Evogliptin Suppresses Calcific Aortic Valve Disease by Attenuating Inflammation, Fibrosis, and Calcification
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Subjects and Isolation and Culture of Human Valve Interstitial Cells
2.2. Mice
2.3. Rabbits
2.4. Osteogenic Differentiation
2.5. Alizarin Red Staining
2.6. Calcium Assay
2.7. DPP-4 Activity Assay
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Fluorescence Reflectance Imaging In Vivo
2.10. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
2.11. Immunohistochemistry
2.12. Echocardiography
2.13. Statistical Analysis
3. Results
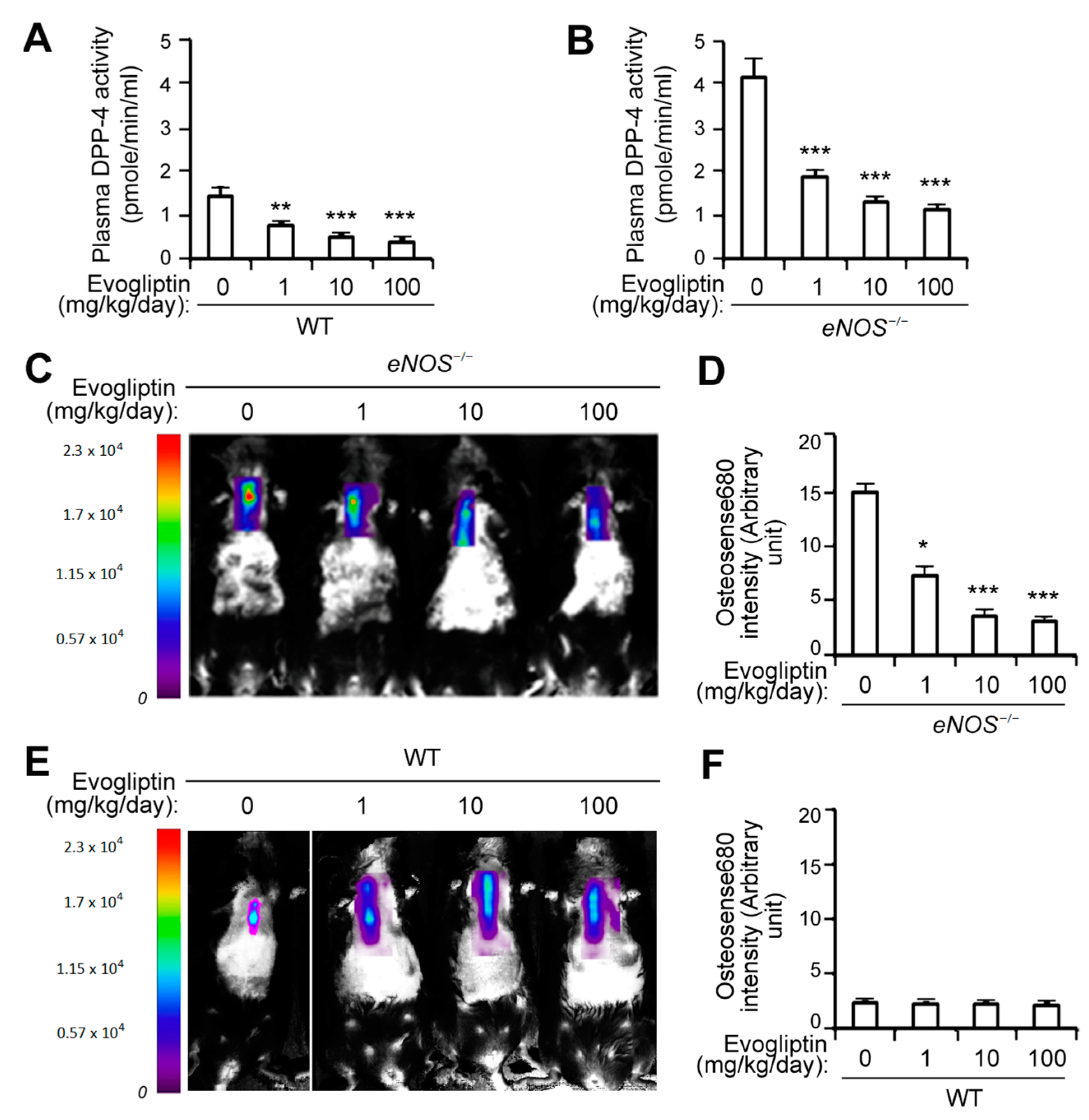
3.1. Evogliptin Suppresses Calcified Lesion Formation in eNOS-/- Mice
3.2. Evogliptin Attenuates the Osteogenic Transition of Aortic Valvular Interstitial Cells in Association with a Reduced Expression of Osteogenesis-Related and Fibrosis-Associated Genes
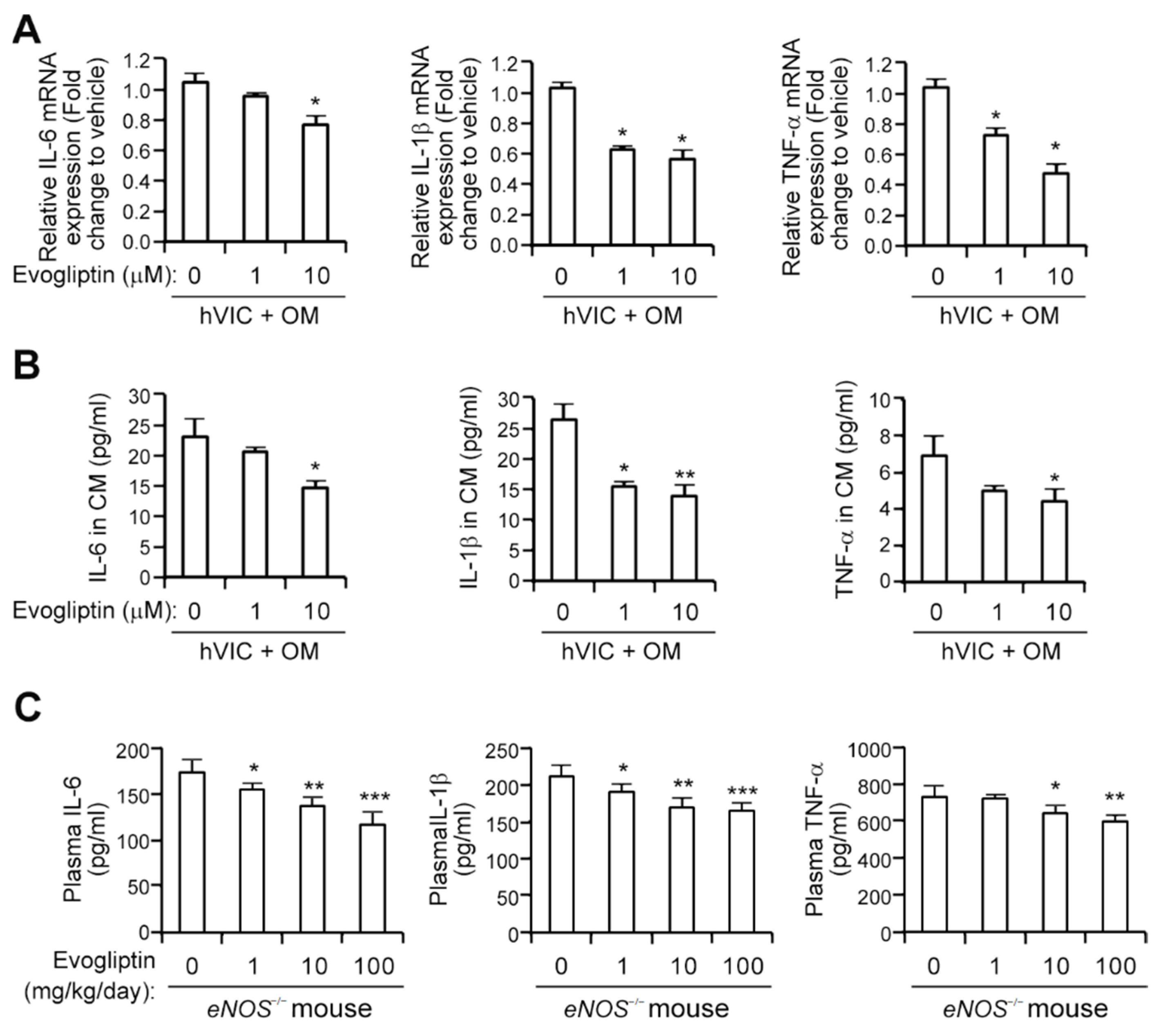
3.3. Evogliptin Attenuates the Expression of Proinflammatory Cytokines
3.4. Evogliptin Attenuates Calcific Aortic Valve Stenosis in a Rabbit Model of CAVD
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Freeman, R.V.; Otto, C.M. Spectrum of calcific aortic valve disease: Pathogenesis, disease progression, and treatment strategies. Circulation 2005, 111, 3316–3326. [Google Scholar] [CrossRef] [PubMed]
- Rabkin-Aikawa, E.; Mayer, J.E., Jr.; Schoen, F.J. Heart valve regeneration. Adv. Biochem. Eng. Biotechnol. 2005, 94, 141–179. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F. Regenerative medicine approach to heart valve replacement. Circulation 2005, 111, 2715–2716. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Otto, C.M.; Kuusisto, J.; Reichenbach, D.D.; Gown, A.M.; O’Brien, K.D. Characterization of the early lesion of ’degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation 1994, 90, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Thyberg, J.; Nilsson, J. Presence of oxidized low density lipoprotein in nonrheumatic stenotic aortic valves. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1218–1222. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, E.; Libby, P. A Rock and a Hard Place: Chiseling Away at the Multiple Mechanisms of Aortic Stenosis. Circulation 2017, 135, 1951–1955. [Google Scholar] [CrossRef] [PubMed]
- Dweck, M.R.; Boon, N.A.; Newby, D.E. Calcific aortic stenosis: A disease of the valve and the myocardium. J. Am. Coll. Cardiol. 2012, 60, 1854–1863. [Google Scholar] [CrossRef]
- Matteucci, E.; Giampietro, O. Dipeptidyl peptidase-4 (CD26): Knowing the function before inhibiting the enzyme. Curr. Med. Chem. 2009, 16, 2943–2951. [Google Scholar] [CrossRef]
- Zhong, J.; Rao, X.; Rajagopalan, S. An emerging role of dipeptidyl peptidase 4 (DPP4) beyond glucose control: Potential implications in cardiovascular disease. Atherosclerosis 2013, 226, 305–314. [Google Scholar] [CrossRef]
- Stonehouse, A.H.; Darsow, T.; Maggs, D.G. Incretin-based therapies. J. Diabetes 2012, 4, 55–67. [Google Scholar] [CrossRef]
- Pratley, R.E.; Salsali, A. Inhibition of DPP-4: A new therapeutic approach for the treatment of type 2 diabetes. Curr. Med Res. Opin. 2007, 23, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Morimoto, K.; Hasegawa, T.; Sasaki, N.; Yamashita, T.; Hirata, K.; Okita, Y.; Okada, K. Orally administered dipeptidyl peptidase-4 inhibitor (alogliptin) prevents abdominal aortic aneurysm formation through an antioxidant effect in rats. J. Vasc. Surg. 2014, 59, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Darsalia, V.; Ortsäter, H.; Olverling, A.; Darlöf, E.; Wolbert, P.; Nyström, T.; Klein, T.; Sjöholm, Å.; Patrone, C. The DPP-4 inhibitor linagliptin counteracts stroke in the normal and diabetic mouse brain: A comparison with glimepiride. Diabetes 2013, 62, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, H.; Zempo, H.; Ogawa, M.; Watanabe, R.; Suzuki, J.; Akazawa, H.; Komuro, I.; Isobe, M. A DPP-4 inhibitor suppresses fibrosis and inflammation on experimental autoimmune myocarditis in mice. PLoS ONE 2015, 10, e0119360. [Google Scholar] [CrossRef] [PubMed]
- Yazbeck, R.; Howarth, G.S.; Abbott, C.A. Dipeptidyl peptidase inhibitors, an emerging drug class for inflammatory disease? Trends Pharmacol. Sci. 2009, 30, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Murakami, T.; Horikawa, H.; Sugiura, M.; Kawashima, K.; Sugita, T. Suppression of arthritis by the inhibitors of dipeptidyl peptidase IV. Int. J. Immunopharmacol. 1997, 19, 15–24. [Google Scholar] [CrossRef]
- Preller, V.; Gerber, A.; Wrenger, S.; Togni, M.; Marguet, D.; Tadje, J.; Lendeckel, U.; Röcken, C.; Faust, J.; Neubert, K.; et al. TGF-beta1-mediated control of central nervous system inflammation and autoimmunity through the inhibitory receptor CD26. J. Immunol. 2007, 178, 4632–4640. [Google Scholar] [CrossRef]
- Lenski, M.; Kazakov, A.; Marx, N.; Böhm, M.; Laufs, U. Effects of DPP-4 inhibition on cardiac metabolism and function in mice. J. Mol. Cell. Cardiol. 2011, 51, 906–918. [Google Scholar] [CrossRef]
- Choi, B.; Lee, S.; Kim, S.M.; Lee, E.J.; Lee, S.R.; Kim, D.H.; Jang, J.Y.; Kang, S.W.; Lee, K.U.; Chang, E.J.; et al. Dipeptidyl Peptidase-4 Induces Aortic Valve Calcification by Inhibiting Insulin-Like Growth Factor-1 Signaling in Valvular Interstitial Cells. Circulation 2017, 135, 1935–1950. [Google Scholar] [CrossRef]
- Lee, S.; Lee, S.A.; Choi, B.; Kim, Y.J.; Oh, S.J.; Choi, H.M.; Kim, E.K.; Kim, D.H.; Cho, G.Y.; Song, J.M.; et al. Dipeptidyl peptidase-4 inhibition to prevent progression of calcific aortic stenosis. Heart (British Cardiac Society) 2020. [Google Scholar] [CrossRef]
- Loddick, S.A.; Liu, X.J.; Lu, Z.X.; Liu, C.; Behan, D.P.; Chalmers, D.C.; Foster, A.C.; Vale, W.W.; Ling, N.; De Souza, E.B. Displacement of insulin-like growth factors from their binding proteins as a potential treatment for stroke. Proc. Natl. Acad. Sci. USA 1998, 95, 1894–1898. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lim, J.; Lu, J.; Pedego, T.M.; Demer, L.; Tintut, Y. Protective Role of Smad6 in Inflammation-Induced Valvular Cell Calcification. J. Cell. Biochem. 2015, 116, 2354–2364. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Glomset, J.; Kariya, B.; Harker, L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc. Natl. Acad. Sci. USA 1974, 71, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Tintut, Y.; Parhami, F.; Boström, K.; Jackson, S.M.; Demer, L.L. cAMP stimulates osteoblast-like differentiation of calcifying vascular cells. Potential signaling pathway for vascular calcification. J. Biol. Chem. 1998, 273, 7547–7553. [Google Scholar] [CrossRef] [PubMed]
- El Accaoui, R.N.; Gould, S.T.; Hajj, G.P.; Chu, Y.; Davis, M.K.; Kraft, D.C.; Lund, D.D.; Brooks, R.M.; Doshi, H.; Zimmerman, K.A.; et al. Aortic valve sclerosis in mice deficient in endothelial nitric oxide synthase. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1302–H1313. [Google Scholar] [CrossRef] [PubMed]
- Sider, K.L.; Blaser, M.C.; Simmons, C.A. Animal models of calcific aortic valve disease. Int. J. Inflamm. 2011, 2011, 364310. [Google Scholar] [CrossRef]
- Du, Y.; Gao, C.; Liu, Z.; Wang, L.; Liu, B.; He, F.; Zhang, T.; Wang, Y.; Wang, X.; Xu, M.; et al. Upregulation of a disintegrin and metalloproteinase with thrombospondin motifs-7 by miR-29 repression mediates vascular smooth muscle calcification. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2580–2588. [Google Scholar] [CrossRef]
- Beazley, K.E.; Eghtesad, S.; Nurminskaya, M.V. Quercetin attenuates warfarin-induced vascular calcification in vitro independently from matrix Gla protein. J. Biol. Chem. 2013, 288, 2632–2640. [Google Scholar] [CrossRef]
- Aikawa, E.; Nahrendorf, M.; Figueiredo, J.L.; Swirski, F.K.; Shtatland, T.; Kohler, R.H.; Jaffer, F.A.; Aikawa, M.; Weissleder, R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation 2007, 116, 2841–2850. [Google Scholar] [CrossRef]
- Zaheer, A.; Lenkinski, R.E.; Mahmood, A.; Jones, A.G.; Cantley, L.C.; Frangioni, J.V. In vivo near-infrared fluorescence imaging of osteoblastic activity. Nat. Biotechnol. 2001, 19, 1148–1154. [Google Scholar] [CrossRef]
- Zaheer, A.; Murshed, M.; De Grand, A.M.; Morgan, T.G.; Karsenty, G.; Frangioni, J.V. Optical imaging of hydroxyapatite in the calcified vasculature of transgenic animals. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, R.; Suzuki, S.; Sakamoto, H.; Miura, N.; Misumi, K.; Miyahara, K. Development of atherosclerotic lesions in cholesterol-loaded rabbits. Exp. Anim. 1999, 48, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A. Cellular mechanisms of aortic valve calcification. Circ. Cardiovasc. Interv. 2012, 5, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Drolet, M.C.; Couet, J.; Arsenault, M. Development of aortic valve sclerosis or stenosis in rabbits: Role of cholesterol and calcium. J. Heart Valve Dis. 2008, 17, 381–387. [Google Scholar]
- Drolet, M.C.; Arsenault, M.; Couet, J. Experimental aortic valve stenosis in rabbits. J. Am. Coll. Cardiol. 2003, 41, 1211–1217. [Google Scholar] [CrossRef]
- Shah, Z.; Kampfrath, T.; Deiuliis, J.A.; Zhong, J.; Pineda, C.; Ying, Z.; Xu, X.; Lu, B.; Moffatt-Bruce, S.; Durairaj, R.; et al. Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation 2011, 124, 2338–2349. [Google Scholar] [CrossRef]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef]
- Pala, L.; Rotella, C.M. The role of DPP4 activity in cardiovascular districts: In vivo and in vitro evidence. J. Diabetes Res. 2013, 2013, 590456. [Google Scholar] [CrossRef]
- Ku, H.C.; Chen, W.P.; Su, M.J. DPP4 deficiency preserves cardiac function via GLP-1 signaling in rats subjected to myocardial ischemia/reperfusion. Naunyn Schmiedeberg Arch. Pharmacol. 2011, 384, 197–207. [Google Scholar] [CrossRef]
- Sauvé, M.; Ban, K.; Momen, M.A.; Zhou, Y.Q.; Henkelman, R.M.; Husain, M.; Drucker, D.J. Genetic deletion or pharmacological inhibition of dipeptidyl peptidase-4 improves cardiovascular outcomes after myocardial infarction in mice. Diabetes 2010, 59, 1063–1073. [Google Scholar] [CrossRef]
- Kim, T.E.; Lim, K.S.; Park, M.K.; Yoon, S.H.; Cho, J.Y.; Shin, S.G.; Jang, I.J.; Yu, K.S. Evaluation of the pharmacokinetics, food effect, pharmacodynamics, and tolerability of DA-1229, a dipeptidyl peptidase IV inhibitor, in healthy volunteers: First-in-human study. Clin. Ther. 2012, 34, 1986–1998. [Google Scholar] [CrossRef] [PubMed]
- Gu, N.; Park, M.K.; Kim, T.E.; Bahng, M.Y.; Lim, K.S.; Cho, S.H.; Yoon, S.H.; Cho, J.Y.; Jang, I.J.; Yu, K.S. Multiple-dose pharmacokinetics and pharmacodynamics of evogliptin (DA-1229), a novel dipeptidyl peptidase IV inhibitor, in healthy volunteers. Drug Des. Dev. Ther. 2014, 8, 1709–1721. [Google Scholar] [CrossRef] [PubMed]
- White, W.B.; Cannon, C.P.; Heller, S.R.; Nissen, S.E.; Bergenstal, R.M.; Bakris, G.L.; Perez, A.T.; Fleck, P.R.; Mehta, C.R.; Kupfer, S.; et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N. Engl. J. Med. 2013, 369, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Green, J.B.; Bethel, M.A.; Armstrong, P.W.; Buse, J.B.; Engel, S.S.; Garg, J.; Josse, R.; Kaufman, K.D.; Koglin, J.; Korn, S.; et al. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Perkovic, V.; Johansen, O.E.; Cooper, M.E.; Kahn, S.E.; Marx, N.; Alexander, J.H.; Pencina, M.; Toto, R.D.; Wanner, C.; et al. Effect of Linagliptin vs Placebo on Major Cardiovascular Events in Adults With Type 2 Diabetes and High Cardiovascular and Renal Risk: The CARMELINA Randomized Clinical Trial. JAMA 2019, 321, 69–79. [Google Scholar] [CrossRef]
- Mohler, E.R., 3rd; Chawla, M.K.; Chang, A.W.; Vyavahare, N.; Levy, R.J.; Graham, L.; Gannon, F.H. Identification and characterization of calcifying valve cells from human and canine aortic valves. J. Heart Valve Dis. 1999, 8, 254–260. [Google Scholar]
- Reinhold, D.; Biton, A.; Pieper, S.; Lendeckel, U.; Faust, J.; Neubert, K.; Bank, U.; Täger, M.; Ansorge, S.; Brocke, S. Dipeptidyl peptidase IV (DP IV, CD26) and aminopeptidase N (APN, CD13) as regulators of T cell function and targets of immunotherapy in CNS inflammation. Int. Immunopharmacol. 2006, 6, 1935–1942. [Google Scholar] [CrossRef]
- Bank, U.; Bohr, U.R.; Reinhold, D.; Lendeckel, U.; Ansorge, S.; Malfertheiner, P.; Tager, M. Inflammatory bowel diseases: Multiple benefits from therapy with dipeptidyl- and alanyl-aminopeptidase inhibitors. Front. Biosci. A J. Virtual Libr. 2008, 13, 3699–3713. [Google Scholar] [CrossRef][Green Version]
- Ikedo, T.; Minami, M.; Kataoka, H.; Hayashi, K.; Nagata, M.; Fujikawa, R.; Higuchi, S.; Yasui, M.; Aoki, T.; Fukuda, M.; et al. Dipeptidyl Peptidase-4 Inhibitor Anagliptin Prevents Intracranial Aneurysm Growth by Suppressing Macrophage Infiltration and Activation. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Mathieu, P.; Bouchareb, R.; Boulanger, M.C. Innate and Adaptive Immunity in Calcific Aortic Valve Disease. J. Immunol. Res. 2015, 2015, 851945. [Google Scholar] [CrossRef]
- Éva Sikura, K.; Combi, Z.; Potor, L.; Szerafin, T.; Hendrik, Z.; Méhes, G.; Gergely, P.; Whiteman, M.; Beke, L.; Fürtös, I.; et al. Hydrogen sulfide inhibits aortic valve calcification in heart via regulating RUNX2 by NF-κB, a link between inflammation and mineralization. J. Adv. Res. 2021, 27, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell 1997, 89, 747–754. [Google Scholar] [CrossRef]
- Nguyen, P.A.; Won, J.S.; Rahman, M.K.; Bae, E.J.; Cho, M.K. Modulation of Sirt1/NF-κB interaction of evogliptin is attributed to inhibition of vascular inflammatory response leading to attenuation of atherosclerotic plaque formation. Biochem. Pharmacol. 2019, 168, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Kanasaki, K.; Shi, S.; Kanasaki, M.; He, J.; Nagai, T.; Nakamura, Y.; Ishigaki, Y.; Kitada, M.; Srivastava, S.P.; Koya, D. Linagliptin-mediated DPP-4 inhibition ameliorates kidney fibrosis in streptozotocin-induced diabetic mice by inhibiting endothelial-to-mesenchymal transition in a therapeutic regimen. Diabetes 2014, 63, 2120–2131. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, B.; Kim, E.-Y.; Kim, J.-E.; Oh, S.; Park, S.-O.; Kim, S.-M.; Choi, H.; Song, J.-K.; Chang, E.-J. Evogliptin Suppresses Calcific Aortic Valve Disease by Attenuating Inflammation, Fibrosis, and Calcification. Cells 2021, 10, 57. https://doi.org/10.3390/cells10010057
Choi B, Kim E-Y, Kim J-E, Oh S, Park S-O, Kim S-M, Choi H, Song J-K, Chang E-J. Evogliptin Suppresses Calcific Aortic Valve Disease by Attenuating Inflammation, Fibrosis, and Calcification. Cells. 2021; 10(1):57. https://doi.org/10.3390/cells10010057
Chicago/Turabian StyleChoi, Bongkun, Eun-Young Kim, Ji-Eun Kim, Soyoon Oh, Si-On Park, Sang-Min Kim, Hyuksu Choi, Jae-Kwan Song, and Eun-Ju Chang. 2021. "Evogliptin Suppresses Calcific Aortic Valve Disease by Attenuating Inflammation, Fibrosis, and Calcification" Cells 10, no. 1: 57. https://doi.org/10.3390/cells10010057
APA StyleChoi, B., Kim, E.-Y., Kim, J.-E., Oh, S., Park, S.-O., Kim, S.-M., Choi, H., Song, J.-K., & Chang, E.-J. (2021). Evogliptin Suppresses Calcific Aortic Valve Disease by Attenuating Inflammation, Fibrosis, and Calcification. Cells, 10(1), 57. https://doi.org/10.3390/cells10010057



