Molecular Remission Using Low-Dose Immunotherapy with Minimal Toxicities for Poor Prognosis IGHV—Unmutated Chronic Lymphocytic Leukemia
Abstract
1. Introduction
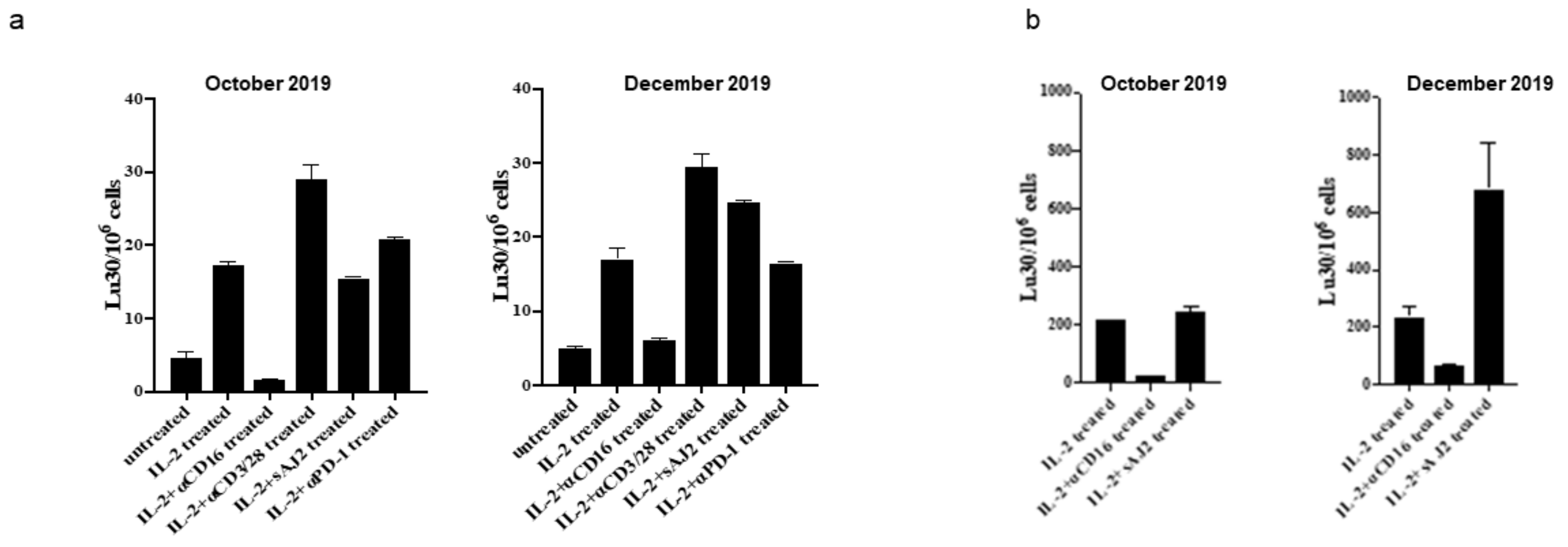
2. Results
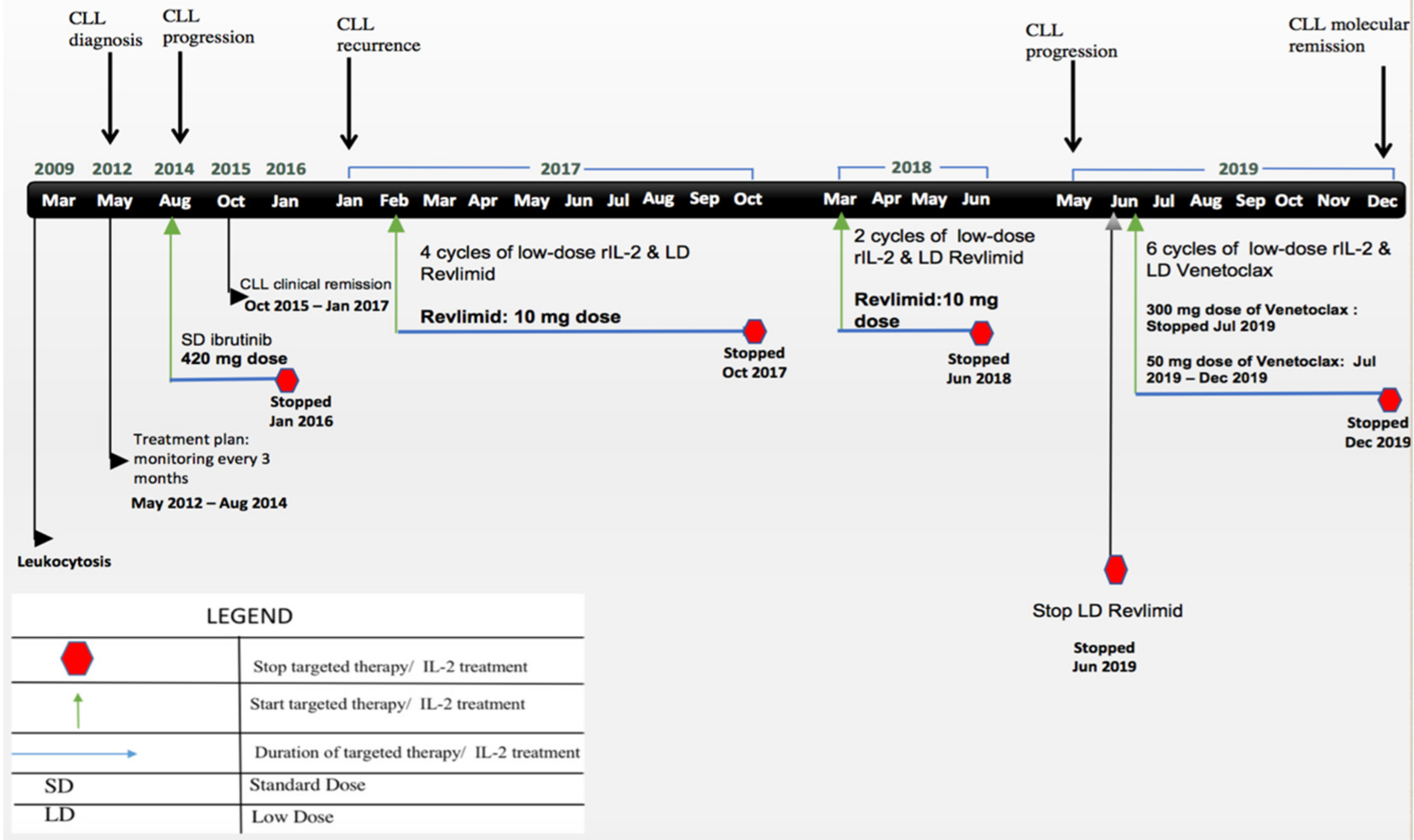
Case Report
3. Discussion
4. Conclusions
5. Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Darwiche, W.; Gubler, B.; Marolleau, J.P.; Ghamlouch, H. Chronic Lymphocytic Leukemia B-Cell Normal Cellular Counterpart: Clues from a Functional Perspective. Front. Immunol. 2018, 9, 683. [Google Scholar] [CrossRef] [PubMed]
- Seifert, M.; Sellmann, L.; Bloehdorn, J.; Wein, F.; Stilgenbauer, S.; Dürig, J.; Küppers, R. Cellular origin and pathophysiology of chronic lymphocytic leukemia. J. Exp. Med. 2012, 209, 2183–2198. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, T.J.; Davis, Z.; Gardiner, A.; Oscier, D.G.; Stevenson, F.K. Unmutated Ig V (H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood 1999, 94, 1848–1854. [Google Scholar] [CrossRef] [PubMed]
- Morrison, V.A. Infectious Complications of Chronic Lymphocytic Leukaemia: Pathogenesis, Spectrum of Infection, Preventive Approaches. Best Pract. Res. Clin. Haematol. 2010, 23, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Dearden, C. Disease-specific complications of chronic lymphocytic leukemia. Hematol. Am. Soc Hematol. Educ. Program. 2008, 1, 450–456. [Google Scholar] [CrossRef]
- Maffei, R.; Bulgarelli, J.; Fiorcari, S.; Bertoncelli, L.; Martinelli, S.; Guarnotta, C.; Castelli, I.; Deaglio, S.; Debbia, G.; De Biasi, S.; et al. The monocytic population in chronic lymphocytic leukemia shows altered composition and deregulation of genes involved in phagocytosis and inflammation. Haematologica 2013, 98, 1115–1123. [Google Scholar] [CrossRef]
- Burger, J.A.; Gribben, J.G. The microenvironment in chronic lymphocytic leukemia (CLL) and other B cell malignancies: Insight into disease biology and new-targeted therapies. Semin Cancer Biol. 2014, 24, 71–81. [Google Scholar] [CrossRef]
- Audrito, V.; Serra, S.; Brusa, D.; Mazzola, F.; Arruga, F.; Vaisitti, T.; Coscia, M.; Maffei, R.; Rossi, D.; Wang, T.; et al. Extracellular nicotinamide phosphoribosyltransferase (NAMPT) promotes M2 macrophage polarization in chronic lymphocytic leukemia. Blood 2015, 125, 111–123. [Google Scholar] [CrossRef]
- DiLillo, D.J.; Weinberg, J.B.; Yoshizaki, A.; Horikawa, M.; Bryant, J.M.; Iwata, Y.; Matsushita, T.; Matta, K.M.; Chen, Y.; Venturi, G.M.; et al. Chronic lymphocytic leukemia and regulatory B cells share IL-10 competence and immunosuppressive function. Leukemia 2013, 27, 170–182. [Google Scholar] [CrossRef]
- Chiorazzi, N.; Fu, S.M.; Montazeri, G.; Kunkel, H.G.; Rai, K.; Gee, T. T cell helper defect in patients with chronic lymphocytic leukemia. J. Immunol. 1979, 122, 1087–1090. [Google Scholar]
- Riches, J.C.; Gribben, J.G. Immunomodulation and immune reconstitution in chronic lymphocytic leukemia. Semin. Hematol. 2014, 51, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Mauri, C.; Bosma, A. Immune regulatory function of B cells. Annu. Rev. Immunol. 2012, 30, 221–241. [Google Scholar] [CrossRef] [PubMed]
- Scheffold, A.; Stilgenbauer, S. Revolution of Chronic Lymphocytic Leukemia Therapy: The Chemo-Free Treatment Paradigm. Curr. Oncol. Rep. 2020, 22, 16. [Google Scholar] [CrossRef] [PubMed]
- Seymour, E.K. Rapid Transitions in the Standard of Care for Chronic Lymphocytic Leukemia (CLL). Oncotarget 2019, 10, 2484–2485. [Google Scholar] [CrossRef]
- Long, M.; Beckwith, K.; Do, P.; Mundy, B.L.; Gordon, A.; Lehman, A.M.; Maddocks, K.J.; Cheney, C.; Jones, J.A.; Flynn, J.M.; et al. Ibrutinib treatment improves T cell number and function in CLL patients. J. Clin. Investig. 2017, 127, 3052–3064. [Google Scholar] [CrossRef]
- Juárez-Salcedo, L.M.; Desai, V.; Dalia, S. Venetoclax: Evidence to date and clinical potential. Drugs Context 2019, 8, 212574. [Google Scholar] [CrossRef]
- Furman, R.R.; Sharman, J.P.; Coutre, S.E.; Cheson, B.D.; Pagel, J.M.; Hillmen, P.; Barrientos, J.C.; Zelenetz, A.D.; Kipps, T.J.; Flinn, I.; et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2014, 370, 997–1007. [Google Scholar] [CrossRef]
- Fürstenau, M.; De Silva, N.; Eichhorst, B.; Hallek, M. Minimal Residual Disease Assessment in CLL: Ready for Use in Clinical Routine? Hemasphere 2019, 3, e287. [Google Scholar] [CrossRef]
- Hofland, T.; Eldering, E.; Kater, A.P.; Tonino, S.H. Engaging Cytotoxic T and NK Cells for Immunotherapy in Chronic Lymphocytic Leukemia. Int. J. Mol. Sci. 2019, 20, 4315. [Google Scholar] [CrossRef]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of Natural Killer Cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef]
- Stabile, H.; Fionda, C.; Gismondi, A.; Santoni, A. Role of Distinct Natural Killer Cell Subsets in Anticancer Response. Front. Immunol. 2017, 8, 293. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Michel, T.; Thérésine, M.; Andrès, E.; Hentges, F.; Zimmer, J. CD56bright natural killer (NK) cells: An important NK cell subset. Immunology 2009, 126, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Jewett, A.; Kos, J.; Kaur, K.; Safaei, T.; Sutanto, C.; Chen, W.; Wong, P.; Namagerdi, A.K.; Fang, C.; Fong, Y.; et al. Natural Killer Cells: Diverse Functions in Tumor Immunity and Defects in Pre-neoplastic and Neoplastic Stages of Tumorigenesis. Mol. Ther. Oncolytics 2019, 16, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.K.; Caudell, E.G.; Smid, C.; Grimm, E.A. IL-2 Activation of NK Cells: Involvement of MKK1/2/ERK but Not P38 Kinase Pathway. J. Immunol. 2000, 164, 6244–6251. [Google Scholar] [CrossRef]
- Fehniger, T.A.; Bluman, E.M.; Porter, M.M.; Mrózek, E.; Cooper, M.A.; VanDeusen, J.B.; Frankel, S.R.; Stock, W.; Caligiuri, M.A. Potential mechanisms of human natural killer cell expansion in vivo during low-dose IL-2 therapy. J. Clin. Investig. 2000, 106, 117–124. [Google Scholar] [CrossRef]
- Maharaj, D.; Vianna, P.; DeCarvalho, G.; Pourkalbassi, D.; Hickey, C.; Gouvea, J. Molecular Remission Using Low-Dose Immunotherapy for Relapsed Refractory Philadelphia Chromosome-Positive Precursor B-Cell Acute Lymphoblastic Leukemia Post-Allogeneic Stem Cell Transplant. Future Sci. OA 2019, 5, FSO380. [Google Scholar] [CrossRef]
- Ramsay, A.G.; Clear, A.J.; Fatah, R.; Gribben, J.G. Multiple inhibitory ligands induce impaired T-cell immunologic synapse function in chronic lymphocytic leukemia that can be blocked with lenalidomide: Establishing a reversible immune evasion mechanism in human cancer. Blood 2012, 120, 1412–1421. [Google Scholar] [CrossRef]
- Ramsay, A.G.; Evans, R.; Kiaii, S.; Svensson, L.; Hogg, N.; Gribben, J.G. Chronic lymphocytic leukemia cells induce defective LFA-1-directed T-cell motility by altering Rho GTPase signaling that is reversible with lenalidomide. Blood 2013, 121, 2704–2714. [Google Scholar] [CrossRef]
- Pittari, G.; Vago, L.; Festuccia, M.; Bonini, C.; Mudawi, D.; Giaccone, L.; Bruno, B. Restoring Natural Killer Cell Immunity against Multiple Myeloma in the Era of New Drugs. Front. Immunol. 2017, 8, 1444. [Google Scholar] [CrossRef]
- Anderson, M.A.; Deng, J.; Seymour, J.F.; Tam, C.; Kim, S.Y.; Fein, J.; Yu, L.; Brown, J.R.; Westerman, D.; Si, E.G.; et al. The BCL2 selective inhibitor venetoclax induces rapid onset apoptosis of CLL cells in patients via a TP53-independent mechanism. Blood 2016, 127, 3215–3224. [Google Scholar] [CrossRef]
- Kaur, K.; Topchyan, P.; Kozlowska, A.K.; Ohanian, N.; Chiang, J.; Maung, P.O.; Park, S.-H.; Ko, M.-W.; Fang, C.; Nishimura, I.; et al. Super-Charged NK Cells Inhibit Growth and Progression of Stem-like/Poorly Differentiated Oral Tumors in Vivo in Humanized BLT Mice; Effect on Tumor Differentiation and Response to Chemotherapeutic Drugs. Oncoimmunology 2018, 7, e1426518. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, H.; Helmi, N.; Abdulaal, W.H.; Zeyadi, M.; Zamzami, M.A.; Wu, W.; Mahmoud, M.M.; Warsi, M.K.; Rasool, M.; Jamal, M.S. Prospects of IL-2 in Cancer Immunotherapy. BioMed Res. Int. 2018, 2018, 9056173. [Google Scholar] [CrossRef] [PubMed]
- Darabi, K.; Kantamnei, S.; Wiernik, P.H. Lenalidomide-induced warm autoimmune hemolytic anemia. J. Clin. Oncol. 2006, 24, e59. [Google Scholar] [CrossRef] [PubMed]
- De Weerdt, I.; Hofland, T.; de Boer, R.; Dobber, J.A.; Dubois, J.; van Nieuwenhuize, D.; Mobasher, M.; de Boer, F.; Hoogendoorn, M.; Velders, G.A.; et al. Distinct Immune Composition in Lymph Node and Peripheral Blood of CLL Patients Is Reshaped during Venetoclax Treatment. Blood Adv. 2019, 3, 2642–2652. [Google Scholar] [CrossRef] [PubMed]
- Seymour, J.F.; Davids, M.S.; Roberts, A.W.; Hallek, M.; Wierda, W.G.; Hillmen, P.; Gerecitano, J.F.; Cerri, E.; Potluri, J.; Kim, S.Y.; et al. Safety Profile of Venetoclax Monotherapy in Patients with Chronic Lymphocytic Leukemia. Blood 2016, 128, 4395. [Google Scholar] [CrossRef]
- He, X.S.; Draghi, M.; Mahmood, K.; Holmes, T.H.; Kemble, G.W.; Dekker, C.L.; Arvin, A.M.; Parham, P.; Greenberg, H.B. T cell-dependent production of IFN-gamma by NK cells in response to influenza A virus. J. Clin. Investig. 2004, 114, 1812–1819. [Google Scholar] [CrossRef]
- Crombie, J.; Davids, M.S. IGHV mutational status testing in chronic lymphocytic leukemia. Am. J. Hematol. 2017, 92, 1393–1397. [Google Scholar] [CrossRef]
- Friedman, D.R.; Sibley, A.B.; Owzar, K.; Chaffee, K.G.; Slager, S.; Kay, N.E.; Hanson, C.A.; Ding, W.; Shanafelt, T.D.; Weinberg, J.B.; et al. Relationship of blood monocytes with chronic lymphocytic leukemia aggressiveness and outcomes: A multi-institutional study. Am. J. Hematol. 2016, 91, 687–691. [Google Scholar] [CrossRef]
- Alhakeem, S.S.; McKenna, M.K.; Oben, K.Z.; Noothi, S.K.; Rivas, J.R.; Hildebrandt, G.C.; Fleischman, R.A.; Rangnekar, V.M.; Muthusamy, N.; Bondada, S. Chronic Lymphocytic Leukemia-Derived IL-10 Suppresses Antitumor Immunity. J. Immunol. 2018, 200, 4180–4189. [Google Scholar] [CrossRef]



| Date of Visit | FISH 13q14.3 (Normal < 3.1%) | Absolute Number of Abnormal CLL Cells * K/μL | Detected Abnormalities |
|---|---|---|---|
| 31 January 2017 | 71% | 9.27 (59%) | ZAP-70 positive Unmutated IGHV: 0.3% |
| 7 April 2017 | 52% | 1.91 (49%) | ZAP-70 positive SF3B1 mutation: detected TP53 deletion: not detected NOTCH1: not detected Unmutated IGHV: 1.7% |
| 8 June 2017 | 51% | 0.27 (16%) | ZAP-70 positive SF3B1 mutation: detected TP53 mutation: not detected NOTCH1: not detected MYD88: not detected Unmutated IGHV: 1.0% |
| 4 August 2017 | 26% | 0.13 (9%) | SF3B1 mutation: detected TP53 mutation: not detected NOTCH1: not detected MYD88: not detected Mutated IGHV: 6.0% |
| 18 October 2017 | Not detected | 0.11 (10%) | SF3B1 mutation: detected TP53 mutation: not detected NOTCH1: not detected MYD88: not detected Mutated IGHV: 9.5% |
| 27 March 2018 | 24% | 0.08 (5%) | SF3B1 mutation: detected TP53 mutation: not detected NOTCH1: not detected MYD88: not detected Unmutated IGHV: 0.34% |
| 5 June 2018 | 21% | 0.62 (27%) | Unmutated IGHV: 0% |
| 17 May 2019 | 93% | 49.6 (79%) | SF3B1 mutation: detected TP53 mutation: not detected NOTCH1: not detected MYD88: not detected Unmutated IGHV: 0.3% |
| 26 August 2019 | 52% | 0.33 (55%) | SF3B1 mutation: detected ATM mutation: detected TP53 mutation: not detected NOTCH1: not detected MYD88: not detected Unmutated IGHV: 0% |
| 3 October 2019 | 7.5% | 0.33 (9.6%) | SF3B1 mutation: detected ATM mutation: detected TP53 mutation: not detected NOTCH1: not detected MYD88: not detected Mutated IGHV: 12.8% |
| 6 December 2019 | Not detected | No abnormal CLL cells (0%) | No mutations |
| 12 March 2020 | Not detected | No abnormal CLL cells (0%) | No mutations |
| 29 September 2020 | Not detected | No abnormal CLL cells (0%) | No mutations |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maharaj, D.; Srinivasan, G.; Abreu, M.M.; Ko, M.-W.; Jewett, A.; Gouvea, J. Molecular Remission Using Low-Dose Immunotherapy with Minimal Toxicities for Poor Prognosis IGHV—Unmutated Chronic Lymphocytic Leukemia. Cells 2021, 10, 10. https://doi.org/10.3390/cells10010010
Maharaj D, Srinivasan G, Abreu MM, Ko M-W, Jewett A, Gouvea J. Molecular Remission Using Low-Dose Immunotherapy with Minimal Toxicities for Poor Prognosis IGHV—Unmutated Chronic Lymphocytic Leukemia. Cells. 2021; 10(1):10. https://doi.org/10.3390/cells10010010
Chicago/Turabian StyleMaharaj, Dipnarine, Gayathri Srinivasan, Maria M. Abreu, Meng-Wei Ko, Anahid Jewett, and Jacqueline Gouvea. 2021. "Molecular Remission Using Low-Dose Immunotherapy with Minimal Toxicities for Poor Prognosis IGHV—Unmutated Chronic Lymphocytic Leukemia" Cells 10, no. 1: 10. https://doi.org/10.3390/cells10010010
APA StyleMaharaj, D., Srinivasan, G., Abreu, M. M., Ko, M.-W., Jewett, A., & Gouvea, J. (2021). Molecular Remission Using Low-Dose Immunotherapy with Minimal Toxicities for Poor Prognosis IGHV—Unmutated Chronic Lymphocytic Leukemia. Cells, 10(1), 10. https://doi.org/10.3390/cells10010010







