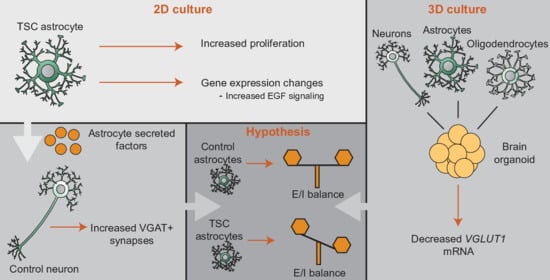
Neuron–Glia Interactions in Tuberous Sclerosis Complex Affect the Synaptic Balance in 2D and Organoid Cultures
Abstract
:1. Introduction
2. Materials and Methods
2.1. iPSC Production
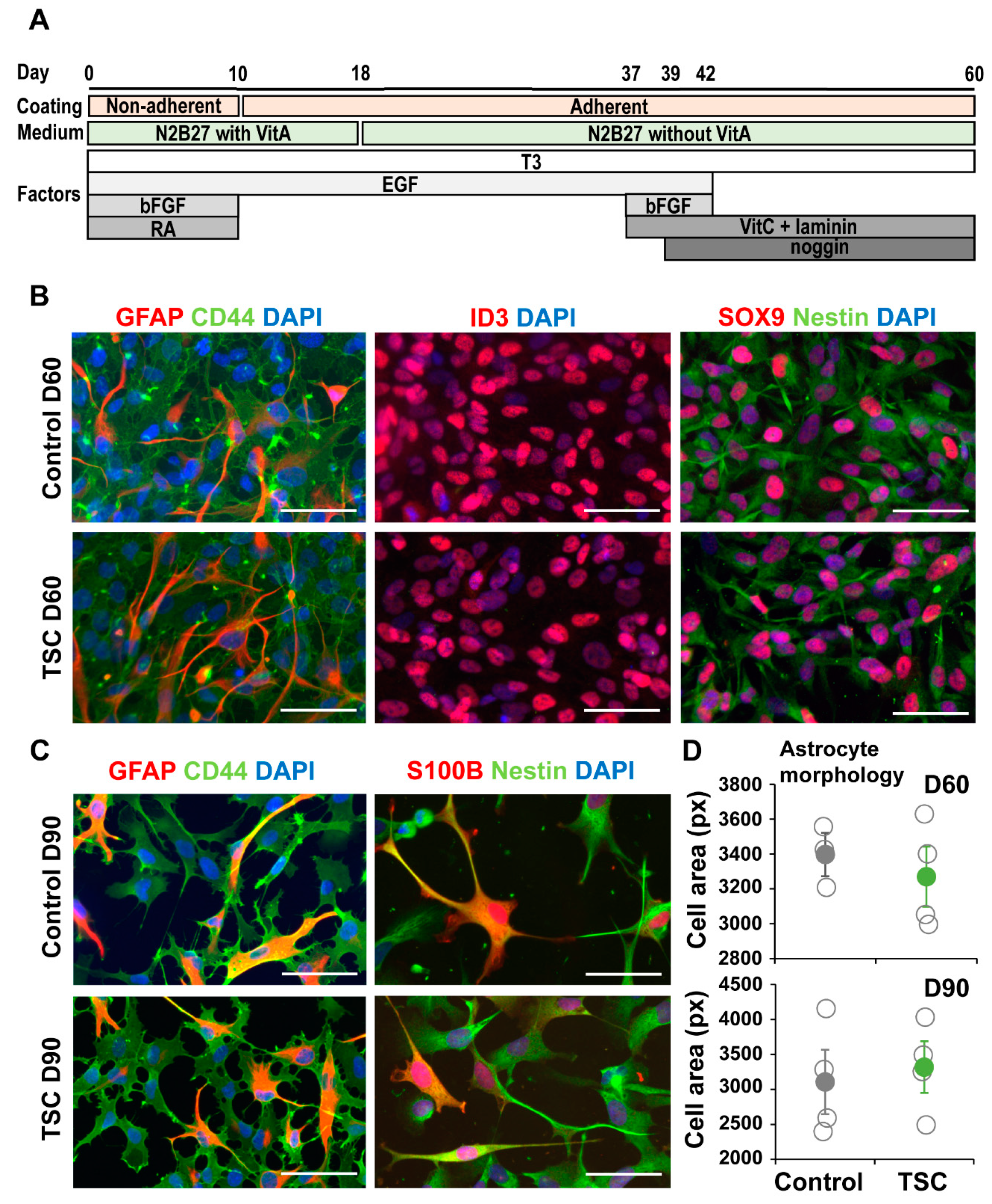
2.2. Astrocyte Differentiation
2.3. Neuronal Differentiation
2.4. Organoid Culture
2.5. Medium Composition
2.6. ACM Collection
2.7. Immunostaining
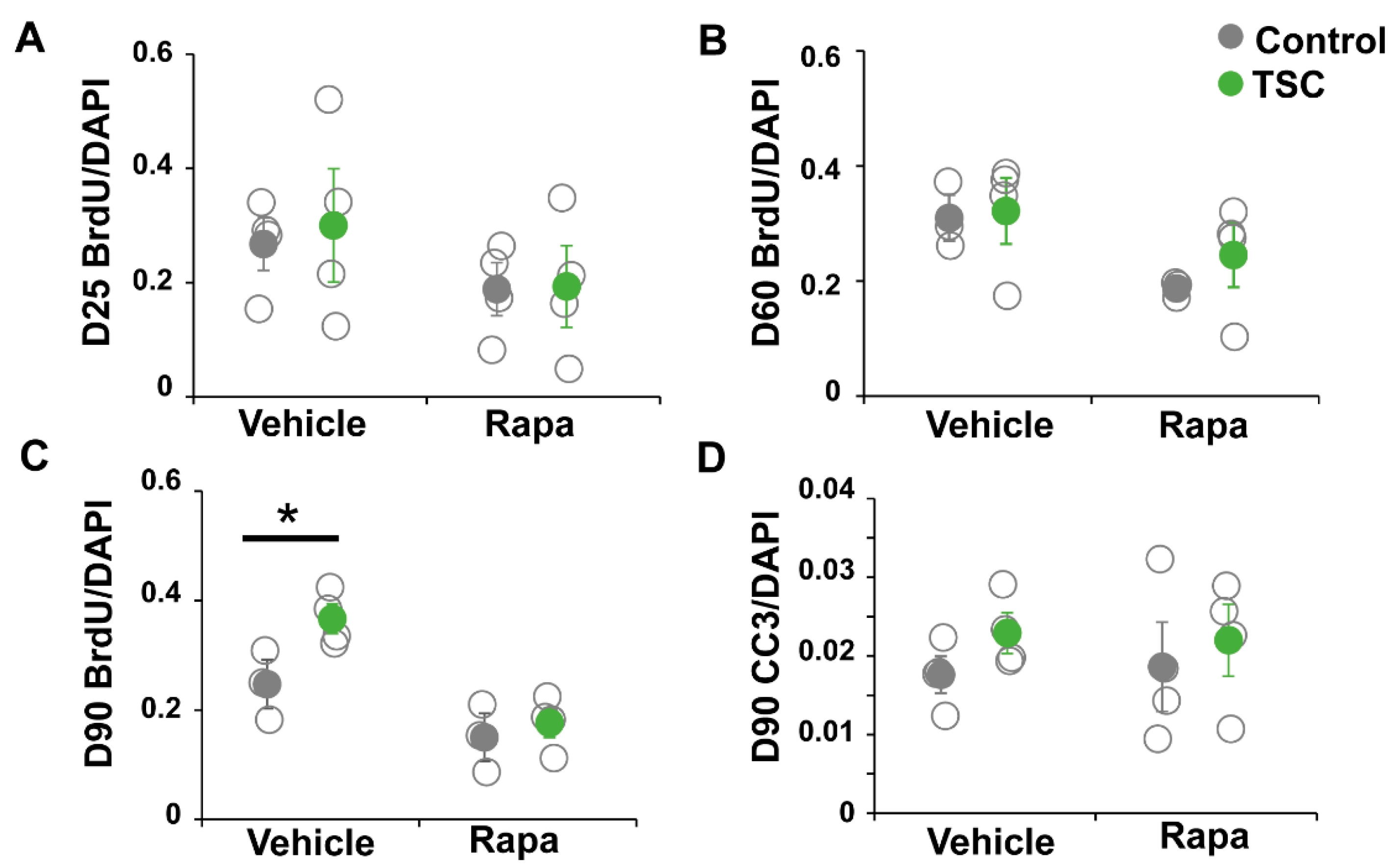
2.8. BrdU Assay
2.9. RNA Isolation
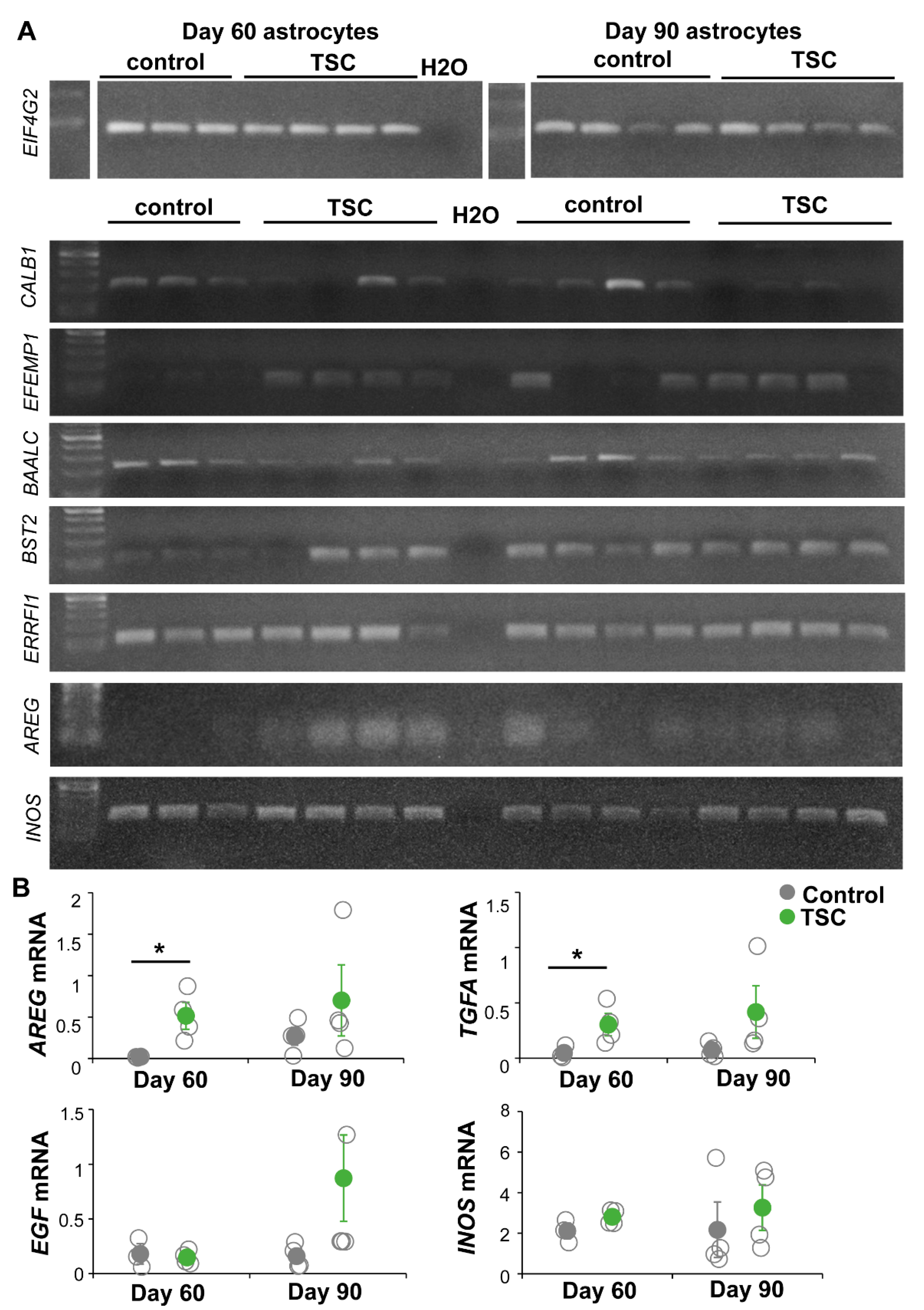
2.10. PCR
2.11. Quantitative PCR
2.12. RNA Sequencing
2.13. Analysis
3. Results
3.1. TSC Astrocytes Show Increased Proliferation
3.2. RNA Sequencing Analysis Revealed Changes in Signaling Pathways and Secreted Factors
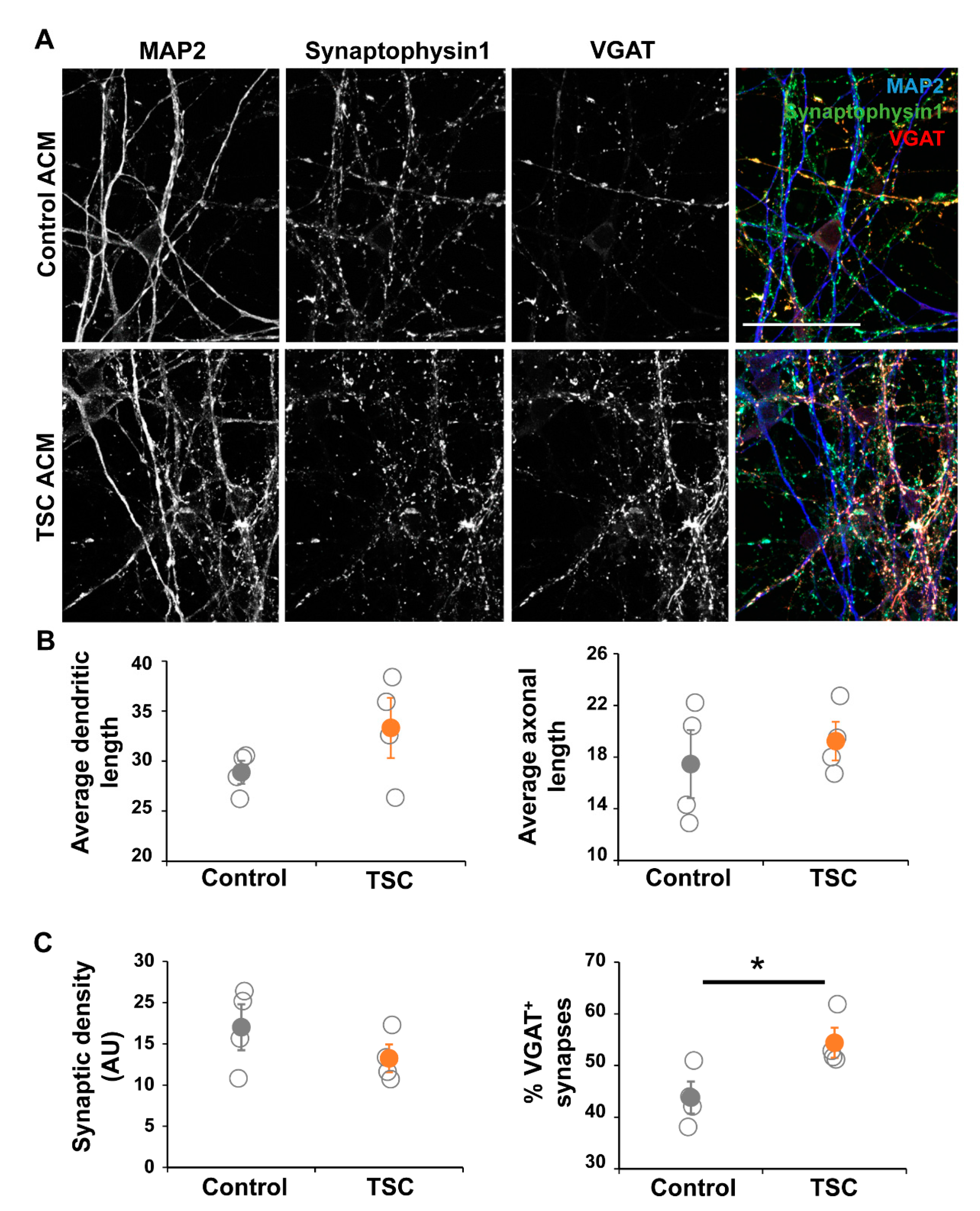
3.3. TSC Astrocytes Affect Neuronal Development
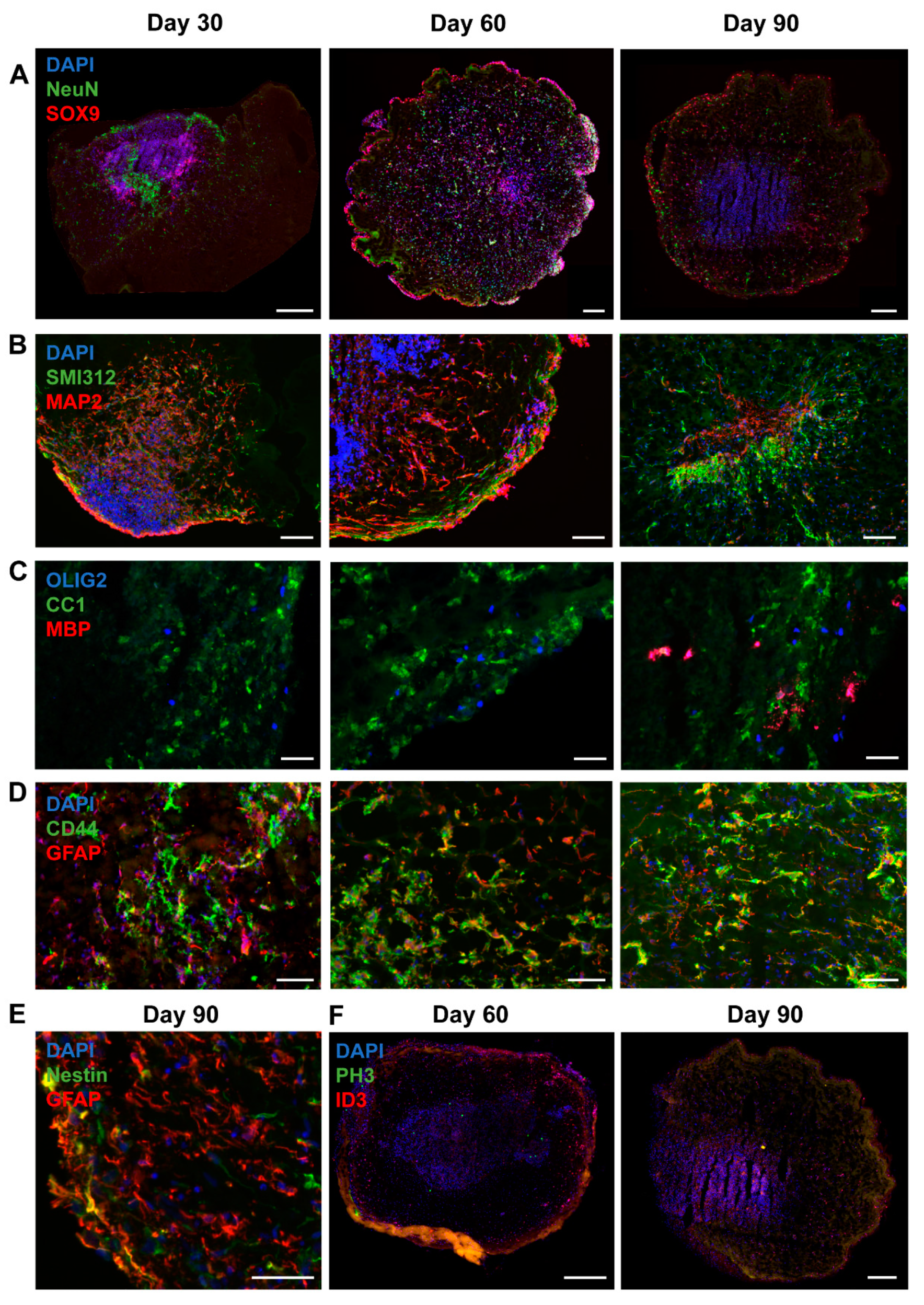
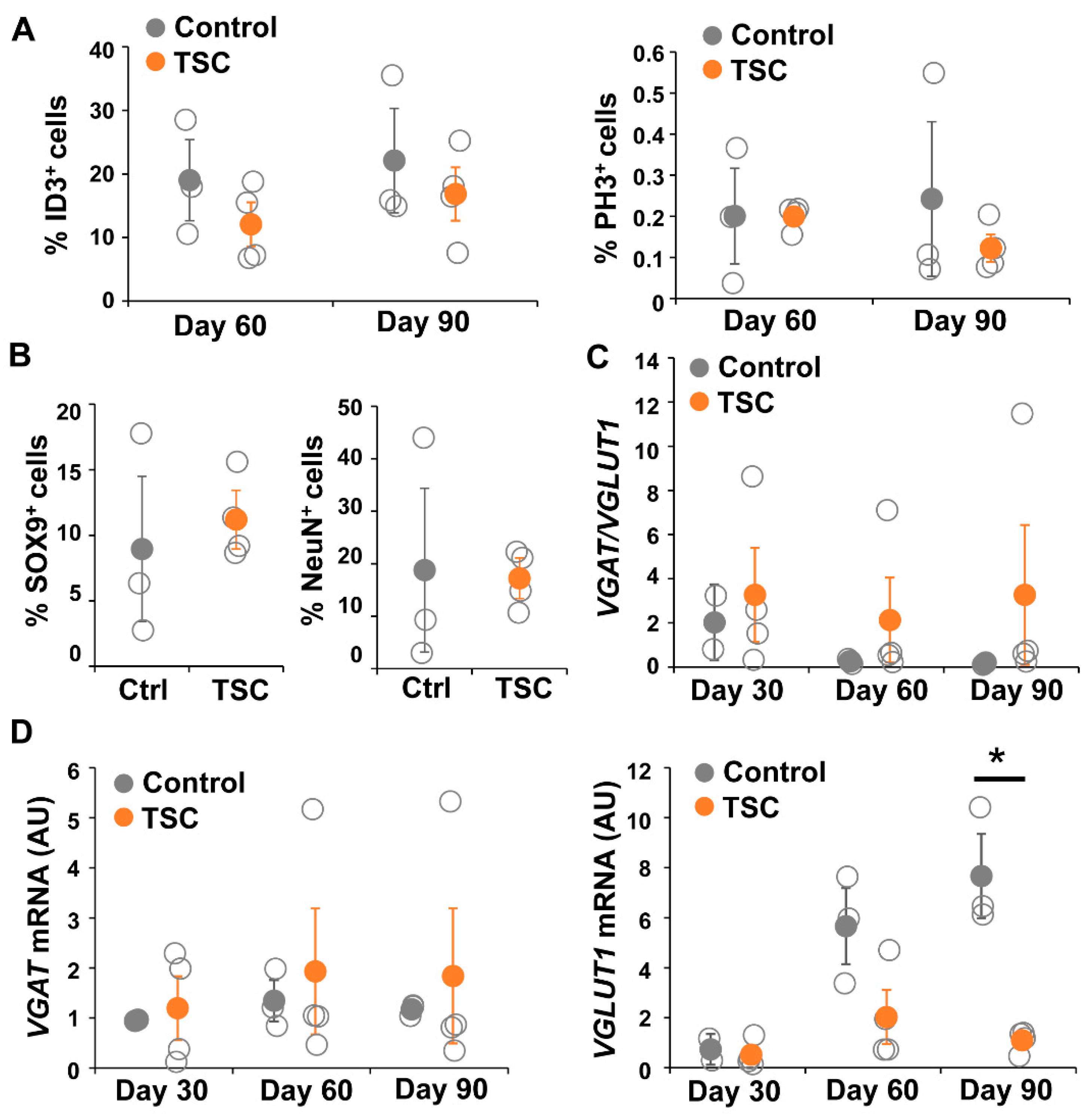
3.4. Organoids Confirm Alterations in Synaptic Balance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Curatolo, P.; Moavero, R.; De Vries, P.J. Neurological and neuropsychiatric aspects of tuberous sclerosis complex. Lancet Neurol. 2015, 14, 733–745. [Google Scholar] [CrossRef]
- Crino, P.B. The mTOR signalling cascade: Paving new roads to cure neurological disease. Nat. Rev. Neurol. 2016, 12, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Boer, K.; Troost, D.; Jansen, F.; Nellist, M.; Ouweland, A.M.V.D.; Geurts, J.J.; Spliet, W.G.; Crino, P.; Aronica, E. Clinicopathological and immunohistochemical findings in an autopsy case of tuberous sclerosis complex. Neuropathology 2008, 28, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Scholl, T.; Mühlebner, A.; Ricken, G.; Mühlebner, A.; Fabing, A.; Samueli, S.; Gröppel, G.; Dorfer, C.; Czech, T.; Hainfellner, J.A.; et al. Impaired oligodendroglial turnover is associated with myelin pathology in focal cortical dysplasia and tuberous sclerosis complex. Brain Pathol. 2017, 27, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Prohl, A.K.; Scherrer, B.; Tomas-Fernandez, X.; Davis, P.E.; Filip-Dhima, R.; Prabhu, S.P.; Peters, J.M.; Bebin, E.M.; Krueger, D.A.; On behalf of the TACERN Study Group; et al. Early white matter development is abnormal in tuberous sclerosis complex patients who develop autism spectrum disorder. J. Neurodev. Disord. 2019, 11, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Clement, E.V.D.P.; Jansen, F.E.; Braun, K.P.J.; Peters, J.M. Update on Drug Management of Refractory Epilepsy in Tuberous Sclerosis Complex. Pediatr. Drugs 2020, 22, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Krueger, D.A.; Sadhwani, A.; Byars, A.W.; De Vries, P.J.; Franz, D.N.; Whittemore, V.H.; Filip-Dhima, R.; Murray, D.; Kapur, K.; Sahin, M. Everolimus for treatment of tuberous sclerosis complex-associated neuropsychiatric disorders. Ann. Clin. Transl. Neurol. 2017, 4, 877–887. [Google Scholar] [CrossRef]
- Overwater, I.E.; Rietman, A.B.; Mous, S.E.; Heus, K.G.C.B.B.; Rizopoulos, D.; Hoopen, L.W.T.; Van Der Vaart, T.; Jansen, F.; Elgersma, Y.; Moll, H.A.; et al. A randomized controlled trial with everolimus for IQ and autism in tuberous sclerosis complex. Neurology 2019, 93, e200–e209. [Google Scholar] [CrossRef] [Green Version]
- Bateup, H.S.; Johnson, C.A.; Denefrio, C.L.; Saulnier, J.L.; Kornacker, K.; Sabatini, B.L. Excitatory/Inhibitory Synaptic Imbalance Leads to Hippocampal Hyperexcitability in Mouse Models of Tuberous Sclerosis. Neuron 2013, 78, 510–522. [Google Scholar] [CrossRef] [Green Version]
- Feliciano, D.M.; Lin, T.V.; Hartman, N.W.; Bartley, C.M.; Kubera, C.; Hsieh, L.S.; Lafourcade, C.; O’Keefe, R.A.; Bordey, A. A circuitry and biochemical basis for tuberous sclerosis symptoms: From epilepsy to neurocognitive deficits. Int. J. Dev. Neurosci. 2013, 31, 667–678. [Google Scholar] [CrossRef] [Green Version]
- Bozzi, Y.; Provenzano, G.; Casarosa, S. Neurobiological bases of autism-epilepsy comorbidity: A focus on excitation/inhibition imbalance. Eur. J. Neurosci. 2018, 47, 534–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.; Lee, J.; Kim, E. Excitation/inhibition imbalance in animal models of autism spectrum disorders. Biol. Psychiatry 2017, 81, 838–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubenstein, J.L.R.; Merzenich, M.M. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003, 2, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Nadadhur, A.G.; Alsaqati, M.; Gasparotto, L.; Cornelissen-Steijger, P.; Van Hugte, E.; Dooves, S.; Harwood, A.J.; Heine, V.M. Neuron-Glia Interactions Increase Neuronal Phenotypes in Tuberous Sclerosis Complex Patient iPSC-Derived Models. Stem Cell Rep. 2019, 12, 42–56. [Google Scholar] [CrossRef] [Green Version]
- Alsaqati, M.; Heine, V.M.; Harwood, A.J. Pharmacological intervention to restore connectivity deficits of neuronal networks derived from ASD patient iPSC with a TSC2 mutation. Mol. Autism 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Zhao, J.-P.; Yoshii, A. Hyperexcitability of the local cortical circuit in mouse models of tuberous sclerosis complex. Mol. Brain 2019, 12, 1–13. [Google Scholar] [CrossRef]
- Haji, N.; Riebe, I.; Aguilar-Valles, A.; Artinian, J.; Laplante, I.; Lacaille, J.-C. Tsc1 haploinsufficiency in Nkx2.1 cells upregulates hippocampal interneuron mTORC1 activity, impairs pyramidal cell synaptic inhibition, and alters contextual fear discrimination and spatial working memory in mice. Mol. Autism 2020, 11, 29. [Google Scholar] [CrossRef]
- Ransom, B.R.; Behar, T.; Nedergaard, M. New roles for astrocytes (stars at last). Trends Neurosci. 2003, 26, 520–522. [Google Scholar] [CrossRef]
- Wong, M.; Ess, K.C.; Uhlmann, E.J.; Jansen, L.A.; Li, W.; Crino, P.B.; Mennerick, S.; Yamada, K.A.; Gutmann, D.H. Impaired glial glutamate transport in a mouse tuberous sclerosis epilepsy model. Ann. Neurol. 2003, 54, 251–256. [Google Scholar] [CrossRef]
- Zeng, L.-H.; Ouyang, Y.; Gazit, V.; Cirrito, J.R.; Jansen, L.A.; Ess, K.C.; Yamada, K.A.; Wozniak, D.F.; Holtzman, D.M.; Gutmann, D.H.; et al. Abnormal glutamate homeostasis and impaired synaptic plasticity and learning in a mouse model of tuberous sclerosis complex. Neurobiol. Dis. 2007, 28, 184–196. [Google Scholar] [CrossRef] [Green Version]
- Jansen, L.A.; Uhlmann, E.J.; Crino, P.B.; Gutmann, D.H.; Wong, M. Epileptogenesis and Reduced Inward Rectifier Potassium Current in Tuberous Sclerosis Complex-1-Deficient Astrocytes. Epilepsia 2005, 46, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zeng, L.-H.; Wong, M. Impaired astrocytic gap junction coupling and potassium buffering in a mouse model of tuberous sclerosis complex. Neurobiol. Dis. 2009, 34, 291–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Short, B.; Kozek, L.; Harmsen, H.; Zhang, B.; Wong, M.; Ess, K.C.; Fu, C.; Naftel, R.; Pearson, M.M.; Carson, R.P. Cerebral aquaporin-4 expression is independent of seizures in tuberous sclerosis complex. Neurobiol. Dis. 2019, 129, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Oberheim, N.A.; Wang, X.; Goldman, S.; Nedergaard, M. Astrocytic complexity distinguishes the human brain. Trends Neurosci. 2006, 29, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Warlich, E.; Kuehle, J.; Cantz, T.; Brugman, M.H.; Maetzig, T.; Galla, M.; Filipczyk, A.; Halle, S.; Klump, H.; Schöler, H.R.; et al. Lentiviral Vector Design and Imaging Approaches to Visualize the Early Stages of Cellular Reprogramming. Mol. Ther. 2011, 19, 782–789. [Google Scholar] [CrossRef]
- Leferink, P.S.; Dooves, S.; Hillen, A.E.J.; Watanabe, K.; Jacobs, G.; Gasparotto, L.; Bsc, P.C.; Van Der Knaap, M.S.; Heine, V.M. Astrocyte Subtype Vulnerability in Stem Cell Models of Vanishing White Matter. Ann. Neurol. 2019, 86, 780–792. [Google Scholar] [CrossRef] [Green Version]
- Nadadhur, A.G.; Leferink, P.S.; Holmes, D.; Hinz, L.; Cornelissen-Steijger, P.; Gasparotto, L.; Heine, V.M. Patterning factors during neural progenitor induction determine regional identity and differentiation potential in vitro. Stem Cell Res. 2018, 32, 25–34. [Google Scholar] [CrossRef]
- Nadadhur, A.G.; Melero, J.E.; Meijer, M.; Schut, D.; Jacobs, G.; Li, K.W.; Hjorth, J.J.J.; Meredith, R.M.; Toonen, R.F.; Van Kesteren, R.E.; et al. Multi-level characterization of balanced inhibitory-excitatory cortical neuron network derived from human pluripotent stem cells. PLoS ONE 2017, 12, e0178533. [Google Scholar] [CrossRef] [Green Version]
- Monzel, A.S.; Smits, L.M.; Hemmer, K.; Hachi, S.; Moreno, E.L.; Van Wuellen, T.; Jarazo, J.; Walter, J.; Brüggemann, I.; Boussaad, I.; et al. Derivation of Human Midbrain-Specific Organoids from Neuroepithelial Stem Cells. Stem Cell Rep. 2017, 8, 1144–1154. [Google Scholar] [CrossRef]
- Blair, J.D.; Hockemeyer, D.; Bateup, H.S. Genetically engineered human cortical spheroid models of tuberous sclerosis. Nat. Med. 2018, 24, 1568–1578. [Google Scholar] [CrossRef]
- Wong, R.W.C.; Guillaud, L. The role of epidermal growth factor and its receptors in mammalian CNS. Cytokine Growth Factor Rev. 2004, 15, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, T.; Nakamura, K.; Yamada, K.; Thanseem, I.; Anitha, A.; Suda, S.; Tsujii, M.; Iwayama, Y.; Hattori, E.; Toyota, T.; et al. SNP analyses of growth factor genes EGF, TGFβ-1, and HGF reveal haplotypic association of EGF with autism. Biochem. Biophys. Res. Commun. 2007, 360, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Soueid, J.; Kourtian, S.; Makhoul, N.J.; Makoukji, J.; Haddad, S.; Ghanem, S.S.; Kobeissy, F.H.; Boustany, R.-M. RYR2, PTDSS1 and AREG genes are implicated in a Lebanese population-based study of copy number variation in autism. Sci. Rep. 2016, 6, srep19088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iseri, E.; Guney, E.; Ceylan, M.F.; Yücel, A.; Aral, A.; Bodur, S.; Sener, S. Increased Serum Levels of Epidermal Growth Factor in Children with Autism. J. Autism Dev. Disord. 2010, 41, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Lesma, E.; Ancona, S.; Sirchia, S.M.; Orpianesi, E.; Grande, V.; Colapietro, P.; Chiaramonte, E.; Di Giulio, A.M.; Gorio, A. TSC 2 epigenetic defect in primary LAM cells. Evidence of an anchorage-independent survival. J. Cell. Mol. Med. 2014, 18, 766–779. [Google Scholar] [CrossRef]
- Lesma, E.; Chiaramonte, E.; Ancona, S.; Orpianesi, E.; Di Giulio, A.M.; Gorio, A. Anti-EGFR Antibody Reduces Lung Nodules by Inhibition of EGFR-Pathway in a Model of Lymphangioleiomyomatosis. BioMed Res. Int. 2015, 2015, 1–14. [Google Scholar] [CrossRef]
- Lesma, E.; Grande, V.; Carelli, S.; Brancaccio, D.; Canevini, M.P.; Alfano, R.M.; Coggi, G.; Di Giulio, A.M.; Gorio, A. Isolation and Growth of Smooth Muscle-Like Cells Derived from Tuberous Sclerosis Complex-2 Human Renal Angiomyolipoma. Am. J. Pathol. 2005, 167, 1093–1103. [Google Scholar] [CrossRef]
- Parker, W.E.; Orlova, K.A.; Heuer, G.G.; Baybis, M.; Aronica, E.; Frost, M.; Wong, M.; Crino, P.B. Enhanced Epidermal Growth Factor, Hepatocyte Growth Factor, and Vascular Endothelial Growth Factor Expression in Tuberous Sclerosis Complex. Am. J. Pathol. 2011, 178, 296–305. [Google Scholar] [CrossRef]
- Eichmüller, O.L.; Corsini, N.S.; Vértesy, Á.; Scholl, T.; Gruber, V.-E.; Peer, A.M.; Chu, J.; Novatchkova, M.; Paredes, M.F.; Feucht, M.; et al. Cerebral organoid model reveals excessive proliferation of human caudal late interneuron progenitors in Tuberous Sclerosis Complex. bioRxiv 2020. [Google Scholar] [CrossRef]
- Mori, K.; Mori, T.; Toda, Y.; Fujii, E.; Miyazaki, M.; Harada, M.; Kagami, S. Decreased benzodiazepine receptor and increased GABA level in cortical tubers in tuberous sclerosis complex. Brain Dev. 2012, 34, 478–486. [Google Scholar] [CrossRef]
- Zucco, A.J.; Pozzo, V.D.; Afinogenova, A.; Hart, R.P.; Devinsky, O.; D’Arcangelo, G. Neural progenitors derived from Tuberous Sclerosis Complex patients exhibit attenuated PI3K/AKT signaling and delayed neuronal differentiation. Mol. Cell. Neurosci. 2018, 92, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cao, J.; Chen, M.; Li, J.; Sun, Y.; Zhang, Y.; Zhu, Y.; Wang, L.; Zhang, C. Abnormal Neural Progenitor Cells Differentiated from Induced Pluripotent Stem Cells Partially Mimicked Development of TSC2 Neurological Abnormalities. Stem Cell Rep. 2017, 8, 883–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winden, K.D.; Sundberg, M.; Yang, C.; Wafa, S.M.; Dwyer, S.; Chen, P.-F.; Buttermore, E.D.; Sahin, M. Biallelic Mutations in TSC2 Lead to Abnormalities Associated with Cortical Tubers in Human iPSC-Derived Neurons. J. Neurosci. 2019, 39, 9294–9305. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari, Y. NKCC1 Chloride Importer Antagonists Attenuate Many Neurological and Psychiatric Disorders. Trends Neurosci. 2017, 40, 536–554. [Google Scholar] [CrossRef]
- Talos, D.M.; Sun, H.; Kosaras, B.; Joseph, A.; Folkerth, R.D.; Poduri, A.; Madsen, J.R.; Black, P.M.; Jensen, F.E.; Black, P.M. Altered inhibition in tuberous sclerosis and type IIb cortical dysplasia. Ann. Neurol. 2011, 71, 539–551. [Google Scholar] [CrossRef] [Green Version]
- Ruffolo, G.; Iyer, A.; Cifelli, P.; Roseti, C.; Mühlebner, A.; Van Scheppingen, J.; Scholl, T.; Hainfellner, J.A.; Feucht, M.; Krsek, P.; et al. Functional aspects of early brain development are preserved in tuberous sclerosis complex (TSC) epileptogenic lesions. Neurobiol. Dis. 2016, 95, 93–101. [Google Scholar] [CrossRef]
- Van Andel, D.M.; Sprengers, J.J.; Oranje, B.; Scheepers, F.E.; Jansen, F.E.; Bruining, H. Effects of bumetanide on neurodevelopmental impairments in patients with tuberous sclerosis complex: An open-label pilot study. Mol. Autism 2020, 11, 1–14. [Google Scholar] [CrossRef]

| Line # | Genotype | Gender | Age | Source |
|---|---|---|---|---|
| 88 | Control | M | 74 d | Amsterdam UMC, Amsterdam, The Netherlands |
| 228 | Control | F | 19 y | Amsterdam UMC, Amsterdam, The Netherlands |
| 233 | TSC1 | M | 17 y | Coriell Institute, Camden, NJ, USA; GM06149 |
| 401 | TSC1 | M | 23 y | Coriell Institute, Camden, NJ, USA; GM02332 |
| 417 | TSC2 | F | 16 y | Coriell Institute, Camden, NJ, USA; GM03958 |
| 420 | Control | M | 21 y | Coriell Institute, Camden, NJ, USA; GM23964 |
| 421 | Control | M | 19 y | Coriell Institute, Camden, NJ, USA; GM23973 |
| 424 | TSC2 | F | 26 y | Coriell Institute, Camden, NJ, USA; GM06102 |
| Target | Host | Dilution | Company | Number |
|---|---|---|---|---|
| BrdU | Rabbit | 1:500 | GeneTex | GTX28091 |
| CC1 | Mouse | 1:1000 | Millipore | OP80 |
| Cleaved Caspase 3 | Rabbit | 1:400 | Cell Signaling | 9661 |
| CD44 | Mouse | 1:100 | Hybridomabank | H4C4 |
| GFAP | Rabbit | 1:1000 | DAKO | Z0334 |
| ID3 | Rabbit | 1:250 | Cell Signaling | 9837 |
| MAP2 | Chicken | 1:5000 | Millipore | AB5543 |
| MBP | Rat | 1:500 | Abcam | Ab7349 |
| Nestin | Mouse | 1:1000 | BD Bioscience | 611658 |
| NeuN | Mouse | 1:500 | Millipore | MAB377 |
| OLIG2 | Rabbit | 1:500 | Millipore | AB9610 |
| PH3 | Mouse | 1:1000 | Cell Signaling | 9706 |
| SOX9 | Rabbit | 1:500 | Cell Signaling | 82630 |
| S100B | Rabbit | 1:1000 | Protein Tech | 15146-AP |
| MAP2 | Chicken | 1:5000 | Millipore | AB5543 |
| SMI312 | Mouse | 1:1000 | Eurogentech | SMI-312P-050 |
| VGAT | Rabbit | 1:500 | Sysy | 131-002 |
| Synaptophysin 1 | Guinea Pig | 1:1000 | Sysy | 101-004 |
| Target | Forward Primer | Reverse Primer |
|---|---|---|
| AREG | GATACTCGGCTCAGGCCATT | ATGGTTCACGCTTCCCAGAG |
| BAALC | TGCACTCGGGCTAAAAGAGA | AATTCAGGTCCAGCAAGGGG |
| BST2 | GGAAGCTGGCACATCTTGGA | CTAACCGTGTTGCCCCATGA |
| CALB1 | GGAAGCATGCCCAAGTGGTATTA | AGCCTTCTTTCGCGCCTGCT |
| EFEMP1 | CTCTGCTAGCTCAAGATTCACA | CAGTGCATTGCGTGTACGTG |
| EGF | TCTACTTGTGTGGGTCCTGC | ATCACTGAGACACCAGCATCC |
| EIF4G2 | AGGACCGCATGTTGGAGATT | TGAGGGGATGGATCCAACTTT |
| ERRFI1 | TGGAGCAGTCGCAGTGAGTTTA | GGAAGCATGCCCAAGTGGTATTA |
| INOS | CGTGGAGACGGGAAAGAAGT | GACCCCAGGCAAGATTTGGA |
| NEUN | TGGCATGACCCTGTACACAC | GCTGCTGCTTCTCTGTAGGG |
| TGFA | TGCCATTCTGGGTACGTTGG | GGACCTGGCAGCAGTGTATC |
| VGAT | GGACTCGTACGTGGCCATAG | AGCTCGATGATCTGCGCTAC |
| VGLUT1 | TTCTGGCTGCTCGTCTCCTA | GGTTCATGAGTTTCGCGCTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dooves, S.; van Velthoven, A.J.H.; Suciati, L.G.; Heine, V.M. Neuron–Glia Interactions in Tuberous Sclerosis Complex Affect the Synaptic Balance in 2D and Organoid Cultures. Cells 2021, 10, 134. https://doi.org/10.3390/cells10010134
Dooves S, van Velthoven AJH, Suciati LG, Heine VM. Neuron–Glia Interactions in Tuberous Sclerosis Complex Affect the Synaptic Balance in 2D and Organoid Cultures. Cells. 2021; 10(1):134. https://doi.org/10.3390/cells10010134
Chicago/Turabian StyleDooves, Stephanie, Arianne J. H. van Velthoven, Linda G. Suciati, and Vivi M. Heine. 2021. "Neuron–Glia Interactions in Tuberous Sclerosis Complex Affect the Synaptic Balance in 2D and Organoid Cultures" Cells 10, no. 1: 134. https://doi.org/10.3390/cells10010134
APA StyleDooves, S., van Velthoven, A. J. H., Suciati, L. G., & Heine, V. M. (2021). Neuron–Glia Interactions in Tuberous Sclerosis Complex Affect the Synaptic Balance in 2D and Organoid Cultures. Cells, 10(1), 134. https://doi.org/10.3390/cells10010134