Neurobiological Basis of Increased Risk for Suicidal Behaviour
Abstract
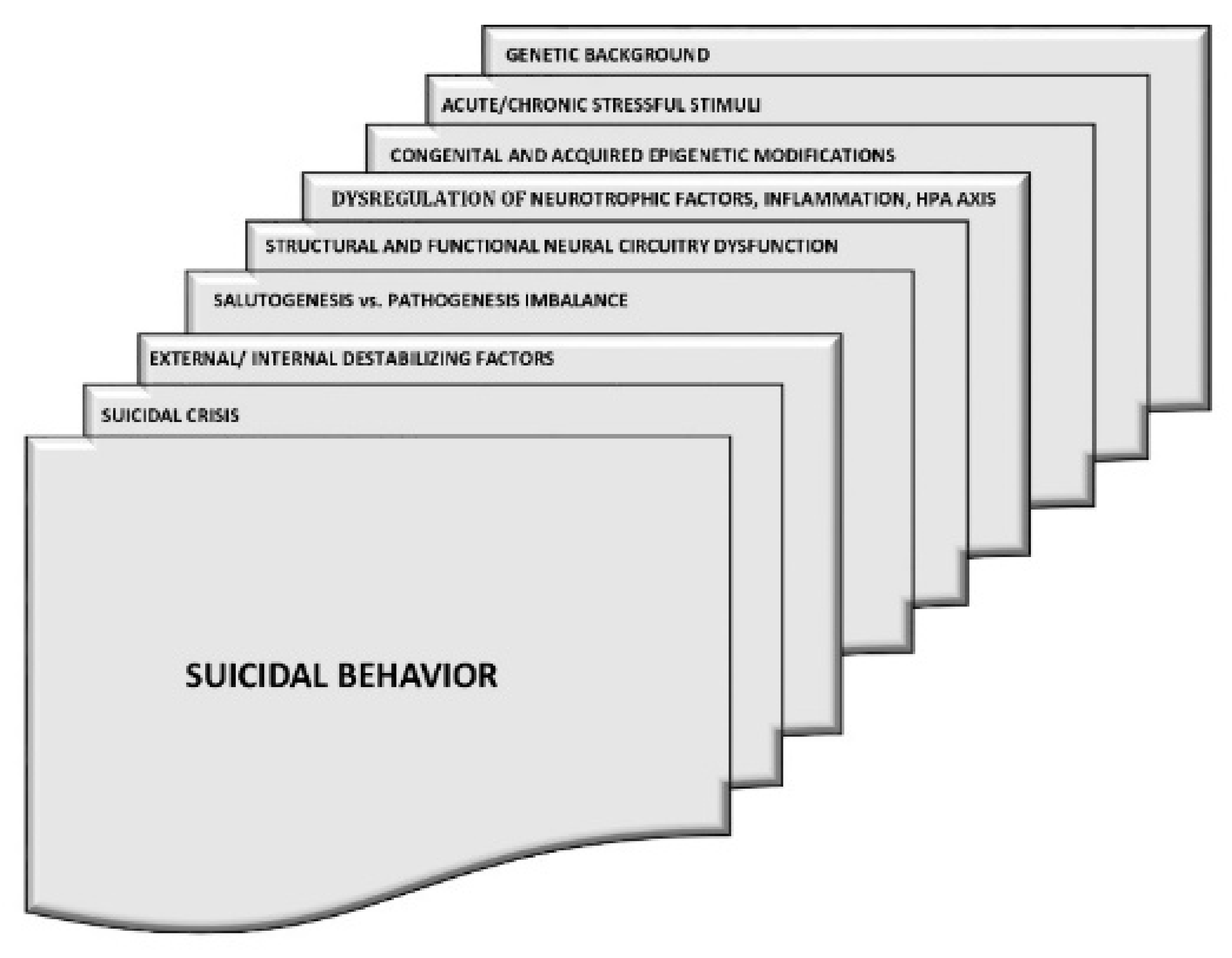
1. The Scope of the Problem
2. The Biological Background of Suicidal Behaviour
2.1. Inflammation and the Kynurenine Pathway
2.2. Serotonin System
2.3. Brain-Derived Neurotrophic Factor (BDNF)
2.4. The Hypothalamic–Pituitary–Adrenal (HPA) Axis
2.5. Glutaminergic and GABAergic Neurotransmission
2.6. Cholesterol
3. Epigenetic Changes
4. Activity of Brain Structures in Suicidal Patients
5. Therapeutic Options to Prevent Suicide
5.1. Lithium
5.2. Clozapine
5.3. Ketamine/Esketamine
6. Drugs That Increase the Risk of Suicidality
7. Substance Misuse
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bryleva, E.Y.; Brundin, L. Kynurenine pathway metabolites and suicidality. Neuropharmacology 2017, 112, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, S. Epidemiology of suicide and the psychiatric perspective. Int. J. Environ. Res. Public Health 2018, 15, 1425. [Google Scholar] [CrossRef]
- Mann, J.J. Neurobiology of suicidal behaviour. Nat. Rev. Neurosci. 2003, 4, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Milner, A.; Sveticic, J.; De Leo, D. Suicide in the absence of mental disorder? A review of psychological autopsy studies across countries. Int. J. Soc. Psychiatry 2012, 59, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Dwivedi, Y. Understanding epigenetic architecture of suicide neurobiology: A critical perspective. Neurosci. Biobehav. Rev. 2017, 72, 10–27. [Google Scholar] [CrossRef] [PubMed]
- De Berardis, D.; Fornaro, M.; Valchera, A.; Cavuto, M.; Perna, G.; Di Nicola, M.; Serafini, G.; Carano, A.; Pompili, M.; Vellante, F.; et al. Eradicating suicide at its roots: Preclinical bases and clinical evidence of the efficacy of ketamine in the treatment of suicidal behaviors. Int. J. Mol. Sci. 2018, 19, 2888. [Google Scholar] [CrossRef]
- Baldessarini, R.J. Epidemiology of suicide: Recent developments. Epidemiol. Psychiatr. Sci. 2019, 29, e71. [Google Scholar] [CrossRef]
- Schmaal, L.; Van Harmelen, A.-L.; Chatzi, V.; Lippard, E.; Toenders, Y.J.; Averill, L.A.; Mazure, C.M.; Blumberg, H.P. Imaging suicidal thoughts and behaviors: A comprehensive review of 2 decades of neuroimaging studies. Mol. Psychiatry 2020, 25, 408–427. [Google Scholar] [CrossRef]
- Bergfeld, I.O.; Mantione, M.; Figee, M.; Schuurman, P.R.; Lok, A.; Denys, D. Treatment-resistant depression and suicidality. J. Affect. Disord. 2018, 235, 362–367. [Google Scholar] [CrossRef]
- Borges, G.; Bagge, C.L.; Cherpitel, C.J.; Conner, K.R.; Orozco, R.; Rossow, I. A meta-analysis of acute use of alcohol and the risk of suicide attempt. Psychol. Med. 2017, 47, 949–957. [Google Scholar] [CrossRef]
- Chesney, E.; Goodwin, G.M.; Fazel, S. Risks of all-cause and suicide mortality in mental disorders: A meta-review. World Psychiatry 2014, 13, 153–160. [Google Scholar] [CrossRef]
- Meltzer, H.Y.; Li, Z.; Kaneda, Y.; Ichikawa, J. Serotonin receptors their key role in drugs to treat schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 1159–1172. [Google Scholar] [CrossRef]
- Mortensen, P.; Agerbo, E.; Erikson, T.; Qin, P.; Westergaard-Nielsen, N. Psychiatric illness and risk factors for suicide in Denmark. Lancet 2000, 355, 9–12. [Google Scholar] [CrossRef]
- Breslau, N. Migraine, suicidal ideation, and suicide attempts. Neurology 1992, 42, 392. [Google Scholar] [CrossRef]
- McFarland, D.; Walsh, L.; Napolitano, S.; Morita, J.; Jaiswal, R. Suicide in patients with cancer: Identifying the risk factors. Oncology 2019, 33, 221–226. [Google Scholar]
- Calati, R.; Nemeroff, C.B.; Lopez-Castroman, J.; Cohen, L.J.; Galynker, I. Candidate biomarkers of suicide crisis syndrome: What to test next? A concept paper. Int. J. Neuropsychopharmacol. 2020, 23, 192–205. [Google Scholar] [CrossRef]
- Jimenez-Trevino, L.; Gonzalez-Blanco, L.; Alvarez-Vazquez, C.; Rodriguez-Revuelta, J.; Martinez, P.A.S. Glutamine and new pharmacological targets to treat suicidal ideation. Curr. Top. Behav. Neurosci. 2020, 46, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Perroud, N.; Baud, P.; Mouthon, D.; Courtet, P.; Malafosse, A. Impulsivity, aggression and suicidal behavior in unipolar and bipolar disorders. J. Affect. Disord. 2011, 134, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Statham, D.J.; Heath, A.C.; Madden, P.A.F.; Bucholz, K.K.; Bierut, L.; Dinwiddie, S.H.; Slutske, W.S.; Dunne, M.; Martin, N. Suicidal behaviour: An epidemiological and genetic study. Psychol. Med. 1998, 28, 839–855. [Google Scholar] [CrossRef]
- Mullins, N.; Bigdeli, T.B.; Børglum, A.D.; Coleman, J.R.I.; Demontis, D.; Mehta, D.; Power, R.A.; Ripke, S.; Stahl, E.A.; Starnawska, A.; et al. GWAS of suicide attempt in psychiatric disorders and association with major depression polygenic risk scores. Am. J. Psychiatry. 2019, 176, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Levey, D.; Polimanti, R.; Cheng, Z.; Zhou, H.; Nuñez, Y.Z.; Jain, S.; He, F.; Sun, X.; Ursano, R.J.; Kessler, R.C.; et al. Genetic associations with suicide attempt severity and genetic overlap with major depression. Transl. Psychiatry 2019, 9, 22. [Google Scholar] [CrossRef]
- Buchman-Schmitt, J.M.; Chu, C.; Michaels, M.S.; Hames, J.L.; Silva, C.; Hagan, C.R.; Ribeiro, J.D.; Selby, E.A.; Joiner, T.E. The role of stressful life events preceding death by suicide: Evidence from two samples of suicide decedents. Psychiatry Res. 2017, 256, 345–352. [Google Scholar] [CrossRef]
- Dwivedi, Y. MicroRNAs in depression and suicide: Recent insights and future perspectives. J. Affect. Disord. 2018, 240, 146–154. [Google Scholar] [CrossRef]
- Ganança, L.; Oquendo, M.A.; Tyrka, A.R.; Cisneros-Trujillo, S.; Mann, J.J.; Sublette, M.E. The role of cytokines in the pathophysiology of suicidal behavior. Psychoneuroendocrinology 2016, 63, 296–310. [Google Scholar] [CrossRef]
- Lengvenyte, A.; Conejero, I.; Courtet, P.; Olié, E. Biological bases of suicidal behaviours: A narrative review. Eur. J. Neurosci. 2019, 10, 14635. [Google Scholar] [CrossRef] [PubMed]
- Lenz, B.; Röther, M.; Bouna-Pyrrou, P.; Mühle, C.; Tektas, O.Y.; Kornhuber, J. The androgen model of suicide completion. Prog. Neurobiol. 2019, 172, 84–103. [Google Scholar] [CrossRef]
- Killgore, W.D.; Cloonan, S.A.; Taylor, E.C.; Allbright, M.C.; Dailey, N.S. Trends in suicidal ideation over the first three months of COVID-19 lockdowns. Psychiatry Res. 2020, 293, 113390. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.; O’Connor, R.C. Suicide in Northern Ireland: Epidemiology, risk factors, and prevention. Lancet Psychiatry 2020, 7, 538–546. [Google Scholar] [CrossRef]
- Allen, L.; Dwivedi, Y. MicroRNA mediators of early life stress vulnerability to depression and suicidal behavior. Mol. Psychiatry 2020, 25, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Orsolini, L.; Latini, R.; Pompili, M.; Serafini, G.; Volpe, U.; Vellante, F.; Fornaro, M.; Valchera, A.; Tomasetti, C.; Fraticelli, S.; et al. Understanding the complex of suicide in depression: From research to clinics. Psychiatry Investig. 2020, 17, 207–221. [Google Scholar] [CrossRef]
- Erhardt, S.; Lim, E.; Linderholm, K.R.; Janelidze, S.; Lindqvist, D.; Samuelsson, M.; Lundberg, K.; Postolache, T.T.; Träskman-Bendz, L.; Guillemin, G.; et al. Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacology 2012, 38, 743–752. [Google Scholar] [CrossRef]
- Sueki, H. Relationship between beck hopelessness scale and suicidal ideation: A short-term longitudinal study. Death Stud. 2020, 17, 1–6. [Google Scholar] [CrossRef]
- Asarnow, J.; McArthur, D.; Hughes, J.; Barbery, V.; Berk, M. Suicide attempt risk in youths: Utility of the harkavy-asnis suicide scale for monitoring risk levels. Suicide Life-Threat. Behav. 2012, 42, 684–698. [Google Scholar] [CrossRef]
- Ofek, H.; Weizman, T.; Apter, A. The child suicide potential scale: Inter-rater reliability and validity in Israeli in-patient adolescents. Isr. J. Psychiatry Relat. Sci. 1998, 35, 253–261. [Google Scholar]
- Pandey, G.N.; Dwivedi, Y. Peripheral biomarkers for suicide. In The Neurobiological Basis of Suicide; Dwivedi, Y., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Millton, UK, 2012. [Google Scholar]
- Miura, H.; Ozaki, N.; Sawada, M.; Isobe, K.; Ohta, T.; Nagatsu, T. A link between stress and depression: Shifts in the balance between the kynurenine and serotonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression. Stress 2008, 11, 198–209. [Google Scholar] [CrossRef]
- Pandey, G.N. Biological basis of suicide and suicidal behavior. Bipolar Disord. 2013, 15, 524–541. [Google Scholar] [CrossRef]
- Niculescu, A.B.; Levey, D.; Phalen, P.; Le-Niculescu, H.; Dainton-Howard, H.; Jain, N.; Belanger, E.; James, A.; George, S.; Weber, H.; et al. Understanding and predicting suicidality using a combined genomic and clinical risk assessment approach. Mol. Psychiatry 2015, 20, 1266–1285. [Google Scholar] [CrossRef]
- Niculescu, A.B.; Le-Niculescu, H.; Levey, D.F.; Phalen, P.L.; Dainton, H.L.; Roseberry, K.; Niculescu, E.M.; Niezer, J.O.; Williams, A.; Graham, D.L.; et al. Precision medicine for suicidality: From universality to subtypes and personalisation. Mol. Psychiatry. 2017, 22, 1250–1273. [Google Scholar] [CrossRef]
- Le-Niculescu, H.; Levey, D.; Ayalew, M.; Palmer, L.; Gavrin, L.M.; Jain, N.; Winiger, E.; Bhosrekar, S.; Shankar, G.; Radel, M.; et al. Discovery and validation of blood biomarkers for suicidality. Mol. Psychiatry 2013, 18, 1249–1264. [Google Scholar] [CrossRef]
- Malik, S.; Kanwar, A.; Sim, L.A.; Prokop, L.J.; Wang, Z.; Benkhadra, K.; Murad, M.H. The association between sleep disturbances and suicidal behaviors in patients with psychiatric diagnoses: A systematic review and meta-analysis. Syst. Rev. 2014, 3, 18. [Google Scholar] [CrossRef]
- Lin, H.-T.; Lai, C.-H.; Perng, H.-J.; Chung, C.-H.; Wang, C.-C.; Chen, W.-L.; Chien, W.-C. Insomnia as an independent predictor of suicide attempts: A nationwide population-based retrospective cohort study. BMC Psychiatry 2018, 18, 117. [Google Scholar] [CrossRef]
- Pigeon, W.R.; Pinquart, M.; Conner, K. Meta-analysis of sleep disturbance and suicidal thoughts and behaviors. J. Clin. Psychiatry 2012, 73, e1160–e1167. [Google Scholar] [CrossRef] [PubMed]
- Amitai, M.; Taler, M.; Ben-Baruch, R.; Lebow, M.; Rotkopf, R.; Apter, A.; Fennig, S.; Weizman, A.; Chen, A. Increased circulatory IL-6 during 8-week fluoxetine treatment is a risk factor for suicidal behaviors in youth. Brain Behav. Immun. 2020, 87, 301–308. [Google Scholar] [CrossRef]
- Cha, C.B.; Franz, P.J.; Guzmán, E.M.; Glenn, C.R.; Kleiman, E.M.; Nock, M.K. Annual research review: Suicide among youth—epidemiology, (potential) etiology, and treatment. J. Child Psychol. Psychiatry 2017, 59, 460–482. [Google Scholar] [CrossRef] [PubMed]
- Keaton, S.A.; Madaj, Z.B.; Heilman, P.; Smart, L.; Grit, J.; Gibbons, R.; Postolache, T.T.; Roaten, K.; Achtyes, E.D.; Brundin, L. An inflammatory profile linked to increased suicide risk. J. Affect. Disord. 2019, 247, 57–65. [Google Scholar] [CrossRef]
- Sudol, K.; Mann, J.J. Biomarkers of sicide attempt behavior: Towards biological model of risk. Curr. Psychiatry. Rep. 2017, 19, 31. [Google Scholar] [CrossRef]
- Brundin, L.; Sellgren, C.M.; Lim, E.; Grit, J.; Palsson, E.; Landen, M.; Samuelsson, M.; Lundgren, K.; Brundin, P.; Fuchs, D.; et al. An enzyme in the kynurenine pathway that governs vulnerability to suicidal behavior by regulating excitotoxicity and neuroinflammation. Transl. Psychiatry 2016, 6, e865. [Google Scholar] [CrossRef]
- Serafini, G.; Parisi, V.M.; Aguglia, A.; Amerio, A.; Sampogna, G.; Fiorillo, A.; Pompili, M.; Amore, M. A specific inflammatory profile underlying suicide risk? Systematic review of the main literature findings. Int. J. Environ. Res. Public Health 2020, 17, 2393. [Google Scholar] [CrossRef] [PubMed]
- Fragoso, Y.D.; Comini-Frota, E.; Lopes, J.S.; Noal, J.S.; Giacomo, M.C.; Gomes, S.; Gonçalves, M.V.M.; da Gama, P.D.; Finkelsztejn, A. Severe depression, suicide attempts, and ideation during the use of interferon beta by patients with multiple sclerosis. Clin. Neuropharmacol. 2010, 33, 312–316. [Google Scholar] [CrossRef]
- Sockalingam, S.; Links, P.S.; Abbey, S.E. Suicide risk in hepatitis C and during interferon-alpha therapy: A review and clinical update. J. Viral Hepat. 2010, 18, 153–160. [Google Scholar] [CrossRef]
- Stuart, M.; Baune, B. Chemokines and chemokine receptors in mood disorders, schizophrenia, and cognitive impairment: A systematic review of biomarker studies. Neurosci. Biobehav. Rev. 2014, 42, 93–115. [Google Scholar] [CrossRef]
- Nassar, A.; Azab, A.N. Effects of lithium on inflammation. ACS Chem. Neurosci. 2014, 5, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, S.; Rheinstein, P. Nonsteroidal anti-inflammatory drugs (NSAIDs) reduce suicidal ideation and depression. Discov. Med 2019, 28, 205–212. [Google Scholar]
- Köhler-Forsberg, O.; Lydholm, C.N.; Hjorthøj, C.; Nordentoft, M.; Mors, O.; Benros, M. Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: Meta-analysis of clinical trials. Acta Psychiatr. Scand. 2019, 139, 404–419. [Google Scholar] [CrossRef]
- Fourrier, C.; Sampson, E.; Mills, N.; Baune, B.T. Anti-inflammatory treatment of depression: Study protocol for a randomised controlled trial of vortioxetine augmented with celecoxib or placebo. Trials 2018, 19, 447. [Google Scholar] [CrossRef]
- Halaris, A.; Cantos, A.; Johnson, K.; Hakimi, M.; Sinacore, J. Modulation of the inflammatory response benefits treatment-resistant bipolar depression: A randomized clinical trial. J. Affect. Disord. 2020, 261, 145–152. [Google Scholar] [CrossRef]
- Hopkins, A.G.; Spiller, H.A.; Kistamgari, S.; Zhu, M.; Michaels, N.L.; Funk, A.R.; Smith, G.A. Suicide-related over-the-counter analgesic exposures reported to United States poison control centers, 2000–2018. Pharmacoepidemiol. Drug. Saf. 2020, 29, 1011–1021. [Google Scholar] [CrossRef]
- Sas, K.; Szabó, E.; Vécsei, L. Mitochondria, oxidative stress and the kynurenine system, with a focus on ageing and neuroprotection. Molecules 2018, 23, 191. [Google Scholar] [CrossRef]
- Tufvesson-Alm, M.; Schwieler, L.; Schwarcz, R.; Goiny, M.; Erhardt, S.; Engberg, G. Importance of kynurenine 3-monooxygenase for spontaneous firing and pharmacological responses of midbrain dopamine neurons: Relevance for schizophrenia. Neuropharmacology 2018, 138, 130–139. [Google Scholar] [CrossRef]
- Hardingham, G.; Fukunaga, Y.; Bading, H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 2002, 5, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Sublette, M.E.; Galfalvy, H.; Fuchs, D.; Lapidus, M.; Grunebaum, M.F.; Oquendo, M.A.; Mann, J.J.; Postolache, T.T. Plasma kynurenine levels are elevated in suicide attempters with major depressive disorder. Brain Behav. Immun. 2011, 25, 1272–1278. [Google Scholar] [CrossRef]
- Savitz, J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef]
- Arango, V.; Underwood, M.; Boldrini, M.; Tamir, H.; Kassir, S.A.; Hsiung, S.-C.; Chen, J.J.-X.; Mann, J.J. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology 2001, 25, 892–903. [Google Scholar] [CrossRef]
- Asberg, M.; Träskman, L.; Thorén, P. 5-HIAA in the cerebrospinal fluid. A biochemical suicide predictor? Arch. Gen. Psychiatry. 1976, 33, 1193–1197. [Google Scholar] [CrossRef] [PubMed]
- Sher, L.; Carballo, J.J.; Grunebaum, M.F.; Burke, A.K.; Zalsman, G.; Huang, Y.-Y.; Mann, J.J.; Oquendo, M.A. A prospective study of the association of cerebrospinal fluid monoamine metabolite levels with lethality of suicide attempts in patients with bipolar disorder. Bipolar Disord. 2006, 8, 543–550. [Google Scholar] [CrossRef]
- Mathews, D.C.; Richards, E.M.; Niciu, M.J.; Ionescu, D.F.; Rasimas, J.J.; Zarate, C.A. Neurobiological aspects of suicide and suicide attempts in bipolar disorder. Transl. Neurosci. 2013, 4, 203–216. [Google Scholar] [CrossRef]
- Antypa, N.; Serretti, A.; Rujescu, D. Serotonergic genes and suicide: A systematic review. Eur. Neuropsychopharmacol. 2013, 23, 1125–1142. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.N.; Rizavi, H.S.; Ren, X.; Dwivedi, Y.; Palkovits, M. Region-specific alterations in glucocorticoid receptor expression in the postmortem brain of teenage suicide victims. Psychoneuroendocrinology 2013, 38, 2628–2639. [Google Scholar] [CrossRef]
- Oquendo, M.A.; Sullivan, G.M.; Sudol, K.; Baca-García, E.; Stanley, B.H.; Sublette, M.E.; Mann, J.J. Toward a biosignature for suicide. Am. J. Psychiatry 2014, 171, 1259–1277. [Google Scholar] [CrossRef]
- Stockmeier, C.A.; Dilley, G.E.; Shapiro, L.A.; Overholser, J.C.; Thompson, P.A.; Meltzer, H.Y. Serotonin receptors in suicide victims with major depression. Neuropsychopharmacology 1997, 16, 162–173. [Google Scholar] [CrossRef]
- Bennett, P.J.; McMahon, W.M.; Watabe, J.; Achilles, J.; Bacon, M.; Coon, H.; Grey, T.; Keller, T.; Tate, D.; Tcaciuc, I.; et al. Tryptophan hydroxylase polymorphisms in suicide victims. Psychiatr. Genet. 2000, 10, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Eisen, R.B.; Perera, S.; Banfield, L.; Anglin, R.; Minuzzi, L.; Samaan, Z. Association between BDNF levels and suicidal behaviour: A systematic review and meta-analysis. Syst. Rev. 2015, 4, 187. [Google Scholar] [CrossRef]
- Autry, A.E.; Monteggia, L.M. Epigenetics in suicide and depression. Biol. Psychiatry 2009, 66, 812–813. [Google Scholar] [CrossRef][Green Version]
- Misztak, P.; Pańczyszyn-Trzewik, P.; Nowak, G.; Sowa-Kućma, M. Epigenetic marks and their relationship with BDNF in the brain of suicide victims. PLoS ONE 2020, 15, e0239335. [Google Scholar] [CrossRef]
- Salas-Magaña, M.; Tovilla-Zárate, C.A.; González-Castro, T.B.; Juárez-Rojop, I.E.; López-Narváez, M.L.; Rodríguez-Pérez, J.M.; Ramírez-Bello, J. Decrease in brain-derived neurotrophic factor at plasma level but not in serum concentrations in suicide behavior: A systematic review and meta-analysis. Brain Behav. 2017, 7, e00706. [Google Scholar] [CrossRef] [PubMed]
- Pompili, M.; Serafini, G.; Innamorati, M.; Möller-Leimkühler, A.M.; Giupponi, G.; Girardi, P.; Tatarelli, R.; Lester, D. The hypothalamic-pituitary-adrenal axis and serotonin abnormalities: A selective overview for the implications of suicide prevention. Eur. Arch. Psychiatry Clin. Neurosci. 2010, 260, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Yerevanian, B.I.; Koek, R.J.; Feusner, J.D.; Hwang, S.; Mintz, J. Antidepressants and suicidal behaviour in unipolar depression. Acta Psychiatr. Scand. 2004, 110, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Turecki, G. Epigenetics and suicidal behavior research pathways. Am. J. Prev. Med. 2014, 47, S144–S151. [Google Scholar] [CrossRef]
- Berardelli, I.; Serafini, G.; Cortese, N.; Fiaschè, F.; O’Connor, R.C.; Pompili, M. The involvement of hypothalamus-pituitary-adrenal (HPA) axis in suicide risk. Brain Sci. 2020, 10, 653. [Google Scholar] [CrossRef]
- Jokinen, J.; Boström, A.E.; Dadfar, A.; Ciuculete, D.M.; Chatzittofis, A.; Åsberg, M.; Schiöth, H.B. Epigenetic changes in the CRH gene are related to severity of suicide attempt and a general psychiatric risk score in adolescents. EBioMedicine 2018, 27, 123–133. [Google Scholar] [CrossRef]
- Merali, Z.; Du, L.; Hrdina, P.; Palkovits, M.; Faludi, G.; Poulter, M.O.; Anisman, H. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA (A) receptor subunits in frontal cortical brain region. J. Neurosci. 2004, 24, 1478–1485. [Google Scholar] [CrossRef]
- Pandey, G.N.; Rizavi, H.S.; Bhaumik, R.; Ren, X. Increased protein and mRNA expression of corticotropin-releasing factor (CRF), decreased CRF receptors and CRF binding protein in specific postmortem brain areas of teenage suicide subjects. Psychoneuroendocrinology 2019, 106, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Gaynor, S.C.; Breen, M.E.; Monson, E.T.; de Klerk, K.; Parsons, M.; DeLuca, A.P.; Scheetz, T.; Zandi, P.P.; Potash, J.B.; Willour, V.L. A targeted sequencing study of glutamatergic candidate genes in suicide attempters with bipolar disorder. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2016, 171, 1080–1087. [Google Scholar] [CrossRef]
- Sowa-Kućma, M.; Szewczyk, B.; Sadlik, K.; Piekoszewski, W.; Trela, F.; Opoka, W.; Poleszak, E.; Pilc, A.; Nowak, G. Zinc, magnesium and NMDA receptor alterations in the hippocampus of suicide victims. J. Affect. Disord. 2013, 151, 924–931. [Google Scholar] [CrossRef]
- Kim, S.; Choi, K.-H.; Baykiz, A.F.; Gershenfeld, H.K. Suicide candidate genes associated with bipolar disorder and schizophrenia: An exploratory gene expression profiling analysis of post-mortem prefrontal cortex. BMC Genom. 2007, 8, 413. [Google Scholar] [CrossRef]
- Sequeira, A.; Mamdani, F.; Ernst, C.; Vawter, M.P.; Bunney, W.E.; Lebel, V.; Rehal, S.; Klempan, T.; Gratton, A.; Benkelfat, C.; et al. Global brain gene expression analysis links glutamatergic and GAB aergic alterations to suicide and major depression. PLoS ONE 2009, 4, e6585. [Google Scholar] [CrossRef]
- Yin, H.; Pantazatos, S.P.; Galfalvy, H.; Huang, Y.Y.; Rosoklija, G.B.; Dwork, A.J.; Burke, A.; Arango, V.; Oquendo, M.A.; Mann, J.J. A pilot integrative genomics study of GABA and glutamate neurotransmitter systems in suicide, suicidal behavior, and major depressive disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2016, 171B, 414–426. [Google Scholar] [CrossRef]
- Poulter, M.O.; Du, L.; Zhurov, V.; Palkovits, M.; Faludi, G.; Merali, Z.; Anisman, H. Altered organization of GABAA receptor mRNA expression in the depressed suicide brain. Front. Mol. Neurosci. 2010, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Galfalvy, H.; Zhang, B.; Tang, W.; Xin, Q.; Li, E.; Xue, X.; Li, Q.; Ye, J.; Yan, N.; et al. Interactions of the GABRG2 polymorphisms and childhood trauma on suicide attempt and related traits in depressed patients. J. Affect. Disord. 2020, 266, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Soreni, N.; Apter, A.; Weizman, A.; Don-Tufeled, O.; Leschiner, S.; Karp, L.; Gavish, M. Decreased platelet peripheral-type benzodiazepine receptors in adolescent inpatients with repeated suicide attempts. Biol. Psychiatry 1999, 46, 484–488. [Google Scholar] [CrossRef]
- Brunner, J.; Parhofer, K.G.; Schwandt, P.; Bronisch, T. Cholesterol, omega-3 fatty acids, and suicide risk: Empirical evidence and pathophysiological hypotheses. Fortschr. Neurol. Psychiatr. 2001, 69, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Kunugi, H.; Takei, N.; Aoki, H.; Nanko, S. Low serum cholesterol in suicide attempters. Biol. Psychiatry 1997, 41, 196–200. [Google Scholar] [CrossRef]
- Sublette, M.E. Lipids and suicide risk. Curr. Top. Behav. Neurosci. 2020, 46, 155–177. [Google Scholar] [PubMed]
- Kim, Y.K.; Myint, A.M. Clinical application of low serum cholesterol as an indicator for suicide risk in major depression. J. Affect. Disord. 2004, 81, 161–166. [Google Scholar] [CrossRef]
- Knowles, E.E.M.; Curran, J.E.; Meikle, P.; Huynh, K.; Mathias, S.R.; Göring, H.H.H.; VandeBerg, J.L.; Mahaney, M.C.; Jalbrzikowski, M.; Mosior, M.K.; et al. Disentangling the genetic overlap between cholesterol and suicide risk. Neuropsychopharmacology 2018, 43, 2556–2563. [Google Scholar] [CrossRef]
- Muldoon, M.F.; Manuck, S.B.; Matthews, K.A. Lowering cholesterol concentrations and mortality: A quantitative review of primary prevention trials. BMJ 1990, 301, 309–314. [Google Scholar] [CrossRef]
- Molero, Y.; Cipriani, A.; Larsson, H.; Lichtenstein, P.; D’Onofrio, B.M.; Fazel, S. Associations between statin use and suicidality, depression, anxiety, and seizures: A Swedish total-population cohort study. Lancet Psychiatry 2020, 7, 982–990. [Google Scholar] [CrossRef]
- Freemantle, E.; Chen, G.G.; Cruceanu, C.; Mechawar, N.; Turecki, G. Analysis of oxysterols and cholesterol in prefrontal cortex of suicides. Int. J. Neuropsychopharmacol. 2013, 16, 1241–1249. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Q.; Chang, C. Epigenetics in health and disease. Adv. Exp. Med. Biol. 2020, 1253, 3–55. [Google Scholar] [CrossRef]
- Kouter, K.; Zupanc, T.; Paska, A.V. Genome-wide DNA methylation patterns in suicide victims: Identification of new candidate genes. Psychiatr. Danub. 2019, 31, 392–396. [Google Scholar] [CrossRef]
- Poulter, M.O.; Du, L.; Weaver, I.; Palkovits, M.; Faludi, G.; Merali, Z.; Szyf, M.; Anisman, H. GABAA receptor promoter hypermethylation in suicide brain: Implications for the involvement of epigenetic processes. Biol. Psychiatry 2008, 64, 645–652. [Google Scholar] [CrossRef]
- Murphy, T.M.; Mullins, N.; Ryan, M.; Foster, T.; Kelly, C.; McClelland, R.; O’Grady, J.; Corcoran, E.; Brady, J.; Reilly, M.; et al. Genetic variation in DNMT3B and increased global DNA methylation is associated with suicide attempts in psychiatric patients. Genes. Brain. Behav. 2013, 12, 125–132. [Google Scholar] [CrossRef]
- Squassina, A.; Niola, P.; Lopez, J.P.; Cruceanu, C.; Pisanu, C.; Congiu, D.; Severino, G.; Ardau, R.; Chillotti, C.; Alda, M.; et al. MicroRNA expression profiling of lymphoblasts from bipolar disorder patients who died by suicide, pathway analysis and integration with postmortem brain findings. Eur. Neuropsychopharmacol. 2020, 34, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Yoshino, Y.; Allen, L.; Prall, K.; Schell, G.; Dwivedi, Y. Exploiting circulating microRNAs as biomarkers in psychiatric disorders. Mol. Diagn. Ther. 2020, 24, 279–298. [Google Scholar] [CrossRef]
- Smalheiser, N.R.; Lugli, G.; Rizavi, H.S.; Torvik, V.I.; Turecki, G.; Dwivedi, Y. MicroRNA expression is down-regulated and reorganised in prefrontal cortex of depressed suicide subjects. PLoS ONE 2012, 7, e33201. [Google Scholar] [CrossRef] [PubMed]
- Klempan, T.A.; Sequeira, A.; Canetti, L.; Lalovic, A.; Ernst, C.; Ffrench-Mullen, J.; Turecki, G. Altered expression of genes involved in ATP biosynthesis and GABAergic neurotransmission in the ventral prefrontal cortex of suicides with and without major depression. Mol. Psychiatry 2007, 14, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.K.; Cohen, J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef]
- Lewis, C.P.; Port, J.D.; Blacker, C.J.; Sonmez, A.I.; Seewoo, B.; Leffler, J.M.; Frye, M.A.; Croarkin, P.E. Altered anterior cingulate glutamatergic metabolism in depressed adolescents with current suicidal ideation. Transl. Psychiatry 2020, 10, 119. [Google Scholar] [CrossRef]
- Zhu, R.; Tian, S.; Wang, H.; Jiang, H.; Wang, X.; Shao, J.; Wang, Q.; Yan, R.; Tao, S.; Liu, H.; et al. Discriminating suicide attempters and predicting suicide risk using altered frontolimbic resting-state functional connectivity in patients with bipolar II disorder. Front. Psychiatry 2020, 11, 597770. [Google Scholar] [CrossRef]
- Guzzetta, F.; Tondo, L.; Centorrino, F.; Baldessarini, R.J. Lithium treatment reduces suicide risk in recurrent major depressive disorder. J. Clin. Psychiatry 2007, 68, 380–383. [Google Scholar] [CrossRef]
- Turecki, G.; Brent, D.A. Suicide and suicidal behaviour. Lancet 2016, 387, 1227–1239. [Google Scholar] [CrossRef]
- Zarate, C.A., Jr.; Brutsche, N.E.; Ibrahim, L.; Franco-Chaves, J.; Diazgranados, N.; Cravchik, A.; Selter, J.; Marquardt, C.A.; Liberty, V.; Luckenbaugh, D.A. Replication of ketamine’s antidepressant efficacy in bipolar depression: A randomised controlled add-on trial. Biol. Psychiatry 2012, 71, 939–946. [Google Scholar] [CrossRef]
- Brown, G.K.; Have, T.T.; Henriques, G.R.; Xie, S.X.; Hollander, J.; Beck, A.T. Cognitive therapy for the prevention of suicide attempts. JAMA 2005, 294, 563–570. [Google Scholar] [CrossRef] [PubMed]
- King, C.A.; Arango, A.; Foster, C.E. Emerging trends in adolescent suicide prevention research. Curr. Opin. Psychol. 2018, 22, 89–94. [Google Scholar] [CrossRef]
- Fazel, S.; Runeson, B. Suicide. N. Engl. J. Med. 2020, 382, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Cwik, M.F.; O’Keefe, V.M.; Haroz, E.E. Suicide in the pediatric population: Screening, risk assessment and treatment. Int. Rev. Psychiatry 2020, 32, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Peltzman, T.; Shiner, B.; Watts, B.V. Effects of electroconvulsive therapy on short-term suicide mortality in a risk-matched patient population. J. ECT 2020, 36, 187–192. [Google Scholar] [CrossRef]
- Calaway, K.; Coshal, S.; Jones, K.; Coverdale, J.; Livingston, R. A systematic review of the safety of electroconvulsive therapy use during the first trimester of pregnancy. J. ECT 2016, 32, 230–235. [Google Scholar] [CrossRef]
- Pompili, M.; Dominici, G.; Giordano, G.; Longo, L.; Serafini, G.; Lester, D.; Amore, M.; Girardi, P. Electroconvulsive treatment during pregnancy: A systematic review. Expert Rev. Neurother. 2014, 14, 1377–1390. [Google Scholar] [CrossRef] [PubMed]
- Pinna, M.; Manchia, M.; Oppo, R.; Scano, F.; Pillai, G.; Loche, A.P.; Salis, P.; Minnai, G.P. Clinical and biological predictors of response to electroconvulsive therapy (ECT): A review. Neurosci. Lett. 2018, 669, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Bioque, M.; Mac-Dowell, K.S.; Meseguer, A.; Macau, E.; Valero, R.; Vieta, E.; Leza, J.C.; Bernardo, M. Effects of electroconvulsive therapy in the systemic inflammatory balance of patients with severe mental disorder. Psychiatry Clin. Neurosci. 2019, 73, 628–635. [Google Scholar] [CrossRef]
- Baldessarini, R.J.; Tondo, L.; Davis, P.; Pompili, M.; Goodwin, F.K.; Hennen, J. Decreased risk of suicides and attempts during long-term lithium treatment: A meta-analytic review. Bipolar Disord. 2007, 8, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Benard, V.; Vaiva, G.; Masson, M.; Geoffroy, P. Lithium and suicide prevention in bipolar disorder. L’Encéphale 2016, 42, 234–241. [Google Scholar] [CrossRef]
- Smith, K.A.; Cipriani, A. Lithium and suicide in mood disorders: Updated meta-review of the scientific literature. Bipolar Disord. 2017, 19, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Wada, A. Lithium and neuropsychiatric therapeutics: Neuroplasticity via. glycogen synthase kinase-3beta, beta-catenin, and neurotrophin cascades. J. Pharmacol. Sci. 2009, 110, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, A.; Hawton, K.; Stockton, S.; Geddes, J.R. Lithium in the prevention of suicide in mood disorders: Updated systematic review and meta-analysis. BMJ 2013, 346, f3646. [Google Scholar] [CrossRef]
- Wilkowska, A.; Wiglusz, M.; Cubała, W.J. Clozapine: Promising treatment for suicidality in bipolar disorder. Psychiatr. Danub 2019, 31, 574–578. [Google Scholar]
- Tondo, L.; Baldessarini, R.J. Antisuicidal effects in mood disorders: Are they unique to lithium? Pharmacopsychiatry 2018, 51, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Malhi, G.S.; Tanious, M.; Das, P.; Coulston, C.M.; Berk, M. Potential mechanisms of action of lithium in bipolar disorder. CNS Drugs 2013, 27, 135–153. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Jope, R.S. Inflammation and lithium: Clues to mechanisms contributing to suicide-linked traits. Transl. Psychiatry 2014, 4, e488. [Google Scholar] [CrossRef] [PubMed]
- Rapaport, M.H.; Manji, H.K. The effects of lithium on ex vivo cytokine production. Biol. Psychiatry 2001, 50, 217–224. [Google Scholar] [CrossRef]
- Bosetti, F.; Rintala, J.; Seemann, R.; Rosenberger, T.; Contreras, M.A.; Rapoport, S.I.; Chang, M.C. Chronic lithium downregulates cyclooxygenase-2 activity and prostaglandin E2 concentration in rat brain. Mol. Psychiatry 2002, 7, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, J.J.; Zarate, C.A., Jr.; Rasimas, J.J. Existing and novel biological therapeutics in suicide prevention. Am. J. Prev. Med. 2014, 47, S195–S203. [Google Scholar] [CrossRef] [PubMed]
- Spivak, B.; Roitman, S.; Vered, Y.; Mester, R.; Graff, E.; Talmon, Y.; Guy, N.; Gonen, N.; Weizman, A. Diminished suicidal and aggressive behavior, high plasma norepinephrine levels, and serum triglyceride levels in chronic neuroleptic-resistant schizophrenic patients maintained on clozapine. Clin. Neuropharmacol. 1998, 21, 245–250. [Google Scholar]
- Vermeulen, J.M.; van Rooijen, G.; van de Kerkhof, M.P.J.; Sutterland, A.L.; Correll, C.U.; de Haan, L. Clozapine and long-term mortality risk in patients with schizophrenia: A systematic review and meta-analysis of studies lasting 1.1–12.5 years. Schizophr. Bull. 2019, 45, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Ceresoli-Borroni, G.; Rassoulpour, A.; Wu, H.-Q.; Guidetti, P.; Schwarcz, R. Chronic neuroleptic treatment reduces endogenous kynurenic acid levels in rat brain. J. Neural Transm. 2006, 113, 1355–1365. [Google Scholar] [CrossRef]
- Pandurangi, A.K.; Buckley, P.F. Inflammation, antipsychotic drugs, and evidence for effectiveness of anti-inflammatory agents in schizophrenia. Curr. Top. Behav. Neurosci. 2019, 44, 227–244. [Google Scholar] [CrossRef]
- Gould, T.D.; Zarate, C.A., Jr.; Thompson, S.M. Molecular pharmacology and neurobiology of rapid-acting antidepressants. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 213–236. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S. Ketamine and rapid-acting antidepressants: A new era in the battle against depression and suicide. F1000Research 2018, 7, 659. [Google Scholar] [CrossRef]
- Ramadan, A.M.; Mansour, I.A. Could ketamine be the answer to treating treatment-resistant major depressive disorder? Gen. Psychiatr. 2020, 33, e100227. [Google Scholar] [CrossRef]
- Veraart, J.K.; Smith-Apeldoorn, S.Y.; Spaans, H.-P.; Kamphuis, J.; Schoevers, R.A. Is ketamine an appropriate alternative to ECT for patients with treatment resistant depression? A systematic review. J. Affect. Disord. 2021, 281, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Ballard, E.D.; Ionescu, D.F.; Vande Voort, J.L.; Niciu, M.J.; Richards, E.M.; Luckenbaugh, D.A.; Brutsché, N.E.; Ameli, R.; Furey, M.L.; Zarate, C.A., Jr. Improvement in suicidal ideation after ketamine infusion: Relationship to reductions in depression and anxiety. J. Psychiatr. Res. 2014, 58, 161–166. [Google Scholar] [CrossRef]
- Matveychuk, D.; Thomas, R.K.; Swainson, J.; Khullar, A.; Mackay, M.-A.; Baker, G.B.; Dursun, S.M. Ketamine as an antidepressant: Overview of its mechanisms of action and potential predictive biomarkers. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320916657. [Google Scholar] [CrossRef]
- Verdonk, F.; Petit, A.-C.; Abdel-Ahad, P.; Vinckier, F.; Jouvion, G.; de Maricourt, P.; De Medeiros, G.F.; Danckaert, A.; Van Steenwinckel, J.; Blatzer, M.; et al. Microglial production of quinolinic acid as a target and a biomarker of the antidepressant effect of ketamine. Brain Behav. Immun. 2019, 81, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Isacsson, G.; Rich, C.L. Antidepressant drugs and the risk of suicide in children and adolescents. Pediatr. Drugs 2014, 16, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Teicher, M.H.; Glod, C.A.; Cole, J.O. Antidepressant drugs and the emergence of suicidal tendencies. Drug Saf. 1993, 8, 186–212. [Google Scholar] [CrossRef]
- Hammad, T.A.; Laughren, T.; Racoosin, J. Suicidality in pediatric patients treated with antidepressant drugs. Arch. Gen. Psychiatry 2006, 63, 332–339. [Google Scholar] [CrossRef]
- Müller-Oerlinghausen, B.; Berghöfer, A. Antidepressants and suicidal risk. J. Clin. Psychiatry 1999, 60, 94–99. [Google Scholar] [PubMed]
- Patorno, E.; Bohn, R.L.; Wahl, P.M.; Avorn, J.; Patrick, A.R.; Liu, J.; Schneeweiss, S. Anticonvulsant medications and the risk of suicide, attempted suicide, or violent death. JAMA 2010, 303, 1401–1409. [Google Scholar] [CrossRef]
- Mula, M.; Sander, J.W. Suicide risk in people with epilepsy taking antiepileptic drugs. Bipolar Disord. 2013, 15, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Fruhbauerova, M.; Comtois, K.A. Addiction counselors and suicide: Education and experience do not improve suicide knowledge, beliefs, or confidence in treating suicidal clients. J. Subst. Abus. Treat. 2019, 106, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Lynch, F.L.; Peterson, E.L.; Lu, C.Y.; Hu, Y.; Rossom, R.C.; Waitzfelder, B.E.; Owen-Smith, A.A.; Hubley, S.; Prabhakar, D.; Williams, L.K.; et al. Substance use disorders and risk of suicide in a general US population: A case control study. Addict. Sci. Clin. Pr. 2020, 15, 14. [Google Scholar] [CrossRef]
- Zhornitsky, S.; Le, T.M.; Dhingra, I.; Adkinson, B.D.; Potvin, S.; Li, C.R. Interpersonal risk factors for suicide in cocaine dependence: Association with self-esteem, personality traits, and childhood abuse. Suicide Life-Threat. Behav. 2020, 50, 867–883. [Google Scholar] [CrossRef]
- Miller, N.S.; Mahler, J.C.; Gold, M.S. Suicide risk associated with drug and alcohol dependence. J. Addict. Dis. 1991, 10, 49–61. [Google Scholar] [CrossRef]
- Arensman, E.; Bennardi, M.; Larkin, C.; Wall, A.; McAuliffe, C.; McCarthy, J.; Williamson, E.; Perry, I. Suicide among young people and adults in Ireland: Method characteristics, toxicological analysis and substance abuse histories compared. PLoS ONE 2016, 11, e0166881. [Google Scholar] [CrossRef] [PubMed]
- Masferrer, L.; Caparrós, B. Risk of suicide and dysfunctional patterns of personality among bereaved substance users. Int. J. Environ. Res. Public Health 2017, 14, 316. [Google Scholar] [CrossRef] [PubMed]
- Lasota, D.; Al-Wathinani, A.; Krajewski, P.; Mirowska-Guzel, D.; Goniewicz, K.; Hertelendy, A.J.; Alhazmi, R.A.; Pawłowski, W.; Khorram-Manesh, A.; Goniewicz, M. Alcohol and the risk of railway suicide. Int. J. Environ. Res. Public Health 2020, 17, 7003. [Google Scholar] [CrossRef] [PubMed]
- Pirkola, S.P.; Suominen, K.; Isometsä, E.T. Suicide in alcohol-dependent individuals: Epidemiology and management. CNS Drugs. 2004, 18, 423–436. [Google Scholar] [CrossRef]
- Borges, G.; Bagge, C.L.; Orozco, R. A literature review and meta-analyses of cannabis use and suicidality. J. Affect. Disord. 2016, 195, 63–74. [Google Scholar] [CrossRef]
- Han, B.; Compton, W.M.; Einstein, E.B.; Volkow, N.D. Associations of suicidality trends with cannabis use as a function of sex and depression status. JAMA Netw. Open 2021, 4, e2113025. [Google Scholar] [CrossRef] [PubMed]
| Risk Factors of Suicide | Matching Treatment That Decreases the Risk of Suicide |
|---|---|
| Emotional traits: aggression, impulsivity, pessimism | Lithium (in depression and bipolar disorder, delayed effect), clozapine (schizophrenia) |
| Early life stress | - |
| Depression | Ketamine/esketamine (in depression, rapid effect), lithium (in depression and bipolar disorder, delayed effect), electroconvulsive therapy, psychotherapy, transcranial magnetic stimulation |
| Schizophrenia | Clozapine |
| Other psychiatric disorders | Psychotherapy |
| Alcohol dependence and other dependence | Treatment of alcohol or substance abuse |
| Prefrontal Cortex | Hippocampus | Peripheral Tissue | |
|---|---|---|---|
| 5-HIAA | - | ↑ | CSF ↓ platelets ↓ |
| Serotonin transporter | ↓ | - | ↓ platelets |
| GABA-A receptor | Contradictory information | ↑ | ↓ |
| CRH | ↑ | ↑ | ↑ |
| CRH receptor type 1 | ↓ | ||
| Cortisol | No data | No data | ↑ plasma, CSF |
| BDNF | ↓ | ↓ | ↓ serum |
| IL-1 | ↑ | No data | ↑ blond |
| IL-6 | ↑ | No data | ↑ blood, CSF |
| IL-8 | - | No data | ↓ blood, CSF |
| Quinolinic acid | ↑ | No data | CSF, blood ↑ |
| Cholesterol | Decrease only in violence | - | ↓ |
| DNA hypermethylation | ↑ | ↑ | ↑ |
| miR-124, miR-139, miR-185, miR-195 | ↑ | No data | No data |
| miR-494, miR-335 | ↓ | No data | No data |
| miR-19a3p | ↑ | No data | Blood mononuclear cells ↑ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wisłowska-Stanek, A.; Kołosowska, K.; Maciejak, P. Neurobiological Basis of Increased Risk for Suicidal Behaviour. Cells 2021, 10, 2519. https://doi.org/10.3390/cells10102519
Wisłowska-Stanek A, Kołosowska K, Maciejak P. Neurobiological Basis of Increased Risk for Suicidal Behaviour. Cells. 2021; 10(10):2519. https://doi.org/10.3390/cells10102519
Chicago/Turabian StyleWisłowska-Stanek, Aleksandra, Karolina Kołosowska, and Piotr Maciejak. 2021. "Neurobiological Basis of Increased Risk for Suicidal Behaviour" Cells 10, no. 10: 2519. https://doi.org/10.3390/cells10102519
APA StyleWisłowska-Stanek, A., Kołosowska, K., & Maciejak, P. (2021). Neurobiological Basis of Increased Risk for Suicidal Behaviour. Cells, 10(10), 2519. https://doi.org/10.3390/cells10102519





