Developing New Anti-Tuberculosis Vaccines: Focus on Adjuvants
Abstract
1. Introduction
2. The BCG Problem and the Design of New TB Vaccines
3. Understanding the Adjuvant’s Immune Role by Understanding TB Immunity
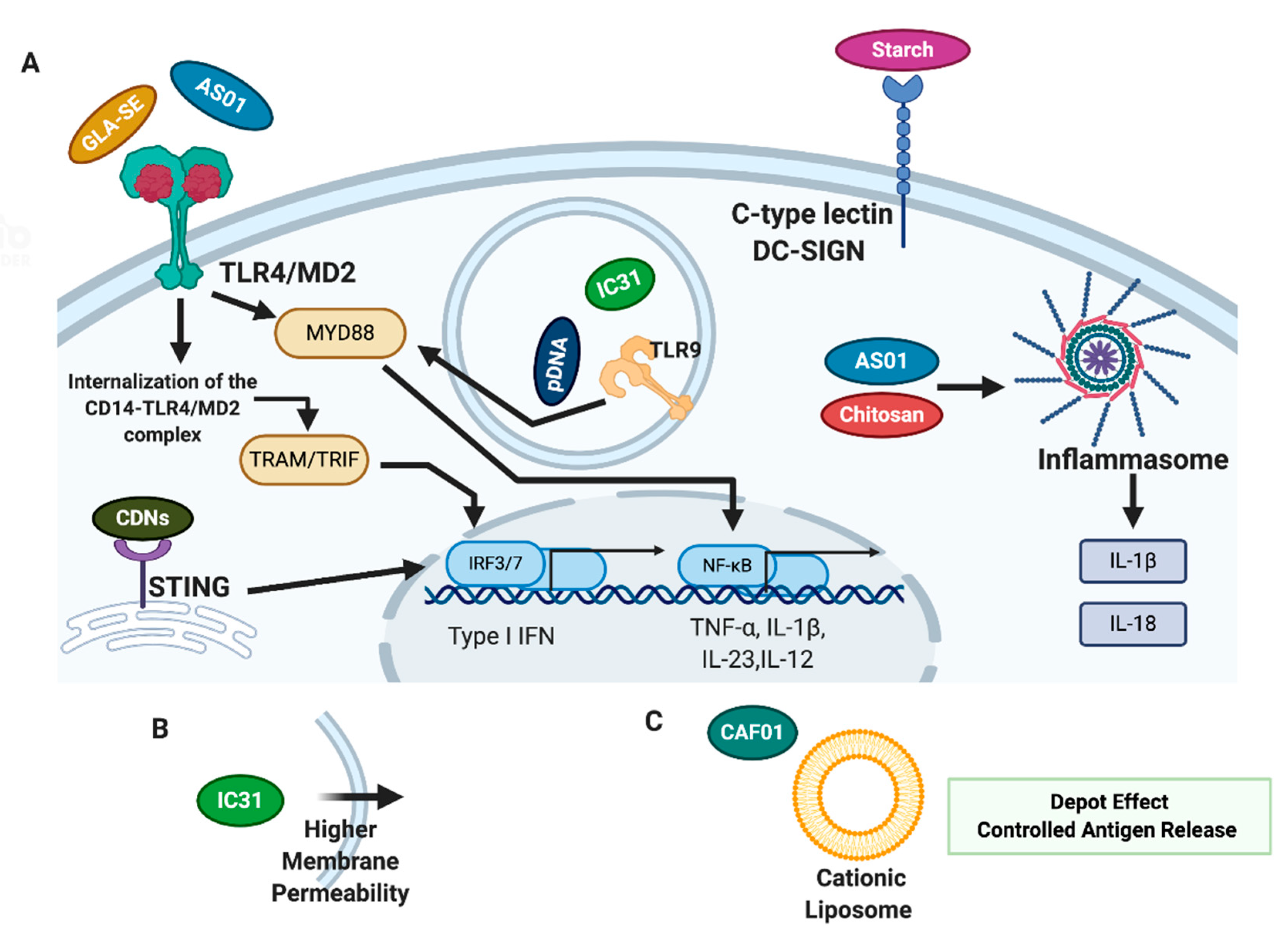
4. Adjuvants in New TB Vaccine Candidates
4.1. Adjuvants in TB Vaccines Currently in Clinical Stage of Development
4.1.1. IC31
4.1.2. GLA-SE
4.1.3. AS01
4.1.4. CAF01
4.2. Adjuvants in TB Vaccines Currently in Preclinical Studies
4.2.1. Starch
4.2.2. Chitosan
4.2.3. Other Adjuvants in Preclinical Studies–Cyclic Dinucleotides and Advax® Formulations
5. Future Perspectives and Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kwon, B.-E.; Ahn, J.-H.; Min, S.; Kim, H.; Seo, J.; Yeo, S.-G.; Ko, H.-J. Development of New Preventive and Therapeutic Vaccines for Tuberculosis. Immune Netw. 2018, 18, e17. [Google Scholar] [CrossRef]
- Furin, J.; Cox, H.; Pai, M. Tuberculosis. Lancet 2019, 393, 1642–1656. [Google Scholar] [CrossRef]
- World Health Organization Global Tuberculosis Report. Available online: https://www.who.int/publications/i/item/9789240013131 (accessed on 3 November 2020).
- Sable, S.B.; Posey, J.E.; Scriba, T.J. Tuberculosis Vaccine Development: Progress in Clinical Evaluation. Clin. Microbiol. Rev. 2019, 33, e00100-19. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Iranshahy, M.; Vahdati-Mashhadian, N.; Sahebkar, A.; Fazly Bazzaz, B.S. Non-antibiotic adjunctive therapy: A promising approach to fight tuberculosis. Pharm. Res. 2019, 146, 104289. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H.E.; Weiner, J.; von Reyn, C.F. Novel approaches to tuberculosis vaccine development. Int. J. Infect. Dis. Ijid Off. Publ. Int. Soc. Infect. Dis. 2017, 56, 263–267. [Google Scholar] [CrossRef]
- Cable, J.; Srikantiah, P.; Crowe, J.E., Jr.; Pulendran, B.; Hill, A.; Ginsberg, A.; Koff, W.; Mathew, A.; Ng, T.; Jansen, K.; et al. Vaccine innovations for emerging infectious diseases—a symposium report. Ann. N. Y. Acad. Sci. 2020, 1462, 14–26. [Google Scholar] [CrossRef]
- Stewart, E.; Triccas, J.A.; Petrovsky, N. Adjuvant Strategies for More Effective Tuberculosis Vaccine Immunity. Microorganisms 2019, 7, 255. [Google Scholar] [CrossRef]
- Martin, C.; Aguilo, N.; Marinova, D.; Gonzalo-Asensio, J. Update on TB Vaccine Pipeline. Appl. Sci. 2020, 10, 2632. [Google Scholar] [CrossRef]
- Bastola, R.; Noh, G.; Keum, T.; Bashyal, S.; Seo, J.-E.; Choi, J.; Oh, Y.; Cho, Y.; Lee, S. Vaccine adjuvants: Smart components to boost the immune system. Arch. Pharm. Res. 2017, 40, 1238–1248. [Google Scholar] [CrossRef]
- Schmidt, S.T.; Pedersen, G.K.; Christensen, D. Rational Design and in vivo Characterization of Vaccine Adjuvants. Ilar J. 2018, 59, 309–322. [Google Scholar] [CrossRef]
- Sarkar, I.; Garg, R.; van Drunen Littel-van den Hurk, S. Selection of adjuvants for vaccines targeting specific pathogens. Expert Rev. Vaccines 2019, 18, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, A.; Tang, J.; Wang, G.; Shi, Y.; Zhan, L.; Qin, C. Tuberculosis vaccine development: From classic to clinical candidates. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1405–1425. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.U.; Knirsch, C.; Anderson, A.S. The role of vaccines in preventing bacterial antimicrobial resistance. Nat. Med. 2018, 24, 10–19. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization WHO Preferred Product Characteristics for New Tuberculosis Vaccines. Available online: https://apps.who.int/iris/handle/10665/273089 (accessed on 3 November 2020).
- Schrager, L.K.; Chandrasekaran, P.; Fritzell, B.H.; Hatherill, M.; Lambert, P.-H.; McShane, H.; Tornieporth, N.; Vekemans, J. WHO preferred product characteristics for new vaccines against tuberculosis. Lancet Infect. Dis. 2018, 18, 828–829. [Google Scholar] [CrossRef]
- Fatima, S.; Kumari, A.; Das, G.; Dwivedi, V.P. Tuberculosis vaccine: A journey from BCG to present. Life Sci. 2020, 252, 117594. [Google Scholar] [CrossRef]
- McShane, H. Insights and challenges in tuberculosis vaccine development. Lancet Respir. Med. 2019, 7, 810–819. [Google Scholar] [CrossRef]
- Lewinsohn, D.A.; Lewinsohn, D.M.; Scriba, T.J. Polyfunctional CD4(+) T Cells As Targets for Tuberculosis Vaccination. Front. Immunol. 2017, 8, 1262. [Google Scholar] [CrossRef]
- Cooper, A.M.; Khader, S.A. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol. Rev. 2008, 226, 191–204. [Google Scholar] [CrossRef]
- Moguche, A.O.; Musvosvi, M.; Penn-Nicholson, A.; Plumlee, C.R.; Mearns, H.; Geldenhuys, H.; Smit, E.; Abrahams, D.; Rozot, V.; Dintwe, O.; et al. Antigen Availability Shapes T Cell Differentiation and Function during Tuberculosis. Cell Host Microbe 2017, 21, 695–706. [Google Scholar] [CrossRef]
- Luabeya, A.K.K.; Kagina, B.M.N.; Tameris, M.D.; Geldenhuys, H.; Hoff, S.T.; Shi, Z.; Kromann, I.; Hatherill, M.; Mahomed, H.; Hanekom, W.A.; et al. First-in-human trial of the post-exposure tuberculosis vaccine H56:IC31 in Mycobacterium tuberculosis infected and non-infected healthy adults. Vaccine 2015, 33, 4130–4140. [Google Scholar] [CrossRef]
- Chikh, G.; Luu, R.; Patel, S.; Davis, H.L.; Weeratna, R.D. Effects of KLK Peptide on Adjuvanticity of Different ODN Sequences. Vaccines 2016, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Faridgohar, M.; Nikoueinejad, H. New findings of Toll-like receptors involved in Mycobacterium tuberculosis infection. Pathog. Glob. Health 2017, 111, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Troy, A.; Esparza-Gonzalez, S.C.; Bartek, A.; Creissen, E.; Izzo, L.; Izzo, A.A. Pulmonary mucosal immunity mediated through CpG provides adequate protection against pulmonary Mycobacterium tuberculosis infection in the mouse model. A role for type I interferon. Tuberculosis 2020, 123, 101949. [Google Scholar] [CrossRef]
- Deshmukh, S.S.; Magcalas, F.W.; Kalbfleisch, K.N.; Carpick, B.W.; Kirkitadze, M.D. Tuberculosis vaccine candidate: Characterization of H4-IC31 formulation and H4 antigen conformation. J. Pharm. Biomed. Anal. 2018, 157, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Norrby, M.; Vesikari, T.; Lindqvist, L.; Maeurer, M.; Ahmed, R.; Mahdavifar, S.; Bennett, S.; McClain, J.B.; Shepherd, B.M.; Li, D.; et al. Safety and immunogenicity of the novel H4:IC31 tuberculosis vaccine candidate in BCG-vaccinated adults: Two phase I dose escalation trials. Vaccine 2017, 35, 1652–1661. [Google Scholar] [CrossRef]
- Nemes, E.; Geldenhuys, H.; Rozot, V.; Rutkowski, K.T.; Ratangee, F.; Bilek, N.; Mabwe, S.; Makhethe, L.; Erasmus, M.; Toefy, A.; et al. Prevention of M. tuberculosis Infection with H4:IC31 Vaccine or BCG Revaccination. N. Engl. J. Med. 2018, 379, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Geldenhuys, H.; Mearns, H.; Miles, D.J.C.; Tameris, M.; Hokey, D.; Shi, Z.; Bennett, S.; Andersen, P.; Kromann, I.; Hoff, S.T.; et al. The tuberculosis vaccine H4:IC31 is safe and induces a persistent polyfunctional CD4 T cell response in South African adults: A randomized controlled trial. Vaccine 2015, 33, 3592–3599. [Google Scholar] [CrossRef] [PubMed]
- Bekker, L.-G.; Dintwe, O.; Fiore-Gartland, A.; Middelkoop, K.; Hutter, J.; Williams, A.; Randhawa, A.K.; Ruhwald, M.; Kromann, I.; Andersen, P.L.; et al. A phase 1b randomized study of the safety and immunological responses to vaccination with H4:IC31, H56:IC31, and BCG revaccination in Mycobacterium tuberculosis-uninfected adolescents in Cape Town, South Africa. EClinicalMedicine 2020, 21. [Google Scholar] [CrossRef]
- Suliman, S.; Luabeya, A.K.K.; Geldenhuys, H.; Tameris, M.; Hoff, S.T.; Shi, Z.; Tait, D.; Kromann, I.; Ruhwald, M.; Rutkowski, K.T.; et al. Dose Optimization of H56:IC31 Vaccine for Tuberculosis-Endemic Populations. A Double-Blind, Placebo-controlled, Dose-Selection Trial. Am. J. Respir. Crit. Care Med. 2018, 199, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.G.; Carter, D.; Casper, C.; Duthie, M.S.; Fox, C.B. Correlates of GLA family adjuvants’ activities. Semin. Immunol. 2018, 39, 22–29. [Google Scholar] [CrossRef]
- Dubois Cauwelaert, N.; Desbien, A.L.; Hudson, T.E.; Pine, S.O.; Reed, S.G.; Coler, R.N.; Orr, M.T. The TLR4 Agonist Vaccine Adjuvant, GLA-SE, Requires Canonical and Atypical Mechanisms of Action for TH1 Induction. PLoS ONE 2016, 11, e0146372. [Google Scholar] [CrossRef]
- Baldwin, S.L.; Reese, V.A.; Huang, P.D.; Beebe, E.A.; Podell, B.K.; Reed, S.G.; Coler, R.N. Protection and Long-Lived Immunity Induced by the ID93/GLA-SE Vaccine Candidate against a Clinical Mycobacterium tuberculosis Isolate. Clin. Vaccine Immunol. 2016, 23, 137–147. [Google Scholar] [CrossRef]
- Cha, S.B.; Kim, W.S.; Kim, J.-S.; Kim, H.; Kwon, K.W.; Han, S.J.; Cho, S.-N.; Coler, R.N.; Reed, S.G.; Shin, S.J. Pulmonary immunity and durable protection induced by the ID93/GLA-SE vaccine candidate against the hyper-virulent Korean Beijing Mycobacterium tuberculosis strain K. Vaccine 2016, 34, 2179–2187. [Google Scholar] [CrossRef]
- Coler, R.N.; Day, T.A.; Ellis, R.; Piazza, F.M.; Beckmann, A.M.; Vergara, J.; Rolf, T.; Lu, L.; Alter, G.; Hokey, D.; et al. The TLR-4 agonist adjuvant, GLA-SE, improves magnitude and quality of immune responses elicited by the ID93 tuberculosis vaccine: First-in-human trial. NPJ Vaccines 2018, 3, 34. [Google Scholar] [CrossRef]
- Larsen, S.E.; Baldwin, S.L.; Orr, M.T.; Reese, V.A.; Pecor, T.; Granger, B.; Dubois Cauwelaert, N.; Podell, B.K.; Coler, R.N. Enhanced Anti-Mycobacterium tuberculosis Immunity over Time with Combined Drug and Immunotherapy Treatment. Vaccines 2018, 6, 30. [Google Scholar] [CrossRef]
- Penn-Nicholson, A.; Tameris, M.; Smit, E.; Day, T.A.; Musvosvi, M.; Jayashankar, L.; Vergara, J.; Mabwe, S.; Bilek, N.; Geldenhuys, H.; et al. Safety and immunogenicity of the novel tuberculosis vaccine ID93 + GLA-SE in BCG-vaccinated healthy adults in South Africa: A randomised, double-blind, placebo-controlled phase 1 trial. Lancet. Respir. Med. 2018, 6, 287–298. [Google Scholar] [CrossRef]
- Kwon, K.W.; Lee, A.; Larsen, S.E.; Baldwin, S.L.; Coler, R.N.; Reed, S.G.; Cho, S.-N.; Ha, S.-J.; Shin, S.J. Long-term protective efficacy with a BCG-prime ID93/GLA-SE boost regimen against the hyper-virulent Mycobacterium tuberculosis strain K in a mouse model. Sci. Rep. 2019, 9, 15560. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef]
- Shi, S.; Zhu, H.; Xia, X.; Liang, Z.; Ma, X.; Sun, B. Vaccine adjuvants: Understanding the structure and mechanism of adjuvanticity. Vaccine 2019, 37, 3167–3178. [Google Scholar] [CrossRef]
- Didierlaurent, A.M.; Laupèze, B.; Di Pasquale, A.; Hergli, N.; Collignon, C.; Garçon, N. Adjuvant system AS01: Helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines 2017, 16, 55–63. [Google Scholar] [CrossRef]
- Penn-Nicholson, A.; Geldenhuys, H.; Burny, W.; van der Most, R.; Day, C.L.; Jongert, E.; Moris, P.; Hatherill, M.; Ofori-Anyinam, O.; Hanekom, W.; et al. Safety and immunogenicity of candidate vaccine M72/AS01E in adolescents in a TB endemic setting. Vaccine 2015, 33, 4025–4034. [Google Scholar] [CrossRef]
- Ji, Z.; Jian, M.; Chen, T.; Luo, L.; Li, L.; Dai, X.; Bai, R.; Ding, Z.; Bi, Y.; Wen, S.; et al. Immunogenicity and Safety of the M72/AS01(E) Candidate Vaccine Against Tuberculosis: A Meta-Analysis. Front. Immunol. 2019, 10, 2089. [Google Scholar] [CrossRef]
- Ullah, I.; Bibi, S.; Ul Haq, I.; Safia; Ullah, K.; Ge, L.; Shi, X.; Bin, M.; Niu, H.; Tian, J.; et al. The Systematic Review and Meta-Analysis on the Immunogenicity and Safety of the Tuberculosis Subunit Vaccines M72/AS01(E) and MVA85A. Front. Immunol. 2020, 11, 1806. [Google Scholar] [CrossRef]
- Van Der Meeren, O.; Hatherill, M.; Nduba, V.; Wilkinson, R.J.; Muyoyeta, M.; Van Brakel, E.; Ayles, H.M.; Henostroza, G.; Thienemann, F.; Scriba, T.J.; et al. Phase 2b Controlled Trial of M72/AS01E Vaccine to Prevent Tuberculosis. N. Engl. J. Med. 2018, 379, 1621–1634. [Google Scholar] [CrossRef]
- Tait, D.R.; Hatherill, M.; Van Der Meeren, O.; Ginsberg, A.M.; Van Brakel, E.; Salaun, B.; Scriba, T.J.; Akite, E.J.; Ayles, H.M.; Bollaerts, A.; et al. Final Analysis of a Trial of M72/AS01E Vaccine to Prevent Tuberculosis. N. Engl. J. Med. 2019, 381, 2429–2439. [Google Scholar] [CrossRef]
- Gillard, P.; Yang, P.-C.; Danilovits, M.; Su, W.-J.; Cheng, S.-L.; Pehme, L.; Bollaerts, A.; Jongert, E.; Moris, P.; Ofori-Anyinam, O.; et al. Safety and immunogenicity of the M72/AS01E candidate tuberculosis vaccine in adults with tuberculosis: A phase II randomised study. Tuberculosis 2016, 100, 118–127. [Google Scholar] [CrossRef]
- Kumarasamy, N.; Poongulali, S.; Bollaerts, A.; Moris, P.; Beulah, F.E.; Ayuk, L.N.; Demoitié, M.-A.; Jongert, E.; Ofori-Anyinam, O. A Randomized, Controlled Safety, and Immunogenicity Trial of the M72/AS01 Candidate Tuberculosis Vaccine in HIV-Positive Indian Adults. Medicine 2016, 95, e2459. [Google Scholar] [CrossRef]
- Pedersen, G.K.; Andersen, P.; Christensen, D. Immunocorrelates of CAF family adjuvants. Semin. Immunol. 2018, 39, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Rodríguez-Rodríguez, C.; Saatchi, K.; Rose, F.; Esposito, T.; Nosrati, Z.; Andersen, P.; Christensen, D.; Häfeli, U.O.; Foged, C. Dual-Isotope SPECT/CT Imaging of the Tuberculosis Subunit Vaccine H56/CAF01: Induction of Strong Systemic and Mucosal IgA and T-Cell Responses in Mice Upon Subcutaneous Prime and Intrapulmonary Boost Immunization. Front. Immunol. 2018, 9, 2825. [Google Scholar] [CrossRef] [PubMed]
- Woodworth, J.S.; Christensen, D.; Cassidy, J.P.; Agger, E.M.; Mortensen, R.; Andersen, P. Mucosal boosting of H56:CAF01 immunization promotes lung-localized T cells and an accelerated pulmonary response to Mycobacterium tuberculosis infection without enhancing vaccine protection. Mucosal Immunol. 2019, 12, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Woodworth, J.S.; Cohen, S.B.; Moguche, A.O.; Plumlee, C.R.; Agger, E.M.; Urdahl, K.B.; Andersen, P. Subunit vaccine H56/CAF01 induces a population of circulating CD4 T cells that traffic into the Mycobacterium tuberculosis-infected lung. Mucosal Immunol. 2017, 10, 555–564. [Google Scholar] [CrossRef]
- Thakur, A.; Ingvarsson, P.T.; Schmidt, S.T.; Rose, F.; Andersen, P.; Christensen, D.; Foged, C. Immunological and physical evaluation of the multistage tuberculosis subunit vaccine candidate H56/CAF01 formulated as a spray-dried powder. Vaccine 2018, 36, 3331–3339. [Google Scholar] [CrossRef]
- Roces, C.B.; Hussain, M.T.; Schmidt, S.T.; Christensen, D.; Perrie, Y. Investigating Prime-Pull Vaccination through a Combination of Parenteral Vaccination and Intranasal Boosting. Vaccines 2019, 8, 10. [Google Scholar] [CrossRef]
- Lu, L.L.; Chung, A.W.; Rosebrock, T.R.; Ghebremichael, M.; Yu, W.H.; Grace, P.S.; Schoen, M.K.; Tafesse, F.; Martin, C.; Leung, V.; et al. A Functional Role for Antibodies in Tuberculosis. Cell 2016, 167, 433–443.e14. [Google Scholar] [CrossRef] [PubMed]
- Santoro, F.; Pettini, E.; Kazmin, D.; Ciabattini, A.; Fiorino, F.; Gilfillan, G.D.; Evenroed, I.M.; Andersen, P.; Pozzi, G.; Medaglini, D. Transcriptomics of the Vaccine Immune Response: Priming with Adjuvant Modulates Recall Innate Responses After Boosting. Front. Immunol. 2018, 9, 1248. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Mendieta, S.; Guillén, D.; Hernández-Pando, R.; Sánchez, S.; Rodríguez-Sanoja, R. Potential of glucans as vaccine adjuvants: A review of the α-glucans case. Carbohydr. Polym. 2017, 165, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Mendieta, S.; Barrios-Payán, J.; Mata-Espinosa, D.; Sánchez, S.; Hernández-Pando, R.; Rodríguez-Sanoja, R. Raw starch microparticles have immunostimulant activity in mice vaccinated with BCG and challenged with Mycobacterium tuberculosis. Vaccine 2017, 35, 5123–5130. [Google Scholar] [CrossRef] [PubMed]
- Raveendran Nair, P.K.; Rodriguez, S.; Ramachandran, R.; Alamo, A.; Melnick, S.J.; Escalon, E.; Garcia, P.I.; Wnuk, S.F.; Ramachandran, C. Immune stimulating properties of a novel polysaccharide from the medicinal plant Tinospora cordifolia. Int. Immunopharmacol. 2004, 4, 1645–1659. [Google Scholar] [CrossRef]
- Nair, P.K.R.; Melnick, S.J.; Ramachandran, R.; Escalon, E.; Ramachandran, C. Mechanism of macrophage activation by (1,4)-α-d-glucan isolated from Tinospora cordifolia. Int. Immunopharmacol. 2006, 6, 1815–1824. [Google Scholar] [CrossRef]
- Dedloff, M.R.; Effler, C.S.; Holban, A.M.; Gestal, M.C. Use of Biopolymers in Mucosally-Administered Vaccinations for Respiratory Disease. Materials 2019, 12, 2445. [Google Scholar] [CrossRef]
- Moreno-Mendieta, S.; Barrera-Rosales, A.; Mata-Espinosa, D.; Barrios-Payán, J.; Sánchez, S.; Hernández-Pando, R.; Rodríguez-Sanoja, R. Raw starch microparticles as BCG adjuvant: Their efficacy depends on the virulence of the infection strains. Vaccine 2019, 37, 5731–5737. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Hu, T. Conjugation with an Inulin–Chitosan Adjuvant Markedly Improves the Immunogenicity of Mycobacterium tuberculosis CFP10-TB10.4 Fusion Protein. Mol. Pharm. 2016, 13, 3626–3635. [Google Scholar] [CrossRef] [PubMed]
- Khademi, F.; Taheri, R.-A.; Yousefi Avarvand, A.; Vaez, H.; Momtazi-Borojeni, A.A.; Soleimanpour, S. Are chitosan natural polymers suitable as adjuvant/delivery system for anti-tuberculosis vaccines? Microb. Pathog. 2018, 121, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Poecheim, J.; Barnier-Quer, C.; Collin, N.; Borchard, G. Ag85A DNA Vaccine Delivery by Nanoparticles: Influence of the Formulation Characteristics on Immune Responses. Vaccines 2016, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Amini, Y.; Tebianian, M.; Mosavari, N.; Fasihi Ramandi, M.; Ebrahimi, S.M.; Najminejad, H.; Dabaghian, M.; Abdollahpour, M. Development of an effective delivery system for intranasal immunization against Mycobacterium tuberculosis ESAT-6 antigen. Artif. Cells Nanomed. Biotechnol. 2017, 45, 291–296. [Google Scholar] [CrossRef]
- Sun, B.; Yu, S.; Zhao, D.; Guo, S.; Wang, X.; Zhao, K. Polysaccharides as vaccine adjuvants. Vaccine 2018, 36, 5226–5234. [Google Scholar] [CrossRef] [PubMed]
- Hellfritzsch, M.; Scherließ, R. Mucosal Vaccination via the Respiratory Tract. Pharmaceutics 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Van Dis, E.; Sogi, K.M.; Rae, C.S.; Sivick, K.E.; Surh, N.H.; Leong, M.L.; Kanne, D.B.; Metchette, K.; Leong, J.J.; Bruml, J.R.; et al. STING-Activating Adjuvants Elicit a Th17 Immune Response and Protect against Mycobacterium tuberculosis Infection. Cell Rep. 2018, 23, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Counoupas, C.; Pinto, R.; Nagalingam, G.; Britton, W.J.; Petrovsky, N.; Triccas, J.A. Delta inulin-based adjuvants promote the generation of polyfunctional CD4(+) T cell responses and protection against Mycobacterium tuberculosis infection. Sci. Rep. 2017, 7, 8582. [Google Scholar] [CrossRef] [PubMed]
- de Paula Oliveira Santos, B.; Trentini, M.M.; Machado, R.B.; Rúbia Nunes Celes, M.; Kipnis, A.; Petrovsky, N.; Junqueira-Kipnis, A.P. Advax4 delta inulin combination adjuvant together with ECMX, a fusion construct of four protective mTB antigens, induces a potent Th1 immune response and protects mice against Mycobacterium tuberculosis infection. Hum. Vaccin. Immunother. 2017, 13, 2967–2976. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Manjaly Thomas, Z.-R.; McShane, H. Aerosol immunisation for TB: Matching route of vaccination to route of infection. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 175–181. [Google Scholar] [CrossRef]
- Ahmed, M.; Smith, D.M.; Hamouda, T.; Rangel-Moreno, J.; Fattom, A.; Khader, S.A. A novel nanoemulsion vaccine induces mucosal Interleukin-17 responses and confers protection upon Mycobacterium tuberculosis challenge in mice. Vaccine 2017, 35, 4983–4989. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Wang, X.; Fan, X. A New Adjuvant MTOM Mediates Mycobacterium tuberculosis Subunit Vaccine to Enhance Th1-Type T Cell Immune Responses and IL-2(+) T Cells. Front. Immunol. 2017, 8, 585. [Google Scholar] [CrossRef] [PubMed]
| Target Population | Adolescents and adults |
| Outcome Measure and Efficacy | 50% or greater efficacy in preventing confirmed pulmonary TB |
| Duration of protection | Ten years or more |
| Safety | Favourable safety profile, even for high-risk groups as HIV patients |
| Schedule | Less than three doses to achieve primary immunization and booster preferentially after 10 years or more |
| Co-administration | Safe and without interactions with other vaccines administrated to the same population |
| Immunogenicity | Characterization of immune markers and concomitant development of correlate of protection of a TB vaccine |
| Programmatic Suitability and Prequalification | Should meet requirements of WHO suitability of vaccines—vaccine presentation, packaging, thermostability, formulation and disposal |
| Value Proposition | Favourable cost-effectiveness and affordable price |
| Adjuvant System | Components | Proposed Mechanism of Action | Type of Immune Response | Vaccine Candidate | Immunization Strategy | Adm. Route | Ref |
|---|---|---|---|---|---|---|---|
| IC31 | KLK, ODN1a | TLR9 activation (ODN1a) Enhanced delivery of ODN1a to the endosome, enhanced antigen presentation (KLK) | Th1–Polyfunctional T-cells producing IFN-γ, IL-2 and TNF-α | H4:IC31 H56:IC31 | Prophylactic Prophylactic, Post-Exposure | I.M. I.M. | [22,23,26,27,28,29,30] |
| GLA-SE | GLA in a Squalene oil-in-water emulsion | TLR4 activation | Th1–Polyfunctional T-cells producing IFN-γ, IL-2 and TNF-α Antigen-specific IgG1 and IgG3 production | ID93: GLA-SE | Prophylactic, BCG booster, Therapeutic | I.M. | [32,33,34,35,36,37,38,39] |
| AS01 | MPL, QS-21 | TLR4 activation (MPL) Induction of NLRP3 inflammasome (QS-21) | Th1–Polyfunctional T-cells producing IFN-γ, IL-2 and TNF-α | M72:AS01E | Post-exposure, BCG booster | I.M. | [42,43,44,45,46,47,48,49] |
| CAF01 | DDA, TDB | MINCLE activation Depot Effect Controlled release of the antigen | Th17–T-cells expressing IL-17 Th1 IgA response | H56:CAF01 | Prophylactic Homologous Boosting | S.C. I.N. | [50,51,52,53,54,55,57] |
| Adjuvant System | Components | Proposed Mechanism of Action | Type of Immune Response | Adm. Route | Ref |
|---|---|---|---|---|---|
| Starch Microparticles | C-type lectin DC specific ICAM-3-grabbing nonintegrin receptor activation Increase in phagocytosis and macrophages activation TLR6 signaling | Th1 | I.N. | [58,59,60,61,62,63] | |
| Chitosan | Inflammasome activation Mucoadhesive, ability to penetrate between cells, controlled release of the antigen, improved cell uptake | Th1 –IFN-γ production, IgG2c Th2 Th17 | I.M. | [65,68] | |
| TMC nanoparticles | TMC | DC maturation Increase in antigen’s intranasal residence Increase in the antigen’s uptake | Th1 Th2 Antigen-specific antibody production | I.N. (TMC-ESAT-6) | [68] |
| TMC Plasmid DNA, Muramyl peptide | DC maturation TLR9 activation (Plasmid DNA) NOD-like receptor 2 activation (muramyl peptide) | Th1 –IFN-γ production, IgG2c | I.M. | [67] | |
| Chitosan-Inulin | Chitosan Inulin | Increase antigen’s exposure to immune cells. Decrease in renal clearance and in proteolytic digestion | Th1- Polyfunctional T-cells producing IFN-γ, IL-2 and TNF-α Th2–T-cells producing IL4 Antigen-specific antibodies–IgG1 and IgG2b | S.C. | [64] |
| CDN-AddaVax® | CDNs Addavax® (oil-in-water emulsion) | STING activation. (CDNs) Enhanced T-cell and B-cell activation (AddaVax®) | Th17 Th1 Th2 | S.C. I.N. | [70] |
| Advax®-CpG | Delta-inulin micropaticles (Advax®) CpG | Enhanced phagocytosis and cell recruitment. (AddaVax®) Enhanced T and B cell activation. (AddaVax®) TLR9 activation (CpG) | Th1–Polyfunctional T-cells producing IFN-γ, IL-2 and TNF-α | I.M. (CysVac2) | [71] |
| Advax®-CpG- murabutide | Delta-inulin micropaticles (Advax®) CpG Muramyl dipetide (murabutide) | Enhanced phagocytosis and cell recruitment. (AddaVax®) T and B cell activation. (AddaVax®) TLR9 activation (CpG) NOD-like receptor 2 activation (muramyl peptide) | Th1–IgG2a and IgG1 production with a IgG2a bias. | I.M. | [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco, A.R.; Peri, F. Developing New Anti-Tuberculosis Vaccines: Focus on Adjuvants. Cells 2021, 10, 78. https://doi.org/10.3390/cells10010078
Franco AR, Peri F. Developing New Anti-Tuberculosis Vaccines: Focus on Adjuvants. Cells. 2021; 10(1):78. https://doi.org/10.3390/cells10010078
Chicago/Turabian StyleFranco, Ana Rita, and Francesco Peri. 2021. "Developing New Anti-Tuberculosis Vaccines: Focus on Adjuvants" Cells 10, no. 1: 78. https://doi.org/10.3390/cells10010078
APA StyleFranco, A. R., & Peri, F. (2021). Developing New Anti-Tuberculosis Vaccines: Focus on Adjuvants. Cells, 10(1), 78. https://doi.org/10.3390/cells10010078






