Dissecting the T Cell Response: Proliferation Assays vs. Cytokine Signatures by ELISPOT
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Isolation
2.2. Soluble Antigen Specific T Cell IFN-γ and IL-2 ELISPOT Assay
2.3. Allogeneic T Cell Cytokine Producing Assay
2.4. 3H-Thymidine Incorporation Proliferation Assay
2.5. Proliferation by CFSE Dye Dilution Method
2.6. Statistical Analysis
3. Results
3.1. Antigen-Specific CD4 and CD8 Memory Cells Display Dissociated Production of IL-2 and IFN-γ
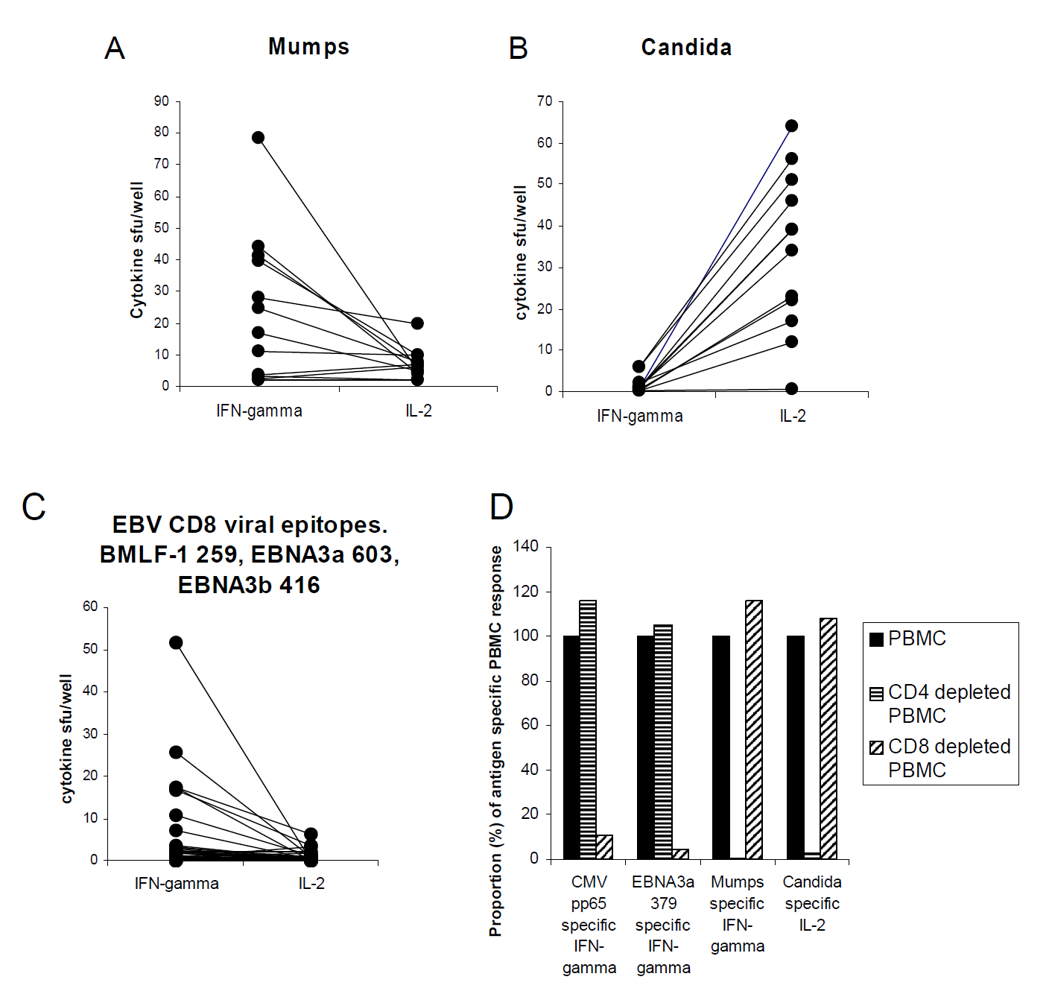
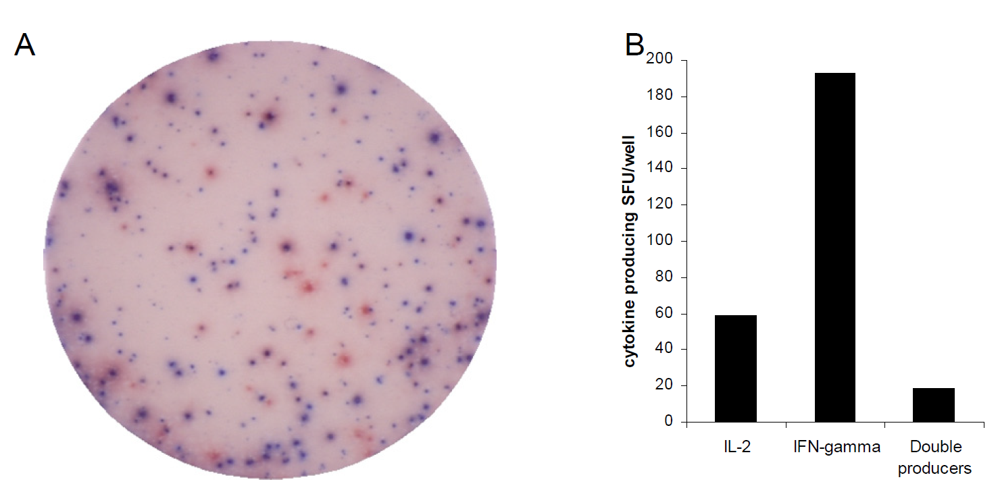
3.2. Allo-Antigen-Specific T Cells Show Dissociated IL-2-IFN-γ Production
| Stimulator × Responder | IL-2 | IFN-γ |
|---|---|---|
| A × B | 20 | 77 |
| A × C | 19 | 47 |
| A × D | 28 | 31 |
| B × A | 11 | 26 |
| B × C | 23 | 50 |
| B × D | 21 | 31 |
| C × A | 4 | 19 |
| C × B | 15 | 21 |
| C × D | 10 | 8 |
| D × A | 9 | 14 |
| D × B | 22 | 40 |
| D × C | 23 | 45 |
| Mean * | 17.1 | 34.1 |

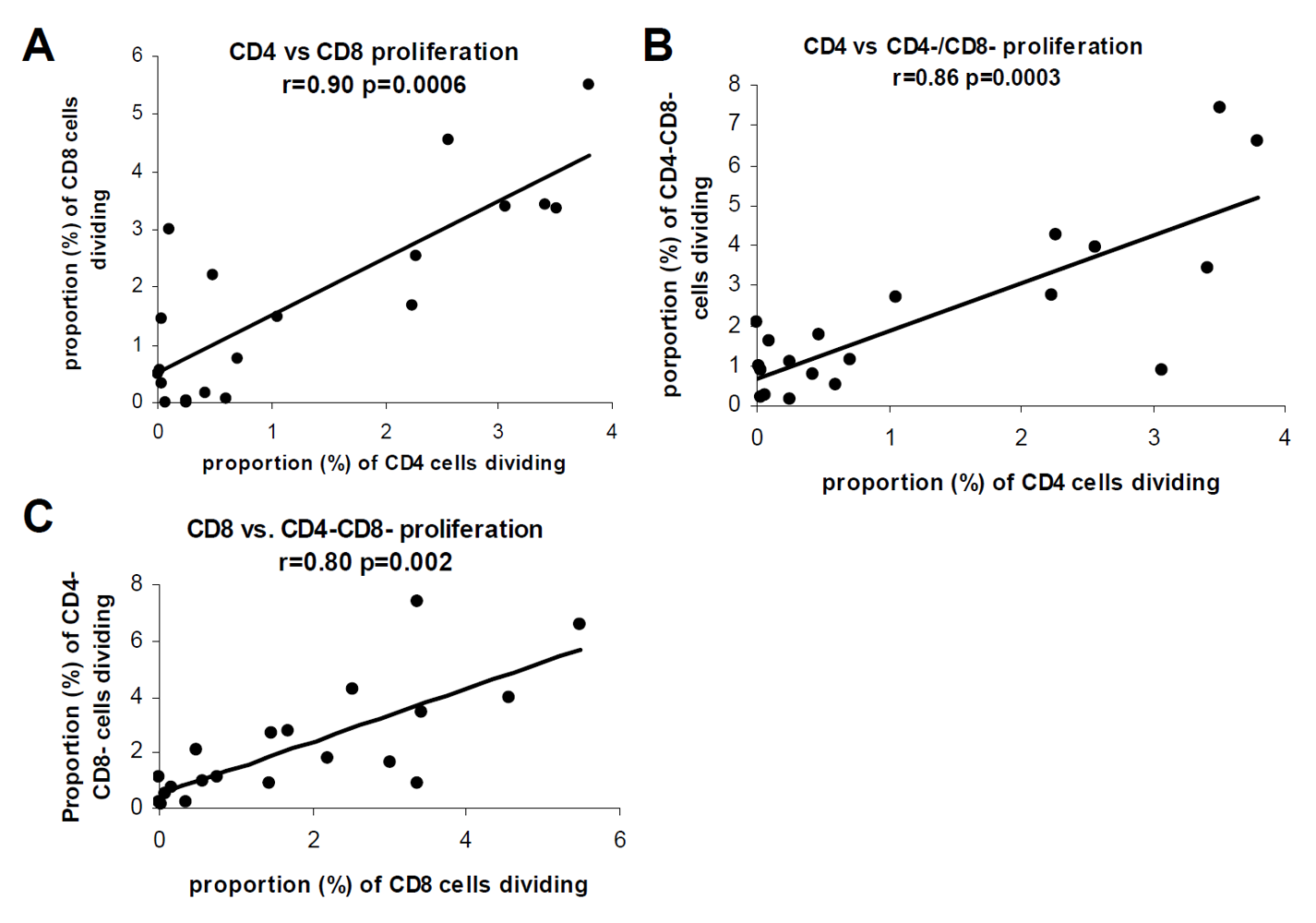
3.3. The Allogeneic Proliferative Response Correlates with the Frequency of IL-2, but not with IFN-γ Producing T Cells
| Compared parameters | 2-way MLR | CD4 allogeneic response | CD8 allogeneic response |
|---|---|---|---|
| 20 h IL-2 vs. proliferation | r = 0.59 * | R = 0.54 * | R = 0.24 |
| 20 h IFN-γ vs. proliferation | r = 0.52 * | R = 0.37 | R = 0.23 |
| 72 h IL-2 vs. proliferation | r = 0.73 ** | R = 0.62 ** | ND |
| 72 h IFN-γ vs. proliferation | r = 0.37 | R = 0.43 | ND |
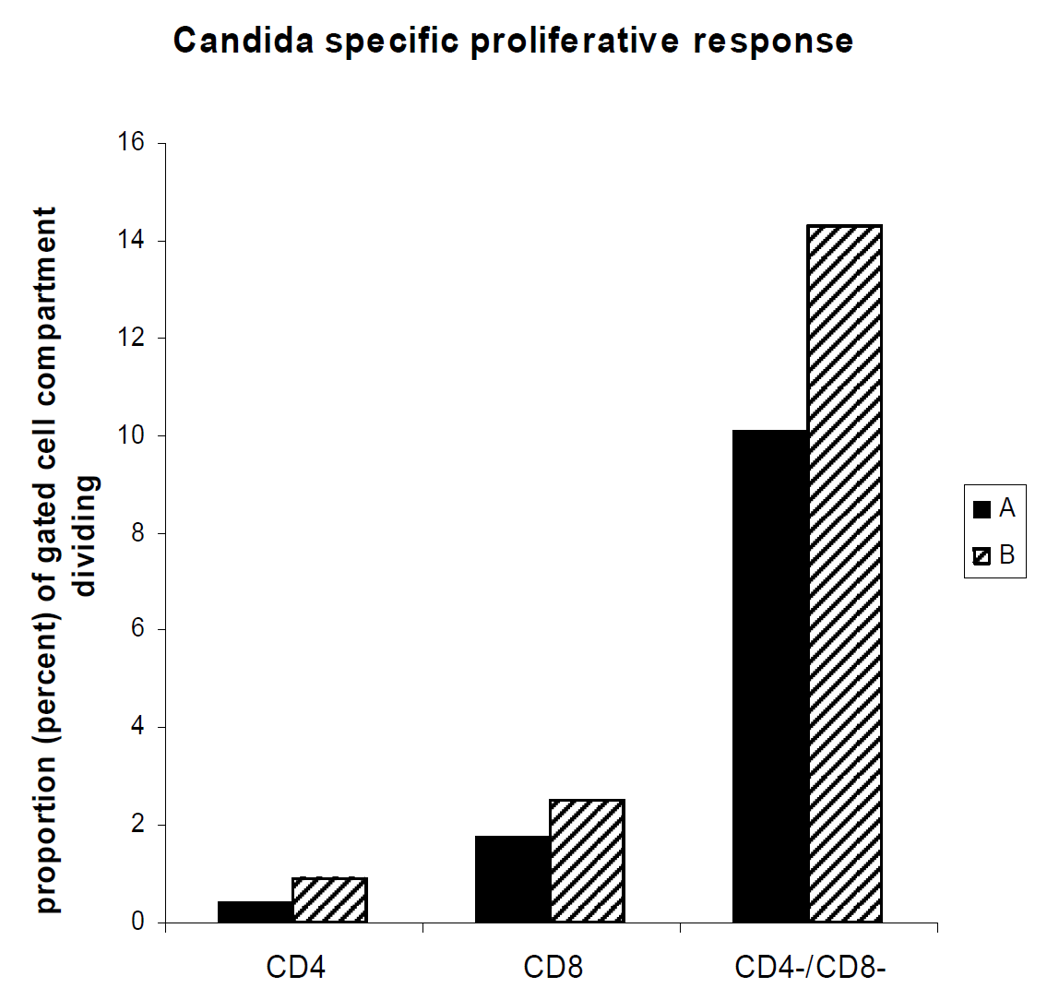
3.4. The Proliferative Response Entails a Substantial Non-T Cell Bystander Component
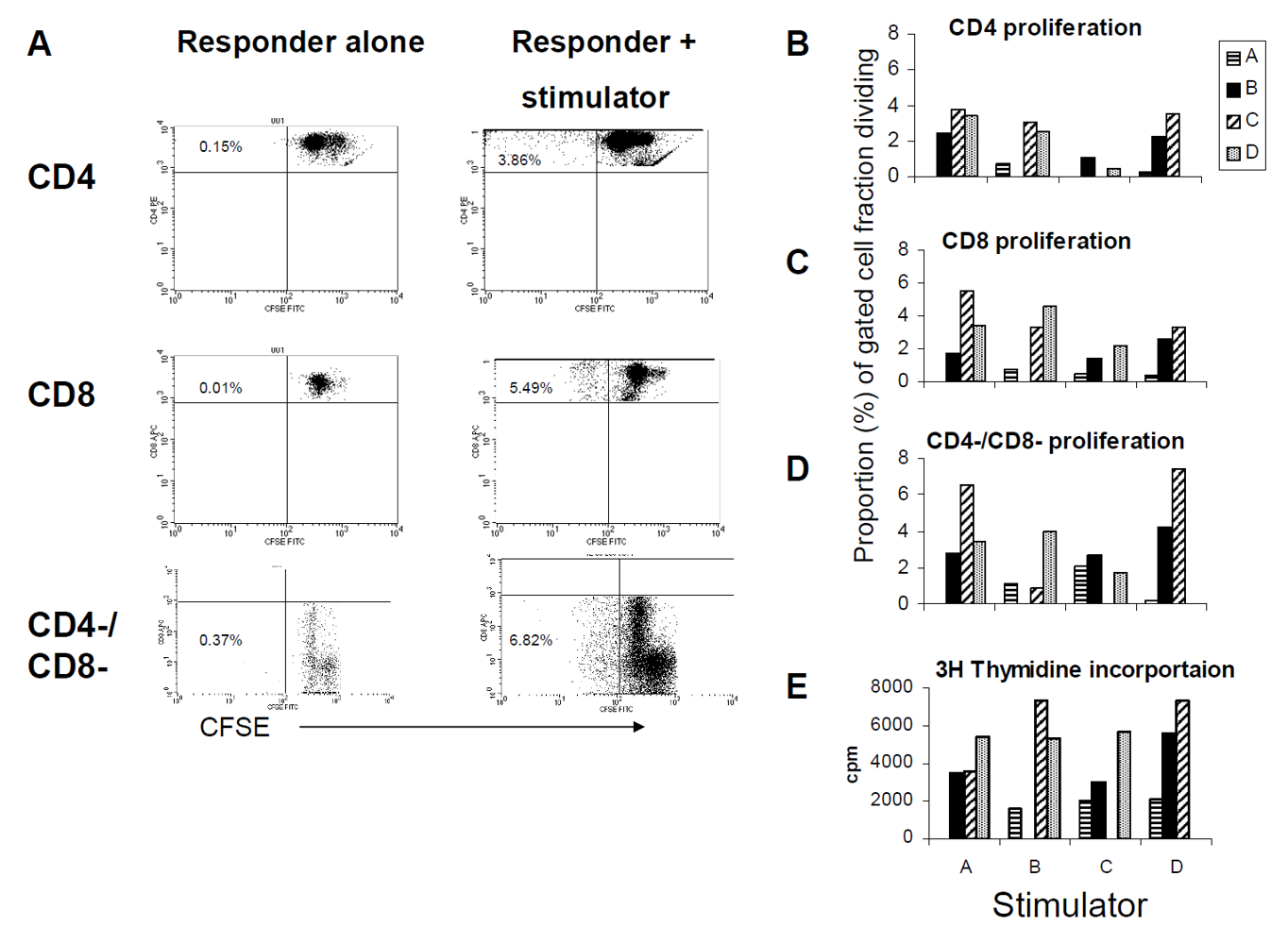
4. Discussion
Conclusions
Acknowledgements
References
- Heeger, P.S. T-cell allorecognition and transplant rejection: A summary and update. Am. J. Transplant. 2003, 3, 525–533. [Google Scholar] [CrossRef]
- D’Orsogna, L.J.; Roelen, D.L.; Doxiadis, I.I.; Claas, F.H. TCR cross-reactivity and allorecognition: New insights into the immunogenetics of allorecognition. Immunogenetics 2012, 64, 77–85. [Google Scholar] [CrossRef]
- Wood, K.J.; Goto, R. Mechanisms of rejection: Current perspectives. Transplantation 2012, 93, 1–10. [Google Scholar] [CrossRef]
- Heeger, P.; Greenspan, N.; Kuhlenschmidt, S.; Dejelo, C.; Hricik, D.; Schulak, J.; Tary-Lehmann, M. Pretransplant frequency of donor-specific, interferon gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of post transplant rejection episodes. J. Immunol. 1999, 163, 2267–2275. [Google Scholar]
- Poggio, E.D.; Clemente, M.; Riley, J.; Roddy, M.; Greenspan, N.S.; Dejelo, C.; Najafian, N.; Sayegh, M.H.; Hricik, D.E.; Heeger, P.S. Alloreactivity in renal transplant recipients with and without chronic allograft nephropathy. J. Am. Soc. Nephrol. 2004, 15, 1952–1960. [Google Scholar] [CrossRef]
- Kwun, J.; Knechtle, S.J.; Hu, H. Determination of the functional status of alloreactive T cells by interferon-gamma kinetics. Transplantation 2006, 81, 590–598. [Google Scholar] [CrossRef]
- Nickel, P.; Presber, F.; Bold, G.; Biti, D.; Schönemann, C.; Tullius, S.G.; Volk, H.D.; Reinke, P. Enzyme-linked immunosorbent spot assay for donor-reactive interferon-gamma-producing cells identifies T-cell presensitization and correlates with graft function at 6 and 12 months in renal-transplant recipients. Transplantation 2004, 78, 1640–1646. [Google Scholar]
- Pantenburg, B.; Heinzel, F.; Das, L.; Heeger, P.S.; Valujskikh, A. T cells primed by Leishmania major infection cross-react with alloantigens and alter the course of allograft rejection. J. Immunol. 2002, 169, 3686–3693. [Google Scholar]
- Jameson, S.C.; Masopust, D. Diversity in T cell memory: An embarrassment of riches. Immunity 2009, 31, 859–871. [Google Scholar] [CrossRef]
- Sallusto, F.; Geginat, J.; Lanzavecchia, A. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu. Rev. Immunol. 2004, 22, 745–763. [Google Scholar] [CrossRef]
- Matesic, D.; Lehmann, P.; Heeger, P. High-resolution characterization of cytokine-producing alloreactivity in naive and allograft-primed mice. Transplantation 1998, 65, 906–914. [Google Scholar] [CrossRef]
- Mempel, T.R.; Henrickson, S.E.; von Andrian, U.H. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 2004, 427, 154–159. [Google Scholar]
- Divekar, A.A.; Zaiss, D.M.; Lee, F.E.; Liu, D.; Topham, D.J.; Sijts, A.J.; Mosmann, T.R. Protein vaccines induce uncommitted IL-2-secreting human and mouse CD4 T cells, whereas infections induce more IFN-gamma-secreting cells. J. Immunol. 2006, 176, 1465–1473. [Google Scholar]
- Forsthuber, T.; Yip, H.C.; Lehmann, P.V. Induction of TH1 and TH2 immunity in neonatal mice [see comments]. Science 1996, 271, 1728–1730. [Google Scholar]
- Anthony, D.D.; Post, A.B.; Valdez, H.; Peterson, D.L.; Murphy, M.; Heeger, P.S. ELISPOT analysis of hepatitis C virus protein-specific IFN-gamma- producing peripheral blood lymphocytes in infected humans with and without cirrhosis. Clin. Immunol. 2001, 99, 232–240. [Google Scholar] [CrossRef]
- Karulin, A.; Hesse, M.; Tary-Lehmann, M.; Lehmann, P. Single-cytokine-producing CD4 memory cells predominate in type 1 and type 2 immunity. J. Immunol. 2000, 164, 1862–1872. [Google Scholar]
- Anthony, D.D.; Valdez, H.; Post, A.B.; Carlson, N.L.; Heeger, P.S.; Lehmann, P.V. Comprehensive determinant mapping of the hepatitis-C-specific CD8 cell repertoire reveals unpredicted immune hierarchy. Clin. Immunol. 2002, 103, 264–276. [Google Scholar]
- Jenkins, M.K.; Khoruts, A.; Ingulli, E.; Mueller, D.L.; McSorley, S.J.; Reinhardt, R.L.; Itano, A.; Pape, K.A. In vivo activation of antigen-specific CD4 T cells. Annu. Rev. Immunol. 2001, 19, 23–45. [Google Scholar] [CrossRef]
- Gattinoni, L.; Lugli, E.; Ji, Y.; Pos, Z.; Paulos, C.M.; Quigley, M.F.; Almeida, J.R.; Gostick, E.; Yu, Z.; Carpenito, C.; Wang, E.; Douek, D.C.; Price, D.A.; June, C.H.; Marincola, F.M.; Roederer, M.; Restifo, N.P. A human memory T cell subset with stem cell-like properties. Nat. Med. 2011, 17, 1290–1297. [Google Scholar]
- Bucy, R.P.; Karr, L.; Huang, G.Q.; Li, J.; Carter, D.; Honjo, K.; Lemons, J.A.; Murphy, K.M.; Weaver, C.T. Single cell analysis of cytokine gene coexpression during CD4+ T-cell phenotype development. Proc. Natl. Acad. Sci. USA 1995, 92, 7565–7569. [Google Scholar]
- Brehm, M.A.; Daniels, K.A.; Welsh, R.M. Rapid production of TNF-alpha following TCR engagement of naive CD8 T cells. J. Immunol. 2005, 175, 5043–5049. [Google Scholar]
- Veiga-Fernandes, H.; Walter, U.; Bourgeois, C.; McLean, A.; Rocha, B. Response of naive and memory CD8+ T cells to antigen stimulation in vivo. Nat. Immunol. 2000, 1, 47–53. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Anthony, D.D.; Milkovich, K.A.; Zhang, W.; Rodriguez, B.; Yonkers, N.L.; Tary-Lehmann, M.; Lehmann, P.V. Dissecting the T Cell Response: Proliferation Assays vs. Cytokine Signatures by ELISPOT. Cells 2012, 1, 127-140. https://doi.org/10.3390/cells1020127
Anthony DD, Milkovich KA, Zhang W, Rodriguez B, Yonkers NL, Tary-Lehmann M, Lehmann PV. Dissecting the T Cell Response: Proliferation Assays vs. Cytokine Signatures by ELISPOT. Cells. 2012; 1(2):127-140. https://doi.org/10.3390/cells1020127
Chicago/Turabian StyleAnthony, Donald D., Kimberly A. Milkovich, Wenji Zhang, Benigno Rodriguez, Nicole L. Yonkers, Magdalena Tary-Lehmann, and Paul V. Lehmann. 2012. "Dissecting the T Cell Response: Proliferation Assays vs. Cytokine Signatures by ELISPOT" Cells 1, no. 2: 127-140. https://doi.org/10.3390/cells1020127
APA StyleAnthony, D. D., Milkovich, K. A., Zhang, W., Rodriguez, B., Yonkers, N. L., Tary-Lehmann, M., & Lehmann, P. V. (2012). Dissecting the T Cell Response: Proliferation Assays vs. Cytokine Signatures by ELISPOT. Cells, 1(2), 127-140. https://doi.org/10.3390/cells1020127





