Enzyme Linked Immuno-Spot; a Useful Tool in the Search for Elusive Immune Markers in Common Pediatric Immunological Diseases
Abstract
:1. Introduction
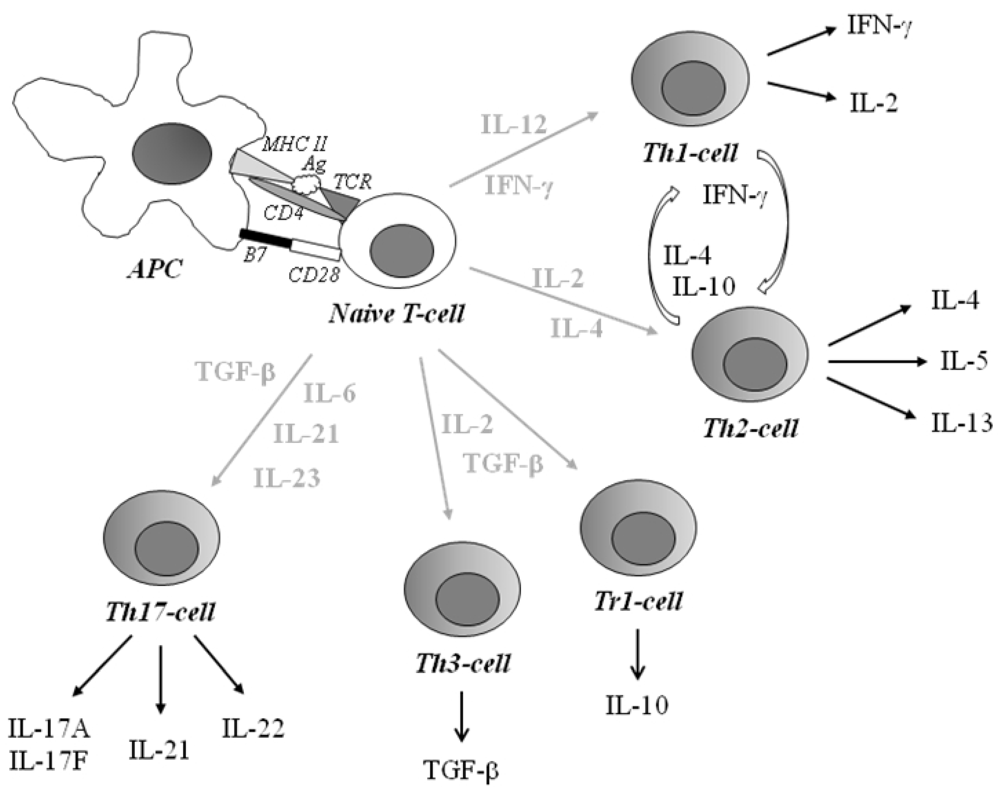
2. Common Pediatric Immunological Diseases
2.1. Type 1 Diabetes
Immunological Aspects of Type 1 Diabetes
2.2. Allergic Disease
Immunological Aspects of Allergic Disease
2.3. Celiac Disease
Immunological Aspects of Celiac Disease
3. Immunological Methods Suitable for Detection of Cytokines
4. Enzyme Linked Immuno-Spot (ELISPOT)
4.1. Methodological Principal
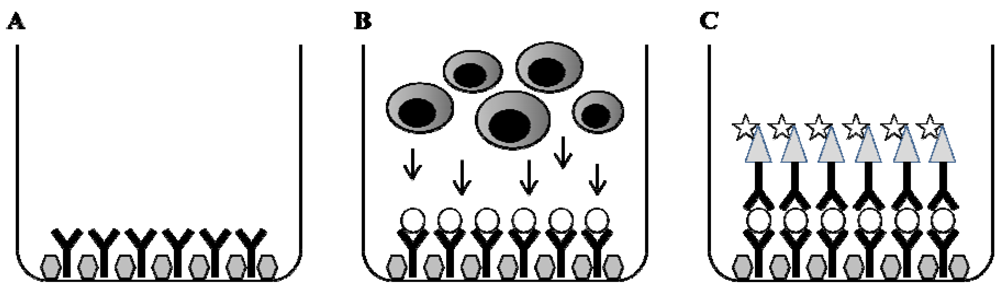
4.2. The Use of ELISPOT in Search for Immune Markers in Pediatric Immunological Diseases
4.3. Methodological Aspects of ELISPOT
5. Concluding Remarks
References
- Von Boehmer, H. Positive selection of lymphocytes. Cell 1994, 76, 219–228. [Google Scholar] [CrossRef]
- Theofilopoulos, A.N. The basis of autoimmunity: Part I. Mechanisms of aberrant self-recognition. Immunol. Today 1995, 16, 90–98. [Google Scholar] [CrossRef]
- Romagnani, S. Lymphokine production by human T cells in disease states. Annu. Rev. Immunol. 1994, 12, 227–257. [Google Scholar] [CrossRef]
- Péne, J.; Rousset, F.; Briere, F.; Chrétien, I.; Bonnefoy, J.Y.; Spits, H.; Yokota, T.; Arai, N.; Arai, K.I. Banchereau J IgE production by normal human lyphocytes is induced by interleukin 4 and suppressed by interferon γ and α and prostaglandin E2. Proc. Natl. Acad. Sci. 1988, 85, 6880–6884. [Google Scholar]
- Mosmann, T.R.; Coffman, R.L. TH1 and TH2 cells: Different patterns of lymphokine secretion lead to different functional properties. Ann. Rev. Immunol. 1989, 7, 145–173. [Google Scholar] [CrossRef]
- Fiorentino, D.F.; Bond, M.W.; Mosmann, T.R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 1989, 170, 2081–2095. [Google Scholar] [CrossRef]
- Mosmann, T.R.; Sad, S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today 1996, 17, 138–146. [Google Scholar] [CrossRef]
- Roncarolo, M.G.; Baccetta, R.; Bordignon, C.; Narula, S.; Levings, MK. Type 1 T regulatory cells. Immunol. Rev. 2001, 182, 68–79. [Google Scholar]
- Weiner, H.L. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol. Rev. 2001, 182, 207–214. [Google Scholar]
- Cua, D.J. Interleukin23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 2003, 421, 744–748. [Google Scholar] [CrossRef]
- Langrish, C.L. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005, 201, 233–240. [Google Scholar] [CrossRef]
- Bettelli, E.; Oukka, M.; Kuchroo, V.K. Th-17 cells in the circle of immunity and autoimmunity. Nature Immunol. 2007, 8, 345–350. [Google Scholar]
- Bullens, D.M.A.; Kasran, A.; Peng, X.; Lorré, K.; Ceuppens, J.L. Effects of anti-IL-4 receptor monoclonal antibody on in vitro T cell cytokine levels: IL-4 production by T cells from non-atopic donors. Clin. Exp. Immunol. 1998, 113, 320–326. [Google Scholar] [CrossRef]
- Castano, L.; Eisenbarth, G.S. Type-I diabetes: A chronic autoimmune disease of human, mouse, and rat. Annu. Rev. Immunol. 1990, 8, 647–679. [Google Scholar] [CrossRef]
- Bach, J.-F. Insulin-dependent diabetes mellitus as an autoimmune disease. Endoc. Rev. 1994, 15, 516–542. [Google Scholar]
- Atkinson, M.A.; Maclaren, N.K. The pathogenesis of insulin-dependent diabetes mellitus. N. Engl. J. Med. 1994, 331, 1428–1436. [Google Scholar] [CrossRef]
- Berhan, Y.; Waernbaum, I.; Lind, T.; Möllsten, A.; Dahlqvist, G. Thirty years of prospective nationwide incidence of childhood tyep 1 diabetes. Diabetes 2011, 60, 577–581. [Google Scholar] [CrossRef]
- The DIAMOND project group. Incidence and trends of childhood type 1 diabetes worldwide 1990–1999. Diabet. Med. 2006, 23, 857–866. [CrossRef]
- Eisenbarth, G.S. Type I diabetes mellitus. A chronic autoimmune disease. N. Engl. J. Med. 1986, 314, 1360–1368. [Google Scholar] [CrossRef]
- Bradley, L.M.; Asensio, V.C.; Schioetz, L.K.; Harbertson, J.; Krahl, T.; Patstone, G.; Woolf, N.; Campbell, I.L.; Sarvetnick, N. Islet-specific Th1, but not Th2, cells secrete multiple chemokines and promote rapid induction of autoimmune diabetes. J. Immunol. 1999, 162, 2511–2520. [Google Scholar]
- Berman, M.A.; Sandborg, C.I.; Wang, Z.; Imfeld, K.L.; Zaldivar, F.J.; Dadufalza, V.; Buckingham, B.A. Decreased IL-4 production in new onset type I insulin-dependent diabetes mellitus. J. Immunol. 1996, 157, 4690–4696. [Google Scholar]
- Karlsson, M.G.E.; Lawesson, S.S.; Ludvigsson, J. Th1-like dominance in high-risk first-degree relatives of type 1 diabetic patients. Diabetologia. 2000, 43, 742–749. [Google Scholar] [CrossRef]
- Halminen, M.; Simell, O.; Knip, M.; Ilonen, J. Cytokine expression in unstimulated PBMC of children with type 1 diabetes and subjects positive for diabetes-associated antibodies. Scand. J. Immunol. 2001, 53, 510–513. [Google Scholar]
- Stechova, K.; Bohmova, K.; Vrabelova, Z.; Sepa, A.; Stadlerova, G.; Zacharovova, K.; Faresjö, M. High T-helper-1 cytokines but low T-helper-3 cytokines, inflammatory cytokines and chemokines in children with high risk of developing type 1 diabetes. Diabetes Metab. Res. Rev. 2007, 23, 462–471. [Google Scholar] [CrossRef]
- Ryden, A.; Stechova, K.; Durilova, M.; Faresjö, M. Switch from a dominant Th1-associated immune profile during the pre-diabetic phase in favour of a temporary increase of a Th3-associated and inflammatory immune profile at the onset of type 1 diabetes. Diabetes Metab. Res. Rev. 2009, 25, 335–343. [Google Scholar] [CrossRef]
- Spergel, J.M.; Paller, A.S. Atopic dermatitis and the atopic march. J. Allergy Clin. Immunol. 2003, 112, S118–S127. [Google Scholar] [CrossRef]
- Asher, M.I.; Montefort, S.; Björksten, B.; Lai, C.K.W.; Strachan, D.P.; Weiland, S.K.; Williams, H. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phase one and three repeat multicountry cross-sectional surveys. Lancet. 2006, 368, 733–743. [Google Scholar]
- Romagnani, S. Regulation of the development of type 2 T-helper cells in allergy. Curr. Opin. Immunol. 1994, 6, 838–846. [Google Scholar] [CrossRef]
- Egan, R.W.; Umland, S.P.; Cuss, F.M.; Chapman, R.W. Biology of interleukin-5 and its relevance to allergic disease. Allergy. 1996, 51, 71–81. [Google Scholar]
- Kay, A.B. Allergy and allergic disease—First of two parts. N. Engl. J. Med. 2001, 344, 30–37. [Google Scholar] [CrossRef]
- Trier, J.S. Celiac sprue. N. Engl. J. Med. 1991, 325, 1709–1719. [Google Scholar] [CrossRef]
- Burgin-Wolff, A.; Gaze, H.; Hadziselimovic, F.; Huber, H.; Lentze, M.J.; Nusslé, D.; Reymond-Berthet, C. Antigliadin and antiendomysium antibody determination for coeliac disease. Arch. Dis. Child. 1991, 66, 941–947. [Google Scholar] [CrossRef]
- Van de Wal, Y.; Kooy, Y.; van Veelen, P.; Pena, S.; Mearin, L.; Papadopoulos, G.; Koning, F. Selective deamidation by tissue transglutaminase strongly enhances gliadin-specific T cell reactivity. J. Immunol. 1998, 161, 1585–1588. [Google Scholar]
- Molberg, Ö; Mcadam, S.N.; Körner, R.; Quarsten, H.; Kristiansen, C.; Madsen, L.; Fugger, L.; Scott, H. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat. Med. 1998, 4, 713–717. [Google Scholar] [CrossRef]
- Nilsen, E.M.; Jahnsen, F.L.; Lundin, K.E.A.; Johansen, F.E.; Fausa, O.; Sollid, L.M.; Jahnsen, J.; Scott, H. Brandtzaeg P, Gluten induces an intestinal cytokine response strongly dominated by interferon-γ in patients with celiac disease. Gastroenterology 1998, 115, 551–563. [Google Scholar] [CrossRef]
- Lahat, N.; Shapiro, S.; Karban, A.; Gerstein, R.; Kinarty, A.; Lerner, A. Cytokine profile in coeliac disease. Scand. J. Immunol. 1999, 49, 441–446. [Google Scholar] [CrossRef]
- Monteleone, I.; Monteleone, G.; Del Vecchio Blanco, G.; Vavassori, P.; Cucchiara, S.; MacDonald, T.T.; Pallone, F. Regulation of the T helper cell type 1 transcription factor T-bet in coeliac disease mucosa. Gut. 2004, 53, 1090–1095. [Google Scholar] [CrossRef]
- Lahdenperä, A.; Ludvigsson, J.; Fälth-Magnusson, K.; Högberg, L.; Vaarala, O. The effect of glute-free diet on Th1-Th2-Th3-assocaited intestinal immune repsonses in celiac disease. Scand. J. Gastroent. 2011, 46, 538–549. [Google Scholar] [CrossRef]
- De Jager, W.; Te Velthuis, H.; Prakken, B.J.; Kuis, W.; Rijkers, G.T. Simultaneous detection of 15 human cytokines in a single sample of stimulated peripheral blood mononuclear cells. Clin. Diagn. Lab. Immunol. 2003, 10, 133–139. [Google Scholar]
- Lagrelius, M.; Jones, P.; Franck, K.; Gaines, H. Cytokine detection by multiplex technology useful for assessing antigen specific cytokine profiles and kinetics in whole blood cultured up to seven days. Cytokine 2006, 33, 156–165. [Google Scholar] [CrossRef]
- Czerkinsky, C.C.; Tarkowski, A.; Nilsson, L.-Å.; Ouchterlony, Ö.; Nygren, H.; Gretzer, C. Reverse enzyme-linked immunospot assay (RELISPOT) for the detection of cells secreting immunoreactive substances. J. Immunol. Methods 1984, 72, 489–496. [Google Scholar] [CrossRef]
- Czerkinsky, C.; Andersson, G.; Ekre, H.-P.; Nilsson, L.-Å.; Klareskog, L.; Ouchterlony, Ö. Reverse ELISPOT assay for clonal analysis of cytokine production. J. Immunol. Methods. 1988, 110, 29–36. [Google Scholar] [CrossRef]
- Hedman, M.; Ludvigsson, J.; Karlsson Faresjö, M.G.E. Nicotinamide reduces high secretion of IFN-γ in high-risk relatives even though it does not prevent type 1 diabetes. J. Interferon Cytokine Res. 2006, 26, 207–213. [Google Scholar] [CrossRef]
- Petrich de Marquesini, L.G.; Fu, J.; Connor, K.J.; Bishop, A.J.; McLintock, N.E.; Wong, F.S.; Dayan, C.M. IFN-γ and IL-10 islet-antigen-specific T cell responses in autoantibody-negative first-degree relatives of patients with type 1 diabetes. Diabetologia 2010, 53, 1451–1460. [Google Scholar] [CrossRef]
- Nilsson, C.; Larsson, A.K.; Höglind, A.; Gabrielsson, S.; Troye-Blomberg, M.; Lilja, G. Low numbers of interleukin-12-producing cord blood mononuclear cells and immunoglobulin E sensitization in early childhood. Clin. Exp. Allergy 2004, 34, 373–380. [Google Scholar]
- Larsson, A.K.; Nilsson, C.; Höglind, A.; Sverremark-Ekström, E.; Lilja, G.; Troye-Blomberg, M. Relationship between maternal and child cytokine responses to allergen and phytohaemagglutinin 2 years after delivery. Clin. Exp. Immunol. 2006, 144, 401–408. [Google Scholar] [CrossRef]
- Bordignon, V.; Sinagra, J.L.; Trento, E.; Pietravalle, M.; Capitanio, B.; Fei, P.C. Antigen specific cytokine response in pediatric patients with atopic dermatitis. Ped. Allergy Immunol. 2005, 16, 113–120. [Google Scholar] [CrossRef]
- Hansson, T.; Dannaeus, A.; Klareskog, L. Cytokine-producing cells in peripheral blood of children with coeliac disease secrete cytokines with a type 1 profile. Clin. Exp. Immunol. 1999, 116, 246–250. [Google Scholar] [CrossRef]
- Salvati, V.M.; Mazzarella, G.; Gianfrani, C.; Levings, M.K.; Stefanile, R.; de Giulio, B.; Laquinto, G.; Giardullo, N.; Auricchio, S. Roncarolo MG, Recombinant human interleukin 10 suppresses gliadin dependent T cell activation in ex vivo cultured coeliac intestinal mucosa. Gut 2005, 54, 46–53. [Google Scholar] [CrossRef]
- Anderson, R.P.; van Heel, D.A.; Tye-Din, J.A.; Barnardo, M.; Salio, M.; Jewell, D.P.; Hill, A.V.S. T cells in peripheral blood after gluten challenge in coeliac disease. Gut 2005, 54, 1217–1223. [Google Scholar]
- Nilsson, L.; Kivling, A.; Jalmelid, M.; Magnusson, F.M.; Faresjö, M. Combinations of common chronic paediatric diseases deviate the immune response in diverging directions. Clin. Exp. Immunol. 2006, 146, 433–442. [Google Scholar] [CrossRef]
- Ekerfelt, C.; Ernerudh, J.; Jenmalm, M.C. Detection of spontaneous and antigen-induced human interleukin-4 responses in vitro: comparison of ELISPOT, a novel ELISA and real-time RT-PCR. J. Immunol. Meth. 2002, 260, 55–67. [Google Scholar] [CrossRef]
- Kabilan, L.; Andersson, G.; Lolli, F.; Ekre, H.-P.; Olsson, T.; Troye-Blomberg, M. Detection of intracellular expression and secretion of interferon-gamma at the single-cell level after activation of human T cells with tetanus toxoid in vitro. Eur. J. Immunol. 1990, 20, 1085–1089. [Google Scholar]
- Schloot, N.C.; Meierhoff, G.; Karlsson Faresjö, M.; Ott, P.A.; Putnam, A.; Lehmann, P.V.; Gottlieb, P.; Roep, B.O.; Peakman, M.; Tree, T. Comparison of cytokine ELISpot assay formats for the detection of islet antigen autoreactive T cells. Report of the Third Immunology of Diabetes Society T-cell Workshop. J. Autoimmunity 2003, 21, 365–376. [Google Scholar] [CrossRef]
- Helms, T.; Boehm, B.O.; Asaad, R.J.; Trezza, R.P.; Lehmann, P.V.; Tary-Lehmann, M. Direct visualization of cytokine-producing recall antigen-specific CD4 memory T cells in healthy individuals and HIV patients. J. Immunol. 2000, 164, 3723–3732. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Faresjö, M. Enzyme Linked Immuno-Spot; a Useful Tool in the Search for Elusive Immune Markers in Common Pediatric Immunological Diseases. Cells 2012, 1, 141-152. https://doi.org/10.3390/cells1020141
Faresjö M. Enzyme Linked Immuno-Spot; a Useful Tool in the Search for Elusive Immune Markers in Common Pediatric Immunological Diseases. Cells. 2012; 1(2):141-152. https://doi.org/10.3390/cells1020141
Chicago/Turabian StyleFaresjö, Maria. 2012. "Enzyme Linked Immuno-Spot; a Useful Tool in the Search for Elusive Immune Markers in Common Pediatric Immunological Diseases" Cells 1, no. 2: 141-152. https://doi.org/10.3390/cells1020141
APA StyleFaresjö, M. (2012). Enzyme Linked Immuno-Spot; a Useful Tool in the Search for Elusive Immune Markers in Common Pediatric Immunological Diseases. Cells, 1(2), 141-152. https://doi.org/10.3390/cells1020141



