Jerusalem Artichoke (Helianthus tuberosus L.): A Versatile and Sustainable Crop for Renewable Energy Production in Europe
Abstract
:1. Introduction
2. Photosynthesis and Assimilate Allocation Strategy
3. Crop Growth and Yield
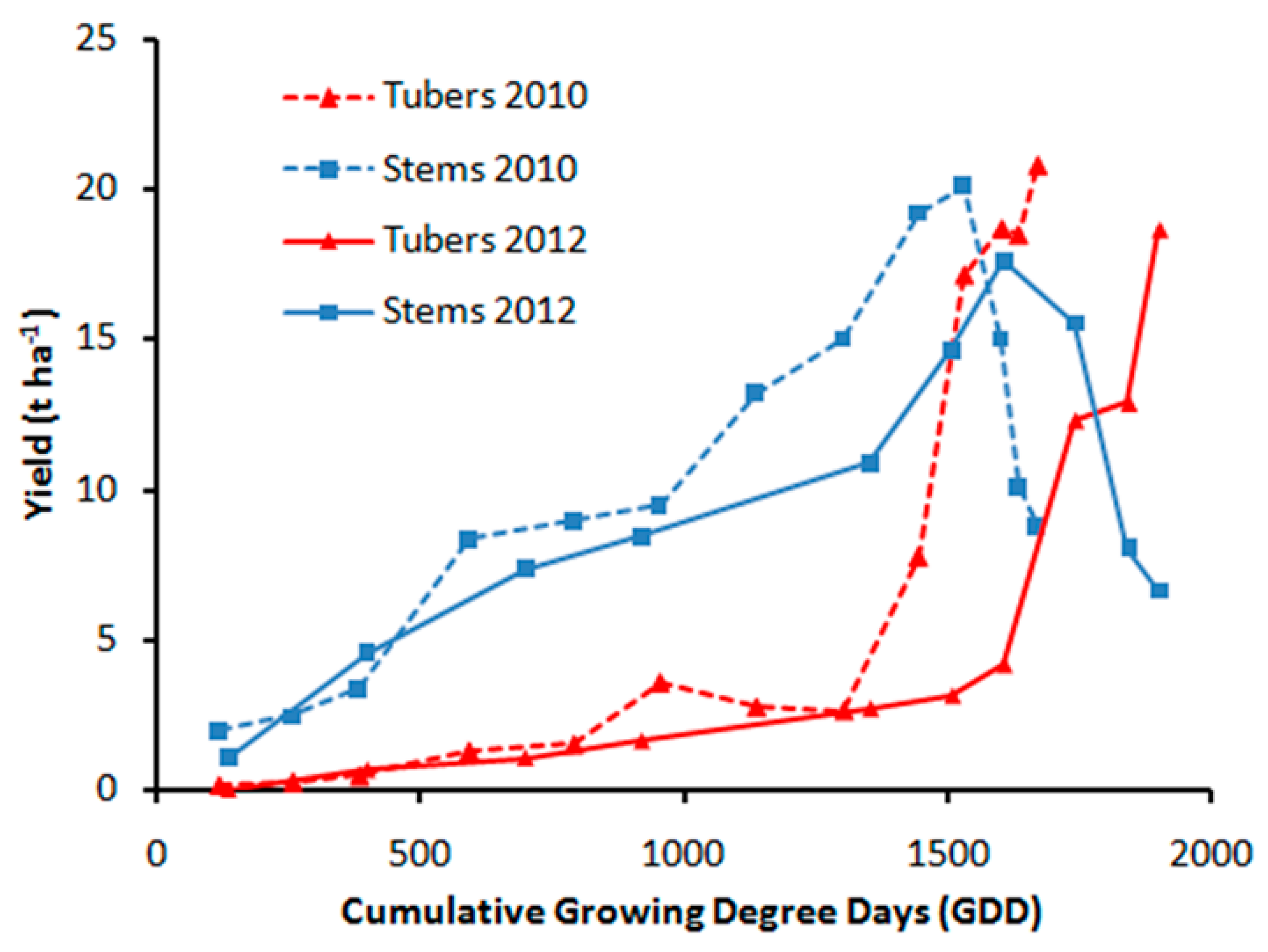
3.1. Biomass Distribution and Leaf Growth
3.2. Biological Yield and Harvest Index
4. Abiotic Factors Affecting Yield
4.1. Soil Type
4.2. Drought
4.3. Salinity
4.4. Radiation
4.5. Temperature
4.6. Photoperiod
4.7. Wind
5. Biotic Factors Affecting Yield
6. Genetic Resources
Germplasm and Diversity
7. Agronomic Strategies
7.1. Planting
7.1.1. Planting Date
7.1.2. Planting Density
7.2. Weeding
7.3. Growth Regulators
7.4. Fertilization
7.5. Irrigation
7.6. Harvesting
7.7. Other Factors
8. Sustainability of JA as Feedstock for Biofuel Production
8.1. JA Ethanol Potential in Comparison with Other Energy Crops
8.2. Life Cycle Assessment of the Impact of JA Cultivation
9. Conclusions and Future Perspectives
- yield performance on abandoned or severely degraded lands of Europe using a range of external inputs (e.g., zero, low, medium, high) and a combination of agronomic practices (i.e., planting date and density, cultivar selection, etc.);
- LCA of JA cultivation using information coming from the above-mentioned field trials;
- techno-economic assessment of biofuel pathways based on JA in comparison to other low ILUC options;
- identification and classification of suitable germplasm to enhance breeding efficiency for the traits of interest (i.e., biomass and sugar yield, WUE, RUE, etc.).
Author Contributions
Funding
Conflicts of Interest
References
- Swanton, C.J.; Clements, D.R.; Moore, M.J.; Cavers, P.B. The biology of Canadian weeds. 101. Helianthus tuberosus L. Can. J. Plant Sci. 1992, 72, 1367–1382. [Google Scholar] [CrossRef]
- Kays, S.J.; Nottingham, S.F. Biology and Chemistry of Jerusalem Artichoke: Helianthus tuberosus L.; CRC Press/Taylor & Francis Group: London, UK, 2008; ISBN 9781420044959. [Google Scholar]
- Pimsaen, W.; Jogloy, S.; Suriharn, B.; Kesmala, T.; Pensuk, V.; Patanothai, A. Genotype by Environment (GxE) Interactions for Yield Components of Jerusalem Artichoke (Helianthus tuberosus L.). Asian J. Plant Sci. 2010, 9, 11–19. [Google Scholar] [CrossRef]
- Denoroy, P. The crop physiology of Helianthus tuberosus L.: A model orientated view. Biomass Bioenergy 1996, 11, 11–32. [Google Scholar] [CrossRef]
- Barloy, J. Identification Criteria for Jerusalem Artichoke Clones. In Topinambur (Jerusalem artichoke); Grassi, G., Gosse, G., Eds.; Report No. EUR 11855 EN-FR-IT of the EEC; Elsevier: Amsterdam, The Netherlands, 1988; pp. 125–136. [Google Scholar]
- Ma, X.Y.; Zhang, L.H.; Shao, H.B.; Xu, G.; Zhang, F.; Ni, F.T.; Brestic, M. Jerusalem artichoke (Helianthus tuberosus), a medicinal salt-resistant plant has high adaptability and multiple-use values. J. Med. Plants Res. 2011, 5, 1272–1279. [Google Scholar]
- Volk, G.M.; Richards, K. Preservation methods for jerusalem artichoke cultivars. HortScience 2006, 41, 80–83. [Google Scholar] [CrossRef]
- Danieli, P.P.; Primi, R.; Ronchi, B.; Ruggeri, R.; Rossini, F.; del Puglia, S.; Cereti, C.F. The potential role of spineless safflower (Carthamus tinctorius L. var. inermis) as fodder crop in central Italy. Ital. J. Agron. 2011, 6, 19–22. [Google Scholar] [CrossRef]
- Hay, R.K.M.; Offer, R.W. Helianthus tuberosus as an alternative forage crop for cool maritime regions: A preliminary study of the yield and nutritional quality of shoot tissues from perennial stands. J. Sci. Food Agric. 1992, 60, 213–221. [Google Scholar] [CrossRef]
- Li, G.; Kemp, P.D. Forage Chicory (Cichorium intybus L.): A Review of Its Agronomy and Animal Production. Adv. Agron. 2005, 88, 187–222. [Google Scholar]
- Rossini, F.; Loreti, P.; Provenzano, M.E.; De Santis, D.; Ruggeri, R. Agronomic performance and beer quality assessment of twenty hop cultivars grown in central Italy. Ital. J. Agron. 2016, 11, 180–187. [Google Scholar] [CrossRef]
- Yang, L.; He, Q.S.; Corscadden, K.; Udenigwe, C.C. The prospects of Jerusalem artichoke in functional food ingredients and bioenergy production. Biotechnol. Rep. 2015, 5, 77–88. [Google Scholar] [CrossRef]
- Araújo, K.; Mahajan, D.; Kerr, R.; Silva, M.D. Global Biofuels at the Crossroads: An Overview of Technical, Policy, and Investment Complexities in the Sustainability of Biofuel Development. Agriculture 2017, 7, 32. [Google Scholar] [CrossRef]
- Sayre, R. Microalgae: The Potential for Carbon Capture. Bioscience 2010, 60, 722–727. [Google Scholar] [CrossRef]
- Davis, S.C.; Parton, W.J.; Del Grosso, S.J.; Keough, C.; Marx, E.; Adler, P.R.; Delucia, E.H. Impact of second-generation biofuel agriculture on greenhouse-gas emissions in the corn-growing regions of the US. Front. Ecol. Environ. 2012, 10, 69–74. [Google Scholar] [CrossRef]
- Valentine, J.; Clifton-Brown, J.; Hastings, A.; Robson, P.; Allison, G.; Smith, P. Food vs. fuel: The use of land for lignocellulosic “next generation” energy crops that minimize competition with primary food production. GCB Bioenergy 2012, 4, 1–19. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Swain, M.R. Bioethanol Production from Corn and Wheat: Food, Fuel, and Future. In Bioethanol Production from Food Crops; Ray, R., Ramachandran, S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 45–79. ISBN 9780128137666. [Google Scholar]
- Monti, A.; Amaducci, M.T.; Venturi, G. Growth response, leaf gas exchange and fructans accumulation of Jerusalem artichoke (Helianthus tuberosus L.) as affected by different water regimes. Eur. J. Agron. 2005, 23, 136–145. [Google Scholar] [CrossRef]
- Baldini, M.; Danuso, F.; Monti, A.; Amaducci, M.T.; Stevanato, P.; De Mastro, G. Chicory and Jerusalem artichoke productivity in different areas of Italy, in relation to water availability and time of harvest. Ital. J. Agron. 2006, 1, 291–307. [Google Scholar] [CrossRef]
- Rebora, C.; Lelio, H.; Gómez, L.; Ibarguren, L. Waste-Water Use in Energy Crops Production. In Waste Water —Treatment and Reutilization; García Einschlag, F.S., Ed.; InTech: London, UK, 2011; pp. 361–374. [Google Scholar]
- Lamascese, N.; Losavio, N.; Ventrella, D.; Vonella, A.V. Relazione tra radiazione e accrescimento del topinambur (Helianthus tuberosus L.) coltivato nell’area metapontina. Riv. Agron. 1998, 32, 141–146. [Google Scholar]
- Somda, Z.C.; McLaurin, W.J.; Kays, S.J. Jerusalem artichoke growth, development, and field storage. II. Carbon and nutrient element allocation and redistribution. J. Plant Nutr. 1999, 22, 1315–1334. [Google Scholar] [CrossRef]
- McLaurin, W.J.; Somda, Z.C.; Kays, S.J. Jerusalem artichoke growth, development, and field storage. I. Numerical assessment of plant part development and dry matter acquisition and allocation. J. Plant Nutr. 1999, 22, 1303–1313. [Google Scholar] [CrossRef]
- Incoll, L.D.; Neales, T.F. The stem as a temporary sink before tuberization in Helianthus tuberosus L. J. Exp. Bot. 1970, 21, 469–476. [Google Scholar] [CrossRef]
- Swanton, C.J.; Cavers, P.B. Biomass and nutrient allocation patterns in Jerusalem artichoke (Helianthus tuberosus). Can. J. Bot. 1989, 67, 2880–2887. [Google Scholar] [CrossRef]
- Kocsis, L.; Liebhard, P.; Praznik, W. Effect of seasonal changes on content and profile of soluble carbohydrates in tubers of different varieties of Jerusalem artichoke (Helianthus tuberosus L.). J. Agric. Food Chem. 2007, 55, 9401–9408. [Google Scholar] [CrossRef] [PubMed]
- Zubr, J.; Pedersen, H.S. Characteristics of growth and development of different Jerusalem artichoke cultivars. Stud. Plant Sci. 1993, 3, 11–19. [Google Scholar]
- Gao, K.; Zhu, T.; Han, G. Water and nitrogen interactively increased the biomass production of Jerusalem artichoke (Helianthus tuberosus L.) in semi-arid area. Afr. J. Biotechnol. 2011, 10, 6466–6472. [Google Scholar] [CrossRef]
- Meijer, W.J.M.; Mathijssen, E.W.J.M. Analysis of crop performance in research on inulin, fibre and oilseed crops. Ind. Crops Prod. 1996, 5, 253–264. [Google Scholar] [CrossRef]
- Liu, Z.X.; Han, L.P.; Yosef, S.; Xie, G.H. Genetic Variation and Yield Performance of Jerusalem Artichoke Germplasm Collected in China. Agric. Sci. China 2011, 10, 668–678. [Google Scholar] [CrossRef]
- Tuck, G.; Glendining, M.J.; Smith, P.; House, J.I.; Wattenbach, M. The potential distribution of bioenergy crops in Europe under present and future climate. Biomass Bioenergy 2006, 30, 183–197. [Google Scholar] [CrossRef]
- Kosaric, N.; Cosentino, G.P.; Wieczorek, A.; Duvnjak, Z. The Jerusalem artichoke as an agricultural crop. Biomass 1984, 5, 1–36. [Google Scholar] [CrossRef]
- Ruf, T.; Audu, V.; Holzhauser, K.; Emmerling, C. Bioenergy from Periodically Waterlogged Cropland in Europe: A First Assessment of the Potential of Five Perennial Energy Crops to Provide Biomass and Their Interactions with Soil. Agronomy 2019, 9, 374. [Google Scholar] [CrossRef]
- De Mastro, G.; Manolio, G.; Marzi, V. Jerusalem artichoke (Helianthus tuberosus L.) and chicory (Cichorium intybus L.): Potential crops for inulin production in the mediterranean area. Acta Hortic. 2004, 629, 365–374. [Google Scholar] [CrossRef]
- Puangbut, D.; Jogloy, S.; Vorasoot, N.; Srijaranai, S.; Holbrook, C.C.; Patanothai, A. Variation of inulin content, inulin yield and water use efficiency for inulin yield in Jerusalem artichoke genotypes under different water regimes. Agric. Water Manag. 2015, 152, 142–150. [Google Scholar] [CrossRef]
- Conde, J.R.; Tenorio, J.L.; Rodríguez-Maribona, B.; Ayerbet, L. Tuber yield of Jerusalem artichoke (Helianthus Tuberosus L.) in relation to water stress. Biomass Bioenergy 1991, 1, 137–142. [Google Scholar] [CrossRef]
- Dias, N.S.; Ferreira, J.F.S.; Liu, X.; Suarez, D.L. Jerusalem artichoke (Helianthus tuberosus, L.) maintains high inulin, tuber yield, and antioxidant capacity under moderately-saline irrigation waters. Ind. Crops Prod. 2016, 94, 1009–1024. [Google Scholar] [CrossRef]
- Klug-Andersen, S. Jerusalem artichoke: A vegetable crop growth regulation and cultivars. Acta Hortic. 1992, 318, 145–152. [Google Scholar] [CrossRef]
- Murai, M.; Yoshida, S. Evidence for the cell wall involvement in temporal changes in freezing tolerance of Jerusalem artichoke (Helianthus tuberosus L.) tubers during cold acclimation. Plant Cell Physiol. 1998, 39, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Soja, G.; Dersch, G. Plant development and hormonal status in the Jerusalem artichoke (Helianthus tuberosus L.). Ind. Crops Prod. 1992, 1, 219–228. [Google Scholar] [CrossRef]
- Serieys, H.; Souyris, I.; Gil, A.; Poinso, B.; Bervillé, A. Diversity of Jerusalem artichoke clones (Helianthus tuberosus L.) from the INRA-Montpellier collection. Genet. Resour. Crop Evol. 2010, 57, 1207–1215. [Google Scholar] [CrossRef]
- Slimestad, R.; Seljaasen, R.; Meijer, K.; Skar, S.L. Norwegian-grown Jerusalem artichoke (Helianthus tuberosus L.): Morphology and content of sugars and fructo-oligosaccharides in stems and tubers. J. Sci. Food Agric. 2010, 90, 956–964. [Google Scholar]
- Labergh, C.; Sackston, W.E. Adaptability and diseases of Jerusalem artichoke (Helianthus tuberosus) in Quebec. Can. J. Plant Sci. 1987, 67, 349–353. [Google Scholar] [CrossRef]
- Viriyasuthee, W.; Saksirirat, W.; Saepaisan, S.; Gleason, M.L. Variability of Alternaria Leaf Spot Resistance in Jerusalem Artichoke (Helianthus Tuberosus L.) Accessions Grown in a Humid Tropical Region. Agronomy 2019, 9, 268. [Google Scholar] [CrossRef]
- Zhao, Y.-Q.; Zhang, D.M.; Zhang, L.-J.; Yu, X.Y.; Yu, H.R.; Shi, K.; Gao, K. First Report of Brown Spot on Jerusalem Artichoke (Helianthus tuberosus) Caused by Bipolaris zeae in China. Plant Dis. 2017, 101, 2146. [Google Scholar] [CrossRef]
- Duke, J.A. Handbook of Energy Crops. Available online: https://hort.purdue.edu/newcrop/duke_energy/Helianthus_tuberosus.html (accessed on 21 July 2019).
- Kongsted, A.G.; Horsted, K.; Hermansen, J.E. Free-range pigs foraging on Jerusalem artichokes (Helianthus tuberosus L.)—Effect of feeding strategy on growth, feed conversion and animal behaviour. Acta Agric. Scand. Sect. A Anim. Sci. 2013, 63, 76–83. [Google Scholar] [CrossRef]
- Breton, C.; Kiru, S.D.; Bervillé, A.; Anushkevich, N.Y. Breeding of Jerusalem artichoke with the desired traits for different directions of use: Retrospective, approaches, and prospects. Agric. Biol. 2017, 52, 940–951. [Google Scholar] [CrossRef]
- Long, X.H.; Shao, H.B.; Liu, L.; Liu, L.P.; Liu, Z.P. Jerusalem artichoke: A sustainable biomass feedstock for biorefinery. Renew. Sustain. Energy Rev. 2016, 54, 1382–1388. [Google Scholar] [CrossRef]
- Baldini, M.; Danuso, F.; Turi, M.; Vannozzi, G.P. Evaluation of new clones of Jerusalem artichoke (Helianthus tuberosus L.) for inulin and sugar yield from stalks and tubers. Ind. Crops Prod. 2004, 19, 25–40. [Google Scholar] [CrossRef]
- Diederichsen, A. Phenotypic diversity of Jerusalem artichoke (Helianthus tuberosus L.) germplasm preserved by the Canadian genebank. Helia 2010, 33, 1–16. [Google Scholar] [CrossRef]
- Kiru, S.; Nasenko, I. Use of genetic resources from Jerusalem artichoke collection of N. Vavilov Institute in breeding for bioenergy and health security. Agron. Res. 2010, 8, 625–632. [Google Scholar]
- Liu, Z.X.; Spiertz, J.H.J.; Sha, J.; Xue, S.; Xie, G.H. Growth and yield performance of Jerusalem artichoke clones in a semiarid region of China. Agron. J. 2012, 104, 1538–1546. [Google Scholar] [CrossRef]
- Kantar, M.B.; Baute, G.J.; Bock, D.G.; Rieseberg, L.H. Genomic variation in Helianthus: Learning from the past and looking to the future. Brief. Funct. Genom. Proteom. 2014, 13, 328–340. [Google Scholar] [CrossRef]
- Atlagić, J.; Dozet, B.; ŠKorić, D. Meiosis and Pollen Viability in Helianthus tuberosus L. and its Hybrids with Cultivated Sunflower. Plant Breed. 1993, 111, 318–324. [Google Scholar] [CrossRef]
- Breton, C.; Serieys, H.; Bervillé, A. Gene transfer from wild Helianthus to sunflower: Topicalities and limits. Oléagineux Corps Gras Lipides 2010, 17, 104–114. [Google Scholar] [CrossRef]
- Wangsomnuk, P.P.; Khampa, S.; Jogloy, S. Exogenous supplementation of growth regulators and temperature improves germination of dormant Jerusalem artichoke (Helianthus tuberosus L.) Seeds under in vitro and in vivo conditions. J. Appl. Biol. Sci. 2015, 9, 23–30. [Google Scholar]
- Puttha, R.; Jogloy, S.; Suriharn, B.; Wangsomnuk, P.P.; Kesmala, T.; Patanothai, A. Variations in morphological and agronomic traits among Jerusalem artichoke (Helianthus tuberosus L.) accessions. Genet. Resour. Crop Evol. 2013, 60, 731–746. [Google Scholar] [CrossRef]
- Wangsomnuk, P.P.; Khampa, S.; Wangsomnuk, P.; Jogloy, S.; Mornkham, T.; Ruttawat, B.; Patanothai, A.; Fu, Y.B. Genetic diversity of worldwide Jerusalem artichoke (Helianthus tuberosus) germplasm as revealed by RAPD markers. Genet. Mol. Res. 2011, 10, 4012–4025. [Google Scholar] [CrossRef] [PubMed]
- Ceoloni, C.; Kuzmanović, L.; Ruggeri, R.; Rossini, F.; Forte, P.; Cuccurullo, A.; Bitti, A. Harnessing genetic diversity of wild gene pools to enhance wheat crop production and sustainability: Challenges and opportunities. Diversity 2017, 9, 55. [Google Scholar] [CrossRef]
- Kuzmanović, L.; Ruggeri, R.; Able, J.A.; Bassi, F.M.; Maccaferri, M.; Tuberosa, R.; De Vita, P.; Rossini, F.; Ceoloni, C. Yield of chromosomally engineered durum wheat-Thinopyrum ponticum recombinant lines in a range of contrasting rain-fed environments. Field Crops Res. 2018, 228, 147–157. [Google Scholar] [CrossRef]
- Rahman, H.; Bennett, R.A.; Séguin-Swartz, G. Broadening genetic diversity in Brassica napus canola: Development of canola-quality spring B. napus from B. napus×B. oleracea var. alboglabra interspecific crosses. Can. J. Plant Sci. 2015, 95, 29–41. [Google Scholar] [CrossRef]
- Zhang, J.; Percy, R.G.; McCarty, J.C. Introgression genetics and breeding between Upland and Pima cotton: A review. Euphytica 2014, 198, 1–12. [Google Scholar] [CrossRef]
- Lakić, Ž.; Balalić, I.; Nožinić, M. Genetic variability for yield and yield components in jerusalem artichoke (Helianthus tuberosus L.). Genetika 2018, 50, 45–57. [Google Scholar] [CrossRef]
- Curt, M.D.; Aguado, P.; Sanz, M.; Sánchez, G.; Fernández, J. Clone precocity and the use of Helianthus tuberosus L. stems for bioethanol. Ind. Crops Prod. 2006, 24, 314–320. [Google Scholar] [CrossRef]
- Lv, S.; Wang, R.; Xiao, Y.; Li, F.; Mu, Y.; Lu, Y.; Gao, W.; Yang, B.; Kou, Y.; Zeng, J.; et al. Growth, yield formation, and inulin performance of a non-food energy crop, Jerusalem artichoke (Helianthus tuberosus L.), in a semi-arid area of China. Ind. Crops Prod. 2019, 134, 71–79. [Google Scholar] [CrossRef]
- Kays, S.J.; Kultur, F. Genetic variation in Jerusalem artichoke (Helianthus tuberosus L.) flowering date and duration. HortScience 2005, 40, 1675–1678. [Google Scholar] [CrossRef]
- Puttha, R.; Jogloy, S.; Wangsomnuk, P.P.; Srijaranai, S.; Kesmala, T.; Patanothai, A. Genotypic variability and genotype by environment interactions for inulin content of Jerusalem artichoke germplasm. Euphytica 2012, 183, 119–131. [Google Scholar] [CrossRef]
- Janket, A.; Jogloy, S.; Vorasoot, N.; Kesmala, T.; Holbrook, C.C.; Patanothai, A. Genetic diversity of water use efficiency in jerusalem artichoke (Helianthus tuberosus L.) germplasm. Aust. J. Crop Sci. 2013, 7, 1670–1681. [Google Scholar]
- Ruttanaprasert, R.; Banterng, P.; Jogloy, S.; Vorasoot, N.; Kesmala, T.; Kanwar, R.S.; Holbrook, C.C.; Patanothai, A. Genotypic variability for tuber yield, biomass, and drought tolerance in Jerusalem artichoke germplasm. Turk. J. Agric. For. 2014, 38, 570–580. [Google Scholar] [CrossRef]
- Ruttanaprasert, R.; Jogloy, S.; Vorasoot, N.; Kesmala, T.; Kanwar, R.S.; Holbrook, C.C.; Patanothai, A. Effects of water stress on total biomass, tuber yield, harvest index and water use efficiency in Jerusalem artichoke. Agric. Water Manag. 2016, 166, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Brkljača, J.; Bodroža-Solarov, M.; Krulj, J.; Terzić, S.; Mikić, A.; Marjanović Jeromela, A. Quantification of inulin content in selected accessions of Jerusalem artichoke (Helianthus tuberosus L.). Helia 2014, 37, 105–112. [Google Scholar] [CrossRef]
- Terzic, S.; Atlagic, J. Nitrogen and sugar content variability in tubers of Jerusalem artichoke (Helianthus tuberosus). Genetika 2009, 41, 289–295. [Google Scholar] [CrossRef]
- Dozet, B.; Marinković, R.; Vasić, D.; Marjanović, A. Genetic similarity of the Jerusalem artichoke populations (Helianthus tuberosus L.) collected in Montenegro. Helia 1993, 16, 41–48. [Google Scholar]
- Sennoi, R.; Jogloy, S.; Saksirirat, W.; Kesmala, T.; Patanothai, A. Genotypic variation of resistance to southern stem rot of Jerusalem artichoke caused by Sclerotium rolfsii. Euphytica 2013, 190, 415–424. [Google Scholar] [CrossRef]
- Junsopa, C.; Jogloy, S.; Saksirirat, W.; Songsri, P.; Kesmala, T.; Shew, B.B. Genotypic diversity of Jerusalem artichoke for resistance to stem rot caused by Sclerotium rolfsii under field conditions. Euphytica 2017, 213, 164. [Google Scholar] [CrossRef]
- Wangsomnuk, P.P.; Khampa, S.; Jogloy, S.; Srivong, T.; Patanothai, A.; Fu, Y.-B. Assessing Genetic Structure and Relatedness of Jerusalem Artichoke (Helianthus tuberosus L.) Germplasm with RAPD, ISSR and SRAP Markers. Am. J. Plant Sci. 2011, 2, 753–764. [Google Scholar] [CrossRef]
- Zhao, M.; Han, R.; Li, L. ISSR marker analysis on genetic diversity of twenty-four cultivars (lines) of Helianthus tuberosus. J. Plant Resour. Environ. 2013, 22, 44–49. [Google Scholar]
- Kou, Y.X.; Zeng, J.; Liu, J.Q.; Zhao, C.M. Germplasm diversity and differentiation of Helianthus tuberosus L. revealed by AFLP marker and phenotypic traits. J. Agric. Sci. 2014, 152, 779–789. [Google Scholar] [CrossRef]
- Jung, W.Y.; Lee, S.S.; Park, H.J.; Kim, C.W.; Kwon, S.; Jeon, J.; Kim, H.; Cho, H.S. Comparative transcriptome profiling and SSR marker identification in three Jerusalem artichoke (Helianthus tuberosus L.) cultivars exhibiting phenotypic variation. Plant Biotechnol. Rep. 2016, 10, 447–461. [Google Scholar] [CrossRef]
- Mornkham, T.; Wangsomnuk, P.P.; Mo, X.C.; Francisco, F.O.; Gao, L.Z.; Kurzweil, H. Development and characterization of novel EST-SSR markers and their application for genetic diversity analysis of Jerusalem artichoke (Helianthus tuberosus L.). Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Sun, X.; Jiang, X.; Wang, L.; Tian, J.; Li, L.; Zhao, M.; Zhong, Q. Characterization of the Tibet plateau Jerusalem artichoke (Helianthus tuberosus L.) transcriptome by de novo assembly to discover genes associated with fructan synthesis and SSR analysis. Hereditas 2019, 156. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhong, Q.; Tian, J.; Wang, L.; Zhao, M.; Li, L.; Sun, X. Characterization and development of EST-SSR markers to study the genetic diversity and populations analysis of Jerusalem artichoke (Helianthus tuberosus L.). Genes Genom. 2018, 40, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Bock, D.G.; Kane, N.C.; Ebert, D.P.; Rieseberg, L.H. Genome skimming reveals the origin of the Jerusalem Artichoke tuber crop species: Neither from Jerusalem nor an artichoke. New Phytol. 2014, 201, 1021–1030. [Google Scholar] [CrossRef]
- Min, C.W.; Jung, W.Y.; Park, H.J.; Moon, K.-B.; Ko, H.; Sohn, J.-H.; Jeon, J.-H.; Kim, H.-S.; Gupta, R.; Kim, S.T.; et al. Label-free quantitative proteomic analysis determines changes in amino acid and carbohydrate metabolism in three cultivars of Jerusalem artichoke tubers. Plant Biotechnol. Rep. 2019, 13, 111–122. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Sousa, L.; Cabanas, J.E.; Arrobas, M. Tuber yield and leaf mineral composition of Jerusalem artichoke (Helianthus tuberosus L.) grown under different cropping practices. Span. J. Agric. Res. 2007, 5, 545–553. [Google Scholar] [CrossRef]
- Youngen, J.A. Jerusalem Artichoke Trials in Southern Oregon; Oregon State University: Corvallis, OR, USA, 1992. [Google Scholar]
- Puangbut, D.; Jogloy, S.; Vorasoot, N.; Srijaranai, S.; Kesmala, T.; Holbrook, C.C.; Patanothai, A. Influence of planting date and temperature on inulin content in Jerusalem artichoke (Helianthus tuberosus L.). Aust. J. Crop Sci. 2012, 6, 1159–1165. [Google Scholar]
- Matías, J.; González, J.; Cabanillas, J.; Royano, L. Influence of NPK fertilisation and harvest date on agronomic performance of Jerusalem artichoke crop in the Guadiana Basin (Southwestern Spain). Ind. Crops Prod. 2013, 48, 191–197. [Google Scholar] [CrossRef]
- Paixão, S.M.; Alves, L.; Pacheco, R.; Silva, C.M. Evaluation of Jerusalem artichoke as a sustainable energy crop to bioethanol: Energy and CO2eq emissions modeling for an industrial scenario. Energy 2018, 150, 468–481. [Google Scholar] [CrossRef]
- Rossini, F.; Provenzano, M.E.; Ruggeri, R. Tuber and stalk yield of Jerusalem artichoke clones as affected by planting density. In Proceedings of the 20th European Biomass Conference and Exhibition, Milan, Italy, 18–22 June 2012; pp. 604–607. [Google Scholar]
- Schittenhelm, S. Agronomic Performance of Root Chicory, Jerusalem Artichoke, and Sugarbeet in Stress and Nonstress Environments. Crop Sci. 1999, 39, 1815–1823. [Google Scholar] [CrossRef]
- Gunnarsson, I.B.; Svensson, S.E.; Johansson, E.; Karakashev, D.; Angelidaki, I. Potential of Jerusalem artichoke (Helianthus tuberosus L.) as a biorefinery crop. Ind. Crops Prod. 2014, 56, 231–240. [Google Scholar] [CrossRef]
- Ruggeri, R.; Provenzano, M.E.; Rossini, F. Effect of mulch on initial coverage of four groundcover species for low input landscaping in a Mediterranean climate. Urban For. Urban Green. 2016, 19. [Google Scholar] [CrossRef]
- Wall, D.A.; Kiehn, F.A.; Friesen, G.H. Tolerance of Columbia Jerusalem artichoke to selective herbicides. Can. J. Plant Sci. 1987, 67, 835–837. [Google Scholar] [CrossRef]
- Losavio, N.; Lamascese, N.; Vonella, A. Water requirements and nitrogen fertilization in Jerusalem artichoke (Helianthus tuberosus L.) grown under Mediterranean conditions. Acta Hortic. 1997, 449, 205–210. [Google Scholar] [CrossRef]
- Dorrell, D.G.; Chubey, B.B. Irrigation, fertilizer, harvest dates and storage effects on the reducing sugar and fructose concentrations of Jerusalem artichoke tubers. Can. J. Plant Sci. 1977, 57, 591–596. [Google Scholar] [CrossRef]
- Saengthongpinit, W.; Sajjaanantakul, T. Influence of harvest time and storage temperature on characteristics of inulin from Jerusalem artichoke (Helianthus tuberosus L.) tubers. Postharvest Biol. Technol. 2005, 37, 93–100. [Google Scholar] [CrossRef]
- Schorr-Galindo, S.; Guiraud, J.P. Sugar potential of different Jerusalem artichoke cultivars according to harvest. Bioresour. Technol. 1997, 60, 15–20. [Google Scholar] [CrossRef]
- Kai, G.; Tie-Xia, Z.; Qi-Bing, W. Nitrogen fertilization, irrigation, and harvest times affect biomass and energy value of Helianthus tuberosus L. J. Plant Nutr. 2016, 39, 1906–1914. [Google Scholar] [CrossRef]
- Acar, R.; Ada, R.; Özköse, A. Effects of different mowing dates of plant top on tuber yield of Jerusalem artichoke (Helianthus tuberosus L.). Afr. J. Biotechnol. 2011, 10, 9036–9040. [Google Scholar] [CrossRef] [Green Version]
- Westley, L.C. The effect of inflorescence bud removal on tuber production in Helianthus tuberosus L. (Asteraceae). Ecology 1993, 74, 2136–2144. [Google Scholar] [CrossRef]
- Gao, K.; Zhang, Z.; Zhu, T.; Tian, X.; Gao, Y.; Zhao, L.; Li, T. The influence of leaf removal on tuber yield and fuel characteristics of Helianthus tuberosus L. in a semi-arid area. Ind. Crops Prod. 2019, 131, 8–13. [Google Scholar] [CrossRef]
- Gao, K.; Zhu, T.X.; Xun, T.; Lin, W.; Yang, G. The influence of root-cutting radius on tuber yield and fuel characteristics of Helianthus tuberosus L. in a semi-arid area. Ind. Crops Prod. 2018, 115, 202–207. [Google Scholar] [CrossRef]
- Haberl, H. Competition for land: A sociometabolic perspective. Ecol. Econ. 2015, 119, 424–431. [Google Scholar] [CrossRef] [Green Version]
- Harvey, M.; Pilgrim, S. The new competition for land: Food, energy, and climate change. Food Policy 2011, 36, S40–S51. [Google Scholar] [CrossRef]
- Tilman, D.; Socolow, R.; Foley, J.A.; Hill, J.; Larson, E.; Lynd, L.; Pacala, S.; Reilly, J.; Searchinger, T.; Somerville, C.; et al. Beneficial Biofuels: The Food, Energy, and Environment. Science 2009, 325, 270–271. [Google Scholar] [CrossRef]
- Qiu, Y.; Lei, P.; Zhang, Y.; Sha, Y.; Zhan, Y.; Xu, Z.; Li, S.; Xu, H.; Ouyang, P. Recent advances in bio-based multi-products of agricultural Jerusalem artichoke resources. Biotechnol. Biofuels 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Renewable Fuel Association Annual World Fuel Ethanol Production. Available online: https://ethanolrfa.org/statistics/annual-ethanol-production/ (accessed on 24 July 2019).
- OECD/FAO Biofuels. OECD-FAO Agricultural Outlook 2018–2027; OECD Publishing: Paris, France, 2018; pp. 191–206. ISBN 978-92-5-130501-0. [Google Scholar]
- Marris, E. Drink the best and drive the rest. Nature 2006, 444, 670–672. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, T.C. Agronomic features, ethanol yields & resource use of four feedstocks for ethanol production in the Philippines. Phillipine J. Crop Sci. 2008, 33, 21–36. [Google Scholar]
- Quintero, J.A.; Montoya, M.I.; Sánchez, O.J.; Giraldo, O.H.; Cardona, C.A. Fuel ethanol production from sugarcane and corn: Comparative analysis for a Colombian case. Energy 2008, 33, 385–399. [Google Scholar] [CrossRef]
- Goldenberg, J.; Guardabassi, P. The potential for first-generation ethanol production from sugarcane. BiofuelsBioprod. Biorefining 2010, 4, 17–24. [Google Scholar] [CrossRef]
- Li, Y.R.; Wei, Y.A. Sugar industry in China: R & D and policy initiatives to meet sugar and biofuel demand of future. Sugar Tech 2006, 8, 203–216. [Google Scholar] [CrossRef]
- Wortmann, C.S.; Liska, A.J.; Ferguson, R.B.; Lyon, D.J.; Klein, R.N.; Dweikat, I. Dryland performance of sweet sorghum and grain crops for biofuel in nebraska. Agron. J. 2010, 102, 319–326. [Google Scholar] [CrossRef]
- Tumbalam, P.; Thelen, K.D.; Adkins, A.; Dale, B.; Balan, V.; Gunawan, C.; Gao, J. Corn stover ethanol yield as affected by grain yield, Bt trait, and environment. Biomass Bioenergy 2016, 85, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Almodares, A.; Hadi, M.R. Production of bioethanol from sweet sorghum: A review. Afr. J. Agric. Res. 2009, 4, 772–780. [Google Scholar]
- Halleux, H.; Lassaux, S.; Renzoni, R.; Germain, A. Comparative Life Cycle Assessment of Two Biofuels Ethanol from Sugar Beet and Rapeseed Methyl Ester. Int. J. LCA 2008, 13, 184–190. [Google Scholar] [CrossRef]
- Barcelos, C.A.; Maeda, R.N.; Maria, L.; Anna, M.S.; Pereira, N. Biomass and Bioenergy Sweet sorghum as a whole-crop feedstock for ethanol production. Biomass Bioenergy 2016, 94, 46–56. [Google Scholar] [CrossRef]
- Rutto, L.K.; Xu, Y.; Brandt, M.; Ren, S.; Kering, M.K. Juice, Ethanol, and Grain Yield Potential of Five Sweet Sorghum (Sorghum bicolor [L.] Moench) Cultivars. J. Sustain. Bioenergy Syst. 2013, 3, 113–118. [Google Scholar] [CrossRef]
- Ekefre, D.E.; Mahapatra, A.K.; Latimore, M.; Bellmer, D.D.; Jena, U.; Whitehead, G.J.; Williams, A.L. Evaluation of three cultivars of sweet sorghum as feedstocks for ethanol production in the Southeast United States. Heliyon 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.R.A.; Abdelhafez, A.A.; Amer, E.A.M. Evaluation of bioethanol production from juice and bagasse of some sweet sorghum varieties. Ann. Agric. Sci. 2015, 60, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Rocha, M.H.; Capaz, R.S.; Lora, E.E.S.; Nogueira, L.A.H.; Leme, M.M.V.; Renó, M.L.G.; Olmo, O.A. Del Life cycle assessment (LCA) for biofuels in Brazilian conditions: A meta-analysis. Renew. Sustain. Energy Rev. 2014, 37, 435–459. [Google Scholar] [CrossRef]
- Sanz Requena, J.F.; Guimaraes, A.C.; Quirós Alpera, S.; Relea Gangas, E.; Hernandez-Navarro, S.; Navas Gracia, L.M.; Martin-Gil, J.; Fresneda Cuesta, H. Life Cycle Assessment (LCA) of the biofuel production process from sunflower oil, rapeseed oil and soybean oil. Fuel Process. Technol. 2011, 92, 190–199. [Google Scholar] [CrossRef]
- Castanheira, É.G.; Grisoli, R.; Coelho, S.; Anderi Da Silva, G.; Freire, F. Life-cycle assessment of soybean-based biodiesel in Europe: Comparing grain, oil and biodiesel import from Brazil. J. Clean. Prod. 2015, 102, 188–201. [Google Scholar] [CrossRef]
- Plöchl, M.; Heiermann, M.; Linke, B.; Schelle, H. Biogas Crops—Part II: Balance of Greenhouse Gas Emissions and Energy from Using Field Crops for Anaerobic Digestion. Agric. Eng. Int. CIGR Ejournal 2009, XI, 1–11. [Google Scholar]
| Location | Planting Date | Plant Density (plants m−2) | Weeding | Fertilization (kg ha−1) | Irrigation | Tuber Yield (t ha−1) | Reference |
|---|---|---|---|---|---|---|---|
| Spain (38°51′ N) | Late March | 3.8 | Chemical | NPK: 0–0–0 | Irrigated: 100% ETM | 7.1–10.7 DM | [89] |
| Late March | 3.8 | Chemical | NPK: 54–108–162 | Irrigated: 100% ETM | 8.2–11.7 DM | ||
| Late March | 3.8 | Chemical | NPK: 108–216–324 | Irrigated: 100% ETM | 7.7–13.3 DM | ||
| Portugal (39°55′ N) | April | 10.0 | n.i. | NPK: 0–0–0 | Rainfed | 40.0 FM | [90] |
| Italy (40°20′ N) | Late March-Early April | 5.7 | Mechanical | NPK: 150–120–0 | Irrigated: 100% ETM | 80 FM | [19] |
| Portugal (41°48′ N) | Mid-March | 2.0, 3.0, 4.0 | Chemical | NPK: 100–20–40 | Irrigated: 460 mm | 14.9, 12.9, 10.6 DM 29.2, 25.1, 23.9 FM | [86] |
| Mid-March | 2.0 and 4.0 | Mechanical | NPK: 50–15–30 | Rainfed | 7.4 and 7.1 DM 24.4 and 24.1 FM | ||
| Italy (42°42′ N) | Late March | 4.0, 6.0, 8.0 | Mechanical | NPK: 120–100–80 | Rainfed-Irrigated * | 11.7, 15.1, 21.6 DM | [91] |
| Late April | 8.0, 10.0 | Mechanical | NPK: 120–100–80 | Rainfed-Irrigated * | 16.4, 17.8 DM | ||
| Italy (44°30′ N) | Mid-April | 7.0 | Mechanical | NPK: 100–100–0 | Rainfed | 61 FM | [19] |
| Italy (45°2′ N) | Mid-March | 3.6 | Mechanical | NPK: 150–100–100 | Irrigated: 100% ETM | 8–17 DM | [50] |
| Germany (52°17′ N) | Early to mid-April | 4.0 | Mechanical | NPK: 0–70–140 + 21MgO | Irrigated | 8.4–10.9 DM | [92] |
| Early to mid-April | 4.0 | Mechanical | NPK: 60–70–140 + 21MgO | Irrigated | 10.0–12.6 DM | ||
| Early to mid-April | 4.0 | Mechanical | NPK: 120–70–140 + 21MgO | Irrigated | 10.6–12.9 DM | ||
| Sweden (55°38′ N) | Mid-May | 3.3 | n.i. | Compost | n.i. | 30–80 FM | [93] |
| Crop | Feedstock | Ethanol Yield (L ha−1) | References |
|---|---|---|---|
| Jerusalem artichoke | Tubers | 1500–11,000 | [2] and references therein |
| Tops | 2835–11,230 | [2] and references therein; [65] | |
| Sugarcane | Whole crop | 2800–8764 | [111,112,113,114,115] |
| Corn | Grains | 2000–6698 | [112,113,114,116] |
| Stovers | 1258–1767 | [117] | |
| Sugar-beet | Roots | 5000–6000 | [114,118,119] |
| Sweet sorghum | Juice | 532–7619 | [116,118,120,121,122,123] |
| Grains | 2370 | [120] | |
| Bagasse | 5333–10,365 | [120,123] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossini, F.; Provenzano, M.E.; Kuzmanović, L.; Ruggeri, R. Jerusalem Artichoke (Helianthus tuberosus L.): A Versatile and Sustainable Crop for Renewable Energy Production in Europe. Agronomy 2019, 9, 528. https://doi.org/10.3390/agronomy9090528
Rossini F, Provenzano ME, Kuzmanović L, Ruggeri R. Jerusalem Artichoke (Helianthus tuberosus L.): A Versatile and Sustainable Crop for Renewable Energy Production in Europe. Agronomy. 2019; 9(9):528. https://doi.org/10.3390/agronomy9090528
Chicago/Turabian StyleRossini, Francesco, Maria Elena Provenzano, Ljiljana Kuzmanović, and Roberto Ruggeri. 2019. "Jerusalem Artichoke (Helianthus tuberosus L.): A Versatile and Sustainable Crop for Renewable Energy Production in Europe" Agronomy 9, no. 9: 528. https://doi.org/10.3390/agronomy9090528






