Effects of Light, Temperature, and Soil Depth on the Germination and Emergence of Conyza canadensis (L.) Cronq.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Germination Tests
2.2. Emergence Tests
2.3. Statistical Analyses
2.4. Modeling Germination Rates as a Function of Temperature
3. Results
3.1. Germination Tests
3.2. Emergence Tests
3.3. Modeling Germination Rates as a Function of Temperature
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clements, D.R.; Ditommaso, A. Climate change and weed adaptation: Can evolution of invasive plants lead to greater range expansion than forecasted? Weed Res. 2011, 51, 227–240. [Google Scholar] [CrossRef]
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. Summary for Policymakers. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC); Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- McDonald, A.; Riha, S.; Ditommaso, A.; DeGaetano, A. Climate change and the geography of weed damage: Analysis of U.S. maize systems suggests the potential for significant range transformations. Agric. Ecosyst. Environ. 2009, 130, 131–140. [Google Scholar] [CrossRef]
- Bonciarelli, U.; Onofri, A.; Benincasa, P.; Farneselli, M.; Guiducci, M.; Pannacci, E.; Tosti, G.; Tei, F. Long-term evaluation of productivity, stability and sustainability for cropping systems in Mediterranean rainfed conditions. Eur. J. Agron. 2016, 77, 146–155. [Google Scholar] [CrossRef]
- Potter, K.J.B.; Kriticos, D.J.; Watt, M.S.; Leriche, A. The current and future potential distribution of Cytisus scoparius: A weed of pastoral systems, natural ecosystems and plantation forestry. Weed Res. 2009, 49, 271–282. [Google Scholar] [CrossRef]
- Har-Edom, O.-L.; Sternberg, M. Invasive species and climate change: Conyza Canadensis (L.) Cronquist as a tool for assessing the invisibility of natural plant communities along an aridity gradient. Biol. Invasions 2010, 12, 1953–1960. [Google Scholar] [CrossRef]
- Graziani, F.; Onofri, A.; Pannacci, E.; Tei, F.; Guiducci, M. Size and composition of weed seedbank in long-term organic and conventional low-input cropping systems. Eur. J. Agron. 2012, 39, 52–61. [Google Scholar] [CrossRef]
- Thuiller, W.; Richardson, D.M.; Rouget, M.; Proches, S.; Wilson, J.R.U. Interactions between environment, species traits, and human uses describe patterns of plant invasions. Ecology 2006, 87, 1755–1769. [Google Scholar] [CrossRef]
- Kathiresan, R.; Gualbert, G. Impact of climate change on the invasive traits of weeds. Weed Biol. Manag. 2016, 16, 59–66. [Google Scholar] [CrossRef]
- Kriticos, D.J.; Sutherst, R.W.; Brown, J.R.; Adkins, S.W.; Maywald, G.F. Climate change and the potential distribution of an invasive alien plant: Acacia nilotica ssp. indica in Australia. J. Appl. Ecol. 2003, 40, 111–124. [Google Scholar] [CrossRef]
- Vidotto, F.; Masin, R.; Pannacci, E.; Mariani, L. Effetti del cambiamento climatico sulla dinamica evolutiva delle malerbe. In Gestione delle Malerbe nelle Colture Agrarie alla Luce delle Attuali Problematiche Legislative, Agronomiche e Ambientali, Proceedings of the XIX Convegno S.I.R.F.I., Bologna, Italy, 17 December 2013; Vidotto, F., de Palo e Aldo Ferrero, F., Eds.; Tipografia Fiordo S.R.L.: Galliate, Italy, 2013. [Google Scholar]
- Weaver, S.E. The biology of Canadian weeds. 115. Conyza canadensis. Can. J. Plant Sci. 2001, 81, 867–875. [Google Scholar] [CrossRef]
- Benvenuti, S. Weed seed movement and dispersal strategies in the agricultural environment. Weed Biol. Manag. 2007, 7, 141–157. [Google Scholar] [CrossRef]
- Wu, H.; Walker, S.; Rollin, M.J.; Tan, D.K.Y.; Robinson, G.; Werth, J. Germination, persistence, and emergence of flaxleaf fleabane (Conyza bonariensis [L.] Cronquist). Weed Biol. Manag. 2007, 7, 192–199. [Google Scholar] [CrossRef]
- Fracchiolla, M.; Stellacci, A.M.; Cazzato, E.; Tedone, L.; Alhajj Ali, S.; De Mastro, G. Effects of Conservative Tillage and Nitrogen Management on Weed Seed Bank after a Seven-Year Durum Wheat—Faba Bean Rotation. Plants 2018, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Zanin, G.; Zuin, M.G.; Catizone, P. Definizione e classificazione delle malerbe. In Malerbologia; Catizone, P., Zanin, G., Eds.; Pàtron Editore: Bologna, Italy, 2001; pp. 23–52. [Google Scholar]
- Hanf, M. The Arable Weeds of Europe with Their Seedlings and Seeds; BASF: Hadleigh, UK, 1983. [Google Scholar]
- Frankton, C.; Mulligan, G.A. Weeds of Canada, No 948; NC Press Limited: Toronto, ON, Canada, 1987. [Google Scholar]
- Tozzi, E.; Beckie, H.; Weiss, R.; Gonzalez-Andujar, J.L.; Storkey, J.; Cici, S.Z.H.; Acker, R.C. Seed germination response to temperature for a range of international populations of Conyza canadensis. Weed Res. 2014, 54, 178–185. [Google Scholar] [CrossRef]
- Thebaud, C.; Abbott, R.J. Characterization of invasive Conyza species (Asteraceae) in Europe: Quantitative trait and isozyme analysis. Am. J. Bot. 1995, 82, 360–368. [Google Scholar] [CrossRef]
- Regehr, D.L.; Bazzaz, F.A. The Population Dynamics of Erigeron Canadensis, A Successional Winter Annual. J. Ecol. 1979, 67, 923–933. [Google Scholar] [CrossRef]
- Bhowmik, P.C.; Bekech, M.M. Horseweed (Conyza canadensis) seed production, emergence, and distribution in no tillage and conventional-tillage corn (Zea mays). Agronomy 1993, 1, 67–71. [Google Scholar]
- Dauer, J.T.; Mortensen, D.A.; Vangessel, M.J. Temporal and Spatial Dynamics of Long-Distance Conyza canadensis Seed Dispersal. J. Appl. Ecol. 2007, 44, 105–114. [Google Scholar] [CrossRef]
- Tsuyuzaki, S.; Kanda, F. Revegetation Patterns and Seedbank Structure on Abandoned Pastures in Northern Japan. Am. J. Bot. 1996, 83, 1422–1428. [Google Scholar] [CrossRef]
- Tremmel, D.C.; Peterson, K.M. Competitive Subordination of a Piedmont Old Field Successional Dominant by an Introduced Species. Am. J. Bot. 1983, 70, 1125–1132. [Google Scholar] [CrossRef]
- Steinmaus, S.J.; Prather, T.S.; Holt, J.S. Estimation of base temperatures for nine weed species. J. Exp. Bot. 2000, 51, 275–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nandula, V.K.; Eubank, T.W.; Poston, D.H.; Koger, C.H.; Reddy, K.N. Factors affecting germination of horseweed (Conyza canadensis). Weed Sci. 2006, 54, 898–902. [Google Scholar] [CrossRef]
- Mulligan, G.A.; Findlay, J.N. Reproductive systems and colonization in Canadian weeds. Can. J. Bot. 1970, 48, 859–860. [Google Scholar] [CrossRef]
- Whittle, C.A.; Duchesne, L.C.; Needham, T. The impact of broadcast burning and fire severity on species composition and abundance of surface vegetation in a jack pine (Pinus banksiana) clear-cut. Forest Ecol. Manag. 1997, 94, 141–148. [Google Scholar] [CrossRef]
- Ohtsuka, T. A comparative review of early herbaceous stages of secondary succession in temperate and tropical regions. Jpn. J. Ecol. 1998, 48, 143–157. [Google Scholar]
- Paula, J.M.; Vargas, L.; Agostinetto, D.; Nohatto, M.A. Management of Glyphosate-Resistant Conyza bonariensis. Planta Daninha 2011, 29, 217–227. [Google Scholar] [CrossRef]
- Kapusta, G. Seedbed Tillage and Herbicide Influence on Soybean (Glycine max) Weed Control and Yield. Weed Sci. 1979, 27, 520–526. [Google Scholar] [CrossRef]
- Buhler, D.D. Population Dynamics and Control of Annual Weeds in Corn (Zea mays) as Influenced by Tillage Systems. Weed Sci. 1992, 40, 241–248. [Google Scholar] [CrossRef]
- Wiese, A.F.; Salisbury, C.D.; Bean, B.W. Downy brome (Bromus tectorum), jointed goatgrass (Aegilops cylindrica) and horseweed (Conyza canadensis) control in fallow. Weed Technol. 1995, 9, 249–254. [Google Scholar] [CrossRef]
- Palma-Bautista, C.; Tahmasebi, B.K.; Fernández-Moreno, P.T.; Rojano-Delgado, A.M.; De la Cruz, R.A.; Prado, R.D. First case of Conyza canadensis from Hungary with multiple resistance to glyphosate and flazasulfuron. Agronomy 2018, 8, 157. [Google Scholar] [CrossRef]
- The International Survey of Herbicide Resistant Weeds (Heap). Available online: www.weedscience.org (accessed on 2 October 2017).
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 2014; pp. 150–162. [Google Scholar]
- Ritz, C.; Pipper, C.B.; Streibig, J.C. Analysis of germination data from agricultural experiments. Eur. J. Agron. 2013, 45, 1–6. [Google Scholar] [CrossRef]
- Faraway, J.J. Extending the Linear Model with R. Generalized Linear, Mixed Effects and Nonparametric Regression Models; Chapman & Hall/CRC: Boca Raton, FL, USA, 2006. [Google Scholar]
- Bretz, F.; Hothorn, T.; Westfall, P.H. Multiple Comparisons Using R.; Chapman & Hall: London, UK, 2011; ISBN 978-1-58488-574-0. [Google Scholar]
- Bradford, K.J. Applications of hydrothermal time to quantifying and modeling seed germination and dormancy. Weed Sci. 2002, 50, 248–260. [Google Scholar] [CrossRef]
- Catara, S.; Cristaudo, A.; Gualtieri, A.; Galesi, R.; Impelluso, C.; Onofri, A. Threshold temperatures for seed germination in nine species of Verbascum (Scrophulariaceae). Seed Sci. Res. 2016, 26, 30–46. [Google Scholar] [CrossRef]
- Masin, R.; Onofri, A.; Gasparini, V.; Zanin, G. Can alternating temperatures be used to estimate base temperature for seed germination? Weed Res. 2017, 57, 390–398. [Google Scholar] [CrossRef]
- Box, G.E.P.; Cox, D.R. An Analysis of Transformations. J. R. Stat. Soc. 1964, 26, 211–252. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R.-project.org/ (accessed on 22 May 2019).
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using, R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- Onofri, A.; Benincasa, P.; Mesgaran, M.B.; Ritz, C. Hydrothermal-time-to-event models for seed germination. Eur. J. Agron. 2018, 101, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Rowse, H.R.; Finch-Savage, W.E. Hydrothermal threshold models can describe the germination response of carrot (Daucus carota) and onion (Allium cepa) seed populations across both sub- and supra-optimal temperatures. New Phytol. 2003, 158, 101–108. [Google Scholar] [CrossRef]
- Forcella, F.; Benech-Arnold, R.L.; Sanchez, R.; Ghersa, C.M. Modeling seedling emergence. Field Crop Res. 2000, 67, 123–139. [Google Scholar] [CrossRef]
- Masin, R.; Loddo, D.; Gasparini, V.; Otto, S. Evaluation of Weed Emergence Model AlertInf for Maize in Soybean. Weed Sci. 2014, 62, 360–369. [Google Scholar] [CrossRef]
- Karlsson, L.M.; Milberg, P. Comparing after-ripening response and germination requirements of Conyza canadensis and C. bonariensis (Asteraceae) through logistic functions. Weed Res. 2007, 47, 433–441. [Google Scholar] [CrossRef]
- Davidson, A.M.; Jennions, M.; Nicotra, A.B. Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecol. Lett. 2011, 14, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Grantz, D.A.; Shrestha, A.; Vu, H. Early vigor and ozone response in horseweed (Conyza canadensis) biotypes differing in glyphosate resistance. Weed Sci. 2008, 56, 224–230. [Google Scholar] [CrossRef]
- Santos, G.; Oliveira, R.S.; Constantin, J.; Francischini, A.C.; Machado, M.F.P.S.; Mangolin, C.A.; Nakajima, J.N. Conyza sumatrensis: A new weed species resistant to glyphosate in the Americas. Weed Biol. Manag. 2014, 14, 106–114. [Google Scholar] [CrossRef]
- Leroux, G.D.; Benoit, D.L.; Banville, S. Effect of crop rotations on weed control, Bidens cernua and Erigeron canadensis populations, and carrot yields in organic soils. Crop Prot. 1996, 15, 171–178. [Google Scholar] [CrossRef]
- Pannacci, E.; Lattanzi, B.; Tei, F. Non-chemical weed management strategies in minor crops: A review. Crop Prot. 2017, 96, 44–58. [Google Scholar] [CrossRef]
- Sanbagavalli, S.; Somasundaram, E.; Marimuthu, S.; Ramesh, C. Stale Seedbed Technique of Weed Management: A Review. Int. J. Agric. Sci. 2016, 8, 3490–3493. [Google Scholar]
- Pittman, K.B.; Barney, J.N.; Flessner, M.L. Horseweed (Conyza canadensis) suppression from cover crop mixtures and fall-applied residual herbicides. Weed Technol. 2019, 33, 303–311. [Google Scholar] [CrossRef]
- Campiglia, E.; Radicetti, E.; Mancinelli, R. Cover crops and mulches influence weed management and weed flora composition in strip-tilled tomato (Solanum lycopersicum). Weed Res. 2015, 55, 416–425. [Google Scholar] [CrossRef]
- Pannacci, E.; Graziani, F.; Tei, F. Seed Filter Extractor: A new instrument for the evaluation of weed seedbank. Soil Tillage Res. 2015, 150, 78–82. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Sadia, S.; Ali, H.H.; Jabran, K.; Peerzada, A.M.; Chauhan, B.S. Biology and management of two important Conyza weeds: A global review. Environ. Sci. Pollut. Res. 2016, 23, 24694–24710. [Google Scholar] [CrossRef] [PubMed]
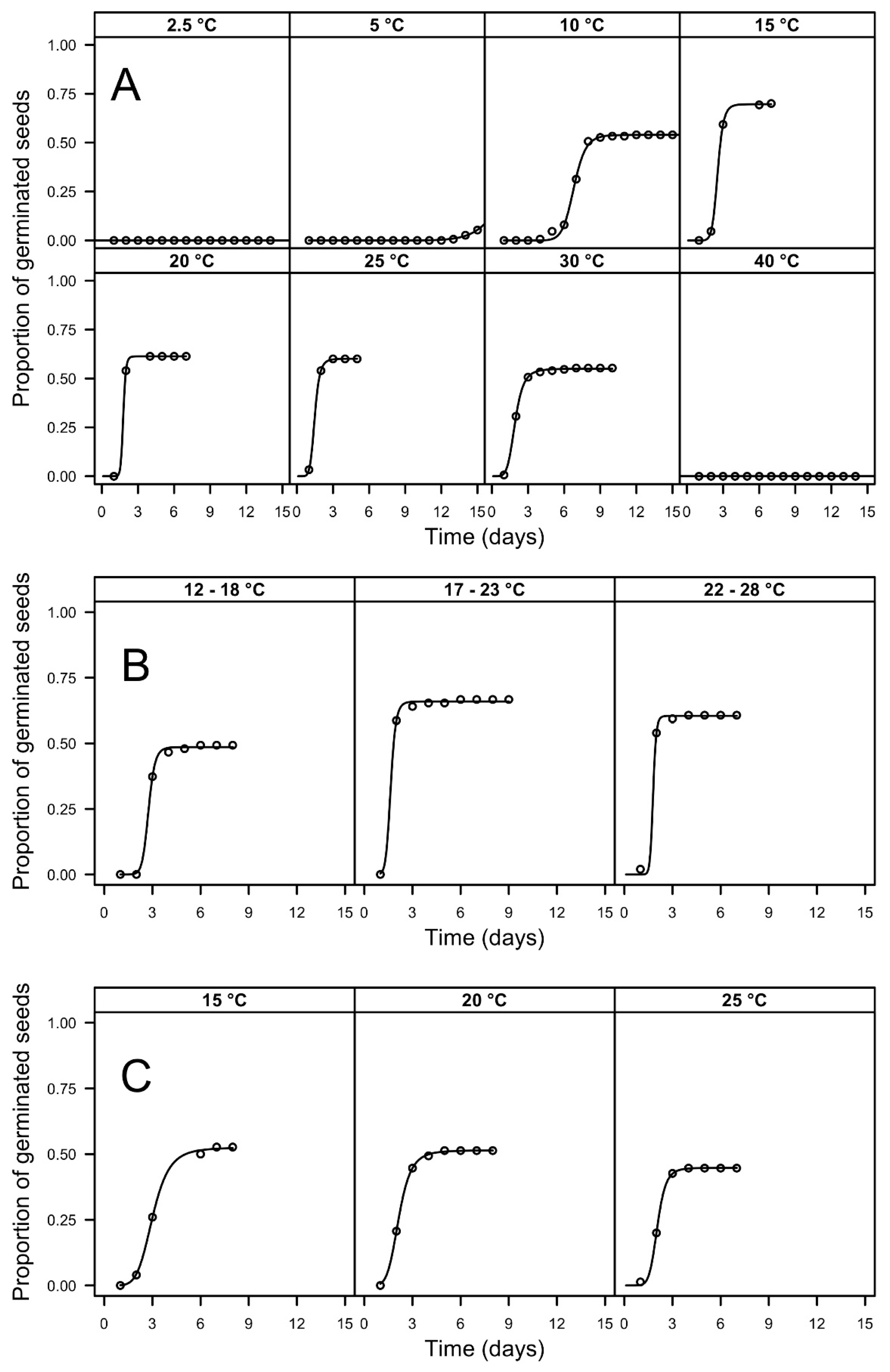


| Temp. | Light Regime (Light/Dark h.) | FGP | T30 (d) | ||
|---|---|---|---|---|---|
| 2.5 | 12/12 | 0.00 (0.00) | a | >16 | - |
| 5 | 12/12 | 0.11 (0.02) | b | >16 | - |
| 10 | 12/12 | 0.54 (0.04) | cde | 6.92 (0.27) | a |
| 15 | 12/12 | 0.70 (0.04) | e | 2.54 (0.10) | c |
| 20 | 12/12 | 0.61 (0.04) | cde | 1.89 (0.09) | f |
| 25 | 12/12 | 0.60 (0.04) | cde | 1.49 (0.06) | g |
| 30 | 12/12 | 0.55 (0.04) | cde | 1.99 (0.08) | ef |
| 40 | 12/12 | 0.00 (0.00) | a | >16 | - |
| 12/18 | 12/12 | 0.49 (0.04) | cd | 2.94 (0.12) | b |
| 17/23 | 12/12 | 0.67 (0.04) | de | 1.55 (0.06) | g |
| 22/28 | 12/12 | 0.61 (0.04) | cde | 1.53 (0.07) | g |
| 15 | 0/24 | 0.53 (0.04) | cde | 3.24 (0.13) | b |
| 20 | 0/24 | 0.51 (0.04) | cd | 2.26 (0.09) | cd |
| 25 | 0/24 | 0.45 (0.04) | c | 2.17 (0.11) | de |
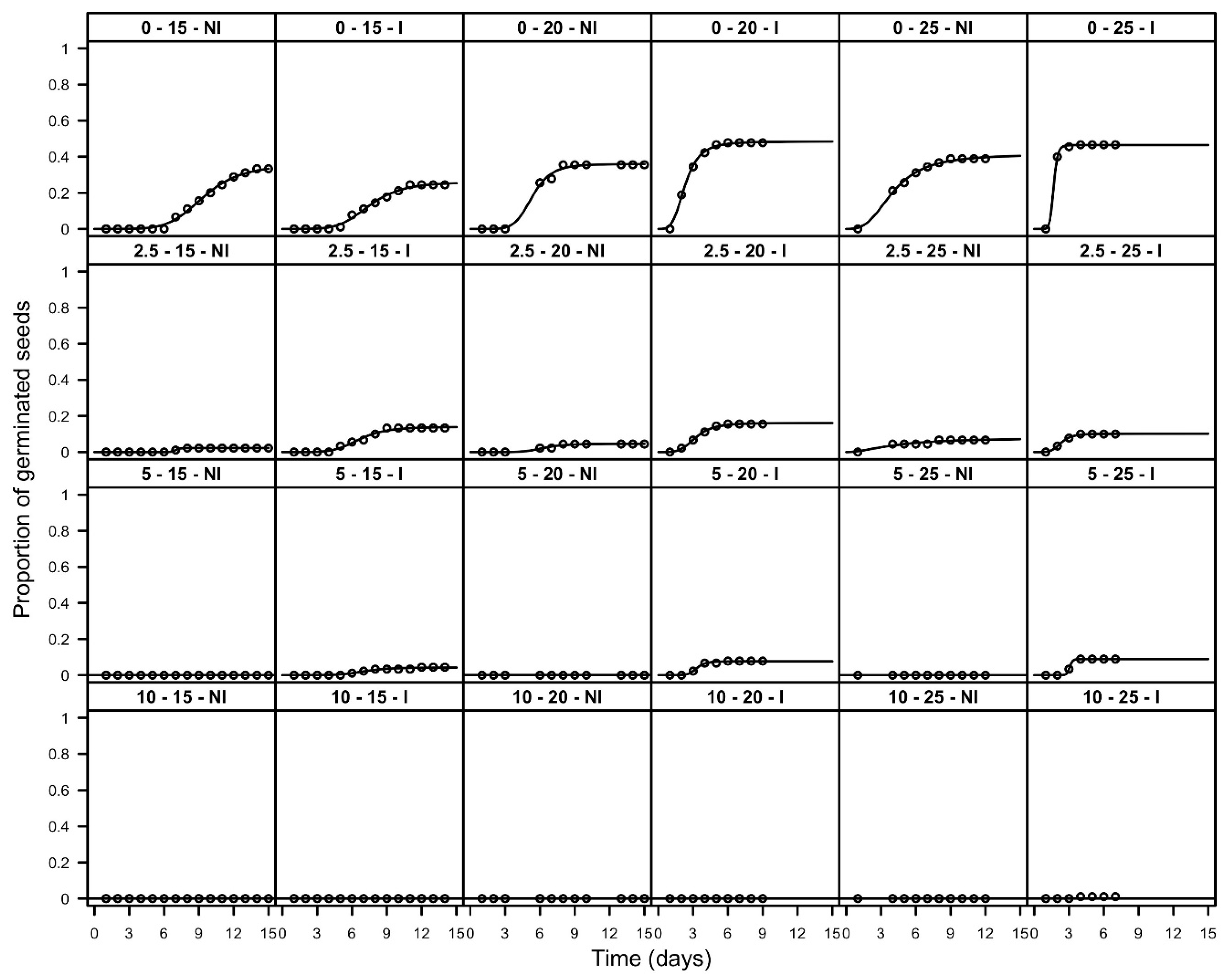
| Depth (mm) | Temperature (°C) | Pre-Treatment | FGP | T30 (d) | ||||
|---|---|---|---|---|---|---|---|---|
| 0 | 15 | NI | 0.34 | (0.03) | a | 11.7 | (2.58) | a |
| 2.5 | 15 | NI | 0.02 | (0.01) | cde | >16 | ||
| 5 | 15 | NI | 0.00 | (0.00) | c | >16 | ||
| 10 | 15 | NI | 0.00 | (0.00) | c | >16 | ||
| 0 | 20 | NI | 0.36 | (0.03) | ab | 8.4 | (1.48) | a |
| 2.5 | 20 | NI | 0.04 | (0.01) | def | >16 | ||
| 5 | 20 | NI | 0.00 | (0.00) | c | >16 | ||
| 10 | 20 | NI | 0.00 | (0.00) | c | >16 | ||
| 0 | 25 | NI | 0.40 | (0.03) | a | 4.7 | (1.04) | ab |
| 2.5 | 25 | NI | 0.07 | (0.01) | dfa | >16 | ||
| 5 | 25 | NI | 0.00 | (0.00) | c | >16 | ||
| 10 | 25 | NI | 0.00 | (0.00) | c | >16 | ||
| 0 | 15 | I | 0.25 | (0.02) | bh | >16 | ||
| 2.5 | 15 | I | 0.13 | (0.02) | g | >16 | ||
| 5 | 15 | I | 0.05 | (0.01) | def | >16 | ||
| 10 | 15 | I | 0.00 | (0.00) | c | >16 | ||
| 0 | 20 | I | 0.48 | (0.03) | a | 2.5 | (0.47) | b |
| 2.5 | 20 | I | 0.16 | (0.02) | gh | >16 | ||
| 5 | 20 | I | 0.08 | (0.01) | dfg | >16 | ||
| 10 | 20 | I | 0.00 | (0.00) | c | >16 | ||
| 0 | 25 | I | 0.47 | (0.03) | a | 1.8 | (0.56) | b |
| 2.5 | 25 | I | 0.10 | (0.02) | fg | >16 | ||
| 5 | 25 | I | 0.09 | (0.02) | fg | >16 | ||
| 10 | 25 | I | 0.01 | (0.01) | ce | >16 | ||
| Percentile | Tb (°C) | ΘT (°C Day) | k | Tc (°C) | ||||
|---|---|---|---|---|---|---|---|---|
| 10th | 6.67 | (0.53) | 20.24 | (1.57) | 0.23 | (0.06) | 33.90 | (0.82) |
| 20th | 6.67 | (0.53) | 21.69 | (1.81) | 0.23 | (0.06) | 33.33 | (0.70) |
| 30th | 6.67 | (0.53) | 22.82 | (2.00) | 0.23 | (0.06) | 32.95 | (0.62) |
| 40th | 6.67 | (0.53) | 24.03 | (2.22) | 0.23 | (0.06) | 32.55 | (0.54) |
| 50th | 6.67 | (0.53) | 24.32 | (2.72) | 0.23 | (0.06) | 30.71 | (0.22) |
| Parameters | Estimate | SE |
|---|---|---|
| Tb | 6.81 | 0.54 |
| θT(0) | 19.64 | 1.48 |
| b1 | 0.073 | 0.037 |
| k | 0.201 | 0.054 |
| Tc(0) | 35.78 | 1.16 |
| b2 | −0.094 | 0.021 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ottavini, D.; Pannacci, E.; Onofri, A.; Tei, F.; Kryger Jensen, P. Effects of Light, Temperature, and Soil Depth on the Germination and Emergence of Conyza canadensis (L.) Cronq. Agronomy 2019, 9, 533. https://doi.org/10.3390/agronomy9090533
Ottavini D, Pannacci E, Onofri A, Tei F, Kryger Jensen P. Effects of Light, Temperature, and Soil Depth on the Germination and Emergence of Conyza canadensis (L.) Cronq. Agronomy. 2019; 9(9):533. https://doi.org/10.3390/agronomy9090533
Chicago/Turabian StyleOttavini, Daniele, Euro Pannacci, Andrea Onofri, Francesco Tei, and Peter Kryger Jensen. 2019. "Effects of Light, Temperature, and Soil Depth on the Germination and Emergence of Conyza canadensis (L.) Cronq." Agronomy 9, no. 9: 533. https://doi.org/10.3390/agronomy9090533
APA StyleOttavini, D., Pannacci, E., Onofri, A., Tei, F., & Kryger Jensen, P. (2019). Effects of Light, Temperature, and Soil Depth on the Germination and Emergence of Conyza canadensis (L.) Cronq. Agronomy, 9(9), 533. https://doi.org/10.3390/agronomy9090533






