Role of Synovial Exosomes in Osteoclast Differentiation in Inflammatory Arthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Human Synovial Fluid
2.2. Exosome Characterization
2.2.1. Exosome Isolation
2.2.2. Transmission Electron Microscopy (TEM)
2.2.3. Size Distribution
2.2.4. Exosome Quantification
2.2.5. Exosome Labeling and Uptake
2.2.6. RANKL-ELISA
2.2.7. Flow Cytometry
2.3. Exosome and Osteoclastogenesis
2.3.1. In Vitro Exosome Functional Assays
2.3.2. Cell Proliferation Assay
2.3.3. TRAP Staining and Activity
2.3.4. Evaluation of Actin Ring Formation
2.4. Statistical Analysis
3. Results
3.1. Characterization of Synovial Exosomes in Inflammatory Arthritis
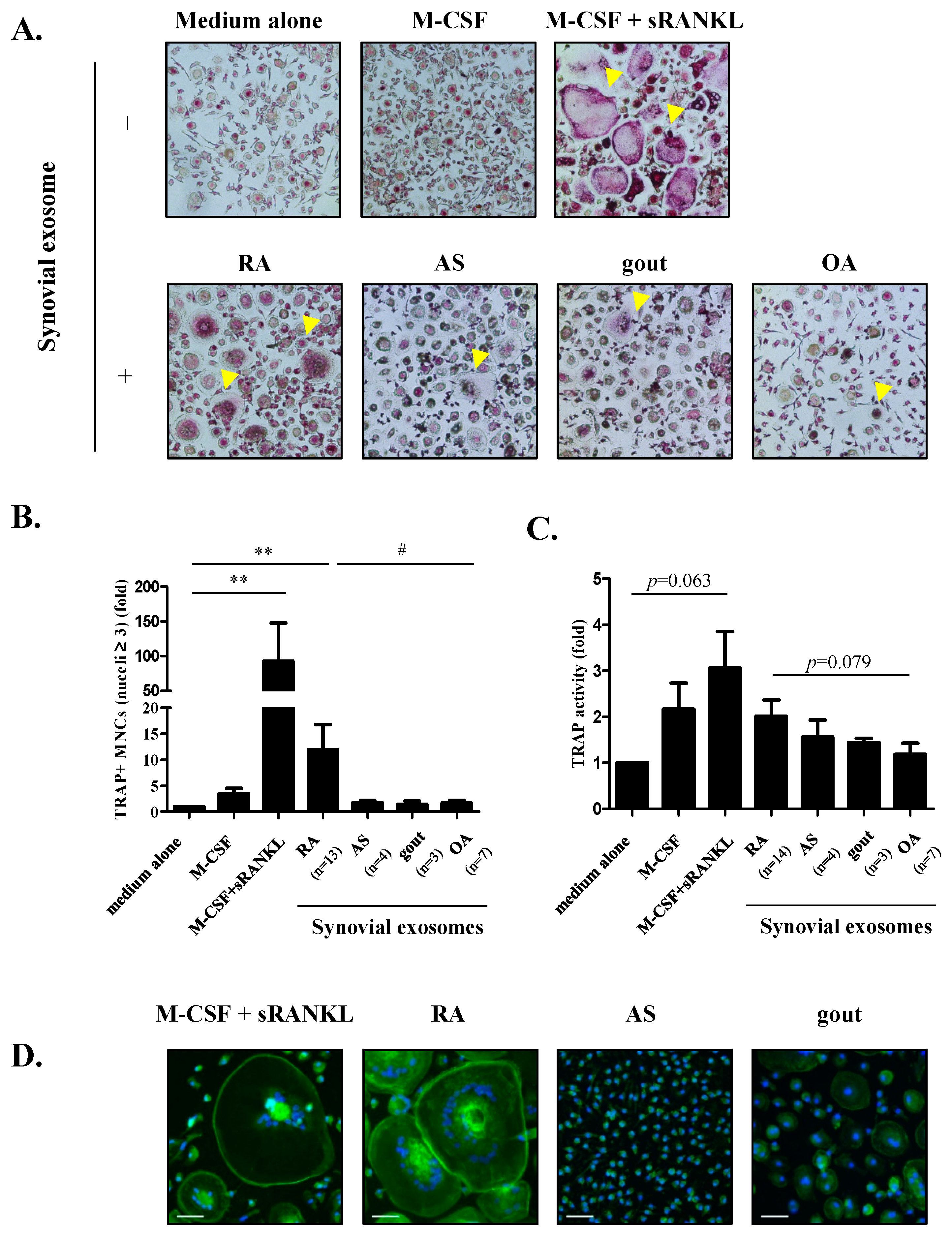
3.2. The Role of Exosomes on Osteoclastogenesis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radner, H.; Ramiro, S.; Buchbinder, R.; Landewe, R.B.; van der Heijde, D.; Aletaha, D. Pain management for inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and other spondylarthritis) and gastrointestinal or liver comorbidity. Cochrane Database Syst. Rev. 2012, 1, CD008951. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, M.; Brennan, F.M.; Maini, R.N. Role of cytokines in rheumatoid arthritis. Annu. Rev. Immunol. 1996, 14, 397–440. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, N.; Sato, K.; Goto, M.; Sasano, M.; Natsuyama, M.; Inoue, K.; Nishioka, K. Augmented interleukin-1 production and HLA-DR expression in the synovium of rheumatoid arthritis patients. Possible involvement in joint destruction. Arthritis Rheum. 1988, 31, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Goldring, S.R.; Purdue, P.E.; Crotti, T.N.; Shen, Z.; Flannery, M.R.; Binder, N.B.; Ross, F.P.; McHugh, K.P. Bone remodelling in inflammatory arthritis. Ann. Rheum. Dis. 2013, 72 (Suppl. 2), ii52–ii55. [Google Scholar] [CrossRef] [PubMed]
- Schett, G. Joint remodelling in inflammatory disease. Ann. Rheum. Dis. 2007, 66 (Suppl. 3), iii42–iii44. [Google Scholar] [CrossRef]
- Appel, H.; Loddenkemper, C.; Miossec, P. Rheumatoid arthritis and ankylosing spondylitis—pathology of acute inflammation. Clin. Exp. Rheumatol. 2009, 27, S15–S19. [Google Scholar] [PubMed]
- McQueen, F.M.; Doyle, A.; Reeves, Q.; Gao, A.; Tsai, A.; Gamble, G.D.; Curteis, B.; Williams, M.; Dalbeth, N. Bone erosions in patients with chronic gouty arthropathy are associated with tophi but not bone oedema or synovitis: New insights from a 3 T MRI study. Rheumatology (Oxford) 2014, 53, 95–103. [Google Scholar] [CrossRef]
- Nakashima, T.; Takayanagi, H. Osteoimmunology: Crosstalk between the immune and bone systems. J. Clin. Immunol. 2009, 29, 555–567. [Google Scholar] [CrossRef]
- Azuma, Y.; Kaji, K.; Katogi, R.; Takeshita, S.; Kudo, A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J. Biol. Chem. 2000, 275, 4858–4864. [Google Scholar] [CrossRef]
- Pfeilschifter, J.; Chenu, C.; Bird, A.; Mundy, G.R.; Roodman, G.D. Interleukin-1 and tumor necrosis factor stimulate the formation of human osteoclastlike cells in vitro. J. Bone Miner. Res. 1989, 4, 113–118. [Google Scholar] [CrossRef]
- Mabilleau, G.; Sabokbar, A. Interleukin-32 promotes osteoclast differentiation but not osteoclast activation. PLoS ONE 2009, 4, e4173. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.H.; Ko, N.Y.; Kim, H.S.; Kim, J.W.; Kim, D.K.; Kim, A.R.; Lee, S.H.; Kim, Y.G.; Lee, C.K.; Lee, S.H.; et al. Interleukin-33 stimulates formation of functional osteoclasts from human CD14(+) monocytes. Cell. Mol. Life Sci. 2010, 67, 3883–3892. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Saa, P.; Yakovleva, O.; de Castro, J.; Vasilyeva, I.; De Paoli, S.H.; Simak, J.; Cervenakova, L. First demonstration of transmissible spongiform encephalopathy-associated prion protein (PrPTSE) in extracellular vesicles from plasma of mice infected with mouse-adapted variant Creutzfeldt-Jakob disease by in vitro amplification. J. Biol. Chem. 2014, 289, 29247–29260. [Google Scholar] [CrossRef] [PubMed]
- Hannafon, B.N.; Ding, W.Q. Intercellular communication by exosome-derived microRNAs in cancer. Int. J. Mol. Sci. 2013, 14, 14240–14269. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Xu, Q. Functions and application of exosomes. Acta Pol. Pharm. 2014, 71, 537–543. [Google Scholar]
- Distler, J.H.; Pisetsky, D.S.; Huber, L.C.; Kalden, J.R.; Gay, S.; Distler, O. Microparticles as regulators of inflammation: Novel players of cellular crosstalk in the rheumatic diseases. Arthritis Rheum. 2005, 52, 3337–3348. [Google Scholar] [CrossRef]
- Song, J.; Kim, D.; Han, J.; Kim, Y.; Lee, M.; Jin, E.J. PBMC and exosome-derived Hotair is a critical regulator and potent marker for rheumatoid arthritis. Clin. Exp. Med. 2015, 15, 121–126. [Google Scholar] [CrossRef]
- György, B.; Szabo, T.G.; Turiak, L.; Wright, M.; Herczeg, P.; Ledeczi, Z.; Kittel, A.; Polgar, A.; Toth, K.; Derfalvi, B.; et al. Im-proved flow cytometric assessment reveals distinct microvesicle (cell-derived microparticle) signatures in joint diseases. PLoS ONE 2012, 7, e49726. [Google Scholar] [CrossRef]
- Raimondi, L.; De Luca, A.; Amodio, N.; Manno, M.; Raccosta, S.; Taverna, S.; Bellavia, D.; Naselli, F.; Fontana, S.; Schillaci, O.; et al. Involvement of multiple myeloma cell-derived exosomes in osteoclast differentiation. Oncotarget 2015, 6, 13772–13789. [Google Scholar] [CrossRef]
- Taverna, S.; Pucci, M.; Giallombardo, M.; Di Bella, M.A.; Santarpia, M.; Reclusa, P.; Gil-Bazo, I.; Rolfo, C.; Alessandro, R. Am-phiregulin contained in NSCLC-exosomes induces osteoclast differentiation through the activation of EGFR pathway. Sci. Rep. 2017, 7, 3170. [Google Scholar] [CrossRef] [PubMed]
- Adamopoulos, I.E.; Danks, L.; Itonaga, I.; Locklin, R.M.; Sabokbar, A.; Ferguson, D.J.; Athanasou, N.A. Stimulation of osteoclast formation by inflammatory synovial fluid. Virchows Arch. 2006, 449, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.E.; Kunkel, S.L.; Burrows, J.C.; Evanoff, H.L.; Haines, G.K.; Pope, R.M.; Strieter, R.M. Synovial tissue macrophage as a source of the chemotactic cytokine IL-8. J. Immunol. 1991, 147, 2187–2195. [Google Scholar] [PubMed]
- Lettesjö, H.; Nordstrom, E.; Strom, H.; Nilsson, B.; Glinghammar, B.; Dahlstedt, L.; Moller, E. Synovial fluid cytokines in patients with rheumatoid arthritis or other arthritic lesions. Scand. J. Immunol. 1998, 48, 286–292. [Google Scholar]
- Yarilina, A.; Xu, K.; Chen, J.; Ivashkiv, L.B. TNF activates calcium-nuclear factor of activated T cells (NFAT)c1 signaling pathways in human macrophages. Proc. Natl. Acad. Sci. USA 2011, 108, 1573–1578. [Google Scholar] [CrossRef]
- Fuller, K.; Murphy, C.; Kirstein, B.; Fox, S.W.; Chambers, T.J. TNFalpha potently activates osteoclasts, through a direct action independent of and strongly synergistic with RANKL. Endocrinology 2002, 143, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Kitaura, H.; Zhou, P.; Ross, F.P.; Teitelbaum, S.L. IL-1 mediates TNF-induced osteoclastogenesis. J. Clin. Invest 2005, 115, 282–290. [Google Scholar] [CrossRef]
- Tanabe, N.; Maeno, M.; Suzuki, N.; Fujisaki, K.; Tanaka, H.; Ogiso, B.; Ito, K. IL-1 alpha stimulates the formation of osteoclast-like cells by increasing M-CSF and PGE2 production and decreasing OPG production by osteoblasts. Life Sci. 2005, 77, 615–626. [Google Scholar] [CrossRef]
- Liu, X.H.; Kirschenbaum, A.; Yao, S.; Levine, A.C. The role of the interleukin-6/gp130 signaling pathway in bone metabolism. Vitam. Horm. 2006, 74, 341–355. [Google Scholar] [CrossRef]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; McShane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S.; et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988, 31, 315–324. [Google Scholar] [CrossRef]
- Savina, A.; Vidal, M.; Colombo, M.I. The exosome pathway in K562 cells is regulated by Rab11. J. Cell Sci. 2002, 115, 2505–2515. [Google Scholar] [PubMed]
- Nguyen, D.G.; Booth, A.; Gould, S.J.; Hildreth, J.E. Evidence that HIV budding in primary macrophages occurs through the exosome release pathway. J. Biol. Chem. 2003, 278, 52347–52354. [Google Scholar] [CrossRef] [PubMed]
- Minkin, C. Bone acid phosphatase: Tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcif. Tissue Int. 1982, 34, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Reeve, J.L.; Zou, W.; Liu, Y.; Maltzman, J.S.; Ross, F.P.; Teitelbaum, S.L. SLP-76 couples Syk to the osteoclast cytoskeleton. J. Immunol. 2009, 183, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- Andreu, Z.; Yanez-Mo, M. Tetraspanins in extracellular vesicle formation and function. Front Immunol. 2014, 5, 442. [Google Scholar] [CrossRef] [PubMed]
- Berckmans, R.J.; Nieuwland, R.; Kraan, M.C.; Schaap, M.C.; Pots, D.; Smeets, T.J.; Sturk, A.; Tak, P.P. Synovial microparticles from arthritic patients modulate chemokine and cytokine release by synoviocytes. Arthritis Res. Ther. 2005, 7, R536–R544. [Google Scholar] [CrossRef]
- Messer, L.; Alsaleh, G.; Freyssinet, J.M.; Zobairi, F.; Leray, I.; Gottenberg, J.E.; Sibilia, J.; Toti-Orfanoudakis, F.; Wachsmann, D. Microparticle-induced release of B-lymphocyte regulators by rheumatoid synoviocytes. Arthritis Res. Ther. 2009, 11, R40. [Google Scholar] [CrossRef] [PubMed]
- Knijff-Dutmer, E.A.; Koerts, J.; Nieuwland, R.; Kalsbeek-Batenburg, E.M.; van de Laar, M.A. Elevated levels of platelet micro-particles are associated with disease activity in rheumatoid arthritis. Arthritis Rheum. 2002, 46, 1498–1503. [Google Scholar] [CrossRef]
- Sugatani, T.; Hruska, K.A. Impaired micro-RNA pathways diminish osteoclast differentiation and function. J. Biol. Chem. 2009, 284, 4667–4678. [Google Scholar] [CrossRef]
- Sugatani, T.; Vacher, J.; Hruska, K.A. A microRNA expression signature of osteoclastogenesis. Blood 2011, 117, 3648–3657. [Google Scholar] [CrossRef]
- Mizoguchi, F.; Murakami, Y.; Saito, T.; Miyasaka, N.; Kohsaka, H. miR-31 controls osteoclast formation and bone resorption by targeting RhoA. Arthritis Res. Ther. 2013, 15, R102. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Chen, C.; He, H.B.; Hu, R.; Zhou, H.D.; Xie, H.; Zhu, W.; Dai, R.C.; Wu, X.P.; Liao, E.Y.; et al. miR-148a regulates osteoclastogenesis by targeting V-maf musculoaponeurotic fibrosarcoma oncogene homolog B. J. Bone Miner. Res. 2013, 28, 1180–1190. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.E.; Kim, J.S.; Shin, J.H.; Moon, K.W.; Park, J.K.; Park, K.S.; Lee, E.Y. Role of Synovial Exosomes in Osteoclast Differentiation in Inflammatory Arthritis. Cells 2021, 10, 120. https://doi.org/10.3390/cells10010120
Song JE, Kim JS, Shin JH, Moon KW, Park JK, Park KS, Lee EY. Role of Synovial Exosomes in Osteoclast Differentiation in Inflammatory Arthritis. Cells. 2021; 10(1):120. https://doi.org/10.3390/cells10010120
Chicago/Turabian StyleSong, Ji Eun, Ji Soo Kim, Ji Hye Shin, Ki Won Moon, Jin Kyun Park, Kyong Soo Park, and Eun Young Lee. 2021. "Role of Synovial Exosomes in Osteoclast Differentiation in Inflammatory Arthritis" Cells 10, no. 1: 120. https://doi.org/10.3390/cells10010120
APA StyleSong, J. E., Kim, J. S., Shin, J. H., Moon, K. W., Park, J. K., Park, K. S., & Lee, E. Y. (2021). Role of Synovial Exosomes in Osteoclast Differentiation in Inflammatory Arthritis. Cells, 10(1), 120. https://doi.org/10.3390/cells10010120




