Effect of Salinity Stress and Microbial Inoculations on Glomalin Production and Plant Growth Parameters of Snap Bean (Phaseolus vulgaris)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Experimental Layout
2.2. Planting Schemes and Data Collection
2.3. Plant Height, Leaf Chlorophyll Content and Bean Yield
2.4. Shoot and Root Dry Biomass
2.5. Estimation of AMF (Arbuscular Mycorrhizal Fungi) Root Colonization
2.6. Glomalin-Related Soil Protein (GRSP) Extraction
2.7. Statistical Analyses
3. Results and Discussion
3.1. Effect of Salinity and Microbial Inoculations on Soil and Plant Parameters
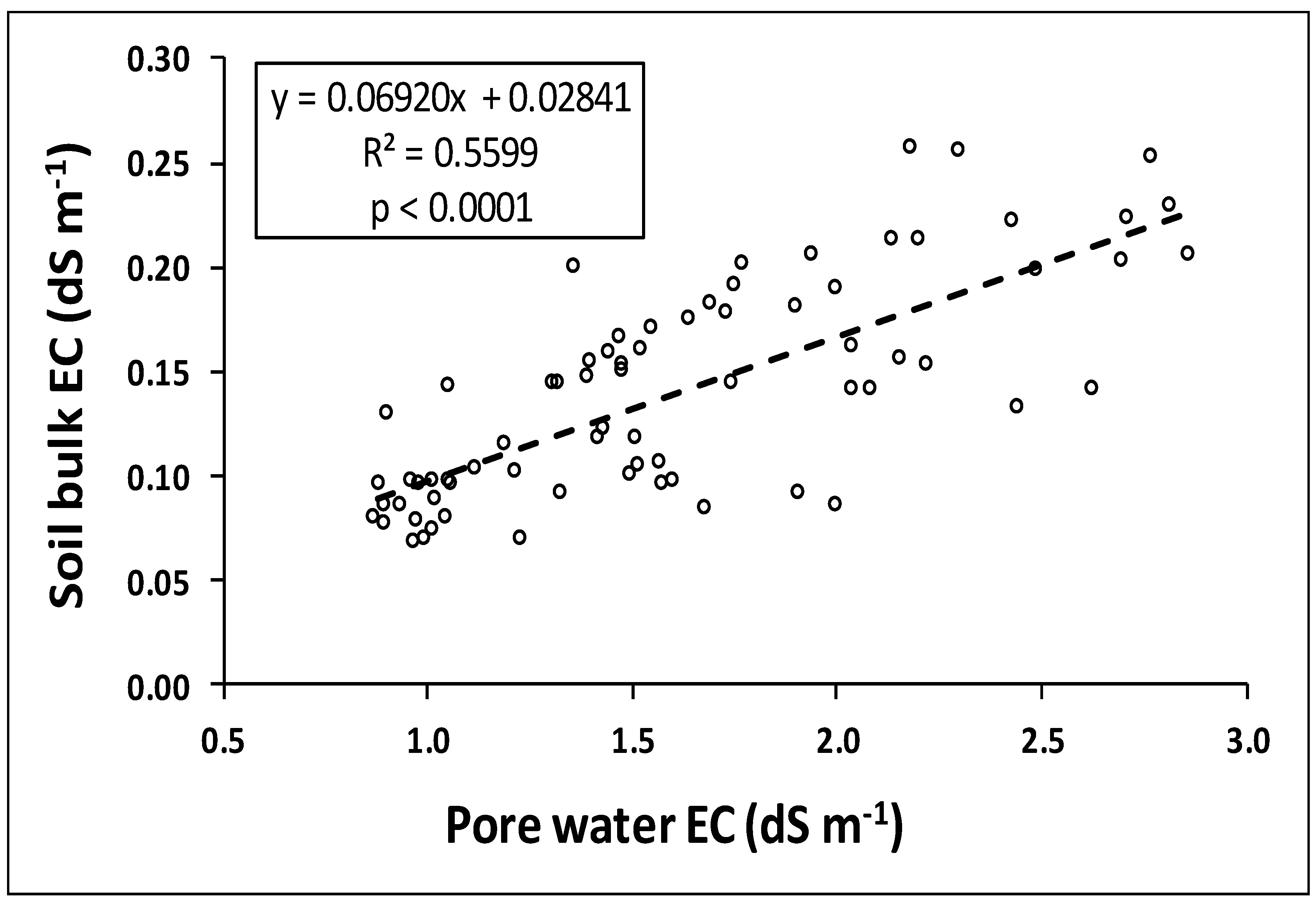
3.2. Analysis of Pore Water EC and Soil Bulk EC
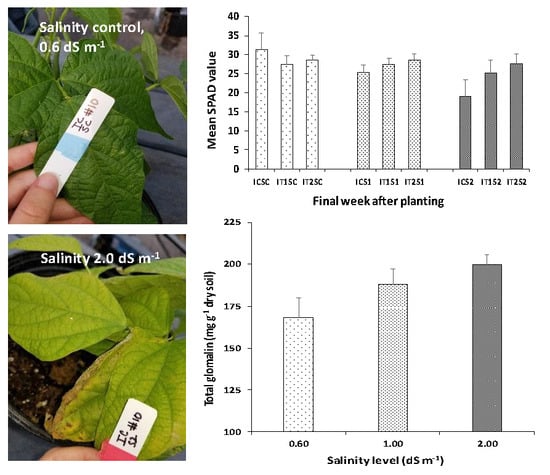
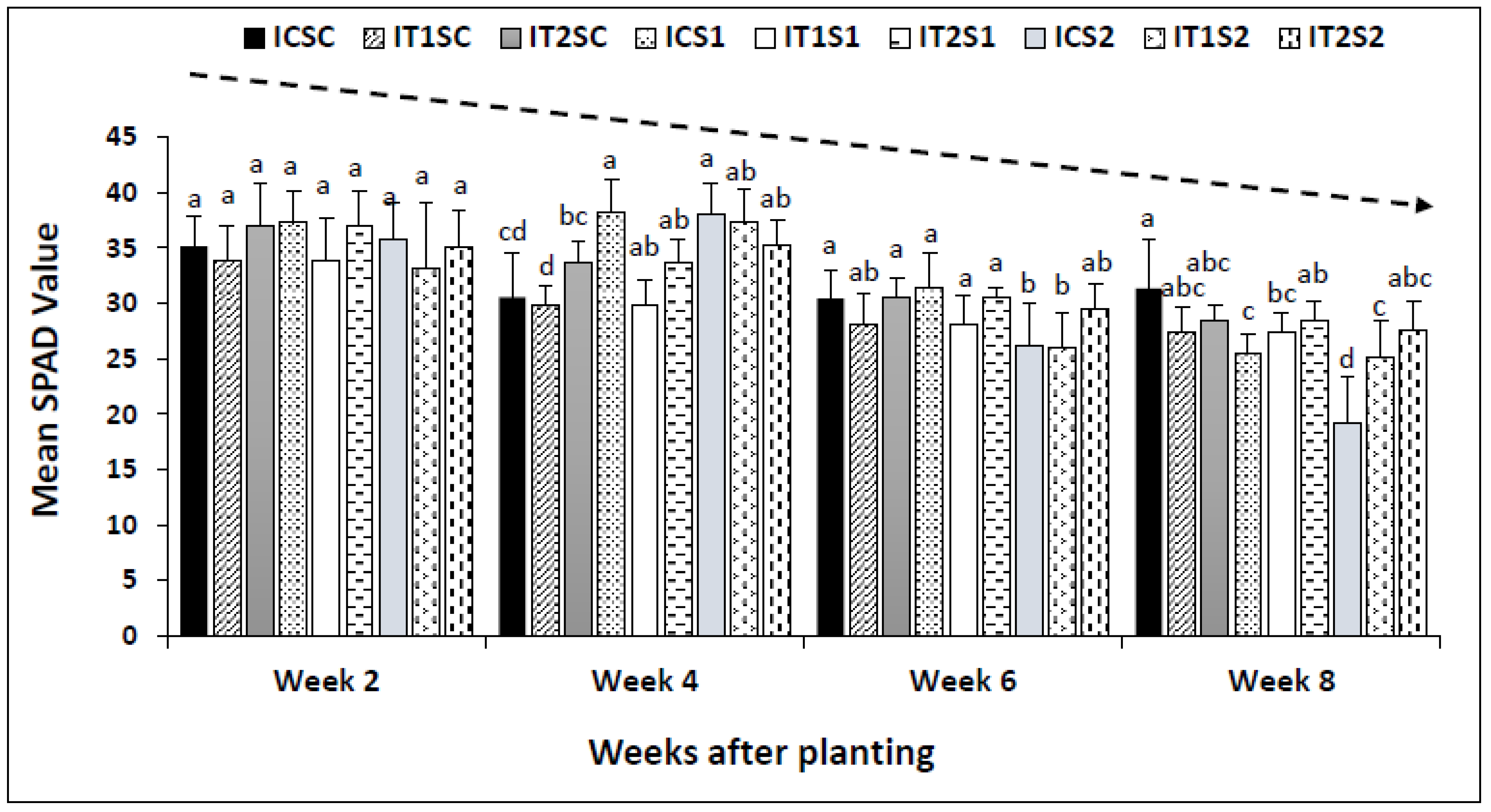
3.3. Analysis of Leaf Chlorophyll Content (SPAD Readings)
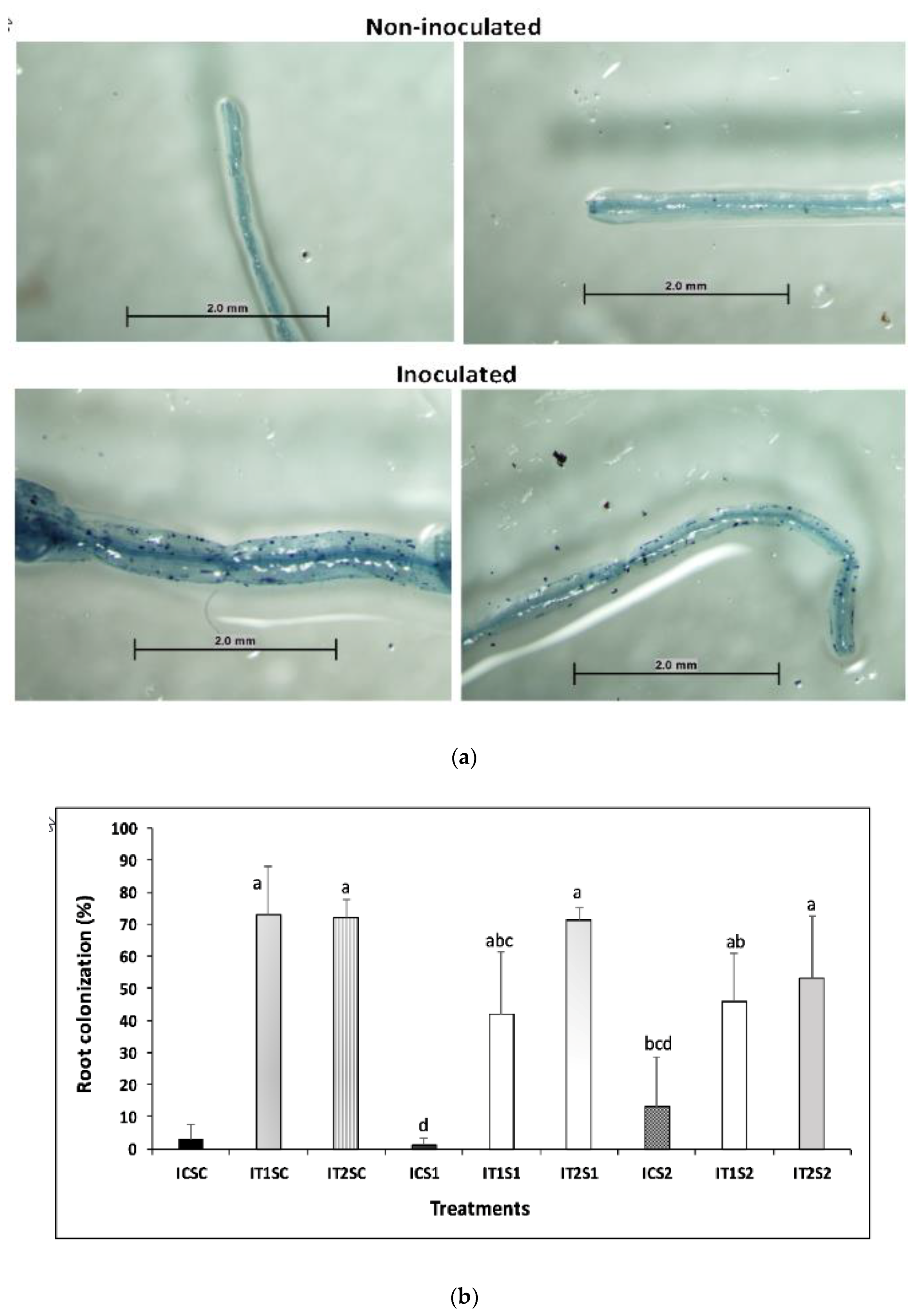
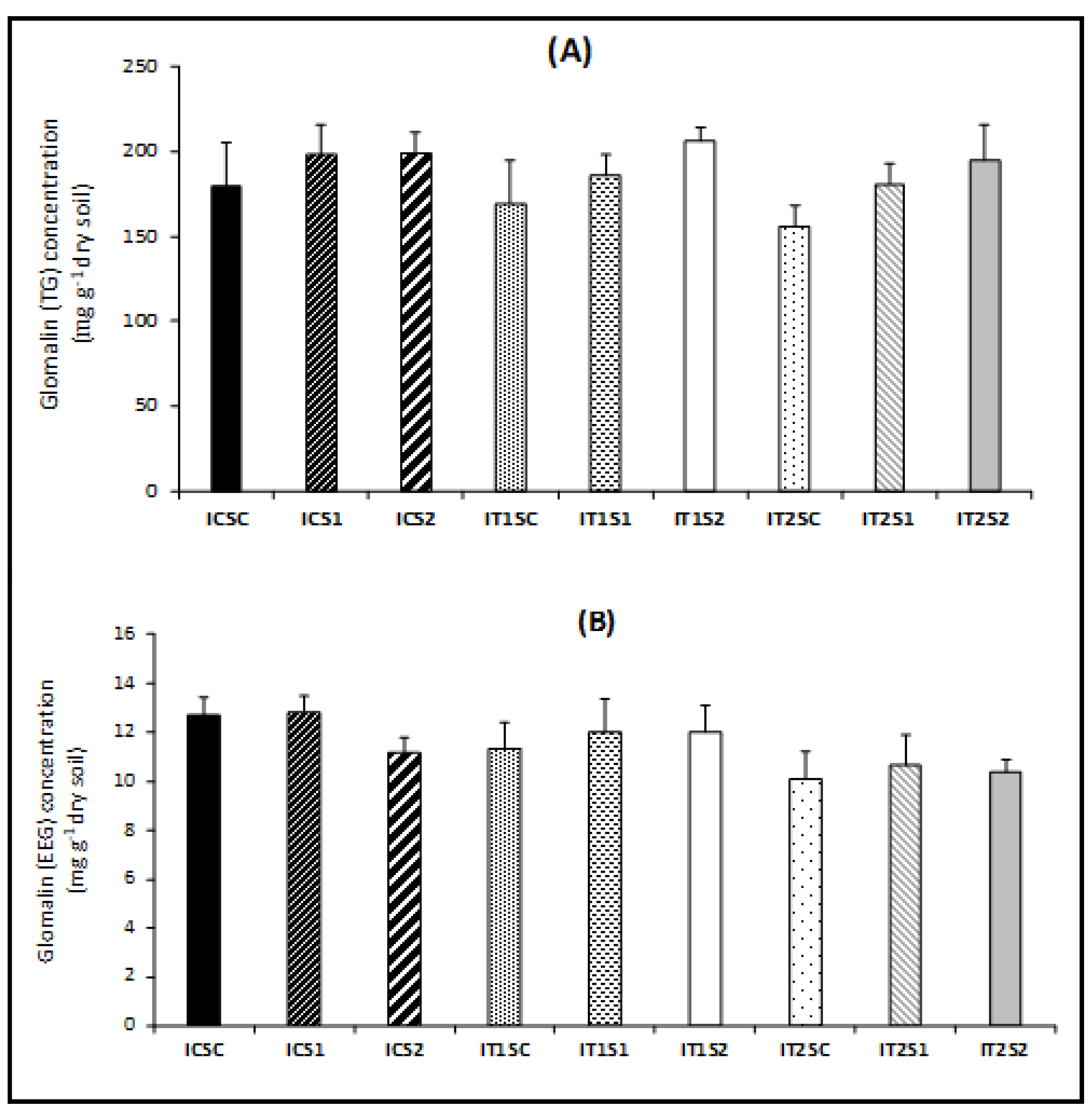
3.4. Root Colonization of AMF and Glomalin Production (TG and EEG)
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| EC | Electrical conductivity |
| GRSP | Glomalin Related Soil Protein |
| EG | Easily Extractable Glomalin |
| AMF | Arbuscular Mycorrhizal Fungi |
| DAP | Days After Planting |
| SPAD | Soil Plant Analytical Development |
| SOC | Soil Organic Carbon |
References
- Food and Agriculture Organization of the United Nations (FAO). Status of the World’s Soil Resources; FAO: Rome, Italy, 2015. [Google Scholar]
- Tanji, K.K. Salinity in the Soil Environment. In Salinity: Environment—Plants—Molecules; Läuchli, A., Lüttge, U., Eds.; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Zhou, M.; Butterbach-Bahl, K.; Vereecken, H.; Brüggemann, N. A meta-analysis of soil salinization effects on nitrogen pools, cycles and fluxes in coastal ecosystems. Glob. Chang. Biol. 2017, 23, 1338–1352. [Google Scholar] [CrossRef] [PubMed]
- Kaleris, V.K.; Ziogas, A.I. The effect of cutoff walls on saltwater intrusion and groundwater extraction in coastal aquifers. J. Hydrol. 2013, 476, 370–383. [Google Scholar] [CrossRef]
- Barlow, P.M.; Reichard, E.G. Saltwater intrusion in coastal regions of North America. J. Hydrol. 2010, 18, 247–260. [Google Scholar] [CrossRef]
- Guha, H.; Panday, S. Impact of Sea Level Rise on Groundwater Salinity in a Coastal Community of South Florida. J. Am. Water Resour. Assoc. 2012, 48, 510–529. [Google Scholar] [CrossRef]
- Price, R.M.; Nuttle, W.K.; Cosby, B.J.; Swart, P.K. Variation and uncertainty in evaporation from a subtropical estuary: Florida Bay. Estuaries Coasts 2007, 30, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Satbenau, E.; Kotun, K. Salinity and Hydrology of Florida Bay: Status and Trends 1990-2009; South Florida Natural Resources Center: Homestead, FL, USA, 2012.
- Numan, M.; Bashir, S.; Khan, Y.; Mumtaz, R.; Shinwari, Z.K.; Khan, A.L.; Khan, A.; AL-Harrasi, A. Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: A review. Microbiol. Res. 2018, 209, 21–32. [Google Scholar] [CrossRef]
- Manchanda, G.; Garg, N. Salinity and its effects on the functional biology of legumes. Acta Physiol. Plant. 2008, 30, 595–618. [Google Scholar] [CrossRef]
- Evelin, H.; Devi, T.S.; Gupta, S.; Kapoor, R. Mitigation of Salinity Stress in Plants by Arbuscular Mycorrhizal Symbiosis: Current Understanding and New Challenges. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Sathya, A.; Vijayabharathi, R.; Varshney, R.K.; Gowda, C.L.L.; Krishnamurthy, L. Plant growth promoting rhizobia: Challenges and opportunities. 3 Biotech 2015, 5, 355–377. [Google Scholar] [CrossRef]
- Porcel, R.; Aroca, R.; Ruiz-Lozano, J.M. Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron. Sustain. Dev. 2012, 32, 181–200. [Google Scholar] [CrossRef]
- Shober, A.L. Soils and Fertilizers for Master Gardeners: Organisms in the Soil. 2009. Available online: https://edis.ifas.ufl.edu/pdffiles/MG/MG45200.pdf (accessed on 10 September 2019).
- Hanson, B.R.; Grattan, S.R.; Fulton, R. Agricultural Salinity and Drainage; Division of Agriculture and Natural Resources, University of California: Davis, CA, USA, 2006. [Google Scholar]
- Freeman, J.H.; Dittmar, P.J.; Vallad, G.E. Commercial Vegetable Production in Florida. In Vegetable Production Handbook of Florida; UF/IFAS Extension Service, University of Florida: Gainesville, FL, USA, 2019; pp. 1–2. [Google Scholar]
- Marella, R.L. Water withdrawals in Florida, 2012. USGS, 2015. Available online: https://pubs.usgs.gov/of/2015/1156/ofr20151156_marella-water-use-2012.pdf (accessed on 10 September 2019).[Green Version]
- Schubert, S.; Neubert, A.; Schierholt, A.; Sümer, A.; Zörb, C. Development of salt-resistant maize hybrids: The combination of physiological strategies using conventional breeding methods. Plant Sci. 2009, 177, 196–202. [Google Scholar] [CrossRef]
- Dodd, I.C.; Pérez-Alfocea, F. Microbial amelioration of crop salinity stress. J. Exp. Bot. 2012, 63, 3415–3428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hack, C.M.; Porta, M.; Schäufele, R.; Grimoldi, A.A. Arbuscular mycorrhiza mediated effects on growth, mineral nutrition and biological nitrogen fixation of Melilotus alba Med. in a subtropical grassland soil. Appl. Soil Ecol. 2019, 134, 38–44. [Google Scholar] [CrossRef]
- Wang, S.; Feng, Z.; Wang, X.; Gong, W. Arbuscular mycorrhizal fungi alter the response of growth and nutrient uptake of snap bean (Phaseolus vulgaris L.) to O 3. J. Environ. Sci. 2011, 23, 968–974. [Google Scholar] [CrossRef]
- Saleh Al-Garni, S.M. Increased heavy metal tolerance of cowpea plants by dual inoculation of an arbuscular mycorrhizal fungi and nitrogen-fixer Rhizobium bacterium. African J. Biotechnol. 2006, 5, 133–142. [Google Scholar]
- Diouf, D.; Duponnois, R.; Ba, A.T.; Neyra, M.; Lesueur, D. Symbiosis of Acacia auriculiformis and Acacia mangium with mycorrhizal fungi and Bradyrhizobium spp. improves salt tolerance in greenhouse conditions. Funct. Plant Biol. 2005, 32, 1143–1152. [Google Scholar] [CrossRef]
- Fox, J.E.; Gulledge, J.; Engelhaupt, E.; Burow, M.E.; McLachlan, J.A. Pesticides reduce symbiotic efficiency of nitrogen-fixing rhizobia and host plants. Proc. Natl. Acad. Sci. USA 2007, 104, 10282–10287. [Google Scholar] [CrossRef] [Green Version]
- Zahran, H.H. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol. Mol. Biol. Rev. 1999, 63, 968–989. [Google Scholar]
- Atieno, M.; Lesueur, D. Opportunities for improved legume inoculants: Enhanced stress tolerance of rhizobia and benefits to agroecosystems. Symbiosis 2019, 77, 191–205. [Google Scholar] [CrossRef]
- Soussi, M.; Santamaría, M.; Ocaña, A.; Lluch, C. Effects of salinity on protein and lipopolysaccharide pattern in a salt-tolerant strain of Mesorhizobium ciceri. J. Appl. Microbiol. 2001, 90, 476–481. [Google Scholar] [CrossRef] [Green Version]
- Deaker, R.; Roughley, R.J.; Kennedy, I.R. Legume seed inoculation technology–A review. Soil Biol. Biochem. 2004, 36, 1275–1288. [Google Scholar] [CrossRef]
- Christy Kala, T.; Mary Christi, R.; Renuka Bai, N. Effect of rhizobium inoculation on the growth and yield of horsegram (Dolichos biflorus Linn). Plant Arch. 2011, 1, 97–99. [Google Scholar]
- Zhang, W.; Wang, C.; Liu, M.; Yu, Y. Integrated reclamation of saline soil nitrogen transformation in the hyphosphere by earthworms and arbuscular mycorrhizal fungus. Appl. Soil Ecol. 2019, 135, 137–146. [Google Scholar] [CrossRef]
- Lǚ, L.H.; Zou, Y.N.; Wu, Q.S. Mycorrhizas Mitigate Soil Replant Disease of Peach Through Regulating Root Exudates, Soil Microbial Population, and Soil Aggregate Stability. Commun. Soil Sci. Plant Anal. 2019, 50, 909–921. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Wang, P.; Wu, Q.H.; Zou, Y.N.; Bao, Q.; Wu, Q.S. Arbuscular mycorrhizas improve plant growth and soil structure in trifoliate orange under salt stress. Arch. Agron. Soil Sci. 2017, 63, 491–500. [Google Scholar] [CrossRef]
- Hu, Z.H.; Zhuo, F.; Jing, S.H.; Li, X.; Yan, T.X.; Lei, L.L.; Lu, R.R.; Zhang, X.F.; Jing, Y.X. Combined application of arbuscular mycorrhizal fungi and steel slag improves plant growth and reduces Cd, Pb accumulation in Zea mays. Int. J. Phytoremediation 2019, 21, 857–865. [Google Scholar] [CrossRef]
- Scholberg, J.M.S.; Locascio, S.J. Growth response of snap bean and tomato as affected by salinity and irrigation method. HortScience 1999, 34, 259–264. [Google Scholar] [CrossRef]
- Hamaoui, B.; Abbadi, J.; Burdman, S.; Rashid, A.; Sarig, S. Effects of inoculation with Azospirillum brasilense on chickpeas (Cicer arietinum) and faba beans (Vicia faba) under different growth conditions. Agronomie 2001, 21, 553–560. [Google Scholar] [CrossRef]
- Freidenreich, A.; Barraza, G.; Jayachandran, K.; Khoddamzadeh, A.A. Precision Agriculture Application for Sustainable Nitrogen Management of Justicia brandegeana Using Optical Sensor Technology. Agriculture 2019, 9, 98. [Google Scholar] [CrossRef]
- Ling, Q.; Huang, W.; Jarvis, P. Use of a SPAD-502 meter to measure leaf chlorophyll concentration in Arabidopsis thaliana. Photosynth. Res. 2011, 107, 209–214. [Google Scholar] [CrossRef]
- Miller, M.H.; McGonigle, T.P.; Swan, J.A.; Evans, D.G.; Fairchild, G.L. A New Method which Gives an Objective Measure of Colonization of Roots by Vesicular-Arbuscular Mycorrhizal Fungi. New Phytol. 1990, 115, 495–501. [Google Scholar]
- Wright, S.F.; Upadhyaya, A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil 1998, 198, 97–107. [Google Scholar] [CrossRef]
- Wang, W.; Zhong, Z.; Wang, Q.; Wang, H.; Fu, Y.; He, X. Glomalin contributed more to carbon, nutrients in deeper soils, and differently associated with climates and soil properties in vertical profiles. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SAS Software, version 9.4; SAS Institute Inc.: Cary, NC, USA, 2013.
- Mori, M.; Di Mola, I.; Chiarandà, F.Q. Salt stress and transplant time in snap bean: Growth and productive behaviour. Int. J. Plant Prod. 2011, 5, 49–64. [Google Scholar]
- Selvakumar, G.; Yi, P.H.; Lee, S.E.; Shagol, C.C.; Han, S.G.; Sa, T.; Chung, B.N. Effects of long-term subcultured Arbuscular mycorrhizal fungi on red pepper plant growth and soil glomalin content. Mycobiology 2018, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Hasamuzzaman, M.; Fujita, M.; Islam, M.N.; Ahamed, K.U.; Nahar, K. Performance of four irrigated rice varieties under different levels of salinity stress. Int. J. Integr. Biol. 2009, 6, 85–90. [Google Scholar]
- De Almeida, W.F.; da Paz, V.P.; de Jesus, A.P.C.; da Silva, J.S.; Gonçalves, K.S.; de Oliveira, A.S. Yield of green beans subjected to continuous and pulse drip irrigation with saline water. Rev. Bras. Eng. Agríc. Ambient. 2018, 22, 476–481. [Google Scholar] [CrossRef]
- Shridhar, B.S.; Author, C.; Shrimant Shridhar, B.; Shridhar, B.S.; Author, C.; Shrimant Shridhar, B. Review: Nitrogen Fixing Microorganisms. Int. J. Microbiol. Res. 2012. [Google Scholar] [CrossRef]
- de Souza, R.; Ambrosini, A.; Passaglia, L.M.P. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef]
- Qin, L.; Jiang, H.; Tian, J.; Zhao, J.; Liao, H. Rhizobia enhance acquisition of phosphorus from different sources by soybean plants. Plant Soil 2011, 349, 25–36. [Google Scholar] [CrossRef]
- Franzini, V.I.; Azcón, R.; Ruiz-Lozano, J.M.; Aroca, R. Rhizobial symbiosis modifies root hydraulic properties in bean plants under non-stressed and salinity-stressed conditions. Planta 2019, 249, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Taïbi, K.; Taïbi, F.; Ait Abderrahim, L.; Ennajah, A.; Belkhodja, M.; Mulet, J.M. Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. South African J. Bot. 2016, 105, 306–312. [Google Scholar] [CrossRef]
- Tester, M.; Davenport, R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.G.; Bai, Y.J.; Kong, C.C.; Bian, B.; Xie, Z.H. Synergistic Interactions Between Salt-tolerant Rhizobia and Arbuscular Mycorrhizal Fungi on Salinity Tolerance of Sesbania cannabina Plants. J. Plant Growth Regul. 2016, 35, 1098–1107. [Google Scholar] [CrossRef]
- Bothe, H. Arbuscular mycorrhiza and salt tolerance of plants. Symbiosis 2012, 58, 7–16. [Google Scholar] [CrossRef]
- Yao, L.; Wu, Z.; Zheng, Y.; Kaleem, I.; Li, C. Growth promotion and protection against salt stress by Pseudomonas putida Rs-198 on cotton. Eur. J. Soil Biol. 2010, 46, 49–54. [Google Scholar] [CrossRef]
- Peng, Z.; He, S.; Sun, J.; Pan, Z.; Gong, W.; Lu, Y.; Du, X. Na + compartmentalization related to salinity stress tolerance in upland cotton (Gossypium hirsutum) seedlings. Sci. Rep. 2016. [Google Scholar] [CrossRef]
- Apte, S.K.; Thomas, J. Possible amelioration of coastal soil salinity using halotolerant nitrogen-fixing cyanobacteria. Plant Soil 1997, 189, 205–211. [Google Scholar] [CrossRef]
- Giri, B.; Kapoor, R.; Mukerji, K.G. Influence of arbuscular mycorrhizal fungi and salinity on growth, biomass, and mineral nutrition of Acacia auriculiformis. Biol. Fertil. Soils 2003, 38, 170–175. [Google Scholar] [CrossRef]
- Al-Karaki, G.N. Nursery inoculation of tomato with arbuscular mycorrhizal fungi and subsequent performance under irrigation with saline water. Sci. Hortic. 2006, 109, 1–7. [Google Scholar] [CrossRef]
- Soliman, A.S.; Shanan, N.T.; Massoud, O.N.; Swelim, D.M. Improving salinity tolerance of Acacia saligna (Labill.) plant by arbuscular mycorrhizal fungi and Rhizobium inoculation. African J. Biotechnol. 2014, 11, 1259–1266. [Google Scholar]
- Valifard, M.; Mohsenzadeh, S.; Kholdebarin, B.; Rowshan, V.; Niazi, A.; Moghadam, A. Effect of salt stress on terpenoid biosynthesis in Salvia mirzayanii: From gene to metabolite. J. Hortic. Sci. Biotechnol. 2019, 94, 389–399. [Google Scholar] [CrossRef]
- Babu, N.; Devaraj, R. High temperature and salt stress response in French bean (Phaseolus vulgaris). Aust. J. Crop Sci. South. Cross J. 2008, 2, 40–48. [Google Scholar]
- Gama, P.B.; Inanaga, S.; Tanaka, K.; Nakazawa, R. Physiological response of common bean (Phaseolus vulgaris L.) seedlings to salinity stress. African J. Biotechnol. 2007, 6, 79–88. [Google Scholar]
- Mortimer, P.E.; Pérez-Fernández, M.A.; Valentine, A.J. The role of arbuscular mycorrhizal colonization in the carbon and nutrient economy of the tripartite symbiosis with nodulated Phaseolus vulgaris. Soil Biol. Biochem. 2008, 40, 1019–1027. [Google Scholar] [CrossRef]
- Wu, F.; Li, J.; Chen, Y.; Zhang, L.; Zhang, Y.; Wang, S.; Shi, X.; Li, L.; Liang, J. Effects of phosphate solubilizing bacteria on the growth, photosynthesis, and nutrient uptake of Camellia oleifera abel. Forests 2019, 10, 348. [Google Scholar] [CrossRef]
- Priya, H.; Prasanna, R.; Ramakrishnan, B.; Bidyarani, N.; Babu, S.; Thapa, S.; Renuka, N. Influence of cyanobacterial inoculation on the culturable microbiome and growth of rice. Microbiol. Res. 2015, 171, 78–89. [Google Scholar] [CrossRef]
- Sheng, M.; Tang, M.; Chen, H.; Yang, B.; Zhang, F.; Huang, Y. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 2008, 18, 287–296. [Google Scholar] [CrossRef]
- Giri, B.; Kapoor, R.; Mukerji, K.G. Improved tolerance of Acacia nilotica to salt stress by arbuscular mycorrhiza, Glomus fasciculatum may be partly related to elevated K/Na ratios in root and shoot tissues. Microb. Ecol. 2007, 54, 753–760. [Google Scholar] [CrossRef]
- Juniper, S.; Abbott, L.K. Soil salinity delays germination and limits growth of hyphae from propagules of arbuscular mycorrhizal fungi. Mycorrhiza 2006, 16, 371–379. [Google Scholar] [CrossRef]
- Wright, S.F.; Upadhyaya, A. Extraction of an abundant and unusual protein from soil and comparison with hyphal protein of arbuscular mycorrhizal fungi. Soil Sci. 1996, 161, 575–586. [Google Scholar] [CrossRef]
- Zou, Y.N.; Srivastava, A.K.; Wu, Q.S.; Huang, Y.M. Glomalin-related soil protein and water relations in mycorrhizal citrus (Citrus tangerina) during soil water deficit. Arch. Agron. Soil Sci. 2014, 60, 1103–1114. [Google Scholar] [CrossRef]
- Hammer, E.C.; Rillig, M.C. The influence of different stresses on glomalin levels in an arbuscular mycorrhizal fungus-salinity increases glomalin content. PLoS ONE 2011, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; He, C.; Huang, L.; Ban, Y.; Tang, M. The effects of arbuscular mycorrhizal fungi on glomalin-related soil protein distribution, aggregate stability and their relationships with soil properties at different soil depths in lead-zinc contaminated area. PLoS ONE 2017, 12, e0182264. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zong, J.; Que, H.; Zhou, Z.; Xiao, M.; Chen, S. Inoculation with arbuscular mycorrhizal fungi increases glomalin-related soil protein content and PAH removal in soils planted with Medicago sativa L. Soil Biol. Biochem. 2017, 115, 148–151. [Google Scholar] [CrossRef]
- Walley, F.L.; Gillespie, A.W.; Adetona, A.B.; Germida, J.J.; Farrell, R.E. Manipulation of rhizosphere organisms to enhance glomalin production and C sequestration: Pitfalls and promises. Can. J. Plant Sci. 2013, 94, 1025–1032. [Google Scholar] [CrossRef]
- Averill, C.; Turner, B.L.; Finzi, A.C. Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature 2014, 505, 543–545. [Google Scholar] [CrossRef]
- Bedini, S.; Pellegrino, E.; Avio, L.; Pellegrini, S.; Bazzoffi, P.; Argese, E.; Giovannetti, M. Changes in soil aggregation and glomalin-related soil protein content as affected by the arbuscular mycorrhizal fungal species Glomus mosseae and Glomus intraradices. Soil Biol. Biochem. 2009, 41, 1491–1496. [Google Scholar] [CrossRef]
- Wright, S.F.; Anderson, R.L. Aggregate stability and glomalin in alternative crop rotations for the central Great Plains. Biol. Fertil. Soils 2000, 31, 249–253. [Google Scholar] [CrossRef]
- Singh, P.K. Role of Glomalin Related Soil Protein Produced by Arbuscular Mycorrhizal Fungi: A Review. Agric. Sci. Res. J. 2012, 2, 119–125. [Google Scholar]
- Smith, S.E.; Read, D. Arbuscular mycorrhizas. In Mycorrhizal Symbiosis; Elsevier: Amsterdam, The Netherlands, 2008; pp. 111–145. [Google Scholar]
- Spagnoletti, F.; Carmona, M.; Gómez, N.E.T.; Chiocchio, V.; Lavado, R.S. Arbuscular mycorrhiza reduces the negative effects of M. phaseolina on soybean plants in arsenic-contaminated soils. Appl. Soil Ecol. 2017, 121, 41–47. [Google Scholar] [CrossRef]
- Rillig, M.C.; Wright, S.F.; Nichols, K.A.; Schmidt, W.F.; Torn, M.S. Large contribution of arbuscular mycorrhizal fungi to soil carbon pools in tropical forest soils. Plant Soil 2001, 233, 167–177. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Miller, R.M. Carbon cycling by arbuscular mycorrhizal fungi in soil-plant systems. Trends Plant Sci. 2003, 8, 407–409. [Google Scholar] [CrossRef]


| Treatment | Soil pH Week 8 | Plant Height (cm) Week 8 | Total Bean Yield (g) | Shoot Dry Weight (g) | Root Dry Weight (g) | Shoot to Root Ratio |
|---|---|---|---|---|---|---|
| ICSC | 6.63 ± 0.48 | 26.48 c ± 2.76 | 44.20 ab ± 7.47 | 5.57 ab ± 0.74 | 1.60 a ± 0.64 | 3.48 |
| IT1SC | 6.52 ± 0.32 | 27.15 c ± 2.91 | 23.93 c ± 7.16 | 3.99 bc ± 0.97 | 1.44 a ± 0.35 | 2.77 |
| IT2SC | 6.76 ± 0.22 | 26.78 c ± 4.91 | 33.29 bc ± 9.71 | 5.61 a ± 1.21 | 1.53 a ± 0.46 | 3.67 |
| ICS1 | 6.36 ± 0.38 | 28.53 bc ± 2.33 | 57.26 a ± 8.00 | 6.53 a ± 1.58 | 1.52 a ± 0.28 | 4.30 |
| IT1S1 | 6.30 ± 0.34 | 31.01 abc ± 2.44 | 27.97 c ± 5.21 | 5.03 abc ± 0.93 | 1.59 a ± 0.25 | 3.16 |
| IT2S1 | 6.60 ±0.18 | 30.83 abc ± 2.66 | 43.57 b ± 10.38 | 6.09 a ± 0.87 | 1.51 a ± 0.22 | 4.03 |
| ICS2 | 6.77 ± 0.37 | 29.52 abc ± 4.15 | 35.49 bc ± 14.83 | 4.96 abc ± 1.29 | 1.27 a ± 0.25 | 3.91 |
| IT1S2 | 6.48 ± 0.22 | 32.31 ab ± 3.74 | 30.36 c ± 8.56 | 3.71 c ± 1.30 | 1.44 a ± 0.29 | 2.58 |
| IT2S2 | 6.60 ± 0.30 | 33.80 a ± 3.95 | 35.36 bc ± 6.03 | 5.19 abc ± 0.71 | 1.42 a ± 0.29 | 3.65 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, C.L.; Dattamudi, S.; Chanda, S.; Jayachandran, K. Effect of Salinity Stress and Microbial Inoculations on Glomalin Production and Plant Growth Parameters of Snap Bean (Phaseolus vulgaris). Agronomy 2019, 9, 545. https://doi.org/10.3390/agronomy9090545
Garcia CL, Dattamudi S, Chanda S, Jayachandran K. Effect of Salinity Stress and Microbial Inoculations on Glomalin Production and Plant Growth Parameters of Snap Bean (Phaseolus vulgaris). Agronomy. 2019; 9(9):545. https://doi.org/10.3390/agronomy9090545
Chicago/Turabian StyleGarcia, Claudia Lyl, Sanku Dattamudi, Saoli Chanda, and Krishnaswamy Jayachandran. 2019. "Effect of Salinity Stress and Microbial Inoculations on Glomalin Production and Plant Growth Parameters of Snap Bean (Phaseolus vulgaris)" Agronomy 9, no. 9: 545. https://doi.org/10.3390/agronomy9090545
APA StyleGarcia, C. L., Dattamudi, S., Chanda, S., & Jayachandran, K. (2019). Effect of Salinity Stress and Microbial Inoculations on Glomalin Production and Plant Growth Parameters of Snap Bean (Phaseolus vulgaris). Agronomy, 9(9), 545. https://doi.org/10.3390/agronomy9090545