Radiation-Activated PI3K/AKT Pathway Promotes the Induction of Cancer Stem-Like Cells via the Upregulation of SOX2 in Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibodies and Reagents
2.2. Cell Culture and Irradiation
2.3. Flow Cytometry and Aldehyde Dehydrogenase (ALDH) Assay
2.4. Fluorescence-Activated Cell Sorting (FACS Sorting)
2.5. Immunocytochemistry (ICC)
2.6. Western Blot Analysis
2.7. Apoptosis Assay
2.8. Colony Formation Assay
2.9. Invasion and Migration Assays
2.10. Tumoursphere-Formation Assay
2.11. Statistical Analysis
3. Results
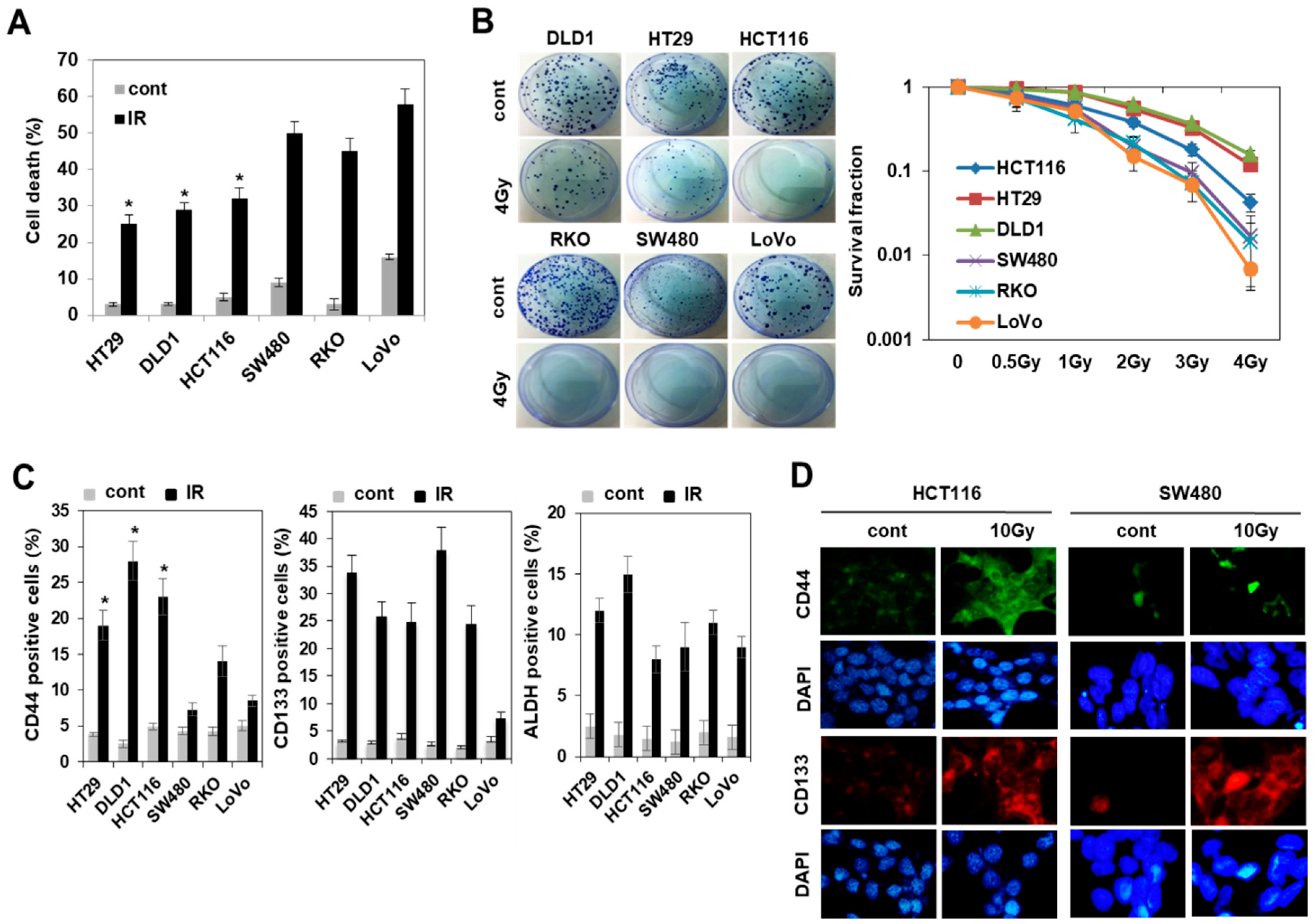
3.1. Radiation Increased the Population of Radioresistant Rather Than Radiosensitive CD44+ Colorectal Cancer Cells
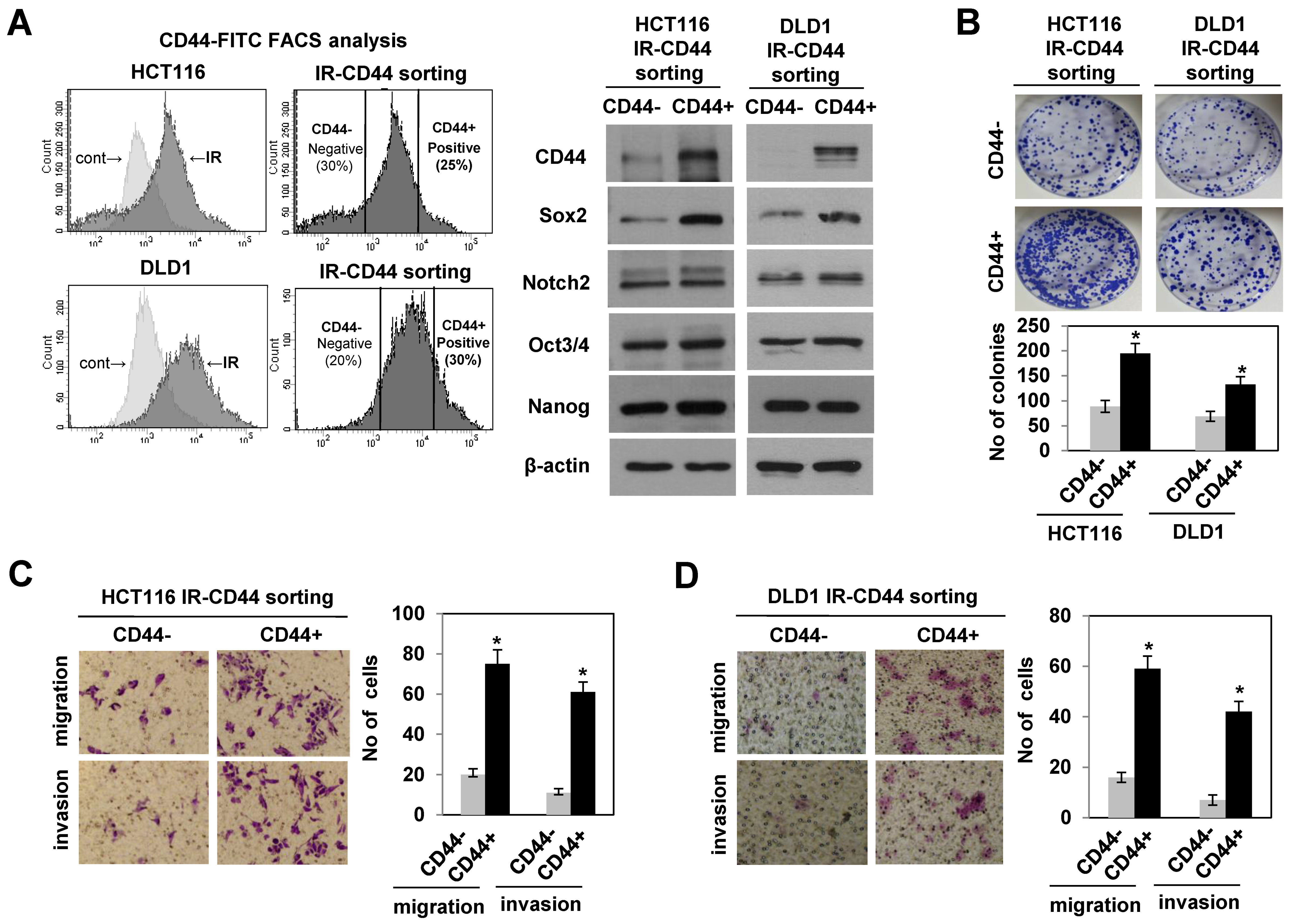
3.2. Radiation-Enriched CD44+ Cells Exhibited the Properties of CSCs Including an Increase in SOX2 Expression
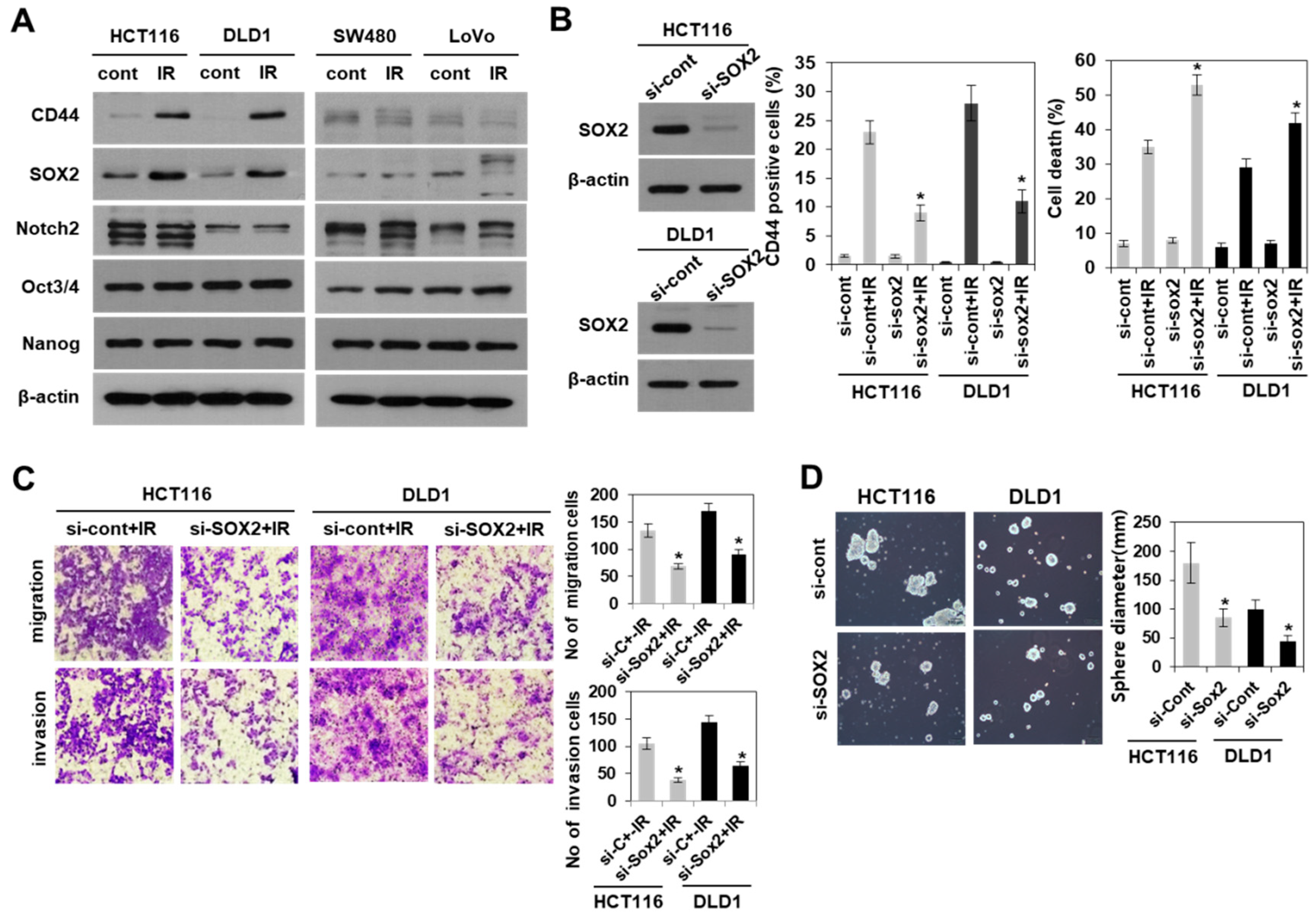
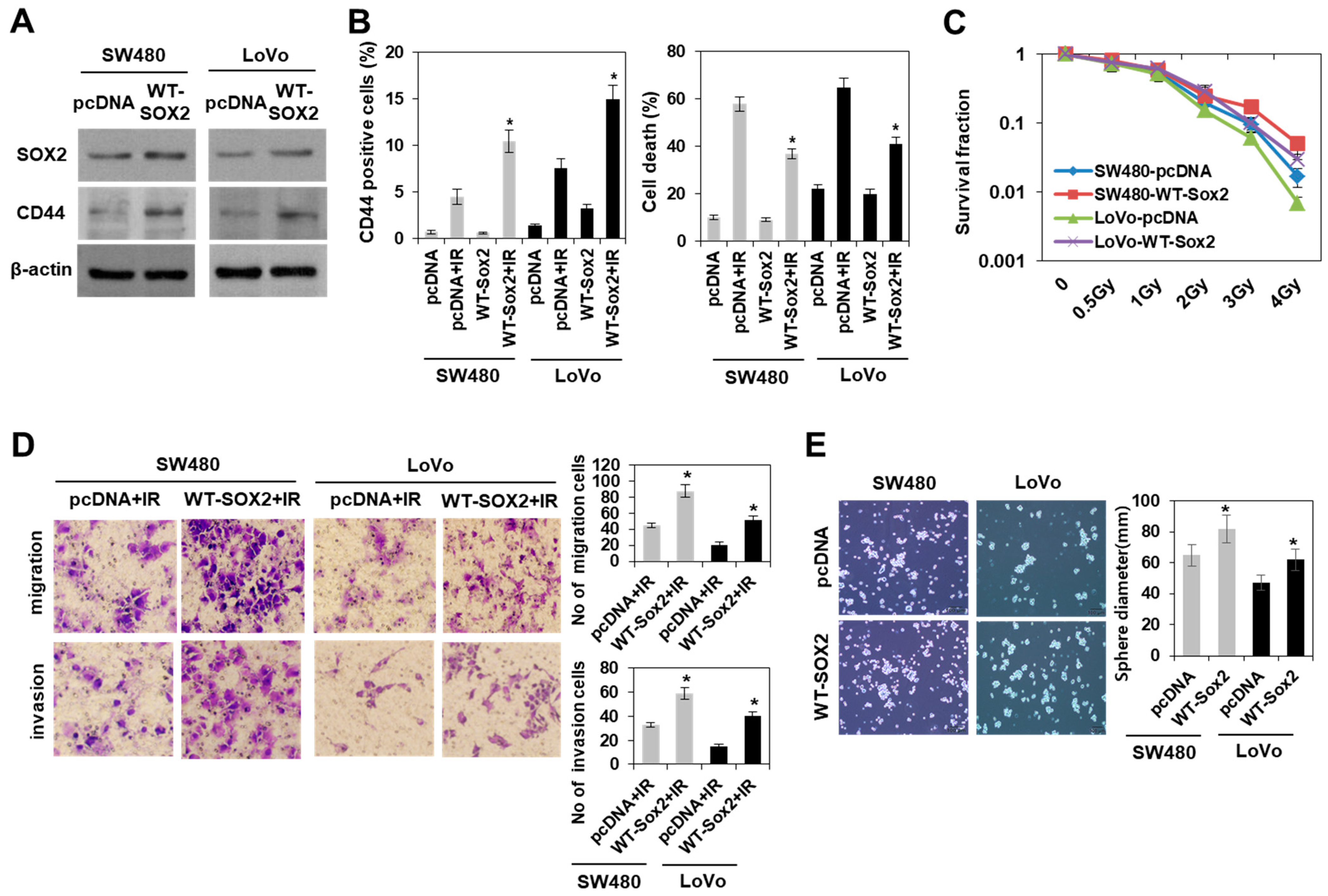
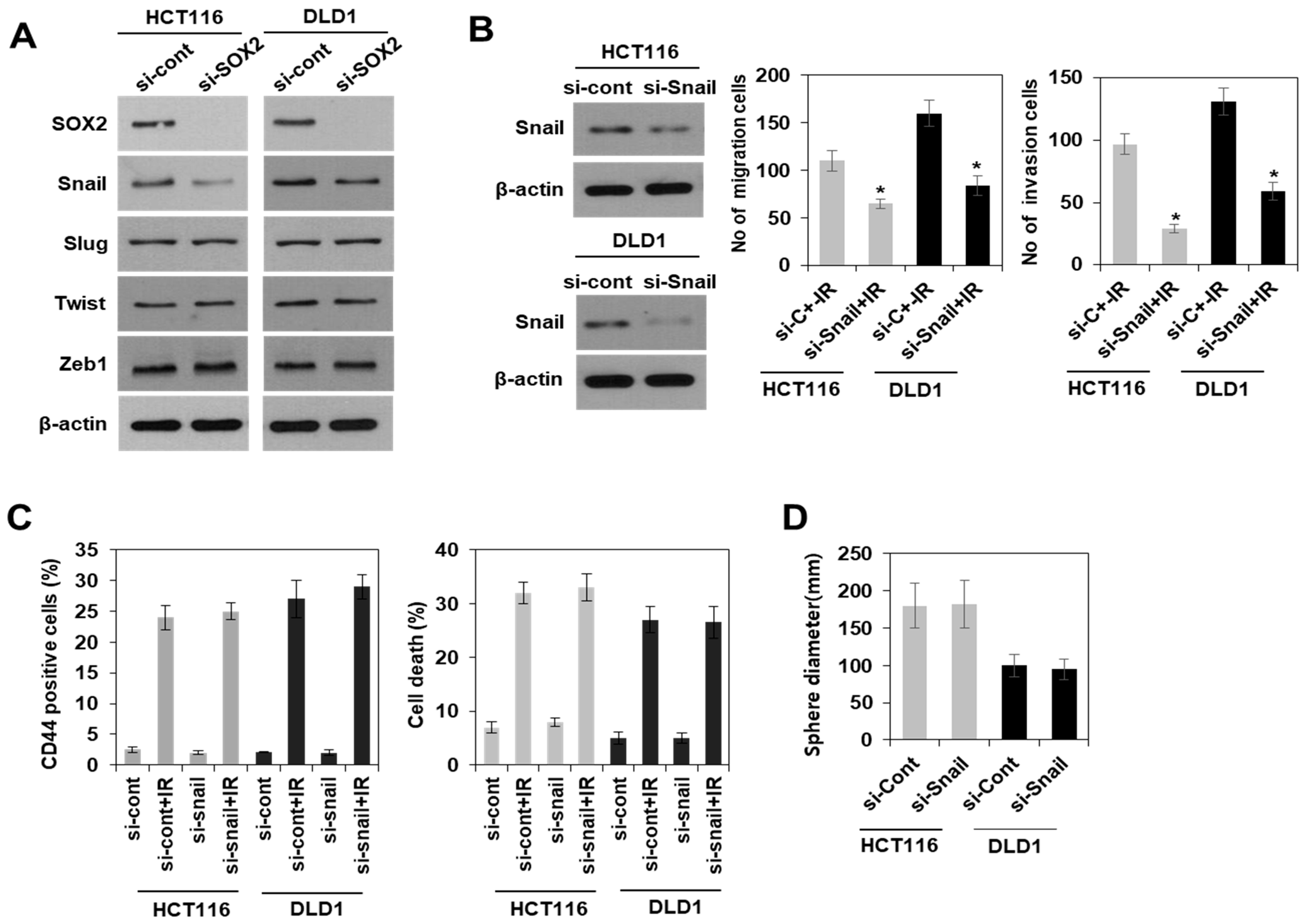
3.3. Modulation of SOX2 Expression in Colorectal Cancer Cells Is Associated with Induction of Colorectal CSCs Following Irradiation
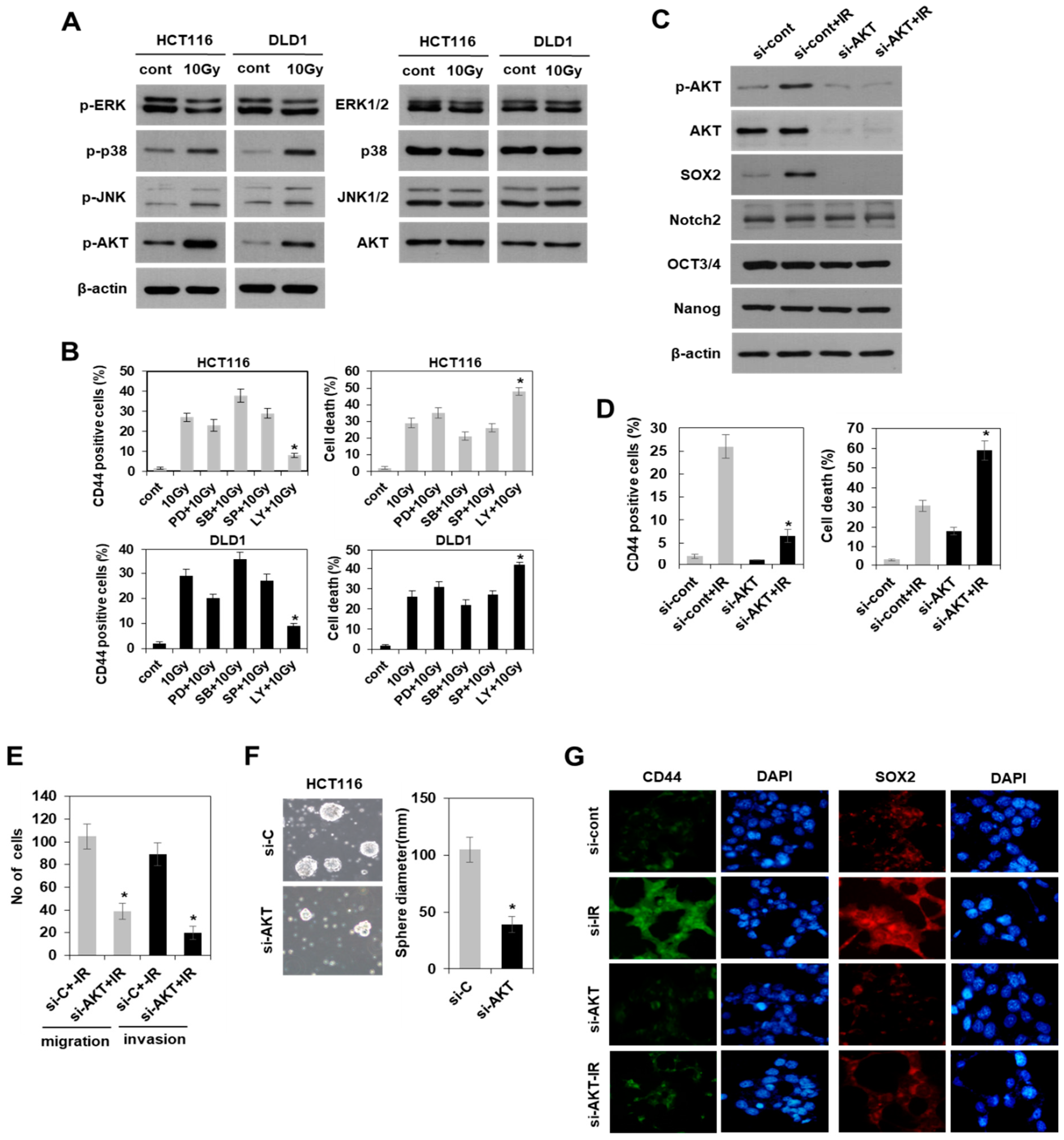
3.4. Radiation-Induced Activation of the PI3K/AKT Pathway, but Not the MAPK Pathway Modulated SOX2-Dependent Induction of Colorectal CSCs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global Cancer Statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Kloor, M.; Pox, C.P. Colorectal Cancer. Lancet 2014, 383, 1490–1502. [Google Scholar] [CrossRef]
- Olivares-Urbano, M.A.; Griñán-Lisón, C.; Marchal, J.A.; Núñez, M.I. CSC Radioresistance: A Therapeutic Challenge to Improve Radiotherapy Effectiveness in Cancer. Cells 2020, 9, 1651. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Hawkins, C.; Clarke, I.D.; Squire, J.A.; Bayani, J.; Hide, T.; Henkelman, R.M.; Cusimano, M.D.; Dirks, P.B. Identification of Human Brain Tumour Initiating Cells. Nature 2004, 432, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, M.; Luo, J.; Zhou, H. Radiotherapy Targeting Cancer Stem Cells “Awakens” Them to Induce Tumour Relapse and Metastasis in Oral Cancer. Int. J. Oral Sci. 2020, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Mishra, L.; Li, S. EMT, CTCs and CSCs in Tumor Relapse and Drug-Resistance. Oncotarget 2015, 6, 10697–10711. [Google Scholar] [CrossRef]
- Zeuner, A.; Todaro, M.; Stassi, G.; De Maria, R. Colorectal Cancer Stem Cells: From the Crypt to the Clinic. Cell Stem Cell 2014, 15, 692–705. [Google Scholar] [CrossRef]
- Szaryńska, M.; Olejniczak, A.; Kobiela, J.; Spychalski, P.; Kmieć, Z. Therapeutic Strategies against Cancer Stem Cells in Human Colorectal Cancer. Oncol. Lett. 2017, 14, 7653–7668. [Google Scholar] [CrossRef]
- Zhou, Y.; Xia, L.; Wang, H.; Oyang, L.; Su, M.; Liu, Q.; Lin, J.; Tan, S.; Tian, Y.; Liao, Q.; et al. Cancer stem cells in progression of colorectal cancer. Oncotarget 2018, 9, 33403–33415. [Google Scholar] [CrossRef]
- Sekido, R.; Lovell-Badge, R. Sex Determination and SRY: Down to a Wink and a Nudge? Trends Genet. 2009, 25, 19–29. [Google Scholar] [CrossRef]
- Wegner, M. All Purpose Sox: The Many Roles of Sox Proteins in Gene Expression. Int. J. Biochem. Cell Biol. 2010, 42, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Boumahdi, S.; Driessens, G.; Lapouge, G.; Rorive, S.; Nassar, D.; Le Mercier, M.; Delatte, B.; Caauwe, A.; Lenglez, S.; Nkusi, E.; et al. SOX2 Controls Tumour Initiation and Cancer Stem-Cell Functions in Squamous-Cell Carcinoma. Nature 2014, 10, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Qian, W.; Zhang, H.; Liang, Y.; Wu, M.; Zhang, Y.; Zhang, X.; Gao, Q.; Li, Y. SOX2 Is a Marker for Stem-Like Tumor Cells in Bladder Cancer. Stem Cell Rep. 2017, 9, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, I.V.; Edin, S.; Eklöf, V.; Öberg, Å.; Palmqvist, R.; Wikberg, M.L. SOX2 Expression Is Associated with a Cancer Stem Cell State and Down-Regulation of CDX2 in Colorectal Cancer. BMC Cancer 2016, 16, 471. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Mizushima, T.; Yokoyama, Y.; Hirose, H.; Wu, X.; Qian, Y.; Ikehata, K.; Miyoshi, N.; Takahashi, H.; Haraguchi, N.; et al. Sox2 Is Associated with Cancer Stem-Like Properties in Colorectal Cancer. Sci. Rep. 2018, 8, 17639. [Google Scholar] [CrossRef]
- Ghisolfi, L.; Keates, A.C.; Hu, X.; Lee, D.K.; Li, C.J. Ionizing Radiation Induces Stemness in Cancer Cells. PLoS ONE 2012, 7, e43628. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, Y.H.; Park, E.H.; Lee, S.J.; Kim, H.; Kim, A.; Lee, S.B.; Shim, S.; Jang, H.; Myung, J.K.; et al. Effects of Metformin and Phenformin on Apoptosis and Epithelial-Mesenchymal Transition in Chemoresistant Rectal Cancer. Cancer Sci. 2019, 110, 2834–2845. [Google Scholar] [CrossRef]
- Cho, M.H.; Park, J.H.; Choi, H.J.; Park, M.K.; Won, H.Y.; Park, Y.J.; Lee, C.H.; Oh, S.H.; Song, Y.S.; Kim, H.S.; et al. DOT1L Cooperates with the c-Myc-p300 Complex to Epigenetically Derepress CDH1 Transcription Factors in Breast Cancer Progression. Nat. Commun. 2015, 6, 7821. [Google Scholar] [CrossRef]
- Du, L.; Wang, H.; He, L.; Zhang, J.; Ni, B.; Wang, X.; Jin, H.; Cahuzac, N.; Mehrpour, M.; Lu, Y.; et al. CD44 Is of Functional Importance for Colorectal Cancer Stem Cells. Clin. Cancer Res. 2008, 14, 6751–6760. [Google Scholar] [CrossRef]
- Dalerba, P.; Dylla, S.J.; Park, I.K.; Liu, R.; Wang, X.; Cho, R.W.; Hoey, T.; Gurney, A.; Huang, E.H.; Simeone, D.M.; et al. Phenotypic Characterization of Human Colorectal Cancer Stem Cells. Proc. Natl. Acad. Sci. USA 2007, 104, 10158–10163. [Google Scholar] [CrossRef]
- Munro, M.J.; Wickremesekera, S.K.; Peng, L.; Tan, S.T.; Itinteang, T. Cancer Stem Cells in Colorectal Cancer: A Review. J. Clin. Pathol. 2018, 71, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Meisel, C.T.; Porcheri, C.; Mitsiadis, T.A. Cancer Stem Cells, Quo Vadis? The Notch Signaling Pathway in Tumor Initiation and Progression. Cells 2020, 9, 1879. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, T. EMT and MET in Metastasis: Where Are the Cancer Stem Cells? Cancer Cell 2012, 22, 699–701. [Google Scholar] [CrossRef]
- Han, X.; Fang, X.; Lou, X.; Hua, D.; Ding, W.; Foltz, G.; Hood, L.; Yuan, Y.; Lin, B. Silencing SOX2 Induced Mesenchymal-epithelial Transition and its Expression Predicts Liver and Lymph Node Metastasis of CRC Patients. PLoS ONE 2012, 7, e41335. [Google Scholar] [CrossRef]
- Shimoda, M.; Sugiura, T.; Imajyo, I.; Ishii, K.; Chigita, S.; Seki, K.; Kobayashi, Y.; Shirasuna, K. The T-Box Transcription Factor Brachyury Regulates Epithelial-Mesenchymal Transition in Association with Cancer Stem-Like Cells in Adenoid Cystic Carcinoma Cells. BMC Cancer 2012, 12, 377. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.; Gao, Y.; Huang, J.; Xu, S.; Wang, B.; Zeng, J.; Fan, J.; Wang, X.; Yue, Y.; Wu, S.; et al. RASAL2, A RAS Gtpase-activating Protein, Inhibits Stemness and Epithelial-Mesenchymal Transition Via MAPK/SOX2 Pathway in Bladder Cancer. Cell Death Dis. 2017, 8, e2600. [Google Scholar] [CrossRef]
- Haston, S.; Pozzi, S.; Carreno, G.; Manshaei, S.; Panousopoulos, L.; Gonzalez-Meljem, J.M.; Apps, J.R.; Virasami, A.; Thavaraj, S.; Gutteridge, A.; et al. MAPK Pathway Control of Stem Cell Proliferation and Differentiation in the Embryonic Pituitary Provides Insights into the Pathogenesis of Papillary Craniopharyngioma. Development 2017, 144, 2141–2152. [Google Scholar] [CrossRef]
- Schaefer, T.; Wang, H.; Mir, P.; Konantz, M.; Pereboom, T.C.; Paczulla, A.M.; Merz, B.; Fehm, T.; Perner, S.; Rothfuss, O.C.; et al. Molecular and Functional Interactions Between AKT and SOX2 in Breast Carcinoma. Oncotarget 2015, 6, 43540–43556. [Google Scholar] [CrossRef]
- Qin, J.; Ji, J.; Deng, R.; Tang, J.; Yang, F.; Feng, G.K.; Chen, W.D.; Wu, X.Q.; Qian, X.J.; Ding, K.; et al. DC120, A Novel AKT Inhibitor, Preferentially Suppresses Nasopharyngeal Carcinoma Cancer Stem-Like Cells by Downregulating Sox2. Oncotarget 2015, 6, 6944–6958. [Google Scholar] [CrossRef][Green Version]
- Kim, M.J.; Byun, J.Y.; Yun, C.H.; Park, I.C.; Lee, K.H.; Lee, S.J. c-Src-p38 Mitogen-Activated Protein Kinase Signaling Is Required for Akt Activation in Response to Ionizing Radiation. Mol. Cancer Res. 2008, 6, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Aruffo, A.; Stamenkovic, I.; Melnick, M.; Underhill, C.B.; Seed, B. CD44 Is the Principal Cell Surface Receptor for Hyaluronate. Cell 1990, 61, 1303–1313. [Google Scholar] [CrossRef]
- Nagano, O.; Saya, H. Mechanism and Biological Significance of CD44 Cleavage. Cancer Sci. 2004, 95, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Sharp, P.A. Regulation of CD44 Alternative Splicing by SRm160 and Its Potential Role in Tumor Cell Invasion. Mol. Cell. Biol. 2006, 26, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Vigetti, D.; Viola, M.; Karousou, E.; Rizzi, M.; Moretto, P.; Genasetti, A.; Clerici, M.; Hascali, V.C.; De Luca, G.; Passi, A. Hyaluronan-CD44-1/2 Regulate Human Aortic Smooth Muscle Cell Motility During Aging. J. Biol. Chem. 2008, 283, 4448–4458. [Google Scholar] [CrossRef]
- Feng, H.L.; Liu, Y.Q.; Yang, L.J.; Bian, X.C.; Yang, Z.L.; Gu, B.; Zhang, H.; Wang, C.J.; Su, X.L.; Zhao, X.M. Expression of CD133 Correlates with Differentiation of Human Colon Cancer Cells. Cancer Biol. Ther. 2010, 9, 216–223. [Google Scholar] [CrossRef]
- Shmelkov, S.V.; Butler, J.M.; Hooper, A.T.; Hormigo, A.; Kushner, J.; Milde, T.; St Clair, R.; Baljevic, M.; White, I.; Jin, D.K.; et al. CD133 Expression Is Not Restricted to Stem Cells, and Both CD133+ and CD133− Metastatic Colon Cancer Cells Initiate Tumors. J. Clin. Investig. 2008, 118, 2111–2120. [Google Scholar] [CrossRef]
- Herreros-Villanueva, M.; Zhang, J.S.; Koenig, A.; Abel, E.V.; Smyrk, T.C.; Bamlet, W.R.; de Narvajas, A.A.; Gomez, T.S.; Simeone, D.M.; Bujanda, L.; et al. SOX2 Promotes Dedifferentiation and Imparts Stem Cell-Like Features to Pancreatic Cancer Cells. Oncogenesis 2013, 2, e61. [Google Scholar] [CrossRef]
- Liu, X.F.; Yang, W.T.; Xu, R.; Liu, J.T.; Zheng, P.S. Cervical Cancer Cells with Positive Sox2 Expression Exhibit the Properties of Cancer Stem Cells. PLoS ONE 2014, 9, e87092. [Google Scholar] [CrossRef]
- François, S.; Usunier, B.; Forgue-Lafitte, M.E.; L’Homme, B.; Benderitter, M.; Douay, L.; Gorin, N.C.; Larsen, A.K.; Chapel, A. Mesenchymal Stem Cell Administration Attenuates Colon Cancer Progression by Modulating the Immune Component within the Colorectal Tumor Microenvironment. Stem Cells Transl. Med. 2019, 8, 285–300. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jeong, E.K.; Ju, M.K.; Jeon, H.M.; Kim, M.Y.; Kim, C.H.; Park, H.G.; Han, S.I.; Kang, H.S. Induction of Metastasis, Cancer Stem Cell Phenotype, and Oncogenic Metabolism in Cancer Cells by Ionizing Radiation. Mol. Cancer 2017, 16, 10. [Google Scholar] [CrossRef]
- Narayanankutty, A. PI 3K/Akt/mTOR Pathway as a Therapeutic Target for Colorectal Cancer: A Review of Preclinical and Clinical Evidence. Curr. Drug Targets 2019, 20, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Rauch, J.; Kolch, W. Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity. Int. J. Mol. Sci. 2020, 21, 1102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.K.; Zhu, Y.L.; Qiu, F.M.; Zhang, T.; Chen, Z.G.; Zheng, S.; Huang, J. Activation of Akt and MAPK Pathways Enhances the Tumorigenicity of CD133+ Primary Colon Cancer Cells. Carcinogenesis 2010, 31, 1376–1380. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-H.; Kim, Y.-H.; Shim, S.; Kim, A.; Jang, H.; Lee, S.-J.; Park, S.; Seo, S.; Jang, W.I.; Lee, S.B.; et al. Radiation-Activated PI3K/AKT Pathway Promotes the Induction of Cancer Stem-Like Cells via the Upregulation of SOX2 in Colorectal Cancer. Cells 2021, 10, 135. https://doi.org/10.3390/cells10010135
Park J-H, Kim Y-H, Shim S, Kim A, Jang H, Lee S-J, Park S, Seo S, Jang WI, Lee SB, et al. Radiation-Activated PI3K/AKT Pathway Promotes the Induction of Cancer Stem-Like Cells via the Upregulation of SOX2 in Colorectal Cancer. Cells. 2021; 10(1):135. https://doi.org/10.3390/cells10010135
Chicago/Turabian StylePark, Ji-Hye, Young-Heon Kim, Sehwan Shim, Areumnuri Kim, Hyosun Jang, Su-Jae Lee, Sunhoo Park, Songwon Seo, Won Il Jang, Seung Bum Lee, and et al. 2021. "Radiation-Activated PI3K/AKT Pathway Promotes the Induction of Cancer Stem-Like Cells via the Upregulation of SOX2 in Colorectal Cancer" Cells 10, no. 1: 135. https://doi.org/10.3390/cells10010135
APA StylePark, J.-H., Kim, Y.-H., Shim, S., Kim, A., Jang, H., Lee, S.-J., Park, S., Seo, S., Jang, W. I., Lee, S. B., & Kim, M.-J. (2021). Radiation-Activated PI3K/AKT Pathway Promotes the Induction of Cancer Stem-Like Cells via the Upregulation of SOX2 in Colorectal Cancer. Cells, 10(1), 135. https://doi.org/10.3390/cells10010135




