase III Alpha-Dependent Phosphatidylinositol 4-Phosphate Production That Is Essential for Its Replication. J. Virol. 2011, 85, 8870–8883. [Google Scholar] [CrossRef] [Green Version]
- Reiss, S.; Rebhan, I.; Backes, P.; Brey, I.R.; Erfle, H.; Matula, P.; Kaderali, L.; Poenisch, M.; Blankenburg, H.; Hiet, M.-S.; et al. Recruitment and Activation of a Lipid Kinase by Hepatitis C Virus NS5A Is Essential for Integrity of the Membranous Replication Compartment. Cell Host Microbe 2011, 9, 32–45. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Hong, Z.; Lin, W.; Shao, R.-X.; Goto, K.; Hsu, V.W.; Chung, R.T. ARF1 and GBF1 Generate a PI4P-Enriched Environment Supportive of Hepatitis C Virus Replication. PLoS ONE 2012, 7, e32135. [Google Scholar] [CrossRef] [Green Version]
- Richards, A.L.; Jackson, W.T. Intracellular Vesicle Acidification Promotes Maturation of Infectious Poliovirus Particles. PLoS Pathog. 2012, 8, e1003046. [Google Scholar] [CrossRef] [Green Version]
- Taylor, M.P.; Burgon, T.B.; Kirkegaard, K.; Jackson, W.T. Role of Microtubules in Extracellular Release of Poliovirus. J. Virol. 2009, 83, 6599–6609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paloheimo, O.; Ihalainen, T.; Tauriainen, S.; Välilehto, O.; Kirjavainen, S.; Niskanen, E.; Laakkonen, J.P.; Hyoty, H.; Vihinen-Ranta, M. Coxsackievirus B3-Induced Cellular Protrusions: Structural Characteristics and Functional Competence. J. Virol. 2011, 85, 6714–6724. [Google Scholar] [CrossRef] [Green Version]
- Mutsafi, Y.; Altan-Bonnet, N. Enterovirus Transmission by Secretory Autophagy. Viruses 2018, 10, 139. [Google Scholar] [CrossRef] [Green Version]
- Fader, C.M.; Colombo, M.I. Autophagy and multivesicular bodies: Two closely related partners. Cell Death Differ. 2009, 16, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Li, X.; Luo, S.; Fan, B.; Zhu, C.; Chen, Z. Coordination and Crosstalk between Autophagosome and Multivesicular Body Pathways in Plant Stress Responses. Cells 2020, 9, 119. [Google Scholar] [CrossRef] [Green Version]
- Hassan, Z.; Hashim, M.J.; Khan, G. Population risk factors for COVID-19 deaths in Nigeria at sub-national level. Pan Afr. Med. J. 2020, 35, 131. [Google Scholar] [CrossRef]
- Schoeman, D.; Fielding, B.C. Coronavirus envelope protein: Current knowledge. Virol. J. 2019, 16, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Brian, D.A.; Baric, R.S. Coronavirus Genome Structure and Replication. Curr. Top. Microbiol. Immunol. 2005, 287, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Wolff, G.; Limpens, R.W.A.L.; Zevenhoven-Dobbe, J.C.; Laugks, U.; Zheng, S.; De Jong, A.W.M.; Koning, R.I.; Agard, D.A.; Grünewald, K.; Koster, A.J.; et al. A molecular pore spans the double membrane of the coronavirus replication organelle. Science 2020, 369, 1395–1398. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, S.G.; Sawicki, D.L.; Younker, D.; Meyer, Y.; Thiel, V.; Stokes, H.; Siddell, S.G. Functional and Genetic Analysis of Coronavirus Replicase-Transcriptase Proteins. PLoS Pathog. 2005, 1, e39. [Google Scholar] [CrossRef]
- Ulasli, M.; Verheije, M.H.; de Haan, C.A.; Reggiori, F. Qualitative and quantitative ultrastructural analysis of the membrane rearrangements induced by coronavirus. Cell Microbiol. 2010, 12, 844–861. [Google Scholar] [CrossRef] [Green Version]
- Ziebuhr, J.; Gorbalenya, A.; Snijder, E. Virus-encoded proteinases and proteolytic processing in the Nidovirales. J. Gen. Virol. 2000, 81, 853–879. [Google Scholar] [CrossRef] [PubMed]
- Reggiori, F.; Monastyrska, I.; Verheije, M.H.; Calì, T.; Ulasli, M.; Bianchi, S.; Bernasconi, R.; de Haan, C.A.; Molinari, M. Coronaviruses Hijack the LC3-I-Positive EDEMosomes, ER-Derived Vesicles Exporting Short-Lived ERAD Regulators, for Replication. Cell Host Microbe 2010, 7, 500–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knoops, K.; Swett-Tapia, C.; Worm, S.H.E.V.D.; Velthuis, A.T.; Koster, A.; Mommaas, A.M.; Snijder, E.J.; Kikkert, M. Integrity of the Early Secretory Pathway Promotes, but Is Not Required for, Severe Acute Respiratory Syndrome Coronavirus RNA Synthesis and Virus-Induced Remodeling of Endoplasmic Reticulum Membranes. J. Virol. 2010, 84, 833–846. [Google Scholar] [CrossRef] [Green Version]
- De Wilde, A.H.; Raj, V.S.; Oudshoorn, D.; Bestebroer, T.M.; Van Nieuwkoop, S.; Limpens, R.; Posthuma, C.C.; Van Der Meer, Y.; Barcena, M.; Haagmans, B.L.; et al. MERS-coronavirus replication induces severe in vitro cytopathology and is strongly inhibited by cyclosporin A or interferon-α treatment. J. Gen. Virol. 2013, 94, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, L.; Howe, A.; Gilchrist, J.B.; Sun, D.; Knight, M.L.; Zanetti-Domingues, L.C.; Bateman, B.; Krebs, A.S.; Chen, L.; Radecke, J.; et al. SARS-CoV-2 Assembly and Egress Pathway Revealed by Correlative Multi-Modal Multi-Scale Cryo-Imaging. Soc. Sci. Res. Netw. 2020. [Google Scholar] [CrossRef]
- Ghosh, S.; Dellibovi-Ragheb, T.A.; Kerviel, A.; Pak, E.; Qiu, Q.; Fisher, M.; Takvorian, P.M.; Bleck, C.; Hsu, V.W.; Fehr, A.R.; et al. β-Coronaviruses Use Lysosomes for Egress Instead of the Biosynthetic Secretory Pathway. Cell 2020, 183, 1520–1535.e14. [Google Scholar] [CrossRef]
- Zhou, X.; Cong, Y.; Veenendaal, T.; Klumperman, J.; Shi, D.; Mari, M.; Reggiori, F. Ultrastructural Characterization of Membrane Rearrangements Induced by Porcine Epidemic Diarrhea Virus Infection. Viruses 2017, 9, 251. [Google Scholar] [CrossRef]
- Schweitzer, B.K.; Chapman, N.M.; Iwen, P.C. Overview of theFlaviviridaeWith an Emphasis on the Japanese Encephalitis Group Viruses. Lab. Med. 2009, 40, 493–499. [Google Scholar] [CrossRef]
- Lindenbach, B.D.; Rice, C.M. Unravelling hepatitis C virus replication from genome to function. Nat. Cell Biol. 2005, 436, 933–938. [Google Scholar] [CrossRef]
- Pierson, T.C.; Diamond, M.S. The continued threat of emerging flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef] [PubMed]
- Esser-Nobis, K.; Romero-Brey, I.; Ganten, T.M.; Gouttenoire, J.; Harak, C.; Klein, R.; Schemmer, P.; Binder, M.; Schnitzler, P.; Moradpour, D.; et al. Analysis of hepatitis C virus resistance to silibinin in vitro and in vivo points to a novel mechanism involving nonstructural protein 4B. Hepatology 2013, 57, 953–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furse, S.; Brooks, N.J.; Seddon, A.; Woscholski, R.; Templer, R.H.; Tate, E.W.; Gaffney, P.; Ces, O. Lipid membrane curvature induced by distearoyl phosphatidylinositol 4-phosphate. Soft Matter. 2012, 8, 3090–3093. [Google Scholar] [CrossRef]
- Amako, Y.; Sarkeshik, A.; Hotta, H.; Yates, J.; Siddiqui, A. Role of Oxysterol Binding Protein in Hepatitis C Virus infection. J. Virol. 2009, 83, 9237–9246. [Google Scholar] [CrossRef] [Green Version]
- Delang, L.; Paeshuyse, J.; Neyts, J. The role of phosphatidylinositol 4-kinases and phosphatidylinositol 4-phosphate during viral replication. Biochem. Pharmacol. 2012, 84, 1400–1408. [Google Scholar] [CrossRef]
- Martín-Acebes, M.A.; Blazquez, A.; de Oya, N.J.; Escribano-Romero, E.; Saiz, J.-C. West Nile Virus Replication Requires Fatty Acid Synthesis but Is Independent on Phosphatidylinositol-4-Phosphate Lipids. PLoS ONE 2011, 6, e24970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Miner, J.J.; Gorman, M.J.; Rausch, K.; Ramage, H.; White, J.P.; Zuiani, A.; Zhang, P.; Fernandez, E.; Zhang, Q.; et al. A CRISPR screen defines a signal peptide processing pathway required by flaviviruses. Nat. Cell Biol. 2016, 535, 164–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanini, F.; Pu, S.-Y.; Bekerman, E.; Einav, S.; Quake, S.R. Single-cell transcriptional dynamics of flavivirus infection. eLife 2018, 7, e32942. [Google Scholar] [CrossRef] [Green Version]
- Marceau, C.D.; Puschnik, A.S.; Majzoub, K.; Ooi, Y.S.; Brewer, S.M.; Fuchs, G.; Swaminathan, K.; Mata, M.A.; Elias, J.E.; Sarnow, P.; et al. Genetic dissection of Flaviviridae host factors through genome-scale CRISPR screens. Nat. Cell Biol. 2016, 535, 159–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heaton, N.S.; Moshkina, N.; Fenouil, R.; Gardner, T.; Aguirre, S.; Shah, P.; Zhao, N.; Manganaro, L.; Hultquist, J.; Noel, J.; et al. Targeting Viral Proteostasis Limits Influenza Virus, HIV, and Dengue Virus Infection. Immunplogy 2016, 44, 46–58. [Google Scholar] [CrossRef] [Green Version]
- Yuan, T.; Lu, J.; Zhang, J.; Zhang, Y.; Chen, L. Spatiotemporal Detection and Analysis of Exocytosis Reveal Fusion “Hotspots” Organized by the Cytoskeleton in Endocrine Cells. Biophys. J. 2015, 108, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Coller, K.E.; Heaton, N.S.; Berger, K.L.; Cooper, J.D.; Saunders, J.L.; Randall, G. Molecular Determinants and Dynamics of Hepatitis C Virus Secretion. PLoS Pathog. 2012, 8, e1002466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, I.-M.; Zhang, W.; Holdaway, H.A.; Li, L.; Kostyuchenko, V.A.; Chipman, P.R.; Kuhn, R.J.; Rossmann, M.G.; Chen, J. Structure of the Immature Dengue Virus at Low pH Primes Proteolytic Maturation. Science 2008, 319, 1834–1837. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lok, S.-M.; Yu, I.-M.; Zhang, Y.; Kuhn, R.J.; Chen, J.; Rossmann, M.G. The Flavivirus Precursor Membrane-Envelope Protein Complex: Structure and Maturation. Science 2008, 319, 1830–1834. [Google Scholar] [CrossRef] [Green Version]
- Teo, S.H.C.; Chu, J.J.H. Cellular Vimentin Regulates Construction of Dengue Virus Replication Complexes through Interaction with NS4A Protein. J. Virol. 2014, 88, 1897–1913. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Gao, N.; Wang, J.-L.; Tian, Y.-P.; Chen, Z.-T.; An, J. Vimentin is required for dengue virus serotype 2 infection but microtubules are not necessary for this process. Arch. Virol. 2008, 153, 1777–1781. [Google Scholar] [CrossRef]
- Egger, D.; Wölk, B.; Gosert, R.; Bianchi, L.; Blum, H.E.; Moradpour, D.; Bienz, K. Expression of Hepatitis C Virus Proteins Induces Distinct Membrane Alterations Including a Candidate Viral Replication Complex. J. Virol. 2002, 76, 5974–5984. [Google Scholar] [CrossRef] [Green Version]
- Targett-Adams, P.; Boulant, S.; McLauchlan, J. Visualization of Double-Stranded RNA in Cells Supporting Hepatitis C Virus RNA Replication. J. Virol. 2008, 82, 2182–2195. [Google Scholar] [CrossRef] [Green Version]
- Heaton, N.S.; Randall, G. Dengue Virus-Induced Autophagy Regulates Lipid Metabolism. Cell Host Microbe 2010, 8, 422–432. [Google Scholar] [CrossRef] [Green Version]
- Tabata, K.; Arimoto, M.; Arakawa, M.; Nara, A.; Saito, K.; Omori, H.; Arai, A.; Ishikawa, T.; Konishi, E.; Suzuki, R.; et al. Unique Requirement for ESCRT Factors in Flavivirus Particle Formation on the Endoplasmic Reticulum. Cell Rep. 2016, 16, 2339–2347. [Google Scholar] [CrossRef] [Green Version]
- Barouch-Bentov, R.; Neveu, G.; Xiao, F.; Beer, M.; Bekerman, E.; Schor, S.; Campbell, J.; Boonyaratanakornkit, J.; Lindenbach, B.; Lu, A.; et al. Hepatitis C Virus Proteins Interact with the Endosomal Sorting Complex Required for Transport (ESCRT) Machinery via Ubiquitination To Facilitate Viral Envelopment. mBio 2016, 7, e01456-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poxviridae. In Fenner’s Veterinary Virology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 157–174.
- Moss, B. Poxvirus Cell Entry: How Many Proteins Does it Take? Viruses 2012, 4, 688–707. [Google Scholar] [CrossRef] [PubMed]
- Parviainen, S.; Autio, K.; Vähä-Koskela, M.; Guse, K.; Pesonen, S.; Rosol, T.; Zhao, F.; Hemminki, A. Incomplete but Infectious Vaccinia Virions Are Produced in the Absence of Oncolysis in Feline SCCF1 Cells. PLoS ONE 2015, 10, e0120496. [Google Scholar] [CrossRef]
- Roberts, K.L.; Smith, G.L. Vaccinia virus morphogenesis and dissemination. Trends Microbiol. 2008, 16, 472–479. [Google Scholar] [CrossRef]
- Sodeik, B.; Doms, R.W.; Ericsson, M.; Hiller, G.; Machamer, C.E.; van ’t Hof, W.; van Meer, G.; Moss, B.; Griffiths, G. Assembly of vaccinia virus: Role of the intermediate compartment between the endoplasmic retic-ulum and the Golgi stacks. J. Cell Biol. 1993, 121, 521–541. [Google Scholar] [CrossRef]
- Herrero-Martínez, E.; Roberts, K.L.; Hollinshead, M.; Smith, G.L. Vaccinia virus intracellular enveloped virions move to the cell periphery on microtubules in the absence of the A36R protein. J. Gen. Virol. 2005, 86, 2961–2968. [Google Scholar] [CrossRef]
- Röttger, S.; Frischknecht, F.; Reckmann, I.; Smith, G.L.; Way, M. Interactions between Vaccinia Virus IEV Membrane Proteins and Their Roles in IEV Assembly and Actin Tail Formation. J. Virol. 1999, 73, 2863–2875. [Google Scholar] [CrossRef] [Green Version]
- Giorgi, C.; Marchi, S.; Pinton, P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat. Rev. Mol. Cell Biol. 2018, 19, 713–730. [Google Scholar] [CrossRef]
- Osellame, L.D.; Blacker, T.S.; Duchen, M.R. Cellular and molecular mechanisms of mitochondrial function. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 711–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, J.; Söderlund-Venermo, M.; Young, N.S. Human Parvoviruses. Clin. Microbiol. Rev. 2017, 30, 43–113. [Google Scholar] [CrossRef] [Green Version]
- Bär, S.; Daeffler, L.; Rommelaere, J.; Nüesch, J.P.F. Vesicular Egress of Non-Enveloped Lytic Parvoviruses Depends on Gelsolin Functioning. PLoS Pathog. 2008, 4, e1000126. [Google Scholar] [CrossRef]
- Gil-Ranedo, J.; Hernando, E.; Valle, N.; Riolobos, L.; Maroto, B.; Almendral, J.M. Differential phosphorylation and n-terminal configuration of capsid subunits in parvovirus assembly and viral trafficking. Virology 2018, 518, 184–194. [Google Scholar] [CrossRef]
- Nuesch, J.P.F.; Rommelaere, J. A viral adaptor protein modulating casein kinase II activity induces cytopathic effects in permissive cells. Proc. Natl. Acad. Sci. USA 2007, 104, 12482–12487. [Google Scholar] [CrossRef] [Green Version]
- Nüesch, J.P.F.; Rommelaere, J. NS1 Interaction with CKIIα: Novel Protein Complex Mediating Parvovirus-Induced Cytotoxicity. J. Virol. 2006, 80, 4729–4739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nüesch, J.P.F.; Bär, S.; Lachmann, S.; Rommelaere, J. Ezrin-Radixin-Moesin Family Proteins Are Involved in Parvovirus Replication and Spreading. J. Virol. 2009, 83, 5854–5863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Añel, A.M.D.; Malhotra, V. PKCη is required for β1γ2/β3γ2- and PKD-mediated transport to the cell surface and the organization of the Golgi apparatus. J. Cell Biol. 2005, 169, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Wilson, D.W. HSV-1 Cytoplasmic Envelopment and Egress. Int. J. Mol. Sci. 2020, 21, 5969. [Google Scholar] [CrossRef] [PubMed]
- Bowman, B.R.; Baker, M.L.; Rixon, F.J.; Chiu, W.; Quiocho, F.A. Structure of the herpesvirus major capsid protein. EMBO J. 2003, 22, 757–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, G.; Fitzmaurice, C.; Naghavi, M.A.; Ahmed, L. Global and regional incidence, mortality and disability-adjusted life-years for Epstein-Barr virus-attributable malignancies, 1990–2017. BMJ Open 2020, 10, e037505. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zhou, S.; Gao, S.; Deng, H. Remodeling of host membranes during herpesvirus assembly and egress. Protein Cell 2019, 10, 315–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigalke, J.M.; Heldwein, E.E. Nuclear Exodus: Herpesviruses Lead the Way. Annu. Rev. Virol. 2016, 3, 387–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marschall, M.; Marzi, A.; Siepen, P.A.D.; Jochmann, R.; Kalmer, M.; Auerochs, S.; Lischka, P.; Leis, M.; Stamminger, T. Cellular p32 Recruits Cytomegalovirus Kinase pUL97 to Redistribute the Nuclear Lamina. J. Biol. Chem. 2005, 280, 33357–33367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farnsworth, A.; Wisner, T.W.; Webb, M.; Roller, R.; Cohen, G.; Eisenberg, R.; Johnson, D.C. Herpes simplex virus glycoproteins gB and gH function in fusion between the virion envelope and the outer nuclear membrane. Proc. Natl. Acad. Sci. USA 2007, 104, 10187–10192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyder, A.; Polcicova, K.; Johnson, D.C. Herpes Simplex Virus gE/gI and US9 Proteins Promote Transport of both Capsids and Virion Glycoproteins in Neuronal Axons. J. Virol. 2008, 82, 10613–10624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mingo, R.M.; Han, J.; Newcomb, W.W.; Brown, J.C. Replication of Herpes Simplex Virus: Egress of Progeny Virus at Specialized Cell Membrane Sites. J. Virol. 2012, 86, 7084–7097. [Google Scholar] [CrossRef] [Green Version]
- Granzow, H.; Weiland, F.; Jöns, A.; Klupp, B.G.; Karger, A.; Mettenleiter, T.C. Ultrastructural analysis of the replication cycle of pseudorabies virus in cell culture: A reassessment. J. Virol. 1997, 71, 2072–2082. [Google Scholar] [CrossRef] [Green Version]
- Gershon, A.A.; Sherman, D.L.; Zhu, Z.; Gabel, C.A.; Ambron, R.T.; Gershon, M.D. Intracellular transport of newly synthesized varicella-zoster virus: Final envelopment in the trans-Golgi network. J. Virol. 1994, 68, 6372–6390. [Google Scholar] [CrossRef] [Green Version]
- Chiu, Y.-F.; Sugden, B.; Chang, P.-J.; Chen, L.-W.; Lin, Y.-J.; Lan, Y.-C.; Lai, C.-H.; Liou, J.-Y.; Liu, S.-T.; Hung, C.-H. Characterization and Intracellular Trafficking of Epstein-Barr Virus BBLF1, a Protein Involved in Virion Maturation. J. Virol. 2012, 86, 9647–9655. [Google Scholar] [CrossRef] [Green Version]
- Bonifacino, J.S.; Dell’Angelica, E.C. Molecular Bases for the Recognition of Tyrosine-based Sorting Signals. J. Cell Biol. 1999, 145, 923–926. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, Z.; Kumar, N.D.; Reggiori, F.; Khan, G. How Viruses Hijack and Modify the Secretory Transport Pathway. Cells 2021, 10, 2535. https://doi.org/10.3390/cells10102535
Hassan Z, Kumar ND, Reggiori F, Khan G. How Viruses Hijack and Modify the Secretory Transport Pathway. Cells. 2021; 10(10):2535. https://doi.org/10.3390/cells10102535
Chicago/Turabian StyleHassan, Zubaida, Nilima Dinesh Kumar, Fulvio Reggiori, and Gulfaraz Khan. 2021. "How Viruses Hijack and Modify the Secretory Transport Pathway" Cells 10, no. 10: 2535. https://doi.org/10.3390/cells10102535
APA StyleHassan, Z., Kumar, N. D., Reggiori, F., & Khan, G. (2021). How Viruses Hijack and Modify the Secretory Transport Pathway. Cells, 10(10), 2535. https://doi.org/10.3390/cells10102535






